ABSTRACT
Approaches to prevent human immunodeficiency virus (HIV-1) transmission are urgently needed. Difficulties in eliciting antibodies that bind conserved epitopes exposed on the unliganded conformation of the HIV-1 envelope glycoprotein (Env) trimer represent barriers to vaccine development. During HIV-1 entry, binding of the gp120 Env to the initial receptor, CD4, triggers conformational changes in Env that result in the formation and exposure of the highly conserved gp120 site for interaction with the coreceptors, CCR5 and CXCR4. The DMJ compounds (+)-DMJ-I-228 and (+)-DMJ-II-121 bind gp120 within the conserved Phe 43 cavity near the CD4-binding site, block CD4 binding, and inhibit HIV-1 infection. Here we show that the DMJ compounds sensitize primary HIV-1, including transmitted/founder viruses, to neutralization by monoclonal antibodies directed against CD4-induced (CD4i) epitopes and the V3 region, two gp120 elements involved in coreceptor binding. Importantly, the DMJ compounds rendered primary HIV-1 sensitive to neutralization by antisera elicited by immunization of rabbits with HIV-1 gp120 cores engineered to assume the CD4-bound state. Thus, small molecules like the DMJ compounds may be useful as microbicides to inhibit HIV-1 infection directly and to sensitize primary HIV-1 to neutralization by readily elicited antibodies.
IMPORTANCE Preventing HIV-1 transmission is a priority for global health. Eliciting antibodies that can neutralize many different strains of HIV-1 is difficult, creating problems for the development of a vaccine. We found that certain small-molecule compounds can sensitize HIV-1 to particular antibodies. These antibodies can be elicited in rabbits. These results suggest an approach to prevent HIV-1 sexual transmission in which a virus-sensitizing microbicide is combined with a vaccine.
INTRODUCTION
Preventing sexual transmission of human immunodeficiency virus type 1 (HIV-1) is critical for altering the course of the global pandemic of AIDS. Currently, approximately 34 million people are living with HIV-1 infection; 2.5 million people are newly infected with the virus annually, and nearly 1.7 million individuals succumb each year to AIDS (1). Hence, there is an urgent need to develop vaccines or other strategies that can prevent HIV-1 transmission.
HIV-1-neutralizing antibodies are an important component of a protective vaccine-induced immune response. Passive administration of HIV-1-neutralizing antibodies protects monkeys from intravenous and mucosal challenge with simian-human immunodeficiency viruses (SHIVs) (2–7). The trimeric envelope glycoprotein (Env) spike on the virion surface is the only HIV-1-specific target accessible to neutralizing antibodies (8–10). The presence of circulating antibodies against a specific region of Env (the gp120 V2 variable region) correlated with the partial protection seen in the RV144 clinical vaccine trial (11–13). Thus, the generation of anti-Env antibodies, particularly neutralizing antibodies, may be critical for a successful HIV-1 vaccine.
The HIV-1 Env spike, which is composed of three gp120 exterior Envs and three gp41 transmembrane Envs, mediates virus entry into host cells (10). The unliganded HIV-1 Env is metastable (14–19). Binding of gp120 to the initial receptor, CD4, triggers Env conformational changes that result in the formation/exposure of two elements: (i) the gp120 binding site for the second receptor, CCR5 or CXCR4, and (ii) the gp41 heptad repeat (HR1) coiled coil (20–29). Binding of gp120 to the CCR5 or CXCR4 coreceptor is thought to induce further Env conformational changes that result in the formation of an energetically stable gp41 six-helix bundle that promotes the fusion of the viral and target cell membranes (18, 19).
As a successful persistent virus, HIV-1 has evolved Env spikes that minimize the elicitation and impact of neutralizing antibodies (10, 30). These features include surface variability, conformational lability, and a heavy coat of glycans (30–34). Most anti-Env antibodies elicited during natural infection do not neutralize HIV-1, and those that do are usually strain restricted, allowing virus escape (30, 35–38). Only after several years of infection in some HIV-1-infected individuals are more broadly neutralizing antibodies generated (37, 39–42). Broadly HIV-1-neutralizing antibodies typically display unusual features that allow binding to the heavily shielded, conserved Env epitopes (39, 43, 44). Some neutralizing antibodies with modest breadth bind Env carbohydrate-dependent epitopes (44–51). The variable and glycosylated features of the HIV-1 Env spike render the elicitation of neutralizing antibodies difficult and have presented extreme challenges to the development of effective Env vaccine immunogens. Even the best current HIV-1 Env immunogens elicit antibodies that inhibit the infection of only the small subset of primary viruses that are more prone to neutralization (44, 52, 53). The sensitivity of HIV-1 strains to antibody neutralization depends upon the integrity of the Env epitope and Env reactivity; the latter property indicates the propensity of unliganded Env to undergo conformational changes (16, 54, 55). A successful HIV-1 vaccine must cover a range of phylogenetically diverse transmitted/founder viruses, most of which have Envs of low reactivity and thus exhibit low sensitivity to neutralization by antibodies (16, 54, 55).
One of the major hurdles facing the development of a successful HIV-1/AIDS vaccine is the requirement to elicit antibodies that recognize conserved elements of the native, unliganded conformation of the HIV-1 Env trimer. These conserved elements are often buried or composed partially or completely of glycans, which render the generation of the cognate antibodies inefficient (30, 32, 33, 44, 56). Two functionally conserved gp120 elements interact with the HIV-1 host cell receptors, CD4 and CCR5 or CXCR4 (57–60). The CD4-binding site (CD4BS) on gp120 is sterically recessed on the HIV-1 Env trimer and surrounded by regions that exhibit interstrain variability and glycosylation (33, 57). Effective neutralizing antibodies directed against the gp120 CD4BS typically engage their epitopes in a manner that does not require the Env trimer to undergo significant conformational changes (16, 61, 62). Indeed, potently neutralizing antibodies directed against multiple conserved HIV-1 Env epitopes generally require minimal conformational change in the unliganded Env trimer for their binding (16).
The vast majority of primary HIV-1 isolates, including transmitted/founder viruses, use CCR5 as a second receptor (24–26, 29, 55). The CCR5-binding site on gp120 consists of a discontinuous surface of the gp120 core and the tip of the V3 loop, both of which are well conserved among primate immunodeficiency viruses (58–60). These elements are not formed and exposed on HIV-1 Env trimers with low envelope reactivity. Antibodies that recognize CD4-induced (CD4i) epitopes in the gp120 core bind near or within the coreceptor-binding site of gp120 (58). Some of these antibodies are specific for CCR5-using HIV-1 variants, whereas other antibodies recognize both CCR5-using and CXCR4-using viruses (63–65). CD4i antibodies are routinely generated in HIV-1-infected humans (66) and can be elicited by HIV-1 gp120 core constructs in which the CD4-bound conformation has been stabilized by disulfide bonds and cavity-filling substitutions (67, 68). Although both the CD4i epitopes and the V3 tip become exposed after HIV-1 binding to cell surface CD4, steric factors (e.g., the target cell membrane) limit the ability of CD4i and V3-directed antibodies to bind their respective epitopes and neutralize the virus (69). Therefore, the neutralizing potency of CD4i and V3-directed antibodies is related to the degree of exposure of these epitopes on the unliganded Env trimer (16, 70). Thus, because of the low Env reactivity of primary and transmitted/founder HIV-1, these viruses are generally inhibited poorly by most CD4i and V3-directed antibodies (16, 54).
There are many ongoing efforts to elicit antibodies that bind the unliganded HIV-1 Env trimer efficiently and neutralize the large fraction of primary transmitted/founder HIV-1 with low Env reactivity (44, 52, 71–73). In this study, we investigated a complementary approach that increases the sensitivity of the HIV-1 virion to antibody neutralization. This approach is based on the observation that induction of the CD4-bound conformation renders primary HIV-1 sensitive to neutralization by CD4i antibodies (66, 70). Hypothetically, any agent that promotes Env conformational changes similar to those induced by CD4 could sensitize HIV-1 to CD4i antibody neutralization. HIV-1 sensitization as a strategy for virus prophylaxis has become feasible as a result of the availability of small-molecule CD4-mimetic compounds. The prototypes of such compounds, NBD-556 and NBD-557, were discovered in a screen for inhibitors of gp120-CD4 interaction (74). NBD-556 and NBD-557 bind in the Phe 43 cavity (75–77), a highly conserved ∼150-Å3 pocket in the gp120 glycoprotein of all HIV-1 strains except those in group O (57). The vestibule of the Phe 43 cavity contains a number of conserved gp120 residues that make critical contacts with CD4 (57). The binding of NBD compounds in the Phe 43 cavity blocks gp120-CD4 interaction and, like the binding of soluble CD4, prematurely triggers the activation of the HIV-1 Env spike (14). The activated state is short-lived (half-life [t1/2] = 5 to 7 min at 37°C), and the bound Env spike rapidly decays into an irreversibly inactivated state (14). Although NBD-556 induces large, entropically unfavorable changes in gp120 conformation (75, 78) and thus binds with only modest affinity (Kd [dissociation constant] = 3 μM), iterative cycles of cocrystallization with gp120 and rational design and synthesis have yielded a number of NBD-556 analogues with improved affinity and antiviral properties (76, 77).
Two compounds, (+)-DMJ-I-228 and (+)-DMJ-II-121 (herein referred to as DMJ compounds), are recently designed and synthesized NBD-556 analogues that have been shown to exhibit improved binding to HIV-1 gp120 and better inhibition of HIV-1 entry (76, 77). The prototypic NBD-556 compound can increase the binding or neutralizing potency of the 17b CD4i antibody weakly and only in laboratory-adapted viruses that have high Env reactivity (15). In contrast, in this report we show that the virus-sensitizing activity of the DMJ compounds is robust and is evident in primary HIV-1 isolates that have low Env reactivity and thus are relatively neutralization resistant. Importantly, we demonstrate the ability of the DMJ compounds to sensitize HIV-1 to neutralization by polyclonal antisera elicited by an engineered gp120 core immunogen.
MATERIALS AND METHODS
Compounds.
All compounds were synthesized as described previously (76, 77, 79). The compounds were analyzed, dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 10 to 20 mM, aliquoted, and stored at −20°C. Each compound was then diluted to 1 mM in serum-free Dulbecco's modified Eagle medium (DMEM) and used for different assays.
Cell lines.
293T human embryonic kidney and Cf2Th canine thymocytes (ATCC) were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum (Sigma) and 100 μg/ml of penicillin-streptomycin (Mediatech, Inc.). Cf2Th cells stably expressing human CCR5 and CD4 were grown in medium supplemented with 0.4 mg/ml of G418 and 0.2 mg/ml of hygromycin (Invitrogen).
Recombinant luciferase viruses.
293T human embryonic kidney cells were cotransfected with plasmids expressing the pCMVΔP1Δenv HIV-1 Gag-Pol packaging construct, the R5 YU2 envelope glycoproteins or the envelope glycoprotein of the control amphotropic murine leukemia virus (A-MLV), and the firefly luciferase-expressing vector at a DNA ratio of 1:1:3 μg using the Effectene transfection reagent (Qiagen). Cotransfection produced recombinant, luciferase-expressing viruses capable of a single round of infection. The virus-containing supernatants were harvested 36 to 40 h after transfection, spun, aliquoted, and frozen at −80°C until further use. The reverse transcriptase (RT) activities of all viruses were measured as described previously (80).
Infection by single-round luciferase viruses.
Cf2Th-CCR5-CD4 target cells were seeded at a density of 6 × 103 cells/well in 96-well luminometer-compatible tissue culture plates (PerkinElmer) 24 h before infection. On the day of infection, NBD-556, (+)-DMJ-I-228, or (+)-DMJ-II-121 (0 to 100 μM) was incubated with recombinant viruses (10,000 RT units) at 37°C for 30 min. In the case of sensitization assays, a constant concentration of compounds was incubated with virus for 30 min at 37°C; then, 17b or other antibodies (0 to 100 μg/ml) were added to the virus-compound mixture and incubated for an additional 30 min at 37°C. The mixtures were then added to the target cells and incubated for 48 h at 37°C; after this time, the medium was removed from each well, and the cells were lysed by the addition of 30 μl of passive lysis buffer (Promega) and three freeze-thaw cycles. An EG&G Berthold LB 96V microplate luminometer was used to measure the luciferase activity of each well after the addition of 100 μl of luciferin buffer (15 mM MgSO4, 15 mM KPO4 [pH 7.8], 1 mM ATP, and 1 mM dithiothreitol) and 50 μl of 1 mM firefly d-luciferin free acid, 99% (Prolume).
Stable core gp120 design.
Based upon available structures of the HIV-1 gp120 core containing the entire V3 region (59), the original gp120 core (57) was redesigned with additional internal changes to stabilize the coreceptor-binding region, as previously described (68). To reduce conformational flexibility and lock the gp120 core into the receptor-bound state, two tactics were used: filling hydrophobic pockets of the core and adding interdomain disulfide pairs. For the first tactic, cavity-filling or “F” changes (T257S and S375W) were designed to fill the Phe 43 cavity; other gp120 cavity-filling substitutions (M95W and A433M) were also included in the stabilized gp120 cores. Four interdomain cysteine pairs (disulfides [DS], or “CC” mutations) were introduced to lock the core into the CD4-bound, coreceptor-binding conformation. These cysteine substitutions specifically involve residues 96 to 275 (DS1; 1st CC), 109 to 428 (DS2; 2nd CC), 123 to 431 (DS3; 3rd CC), and 231 to 267 (DS4; 4th CC). The 2CC gp120 core contains DS1 and DS2; the 3CC gp120 core contains DS1, DS2, and DS3; and the 4CC gp120 core contains all four internal cysteine pairs.
To enhance protein folding and expression, the V1/V2 stem was trimmed and residues were added back to the V3 base β-strands to result in the new V3S unmodified core and the corresponding stable cores 2CC, 3CC, and 4CC (68). To focus the immune response onto the conserved coreceptor-binding site, the immunodominant V1/V2 and V3 hypervariable regions were removed as described below. Previous studies demonstrated that hypervariable region truncations were possible without compromising CD4 binding (81); however, structural analysis suggested that more optimal designs were feasible. The structure of the gp120 core with an intact V3 loop (59) showed that the previously published Gly-Ala-Gly substitution of V3 residues 298 to 329 (to accomplish deletion of V3) removed four hydrogen bonds from β-strand 12 and five hydrogen bonds from β-strand 13. A new substitution (V3S) that retained these hydrogen bonds and added a longer linker was modeled (68). Further structural analysis indicated that additional trimming of the flexible V1/V2 region to eliminate a naturally occurring cysteine pair might facilitate accommodation of additional pairs of stabilizing cysteines elsewhere in the molecule. Accordingly, a more minimal loop (V1/V2b) was modeled with a type II turn connecting strands β2 and β3, replacing nine residues (CVGAGSCNT) with an Ala-Gly-Ala tripeptide.
Protein expression, purification, and characterization.
The stable gp120 cores were expressed by transient transfection of the pcDNA 3.1(−) expression vector into suspended HEK293T cells in serum-free medium (Life Technologies). The stable gp120 cores were purified by 17b affinity columns to a high level of homogeneity, as previously described (68). The folding of the purified stable gp120 cores was assessed by enzyme-linked immunosorbent assay (ELISA), surface plasmon resonance (SPR), and isothermal titration calorimetry (ITC) with the conformational ligands sCD4, 17b, and b12 (68). Protein purity and molecular mass were determined by SDS-PAGE analysis followed by Coomassie blue staining, as well as blue native gel electrophoresis and size exclusion chromatography.
Animal inoculations and analysis of antisera.
Approximately 12-week-old female New Zealand White rabbits were housed at the AAALAC-accredited facilities at Bioqual (Rockville, MD) under specific-pathogen-free conditions. At 4-week intervals, rabbits were inoculated intramuscularly with 50 mg of affinity-purified protein formulated in GlaxoSmithKline adjuvant system AS01B by splitting the protein-adjuvant mix in the two hind legs. Serum was prepared, heat inactivated, and assessed for anti-gp120 ELISA titers and the presence of neutralizing antibodies, as previously described (68, 82).
ITC.
Thermodynamic parameters for the binding of the different inhibitors to gp120 were obtained by ITC using a VP-ITC microcalorimeter from MicroCal/GE Healthcare (Northampton, MA). The titrations were performed at 25°C by injecting 10-μl aliquots of inhibitor solution into the calorimetric cell (volume, ∼1.4 ml) containing gp120 at a concentration of 2 μM. The inhibitor concentration in the syringe was 40 to 60 μM except for NBD-556, which was prepared at a concentration of 125 μM. In all titration experiments, gp120 and the different inhibitors were equilibrated with phosphate-buffered saline (PBS), pH 7.4, with 2% DMSO. The heat evolved upon each injection of inhibitor was obtained by integration of the calorimetric signal. The heat associated with inhibitor binding to gp120 was obtained by subtracting the heat of dilution from the heat of reaction. The enthalpy change (ΔH) and association constant (Ka = 1/Kd) were obtained by nonlinear regression of the data.
Cold inactivation.
Recombinant virus (10,000 RT units) was incubated with either a fixed concentration of the NBD-556 analogues (50 μM for viruses with JR-FL and A-MLV Envs and 20 μM for viruses with YU2 Env) or DMSO on ice at 4°C for the following times: 0, 2, 4, 8, 24, and 48 h. Cf2Th-CCR5-CD4 target cells were seeded at a density of 6 × 103 cells/well in a 96-well luminometer-compatible tissue culture plate (PerkinElmer) 24 h before infection. The mixtures were then added to target cells and incubated for 48 h at 37°C; after this, the medium was removed from each well, and the cells were lysed by the addition of 30 μl of passive lysis buffer (Promega) and three freeze-thaw cycles. An EG&G Berthold LB 96V microplate luminometer was used to measure the luciferase activity of each well after the addition of 100 μl of luciferin buffer (15 mM MgSO4, 15 mM KPO4 [pH 7.8], 1 mM ATP, and 1 mM dithiothreitol) and 50 μl of 1 mM firefly d-luciferin free acid, 99% (Prolume).
RESULTS
Characterization of small-molecule CD4-mimetic compounds.
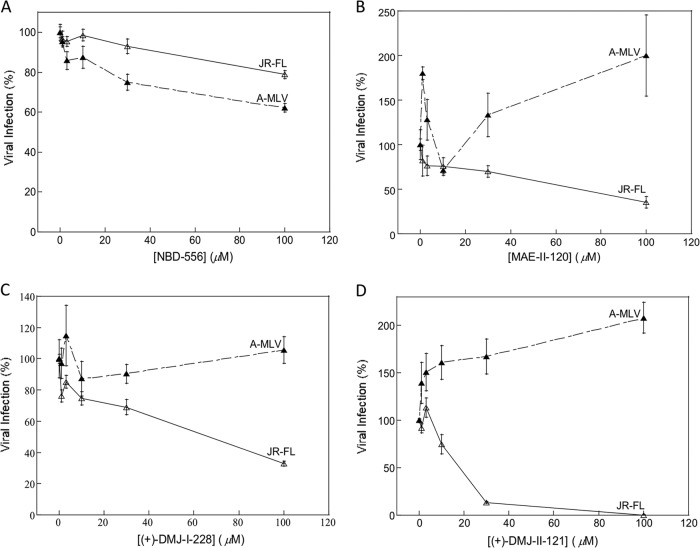
We examined the ability of selected CD4-mimetic compounds to inhibit HIV-1 entry. Two recently identified DMJ compounds, (+)-DMJ-I-228 and (+)-DMJ-II-121 (76, 77), were compared to the parental NBD-556 and an earlier analogue, (±)-MAE-II-120. Recombinant HIV-1 expressing the firefly luciferase gene was pseudotyped with different envelope glycoproteins, either HIV-1 JR-FL Env or, as a control, the amphotropic murine leukemia virus (A-MLV) envelope glycoproteins. The recombinant viruses were incubated with cells expressing CD4 and CCR5 in the presence of different concentrations of the compounds. The DMJ compounds specifically inhibited HIV-1 JR-FL, with an improved potency relative to that of the parental NBD-556 compound (Table 1 and Fig. 1).
TABLE 1.
Properties of small-molecule CD4-mimetic compounds
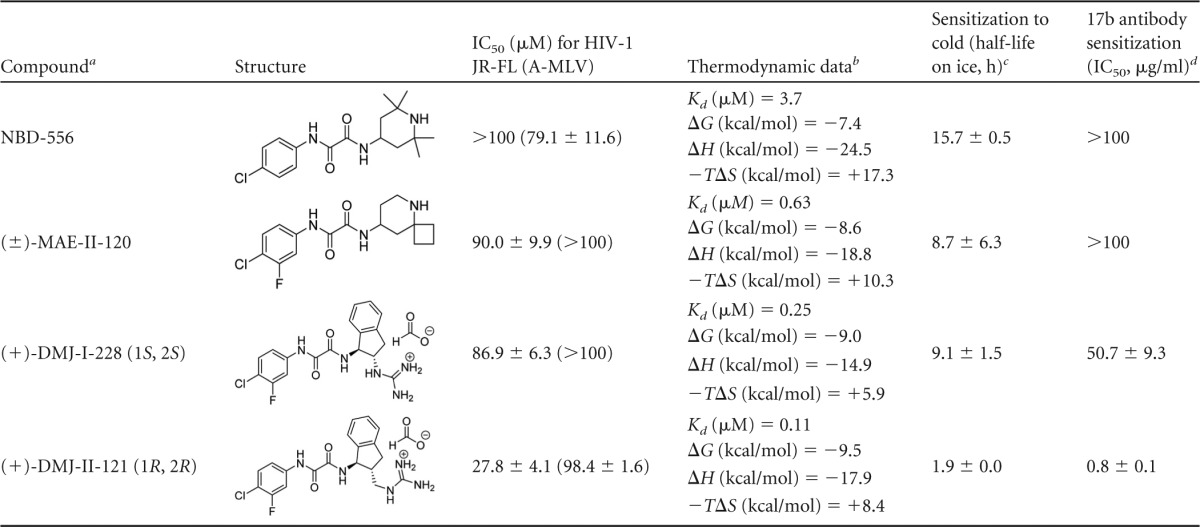
R and S designation refers to the absolute stereochemical configuration at the 1 and 2 positions of the indane ring system in the compound.
The thermodynamic data were determined by isothermal titration calorimetry at 25°C.
The half-life of HIV-1 JR-FL infectivity on ice in the presence of the CD4-mimetic compound (50 μM) is shown. The half-life of HIV-1 JR-FL on ice in DMSO was > 48 h.
The IC50 of the 17b antibody, in μg/ml, is shown for neutralization of HIV-1 JR-FL in the presence of a 50 μM concentration of the compound.
FIG 1.
Effects of NBD-556 and analogues on HIV-1 infection. The infection of Cf2Th-CCR5-CD4 cells by recombinant HIV-1 expressing firefly luciferase was measured in the presence of the indicated concentrations of NBD-556 (A), (±)-MAE-II-120 (B), (+)-DMJ-I-228 (C), and (+)-DMJ-II-121 (D). The viruses contained the HIV-1 JR-FL or A-MLV envelope glycoproteins. The values are represented as a percentage of the level of target cell luciferase observed for each virus in the absence of the compound. The means and standard deviations of the results obtained in triplicate samples are shown. The results are representative of those obtained in 3 to 21 independent experiments.
The thermodynamics of the binding of each compound to the wild-type HIV-1 YU2 gp120 glycoprotein was analyzed by isothermal titration calorimetry. Relative to NBD-556, the DMJ compounds exhibited significant improvement in the ability to bind monomeric gp120 (Table 1). This increase in gp120 binding affinity is largely due to the fact that the entropic contribution to the binding affinity is less unfavorable than for NBD-556 (Table 1). Binding of CD4 and NBD-556 to gp120 is associated with an unusually large enthalpy change (ΔH) that is balanced by a large unfavorable entropic contribution (−TΔS) to the Gibbs free energy of binding (78, 83). This thermodynamic signature results from large-scale conformational structuring and fixation of gp120 by CD4 and NBD-556. Relative to the binding of NBD-556, the binding of the DMJ compounds apparently introduces less order in monomeric gp120.
Previous studies suggest that an increased propensity of the HIV-1 envelope glycoproteins to sample the CD4-bound conformation is associated with increased sensitivity to inactivation following incubation in the cold (84). We examined the half-life on ice of recombinant HIV-1 JR-FL incubated with different NBD-566 analogues. The half-life on ice of HIV-1 JR-FL incubated with DMSO was >48 h. Incubation of HIV-1 JR-FL with the parental NBD-556 compound and earlier analogue (±)-MAE-II-120 resulted in half-lives in the cold of 15.7 and 8.7 h, respectively (Table 1). Incubation with the (+)-DMJ-I-228 and (+)-DMJ-II-121 compounds increased the sensitivity of HIV-1 JR-FL to cold, with half-lives of 9.1 and 1.9 h, respectively (Table 1). Similar results were obtained with HIV-1 YU2 (data not shown). These observations suggest that the DMJ compounds sensitize HIV-1 to cold inactivation and are consistent with the induction of the CD4-bound state in Env by the DMJ compounds.
DMJ compounds sensitize HIV-1 to neutralization by CD4-induced and V3-directed antibodies.
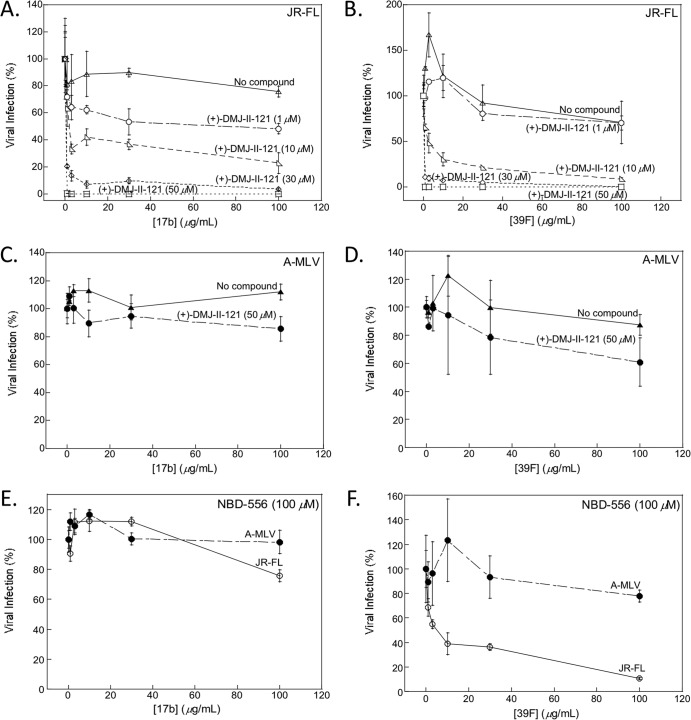
We tested the ability of the DMJ compounds to sensitize HIV-1 to neutralization by antibodies, using the single-round infection of Cf2Th-CCR5-CD4 cells by recombinant HIV-1 encoding firefly luciferase. The recombinant viruses were pseudotyped with the HIV-1 Envs derived from the primary R5 HIV-1 JR-FL and YU2 isolates. Recombinant HIV-1 pseudotyped with the A-MLV Env was used as a control for specificity. HIV-1 neutralization was examined in the presence of subneutralizing concentrations of the CD4-mimetic compounds and different concentrations of monoclonal antibodies. Figure 2 shows the results for antibodies that recognize epitopes known to be induced and/or exposed upon CD4 binding. The 17b antibody recognizes a well-conserved CD4i epitope on the HIV-1 gp120 core that is not formed or exposed in the unliganded Env of primary HIV-1 strains (63, 66, 70). The 39F antibody recognizes a conformation-dependent epitope in the gp120 V3 region of primary R5 HIV-1 (85). In the absence of the DMJ compounds, neither monoclonal antibody inhibited primary HIV-1 infection at concentrations up to 100 μg/ml (Fig. 2A and B and Table 2). (+)-DMJ-II-121 sensitized HIV-1 JR-FL to neutralization by both the 17b and 39F monoclonal antibodies (Fig. 2A and B). For example, in the presence of 30 μM (+)-DMJ-II-121, the 17b antibody inhibited HIV-1 JR-FL entry into cells coexpressing CD4 and CCR5 with a 50% inhibitory concentration (IC50) of 0.6 μg/ml (Fig. 2A). This sensitization was dependent on the (+)-DMJ-II-121 concentration and was specific to HIV-1; there was no significant inhibition of A-MLV by either 17b or 39F antibody in the presence of (+)-DMJ-II-121 (Fig. 2C and D). Both DMJ compounds sensitized HIV-1 YU2 and JR-FL to neutralization by the 17b and 39F antibodies (Fig. 2 and Tables 2 and 3). Only at high concentrations did the parental compound NBD-556 weakly increase the neutralization of HIV-1 JR-FL by the 39F antibody; NBD-556 minimally affected HIV-1 JR-FL neutralization by the 17b antibody (Fig. 2E and F). We conclude that the DMJ compounds render primary HIV-1 isolates sensitive to neutralization by monoclonal antibodies directed against CD4i and V3 epitopes on the gp120 Env.
FIG 2.
(+)-DMJ-II-121 sensitizes HIV-1 JR-FL to neutralization by the 17b and 39F antibodies. (A and B) The neutralization of HIV-1 JR-FL by the CD4-induced antibody 17b (A) and the V3-directed antibody 39F (B) in the presence of the indicated concentrations of (+)-DMJ-II-121 is shown. (C and D) Neutralization of virus with the A-MLV envelope glycoprotein by the 17b (C) or 39F (D) antibody in the presence or absence of 50 μM (+)-DMJ-II-121 is shown. (E and F) Infection by viruses with HIV-1 JR-FL or A-MLV envelope glycoproteins in the presence of the indicated concentrations of 17b (E) or 39F (F) antibody and 100 μM NBD-556. All viral infection data shown are normalized to the level of infection seen in the absence of antibody at the indicated concentrations of (+)-DMJ-II-121 (A to D) or NBD-556 (E and F). The means and standard deviations of the results obtained in triplicate samples are shown. The results are representative of those obtained in 4 to 10 independent experiments.
TABLE 2.
Inhibitory concentrations of the 17b and 39F antibodies for HIV-1 JR-FL, HIV-1 YU2, and A-MLVa
| Antibody or combination | IC50 (μg/ml) for virus |
|||
|---|---|---|---|---|
| JR-FL | YU2 | YU2 S375W | A-MLV | |
| 17b | >100 (n = 23) | >100 (n = 2) | >100 (n = 2) | >100 (n = 25) |
| 39F | >100 (n = 4) | >100 (n = 2) | ND | >100 (n = 4) |
| 17b + 50 μM (+)-DMJ-I-228 | 50.7 ± 9.3 (n = 4) | 18.9 ± 8.4 (n = 4) | >100 (n = 2) | >100 (n = 4) |
| 17b + 50 μM (+)-DMJ-II-121 | 0.8 ± 0.1 (n = 20) | <1 (n = 2) | >100 (n = 1) | >100 (n = 20) |
| 39F + 100 μM (+)-DMJ-I-228 | 0.6 ± 0.1 (n = 2) | <1 (n = 4) | ND | >100 (n = 2) |
| 39F + 50 μM (+)-DMJ-II-121 | 0.5 ± 0.0 (n = 4) | <1 (n = 2) | ND | >100 (n = 4) |
The IC50s of the 17b and 39F antibodies for the indicated viruses in the absence or presence of the indicated concentrations of the DMJ compounds are reported. In pilot experiments, (+)-DMJ-I-228 inhibited HIV-1 JR-FL and HIV-1 YU2 infections with IC50s of 86.9 and 46.7 μM, respectively. (+)-DMJ-II-121 inhibited HIV-1 JR-FL and HIV-1 YU2 with IC50s of 27.8 and 3.6 μM, respectively. Neither DMJ compound inhibited A-MLV infection at 100 μM. The number of experiments is shown in parentheses. ND, not determined.
TABLE 3.
Inhibitory concentrations of antibodies for infection by HIV-1 JR-FL and A-MLV in the absence or presence of 50 μM (+)-DMJ-II-121a
| Antibody | Antibody group | IC50 (μg/ml) for virus at indicated (+)-DMJ-II-121concn |
|||
|---|---|---|---|---|---|
| JR-FL |
A-MLV |
||||
| 0 μM | 50 μM | 0 μM | 50 μM | ||
| 17b | CD4i | >100 | 0.6 ± 0.0 | >100 | 84.7 ± 8.5 |
| CH08 | CD4i | >100 | 1.7 | >100 | >100 |
| VC827 | CD4i | >100 | >100 | >100 | >100 |
| VCT16 | CD4i | >100 | 0.7 | >100 | >100 |
| VCT39 GH | CD4i | >100 | 0.7 | >100 | >100 |
| A32 | C1–C4 | >100 | >100 (80 μM) | >100 | >100 (80 μM) |
| 39F | V3 | >100 | 0.5 ± 0.0 | >100 | >100 |
| 1.4E | V3 | >100 | 0.5 | >100 | >100 |
| 2.1E | V3 | >100 | 0.5 | >100 | >100 |
| CH21 | gp120 conformational | >100 | 25.3 | >100 | >100 |
| F105 | CD4BS | >100 | >100 (80 μM) | >100 | >100 (80 μM) |
| VRC01 | CD4BS | 0.5 | 0.5 | >100 | >100 |
| 2G12 | Glycan | 0.9 ± 0.1 | 1.7 ± 0.3 | 76.9 ± 23.1 | 6.9 ± 3.2 |
| PGT128 | Glycan | 0.3 ± 0.2 | 0.3 ± 0.2 (80 μM) | >100 | 72.4 ± 27.6 (80 μM) |
| PG9 | V1/V2/V3 | >100 | 8.3 ± 0.0 | >100 | 6.7 |
In some experiments, 80 μM (+)-DMJ-II-121 was used, as indicated in parentheses.
Substitution of gp120 Ser 375 with a tryptophan residue fills the Phe 43 cavity (68); this substitution slightly enhances CD4 binding but disrupts the binding of all NBD-556 analogues, including the DMJ compounds. Although the S375W HIV-1 variant is not found in nature, it is useful as a negative control. None of the tested NBD-556 analogues sensitized HIV-1 JR-FL S375W to neutralization by 17b and 39F (Table 2). These results indicate that gp120 binding by the DMJ compounds is critical for their ability to sensitize HIV-1 to neutralization by these antibodies.
We examined the ability of (+)-DMJ-II-121 to sensitize HIV-1 JR-FL to neutralization by a panel of anti-HIV-1 Env monoclonal antibodies (Table 3). The Env epitopes recognized by many of these antibodies have been characterized previously (45, 86, 87). The following antibodies were tested: the 17b, CH08, VC827, VCT16, and VCT39 GH antibodies, which recognize CD4-induced epitopes overlapping the CCR5-binding site (63, 88); the A32 antibody, which recognizes a discontinuous CD4-induced epitope in the C1 and C4 conserved regions of gp120 that does not overlap the CCR5-binding site; antibodies (39F, 1.4E, and 2.1E) directed against the V3 region; antibodies (PGT128 and 2G12) that bind glycan-dependent gp120 outer domain epitopes; and an antibody (PG9) that recognizes quaternary-structure-dependent gp120 epitopes in the V1/V2/V3 region. The neutralization potencies of the CD4i antibodies 17b, CH08, VCT16, and VCT39 GH antibodies increased dramatically when HIV-1 was incubated with 50 μM (+)-DMJ-II-121 (Table 3 and Fig. 3D and E). In addition, (+)-DMJ-II-121 also specifically sensitized HIV-1 JR-FL to neutralization by antibodies directed against the gp120 V3 region, as well as antibody CH21, whose discontinuous gp120 epitope is not yet determined (89). Whereas the CD4-induced and V3 region-directed antibodies specifically neutralized HIV-1 in the presence of (+)-DMJ-II-121, the glycan-directed 2G12 antibody and V1/V2/V3 region-directed PG9 antibody nonspecifically inhibited HIV-1 and the A-MLV negative control in the presence of 50 μM (+)-DMJ-II-121 (Table 3). Together, these results indicate that the DMJ compounds specifically enhance the virus-neutralizing potency of some antibodies targeting HIV-1 Env epitopes that are induced by CD4 binding.
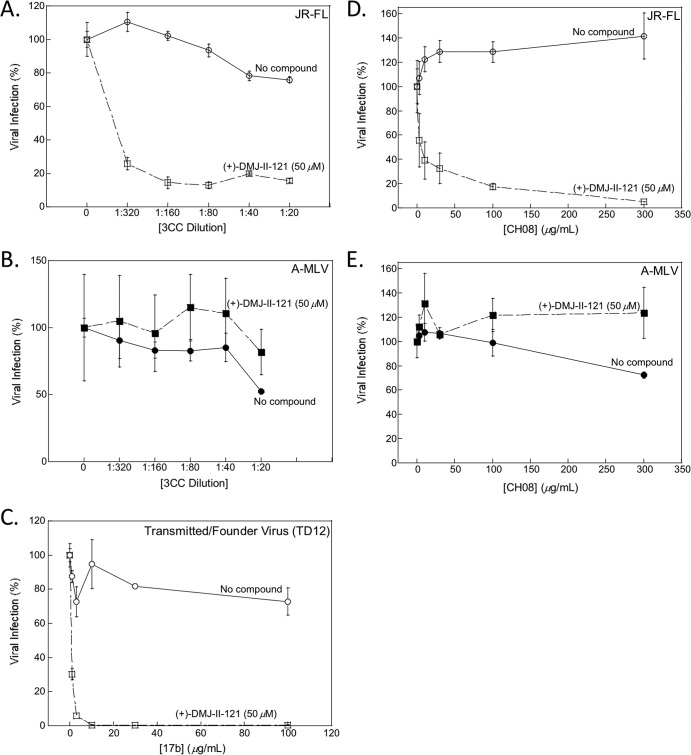
FIG 3.
(+)-DMJ-II-121 sensitization of HIV-1 to neutralization by a CD4-induced antibody from an HIV-1-infected individual and a rabbit antiserum elicited by the 3CC stabilized HIV-1 gp120 core. A concentration of (+)-DMJ-II-121 that reduced infection of HIV-1 JR-FL by approximately 50% was used in these experiments. The effects of increasing concentrations of rabbit antiserum elicited by the 3CC stabilized gp120 core on infection by HIV-1 JR-FL (A) or the A-MLV-pseudotyped HIV-1 control (B) are shown. The infection of the B5 transmitted/founder virus in the presence of the indicated concentrations of the 17b antibody, in the absence and presence of 50 μM (+)-DMJ-II-121, is shown (C). The effects of increasing concentrations of the CD4-induced antibody CH08, which was derived from an HIV-1-infected individual (88), on infection by HIV-1 JR-FL (D) and the A-MLV-pseudotyped HIV-1 control (E), in the absence and presence of 50 μM (+)-DMJ-II-121, are shown. All viral infection data shown are normalized to the level of infection seen in the absence of antibody at a 50 μM concentration of (+)-DMJ-II-121. The means and standard deviations of the results obtained in triplicate samples are shown. The results are representative of those obtained in 2 to 20 independent experiments.
DMJ compounds sensitize HIV-1 to neutralization by antisera elicited by an Env immunogen.
The DMJ compounds were examined to determine if they could sensitize HIV-1 to neutralization by antisera elicited by an Env immunogen. Previous studies demonstrated that CD4i antibodies could be elicited in rabbits immunized with gp120 cores that were modified to stabilize the CD4-bound conformation (68). These stabilized gp120 cores were derived from the laboratory-adapted HIV-1 HXBc2 strain and lack the V1, V2, and V3 variable regions. X-ray crystal structures of gp120 core/two-domain CD4/X5 Fab complexes (59) were used to guide modification of the variable region deletions, substitution of cavity-filling residues, and introduction of two, three, and four potential disulfide bonds (in the respective 2CC, 3CC, and 4CC stabilized cores) (68). In immunized rabbits, the stabilized gp120 cores elicited CD4i antibodies according to the following hierarchy: 4CC > 3CC > 2CC > a core lacking additional disulfide bonds (the V3S core) (68). The ability of (+)-DMJ-II-121 to sensitize HIV-1 JR-FL to neutralization by immune sera from these rabbits was tested. Preimmune sera were also tested, as negative controls. (+)-DMJ-II-121 sensitized HIV-1 JR-FL to neutralization by sera from rabbits immunized with either the 3CC or 4CC stabilized gp120 cores (Table 4 and Fig. 3A). Control recombinant HIV-1 pseudotyped with A-MLV Env was not neutralized by any of the sera in the presence or the absence of (+)-DMJ-II-121 (Fig. 3B). Preimmune sera from each rabbit were used as controls and did not exhibit any neutralization (Table 4). Three of the five sera from rabbits immunized with the 2CC gp120 core neutralized HIV-1 JR-FL only in the presence of (+)-DMJ-II-121 (Table 4). None of the rabbit sera that were elicited by inoculation with the nonstabilized V3S core neutralized HIV-1 JR-FL after incubation with (+)-DMJ-II-121 (Table 4). Thus, neutralization of HIV-1 JR-FL after sensitization by (+)-DMJ-II-121 correlated with the previously reported levels of CD4i antibodies elicited by the different stabilized gp120 cores (68). The 3CC and 4CC gp120 cores better approximate the CD4-bound conformation and more efficiently elicit CD4i antibodies that can be potentiated by (+)-DMJ-II-121 for HIV-1 neutralization.
TABLE 4.
Neutralization titers of sera from rabbits immunized with stabilized and unmodified gp120 cores, tested against HIV-1 JR-FL in the absence or presence of (+)-DMJ-II-121
| Immunogen | Inoculationsb | HIV-1 JR-FL neutralization titer (50% neutralization)a |
|
|---|---|---|---|
| No compound | (+)-DMJ-II-121 (50 μM) | ||
| V3S core (unmodified) | Preimmune | >1:20 (5/5) | >1:20 (5/5) |
| 6 | >1:20 (5/5) | >1:20 (5/5) | |
| 2CC | Preimmune | >1:20 (5/5) | >1:20 (5/5) |
| 6 | >1:20 (5/5) | 1:160 (1/5), 1:80 (1/5), 1:40 (1/5), >1:20 (2/5) | |
| 3CC | Preimmune | >1:20 (4/4) | >1:20 (4/4) |
| 6 | >1:20 (5/5) | 1:320 (3/5), 1:80 (2/5) | |
| 4CC | Preimmune | >1:20 (5/5) | >1:20 (5/5) |
| 4 | >1:20 (5/5) | 1:320 (2/5), 1:80 (2/5), >1:20 (1/5) | |
| 6 | >1:20 (5/5) | 1:320 (1/5), 1:80 (4/5) | |
The numbers in parentheses indicate the numbers of rabbits with the reported serum titer/total number of rabbits in the group.
The numbers indicate the numbers of times the animals were inoculated.
DMJ compounds sensitize HIV-1 transmitted/founder viruses to neutralization by CD4-induced 17b antibody and antisera elicited by a 3CC gp120 core immunogen.
The ability of (+)-DMJ-II-121 to sensitize HIV-1 transmitted/founder viruses to neutralization by the 17b antibody (Table 5) and antisera elicited by the 3CC gp120 core immunogen (Table 6) was examined. In these experiments, the recombinant viruses were pseudotyped with the HIV-1 Envs derived from HIV-1 transmitted/founder viruses (55). Recombinant HIV-1 pseudotyped with the primary R5 HIV-1 JR-FL and the A-MLV Envs served as positive and negative controls, respectively. HIV-1 neutralization was examined in the presence of subneutralizing concentrations of (+)-DMJ-II-121 and different concentrations of the 17b antibody or sera.
TABLE 5.
Inhibitory concentrations of (+)-DMJ-II-121 and the 17b antibody (alone and in the presence of (+)-DMJ-II-121), tested against HIV-1 JR-FL, transmitted/founder viruses, and A-MLVa
| Virus | IC50 of: |
||
|---|---|---|---|
| (+)-DMJ-II-121 (μM) | 17b (μg/ml) | 17b + 50 μM (+)-DMJ-II-121 (μg/ml) | |
| JR-FL | 48.8 ±7.8 | >100 | 0.6 ± 0.0 |
| 4F12 | 4.5 ± 2.2 (n = 2) | >100 (n = 2) | 25.2 ± 24.2 (n = 2) |
| TC4 | 0.9 ± 0.3 | 67.4 ± 32.6 | 34.0 ± 33.0 |
| TD12 | 7.1 ± 2.2 | >100 | <1 |
| TA11 | 3.3 ± 2.7 (n = 2) | >100 (n = 2) | 3.7 ± 2.7 (n = 2) |
| D3 | 59.3 ± 15.9 (n = 2) | >100 (n = 2) | 34.2 ± 33.2 (n = 2) |
| B5 | 46.4 ± 27.1 | 70.6 ± 29.4 | 28.3 ± 24.0 |
| 2010 F5 | 67.4 ± 32.6 | >100 | >100 |
| 0363 C3 | 57.9 ± 24.9 | 93.8 ± 6.2 | 76.9 ± 23.2 |
| 1176 A3 | 63.3 ± 15.9 | 21.4 ± 7.3 | 12.2 ± 6.7 |
| 3A1 | 71.4 ± 14.4 | 96.4 ± 3.6 | 62.4 ± 23.1 |
| TA5 | >100 | 78.1 ± 14.7 | >100 |
| A-MLV | >100 | >100 | >100 |
The IC50s of (+)-DMJ-II-121 and the 17b antibody [alone and in the presence of 50 μM (+)-DMJ-II-121] are reported. The number of experiments is shown in parentheses; unless indicated, the reported IC50 is derived from 3 to 5 independent experiments.
TABLE 6.
Inhibitory concentrations of preimmune sera and sera from rabbits immunized with the 3CC stabilized gp120 core, tested against HIV-1 JR-FL, transmitted/founder viruses, and A-MLV in the presence or absence of (+)-DMJ-II-121
| Virus | IC50 (dilution of serum) |
|||
|---|---|---|---|---|
| Preimmune serum | Stabilized gp120 core sera (3CC) | Preimmune serum + 50 μM (+)-DMJ-II-121 | 3CC sera + 50 μM (+)-DMJ-II-121 | |
| JR-FL | >1:20 | >1:20 | >1:20 | 1:180 |
| TD12 | >1:20 | 1:30 | >1:20 | > 1:320 |
| B5 | >1:20 | >1:20 | >1:20 | 1:160 |
| 3A1 | >1:20 | >1:20 | >1:20 | >1:20 |
| TA5 | >1:20 | >1:20 | >1:20 | 1:180 |
| A-MLV | >1:20 | >1:20 | >1:20 | >1:20 |
The inhibition of the transmitted/founder viruses by (+)-DMJ-II-121 or the 17b antibody alone and the ability of (+)-DMJ-II-121 to sensitize the transmitted/founder viruses to neutralization by the 17b antibody are reported in Table 5. In the absence of (+)-DMJ-II-121, with one exception (1176 A3), the transmitted/founder viruses were negligibly inhibited by the 17b antibody. Of the ten 17b-resistant transmitted/founder HIV-1 isolates, (+)-DMJ-II-121 sensitized six viruses to neutralization by the 17b antibody. These viruses were also sensitive to inhibition by (+)-DMJ-II-121 alone. Three of the transmitted/founder viruses tested were sensitive to (+)-DMJ-II-121 inhibition but were not sensitized by (+)-DMJ-II-121 to 17b neutralization. One transmitted/founder virus (TA5) did not exhibit sensitivity to (+)-DMJ-II-121 alone and was not sensitized to 17b neutralization in the presence of (+)-DMJ-II-121. Thus, a significant fraction of transmitted/founder HIV-1 isolates is sensitive to inhibition by (+)-DMJ-II-121 and can be sensitized to 17b neutralization by this compound.
Four of the transmitted/founder viruses were tested using antisera elicited by the 3CC gp120 core immunogen (Table 6). Two of the transmitted/founder viruses (TD12 and B5) that were sensitive to (+)-DMJ-II-121 inhibition also exhibited increased sensitivity to neutralization by 3CC antisera in the presence of (+)-DMJ-II-121. Unexpectedly, one of the transmitted/founder viruses (TA5) that was not inhibited efficiently by (+)-DMJ-II-121 was neutralized by 3CC-elicited antisera in the presence of (+)-DMJ-II-121. Preimmune sera did not neutralize any of the transmitted/founder viruses in the presence or the absence of (+)-DMJ-II-121. There was no significant inhibition of A-MLV by either 17b or immunized sera in the presence of (+)-DMJ-II-121 (Tables 5 and 6). We conclude that (+)-DMJ-II-121 is able to sensitize a substantial fraction of transmitted/founder HIV-1 isolates to neutralization by antisera elicited by a 3CC gp120 core immunogen.
DISCUSSION
An attractive strategy for preventing HIV-1 acquisition is to generate antibodies in an uninfected individual that potently neutralize a wide range of transmitted/founder HIV-1 variants. Both viral and antibody factors determine HIV-1 neutralization efficiency (16). Transmitted/founder viruses generally exhibit low Env reactivity and thus are relatively resistant to neutralization (16, 54, 55). Antibodies that effectively neutralize these low-reactivity viruses must bind the unliganded Env trimer efficiently, without requiring significant conformational changes in Env (16, 62). The many ongoing efforts to elicit such neutralizing antibodies have yet to succeed (44, 52, 71–73). In this work, we investigated an approach that increases the sensitivity of the HIV-1 virion to some neutralizing antibodies. The approach takes advantage of (i) the natural tendency of HIV-1 Env to make the transition from the unliganded state to the CD4-bound state (17), (ii) the highly conserved nature of the gp120 binding sites for CD4 and CCR5 (57, 58), (iii) the vulnerability to antibody neutralization of the CD4-bound state of Env on a virus that is distant from the target membrane (69, 70), and (iv) the availability of small-molecule CD4-mimetic compounds that exhibit sufficient affinity and breadth (76, 77).
Compared with the parental NBD-556 compound, the newly characterized DMJ compounds bind gp120 with higher affinity, block gp120-CD4 binding more efficiently, and inhibit HIV-1 infection with lower IC50s (76, 77). NBD-556 has been shown to increase the binding of the 17b CD4i antibody to gp120 (78) and has been reported to increase weakly the ability of the 17b CD4i antibody to neutralize laboratory-adapted viruses (15), which have high envelope reactivity (54). Here we demonstrate that the DMJ compounds sensitize primary HIV-1 isolates, which have low envelope reactivity and are relatively neutralization resistant, to inhibition by specific anti-gp120 antibodies. Moreover, we found that in the presence of (+)-DMJ-II-121, multiple primary HIV-1 isolates, including transmitted/founder HIV-1, were sensitive to neutralization by the 17b antibody or antisera elicited by a 3CC gp120 core immunogen. Importantly, the observed sensitization seen with the viruses was dependent on the binding of the DMJ compounds to the viral Env. The DMJ compounds, like their NBD predecessors, do not bind to the S375W variant of HIV-1 Env, where the Phe 43 cavity is filled (67) and therefore unavailable for compound binding. Sensitization of HIV-1 to neutralization by antibodies apparently requires sufficient affinity of the CD4-mimetic compound for Env. In contrast to NBD-556, the DMJ compounds contact the conserved gp120 residue, Asp 368, which makes important contributions to CD4 binding (57). (+)-DMJ-II-121 also has an additional interaction with Met 426 (77). Recent results from alanine scanning have shown that interactions with different residues in gp120 contribute differently to binding affinity and conformational structuring; Asp 368 and, in particular, Met 426 contribute significantly to binding and, to a lesser extent, to conformational structuring (90). The interaction of the DMJ compounds with these residues may explain why the DMJ compounds bind with a smaller entropic penalty than NBD-556. The interaction of the DMJ compounds with gp120 points toward a binding event that is characterized by a better affinity and a coupling to some different conformational changes in Env that are smaller in magnitude and necessary for the sensitization effect.
The CD4i and V3-directed anti-gp120 antibodies neutralized HIV-1 with dramatically improved potency in the presence of the DMJ compounds. These two groups of antibodies recognize gp120 epitopes that share several features: (i) poor formation/exposure on the unliganded HIV-1 Env trimer, (ii) induction by CD4 binding, (iii) involvement in coreceptor binding, and (iv) a high degree of conservation in the components of the epitope that interact with the coreceptor. As the sensitizing effect of the DMJ compounds did not extend to other groups of Env-directed antibodies, sensitization of HIV-1 likely results from the induction of Env conformational changes similar to those induced by CD4. This conclusion is consistent with the documented CD4-mimetic thermodynamic and entry-enhancing properties of NBD-556 analogues (75, 78), as well as the proximity of their gp120 binding site to that of CD4 (17, 75–77). In addition, the sensitization of HIV-1 to CD4i and V3-directed antibodies by different NBD-556 analogues correlated with sensitization of the virus to cold inactivation (Table 1). The sensitivity of HIV-1 variants to cold inactivation is known to increase in proportion to their tendency to assume the CD4-bound conformation (54, 84). Thus, the observed relationship between sensitization of HIV-1 to antibody neutralization and cold inactivation is consistent with different levels of induction of the CD4-bound state by the various NBD-556 analogues. In summary, the sensitization of HIV-1 by the DMJ compounds apparently involves the induction of conformational changes related to those that occur during CD4 binding.
One practical use of the observed sensitization effect might be the enhancement of vaccine efficacy by the DMJ compounds. One frustrating aspect of HIV-1 vaccine development is the difficulty of eliciting antibodies that potently neutralize diverse strains of virus (44, 52, 71–73). Sensitization of HIV-1, including transmitted/founder viruses, by the DMJ compounds results in a virus that is neutralizable by antibodies that can be readily elicited. During HIV-1 infection of humans, CD4i antibodies are elicited early and in a high proportion of infected individuals (66); this suggests that the generation of such antibodies in humans may be achievable by vaccination. Moreover, “stabilized gp120 cores” that have been engineered to assume the CD4-bound state have been demonstrated to raise CD4i antibodies in immunized rabbits (67, 91). In this report, we demonstrate that (+)-DMJ-II-121 sensitizes primary HIV-1 JR-FL and transmitted/founder viruses to polyclonal sera raised by the 3CC and 4CC stabilized cores in multiple rabbits. Thus, the two fundamental components of a prophylactic approach based on HIV-1 sensitization are in place: (i) DMJ compounds that inhibit HIV-1 entry (76, 77) and also sensitize HIV-1 to neutralization by CD4i and V3-directed antibodies and (ii) stabilized gp120 core immunogens that can elicit CD4i antibodies (67). One can thus envision a multicomponent vaccine regimen in which one of the immunogens is a stabilized gp120 core that elicits antibodies against the conserved coreceptor-binding site. DMJ compounds administered orally or in a microbicide formulation could sensitize a range of transmitted/founder viruses to inhibition by the vaccine-elicited antibodies.
This study establishes proof of concept that specific Env-targeted compounds can sensitize primary HIV-1 to antibodies that are present in many HIV-1-infected individuals or that are readily elicited. To identify and overcome the potential challenges associated with practical implementation of these observations, future studies using animal models will be needed.
ACKNOWLEDGMENTS
We thank Yvette McLaughlin and Elizabeth Carpelan for manuscript preparation. We thank Irwin Chaiken and Wayne Hendrickson for valuable discussions and input. We are grateful to Yuxing Li and Richard Wilson for their generous gift of CD4i antibodies, VCT39GH, VC827, and VCT16.
This study was supported by the National Institutes of Health (grants GM56550, AI100645, and AI24755), the International AIDS Vaccine Initiative, and the late William F. McCarty-Cooper. E.F. thanks the National Science Foundation for support (MCB-1157506). N.M. was supported by amfAR grant 107431-45-RFNT, NIH grant AI090682, and a Ragon Institute Innovation Award.
Footnotes
Published ahead of print 2 April 2014
REFERENCES
- 1.United Nations Programme on HIV/AIDS. 2012. UNAIDS report on the global HIV/AIDS epidemic. United Nations Programme on HIV/AIDS, Geneva, Switzerland [Google Scholar]
- 2.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207–210. 10.1038/72318 [DOI] [PubMed] [Google Scholar]
- 3.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204–210. 10.1038/5568 [DOI] [PubMed] [Google Scholar]
- 4.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. 10.1038/nature06106 [DOI] [PubMed] [Google Scholar]
- 5.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 5:e1000433. 10.1371/journal.ppat.1000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruprecht RM. 2009. Passive immunization with human neutralizing monoclonal antibodies against HIV-1 in macaque models: experimental approaches. Methods Mol. Biol. 525:559–566. 10.1007/978-1-59745-554-1_31 [DOI] [PubMed] [Google Scholar]
- 7.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc. Natl. Acad. Sci., U. S. A. 108:11181–11186. 10.1073/pnas.1103012108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allan JS, Coligan JE, Barin F, McLane MF, Sodroski JG, Rosen CA, Haseltine WA, Lee TH, Essex M. 1985. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 228:1091–1094. 10.1126/science.2986290 [DOI] [PubMed] [Google Scholar]
- 9.Robey WG, Safai B, Oroszlan S, Arthur LO, Gonda MA, Gallo RC, Fischinger PJ. 1985. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science 228:593–595. 10.1126/science.2984774 [DOI] [PubMed] [Google Scholar]
- 10.Wyatt R, Sodroski J. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884–1888. 10.1126/science.280.5371.1884 [DOI] [PubMed] [Google Scholar]
- 11.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Anworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, de Souza MS. 2012. The Thai phase III HIV Type 1 Vaccine Trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. MOPH TAVEG Collaboration. AIDS Res. Hum. Retroviruses 28:1444–1457. 10.1089/aid.2012.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rerks-Ngarm S, Pittisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birs DL, Chunsutiwat S, Khamboonruang C, Thongsharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 13.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yaes NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haim H, Si Z, Madani N, Wang L, Courter JR, Princiotto A, Kassa A, DeGrace M, McGee-Estrada K, Mefford M, Gabuzda D, Smith AB, III, Sodroski J. 2009. Soluble CD4 and CD4-mimetic compounds inhibit HIV-1 infection by induction of a short-lived activated state. PLoS Pathog. 5:e1000360. 10.1371/journal.ppat.1000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimura K, Harada S, Shibata J, Hatada M, Yamada Y, Ochiai C, Tamamura H, Matsushita S. 2010. Enhanced exposure of human immunodeficiency virus type 1 primary isolate neutralization epitopes through binding of CD4 mimetic compounds. J. Virol. 84:7558–7568. 10.1128/JVI.00227-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haim H, Salas I, McGee K, Eichelberger N, Winter E, Pacheco B, Sodroski J. 2013. Modeling virus- and antibody-specific factors to predict human immunodeficiency virus neutralization efficiency. Cell Host Microbe 14:547–558. 10.1016/j.chom.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon YD, Finzi A, Wu X, Dogo-isonagie C, Lee CL, Moore LR, Schmidt SD, Stuckey J, Yang Y, Zhou T, Zhu J, Vicic DA, Debnath AIK, Shapiro L, Bewley CA, Mascola JR, Sodroski J, Kwong PD. 2012. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proc. Natl. Acad. Sci. U. S. A. 109:5663–5668. 10.1073/pnas.1112391109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan DC, Fass D, Berger JM, Kim PS. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263–273. 10.1016/S0092-8674(00)80205-6 [DOI] [PubMed] [Google Scholar]
- 19.Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426–430. 10.1038/387426a0 [DOI] [PubMed] [Google Scholar]
- 20.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman JC, Montagnier L. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767–768. 10.1038/312767a0 [DOI] [PubMed] [Google Scholar]
- 21.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763–767. 10.1038/312763a0 [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Gerard N, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A, Desjardins E, Newman W, Gerard C, Sodroski J. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR5. Nature 384:179–183. 10.1038/384179a0 [DOI] [PubMed] [Google Scholar]
- 23.Trkola A, Dragic T, Arthos J, Binley JM, Olson WC, Allaway GP, Cheng-Mayer C, Robinson J, Maddon PJ, Moore JP. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184–187. 10.1038/384184a0 [DOI] [PubMed] [Google Scholar]
- 24.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135–1148. 10.1016/S0092-8674(00)81313-6 [DOI] [PubMed] [Google Scholar]
- 25.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661–666. 10.1038/381661a0 [DOI] [PubMed] [Google Scholar]
- 26.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667–673. 10.1038/381667a0 [DOI] [PubMed] [Google Scholar]
- 27.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85:1149–1158. 10.1016/S0092-8674(00)81314-8 [DOI] [PubMed] [Google Scholar]
- 28.Feng Y, Broder CC, Kennedy PE, Berger EA. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872–877. 10.1126/science.272.5263.872 [DOI] [PubMed] [Google Scholar]
- 29.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. 1996. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955–1958. 10.1126/science.272.5270.1955 [DOI] [PubMed] [Google Scholar]
- 30.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. 10.1038/nature01470 [DOI] [PubMed] [Google Scholar]
- 31.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren P, Robinson J, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678–682. 10.1038/nature01188 [DOI] [PubMed] [Google Scholar]
- 32.McCaffrey RA, Saunders C, Hensel M, Stamatatos L. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 78:3279–3295. 10.1128/JVI.78.7.3279-3295.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705–711. 10.1038/31514 [DOI] [PubMed] [Google Scholar]
- 34.Reitter JN, Means RE, Desrosiers RC. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679–684. 10.1038/nm0698-679 [DOI] [PubMed] [Google Scholar]
- 35.Frost SD, Wrin T, Smith DM, Kosakovsky Pond S, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropouos CJ, Little SJ, Richman DD. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. U. S. A. 102:18514–18519. 10.1073/pnas.0504658102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischer W, Ganusov W, Giorgi EE, Hraber PT, Keele BF, Leitner T, Han CS, Gleasner CD, Green L, Lo CC, Nag A, Wallstrom TC, Wang S, McMichael AJ, Haynes BF, Hahn BH, Perelson AS, Borrow P, Shaw M, Bhattacharya T, Korber BT. 2010. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One 5:e12303. 10.1371/journal.pone.0012303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. 10.1016/j.immuni.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore PL, Ranchobe N, Lambson BE, Gray ES, Cave E, Abrahams MR, Bandawe G, Mlisana K, Abdool Karim SS, Williamson C, Morris L, CAPRISA 002 Study, NIAID Center for HIV/AIDSVaccine Immunology (CHAVI) 2009. Limited neutralizing antibody specificities drive neutralization escape in early HIV-1 subtype C infection. PLoS Pathog. 5:e1000598. 10.1371/journal.ppat.1000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatatos L, Morris L, Burton DR, Mascola JR. 2009. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat. Med. 15:866–870. 10.1038/nm.1949 [DOI] [PubMed] [Google Scholar]
- 40.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L, CAPRISA 002 Study Team 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85:4828–4840. 10.1128/JVI.00198-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wibmer CK, Bhiman JN, Gray ES, Tumba N, Abdool Karim SS, Williamson C, Morris L, Moore PL. 2013. Viral escape from HIV-1 neutralizing antibodies drives increased plasma neutralization breadth through sequential recognition of multiple epitopes and immunotypes. PLoS Pathog. 9:e1003738. 10.1371/journal.ppat.1003738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sather DN, Armann J, Ching LK, Mayrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769. 10.1128/JVI.02036-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu RB, Fu BZ, Gnanapragasam PN, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, Nussenzweig MC. 2013. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153:126–138. 10.1016/j.cell.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R. 2012. A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407. 10.1016/j.chom.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, Depetris R, Katpally U, Marozsan A, Cupo A, Maloveste S, Liu Y, McBride R, Ito Y, Sanders RW, Ogohara C, Paulson JC, Feizi T, Scanlan CN, Wong CH, Moore JP, Olson WC, Ward AB, Poignard P, Schief WR, Burton DR, Wilson IA. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103. 10.1126/science.1213256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker LM, Phogat SK, Chan-Hui PY, Wanger D, Phung P, Goss JL, Wring T, Simek MD, Fling S, Mitchem JL, Lehman JK, Priddy RH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators. Mitro G, Serwanga J, Pozniak A, McPhee D, Manigart O, Mwananyanda L, Karita E, Inwoley A, Jacko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Allen S, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. 10.1126/science.1178746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julian JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators. Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. 10.1038/nature10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, Hoffenberg S, Caulfield M, King CR, Hua Y, Le KM, Khayat R, Deller MC, Clayton T, Tien H, Feizi T, Sanders RW, Paulson JC, Moore JP, Stanfield RL, Burton DR, Ward AB, Wilson IA. 2013. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 20:796–803. 10.1038/nsmb.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doores KJ, Fulton Z, Huber M, Wilson IA, Burton DR. 2010. Antibody 2G12 recognizes di-mannose equivalently in domain- and nondomain-exchanged forms but only binds the HIV-1 glycan shield if domain exchanged. J. Virol. 84:10690–10699. 10.1128/JVI.01110-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calarese DA, Scanlan CN, Zwick MB, Deechongkit S, Mimura Y, Kunert R, Zhu P, Wormald MR, Stanfield RL, Rous KH, Kelly JW, Rudd PM, Dwek RA, Katinger H, Burton DR, Wilson IA. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065–2071. 10.1126/science.1083182 [DOI] [PubMed] [Google Scholar]
- 51.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. 10.1038/nature10696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker BD, Ahmed R, Plotkin S. 2011. Moving ahead an HIV vaccine: use both arms to beat HIV. Nat. Med. 17:1194–1195. 10.1038/nm.2529 [DOI] [PubMed] [Google Scholar]
- 53.Hoxie JA. 2010. Toward an antibody-based HIV-1 vaccine. Annu. Rev. Med. 61:135–152. 10.1146/annurev.med.60.042507.164323 [DOI] [PubMed] [Google Scholar]
- 54.Haim H, Strack B, Kassa A, Madani N, Wang L, Courter JR, Princiotto A, McGee K, Pacheco B, Seaman MS, Smith AB, III, Sodroski J. 2011. Contribution of intrinsic reactivity of the HIV-1 envelope glycoproteins to CD4-independent infection and global inhibitor sensitivity. PLoS Pathog. 7:e1002101. 10.1371/journal.ppat.1002101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557. 10.1073/pnas.0802203105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ping LH, Joseph SB, Anderson JA, Abrahams MR, Salazar-Gonzalez JF, Kincer LP, Treurnicht FK, Arney L, Ojeda S, Zhang M, Keys J, Potter EL, Chu H, Moore P, Salazar M, Iyer S, Jabara C, Kirchherr J, Mapanje C, Ngandu N, Seoighe C, Hoffman I, Gao F, Tang Y, Labranche C, Lee B, Savile A, Vermeulen M, Fiscus S, Morris L, Karim SA, Haynes BF, Shaw GM, Korber BT, Hahn BH, Cohen MS, Montefiori D, Williamson C, Swanstrom R. 2013. Comparison of viral Env proteins from acute and chronic infections with Subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. CAPRISA Acute Infection Study and the Center for HIV-AIDS Vaccine Immunology Consortium. J. Virol. 87:7218–7233. 10.1128/JVI.03577-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. 10.1038/31405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizzuto C, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949–1953. 10.1126/science.280.5371.1949 [DOI] [PubMed] [Google Scholar]
- 59.Huang CC, Tang M, Zhang M-Y, Majeed S, Montabana E, Stanfield RL, Dimitrov D, Korber B, Sodroski J, Wilson IA, Wyatt R, Kwong PD. 2005. Structure of a V3-containing HIV-1 gp120 core. Science 310:1025–1028. 10.1126/science.1118398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang CC, Lam SN, Acharya P, Tang M, Xiang S-H, Shahzad-ul Hassan S, Stanfield RL, Robinson J, Sodroski J, Wilson IA, Wyatt R, Bewley CA, Kwong PD. 2007. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science 317:1930–1934. 10.1126/science.1145373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen L, Kwon YD, Zhou T, Wu X, O'Dell S, Cavacini L, Hessell AJ, Pancera M, Tang M, Xu L, Yang ZY, Zhang MY, Arthos J, Burton DR, Dimitrov DS, Nabel GJ, Posner MR, Sodroski J, Wyatt R, Mascola JR, Kwong PD. 2009. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science 326:1123–1127. 10.1126/science.1175868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mao Y, Wang L, Gu C, Herschhorn A, Désormeaux A, Finzi A, Xiang SH, Sodroski JG. 2013. Molecular architecture of the uncleaved HIV-1 envelope glycoprotein trimer. Proc. Natl. Acad. Sci. U. S. A. 110:12438–12443. 10.1073/pnas.1307382110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thali M, Moore JP, Furman C, Charles M, Ho DD, Robinson J, Sodroski J. 1993. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiang SH, Doka N, Choudhary RK, Sodroski J, Robinson JE. 2002. Characterization of CD4-induced epitopes on the HIV type 1 gp120 envelope glycoprotein recognized by neutralizing human monoclonal antibodies. AIDS Res. Hum. Retroviruses 18:1207–1217. 10.1089/08892220260387959 [DOI] [PubMed] [Google Scholar]
- 65.Xiang SH, Wang L, Abreu M, Huang C-C, Kwong PD, Rosenberg E, Robinson JE, Sodroski J. 2003. Epitope mapping and characterization of a novel CD4-induced human monoclonal antibody capable of neutralizing primary HIV-1 strains. Virology 315:124–134. 10.1016/S0042-6822(03)00521-X [DOI] [PubMed] [Google Scholar]
- 66.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J. Exp. Med. 201:1407–1419. 10.1084/jem.20042510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dey B, Pancera M, Svehla K, Shu Y, Xiang SH, Vainshtein J, Li Y, Sodroski J, Kwong PD, Mascola JR, Wyatt R. 2007. Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: antigenicity, biophysics, and immunogenicity. J. Virol. 81:5579–5593. 10.1128/JVI.02500-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dey B, Svehla K, Xu L, Wycuff D, Zhou T, Voss G, Phogat A, Chakrabarti BK, Li Y, Shaw G, Kwong PD, Nabel GJ, Mascola JR, Wyatt RT. 2009. Structure-based stabilization of HIV-1 gp120 enhances humoral immune responses to the induced co-receptor binding site. PLoS Pathog. 5:e1000445. 10.1371/journal.ppat.1000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labrijn A, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang C-C, Venturi M, Petropoulos C, Wrin T, Dimitrov D, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton D. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 77:10557–10565. 10.1128/JVI.77.19.10557-10565.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan N, Sun Y, Sattentau S, Thali M, Wu D, Denisova G, Gershoni J, Robinson J, Moore J, Sodroski J. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 72:4694–4703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plotkin SA, Robinson HL, Davenport MP. 2012. Mining the mechanisms of an HIV vaccine. Nat. Med. 18:1020–1021. 10.1038/nm.2858 [DOI] [PubMed] [Google Scholar]
- 72.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR. 2013. Rational HIV immunogen design to target specific germline B cell receptors. Science 340:711–716. 10.1126/science.1234150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haynes BF, McElrath MJ. 2013. Progress in HIV-1 vaccine development. Curr. Opin. HIV AIDS 8:326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Q, Ma L, Jiang S, Lu H, Liu S, He Y, Strick N, Neamati N, Debnath AK. 2005. Identification of N-phenyl-N′-(2,2,6,6-tetramethyl-piperidin-4-yl)-oxalamides as a new class of HIV-1 entry inhibitors that prevent gp120 binding to CD4. Virology 339:213–225. 10.1016/j.virol.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 75.Madani N, Schön A, Princiotto AM, LaLonde JM, Courter JR, Soeta T, Ng D, Wang L, Brower ET, Xiang SH, Kwon YD, Huang CC, Wyatt R, Kwong PD, Freire E, Smith AB, III, Sodroski J. 2008. Small-molecule CD4 mimics interact with a highly conserved pocket on HIV-1 gp120. Structure 16:1689–1701. 10.1016/j.str.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaLonde JM, Kwon YD, Jones DM, Sun AW, Courter JR, Soeta T, Kobayashi T, Princiotto AM, Wu X, Schön A, Freire E, Kwong PD, Mascola JR, Sodroski J, Madani N, Smith AB., III 2012. Structure-based design, synthesis, and characterization of dual hotspot small-molecule HIV-1 entry inhibitors. J. Med. Chem. 55:4382–4396. 10.1021/jm300265j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lalonde JM, Le-Khac M, Jones DM, Courter JR, Park J, Schön A, Princiotto AM, Wu X, Mascola JR, Freire E, Sodroski J, Madani N, Hendrickson WA, Smith AB., III 2013. Structure-based design and synthesis of an HIV-1 entry inhibitor exploiting X-ray and thermodynamic characterization. ACS Med. Chem. Lett. 4:338–343. 10.1021/ml300407y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schön A, Madani N, Klein JC, Hubicki A, Ng D, Yang X, Smith AB, III, Sodroski J, Freire E. 2006. Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120. Biochemistry 45:10973–10980. 10.1021/bi061193r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lalonde JM, Elban MA, Courter JR, Sugawara A, Soeta T, Madani N, Princiotto AM, Kwon YD, Kwong PD, Schön A, Freire E, Sodroski J, Smith AB., III 2011. Design, synthesis, and biological evaluation of small molecule inhibitors of CD4-gp120 binding based on virtual screening. Bioorg. Med. Chem. 19:91–101. 10.1016/j.bmc.2010.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rho HM, Poiesz B, Ruscetti FW, Gallo RC. 1981. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology 112:355–360. 10.1016/0042-6822(81)90642-5 [DOI] [PubMed] [Google Scholar]
- 81.Wyatt R, Sullivan N, Thali M, Repke H, Ho D, Robinson J, Posner M, Sodroski J. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis KL, Bibollet-Ruche F, Li H, Decker JM, Kutsch O, Morris L, Salomon A, Pinter A, Hoxie JA, Hahn BH, Kwong PD, Shaw GM. 2009. Human immunodeficiency virus type 2 (HIV-2)/HIV-1 envelope chimeras detect high titers of broadly reactive HIV-1 V3-specific antibodies in human plasma. J. Virol. 83:1240–1259. 10.1128/JVI.01743-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Myszka D, Sweet RW, Hensley P, Brigham-Burke M, Kwong PD, Hendrickson WA, Wyatt R, Sodroski J, Doyle M. 2000. Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. U. S. A. 97:9026–9031. 10.1073/pnas.97.16.9026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kassa A, Finzi A, Pancera M, Courter JR, Smith AB, III, Sodroski J. 2009. Identification of a human immunodeficiency virus type 1 envelope glycoprotein variant resistant to cold inactivation. J. Virol. 83:4476–4488. 10.1128/JVI.02110-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pantophlet R, Wrin T, Cavacini LA, Robinson JE, Burton DR. 2008. Neutralizing activity of antibodies to the V3 loop region of HIV-1 gp120 relative to their epitope fine specificity. Virology 381:251–260. 10.1016/j.virol.2008.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore JP, Sodroski J. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD, Wang LX. 2013. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat. Chem. Biol. 9:521–526. 10.1038/nchembio.1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Friedman J, Alam SM, Shen X, Xia SM, Stewart S, Anasti K, Pollara J, Fouda GG, Yang G, Kelsoe G, Ferrari G, Tomaras GD, Haynes BF, Liao HX, Moody MA, Permar SR. 2012. Isolation of HIV-1-neutralizing mucosal monoclonal antibodies from human colostrum. PLoS One 7:e37648. 10.1371/journal.pone.0037648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J. Infect. Dis. 206:431–441. 10.1093/infdis/jis367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu Y, Schön A, Freire E. 2013. Optimization of CD4/gp120 inhibitors by thermodynamic-guided alanine-scanning mutagenesis. Chem. Biol. Drug Des. 81:72–78. 10.1111/cbdd.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kassa A, Dey AK, Sarkar P, Labranche C, Go EP, Clark DF, Sun Y, Nandi A, Hartog K, Desaire H, Montefiori D, Carfi A, Srivastava IK, Barnett SW. 2013. Stabilizing exposure of conserved epitopes by structure guided insertion of disulfide bond in HIV-1 envelope glycoprotein. PLoS One 8:e76139. 10.1371/journal.pone.0076139 [DOI] [PMC free article] [PubMed] [Google Scholar]