ABSTRACT
Chikungunya virus (CHIKV) is a member of a globally distributed group of arthritogenic alphaviruses that cause weeks to months of debilitating polyarthritis/arthralgia, which is often poorly managed with current treatments. Arthritic disease is usually characterized by high levels of the chemokine CCL2 and a prodigious monocyte/macrophage infiltrate. Several inhibitors of CCL2 and its receptor CCR2 are in development and may find application for treatment of certain inflammatory conditions, including autoimmune and viral arthritides. Here we used CCR2−/− mice to determine the effect of CCR2 deficiency on CHIKV infection and arthritis. Although there were no significant changes in viral load or RNA persistence and only marginal changes in antiviral immunity, arthritic disease was substantially increased and prolonged in CCR2−/− mice compared to wild-type mice. The monocyte/macrophage infiltrate was replaced in CCR2−/− mice by a severe neutrophil (followed by an eosinophil) infiltrate and was associated with changes in the expression levels of multiple inflammatory mediators (including CXCL1, CXCL2, granulocyte colony-stimulating factor [G-CSF], interleukin-1β [IL-1β], and IL-10). The loss of anti-inflammatory macrophages and their activities (e.g., efferocytosis) was also implicated in exacerbated inflammation. Clear evidence of cartilage damage was also seen in CHIKV-infected CCR2−/− mice, a feature not normally associated with alphaviral arthritides. Although recruitment of CCR2+ monocytes/macrophages can contribute to inflammation, it also appears to be critical for preventing excessive pathology and resolving inflammation following alphavirus infection. Caution might thus be warranted when considering therapeutic targeting of CCR2/CCL2 for the treatment of alphaviral arthritides.
IMPORTANCE Here we describe the first analysis of viral arthritis in mice deficient for the chemokine receptor CCR2. CCR2 is thought to be central to the monocyte/macrophage-dominated inflammatory arthritic infiltrates seen after infection with arthritogenic alphaviruses such as chikungunya virus. Surprisingly, the viral arthritis caused by chikungunya virus in CCR2-deficient mice was more severe, prolonged, and erosive and was neutrophil dominated, with viral replication and persistence not being significantly affected. Monocytes/macrophages recruited by CCL2 thus also appear to be important for both preventing even worse pathology mediated by neutrophils and promoting resolution of inflammation. Caution might thus be warranted when considering the use of therapeutic agents that target CCR2/CCL2 or inflammatory monocytes/macrophages for the treatment of alphaviral (and perhaps other viral) arthritides. Individuals with diminished CCR2 responses (due to drug treatment or other reasons) may also be at risk of exacerbated arthritic disease following alphaviral infection.
INTRODUCTION
Although many viruses can cause arthritis (1), few do so with the reliability of the arthritogenic alphaviruses, where symptomatic infection of adults is nearly always associated with rheumatic disease. This group of globally distributed, mosquito-borne, positive-strand RNA viruses includes the Australasian Ross River virus and Barmah Forest virus, the African o'nyong-nyong virus, the American Mayaro virus, the Sindbis virus family (which includes the Scandinavian Ockelbo and Pogosta viruses), and chikungunya virus (CHIKV) (2, 3). CHIKV has caused sporadic outbreaks every 2 to 50 years, which generally have been restricted to Africa and Asia. However, in 2004 to 2012, CHIKV caused the largest outbreak ever recorded for this virus, with an estimated 1.4 million to 6.5 million patients and imported cases being reported in nearly 40 countries, including the United States, Japan, and several European countries (2, 4, 5). CHIKV disease, and alphaviral rheumatic disease generally, is usually self-limiting and characterized by acute and chronic symmetrical peripheral polyarthralgia/polyarthritis, with acute disease often also associated with fever, myalgia, and/or rash. Arthropathy can be debilitating, usually lasts weeks to months, and is generally not erosive but can be protracted (2, 3).
Chemokine (C-C motif) receptor 2 (CCR2) is the receptor for a number of C-C motif chemokines, including CCL2, which is also known as monocyte chemotactic protein 1 (MCP-1). CCL2 recruits monocytes, basophils, and T cells to sites of inflammation and has been implicated as an important mediator in a range of inflammatory diseases, including, inter alia, rheumatoid arthritis (RA), multiple sclerosis, atherosclerosis, asthma, neuropathic pain (6), obesity, diabetes (7), and cancer (8). A range of therapeutic agents that target CCR2 or CCL2 are being developed (6–8), although initial trial results for CCR2 blockade in RA patients have been disappointing (9). Nevertheless, CCR2/CCL2 inhibition may ultimately find utility in the treatment of a range of conditions (6–8). Elevated levels of CCL2 and monocyte/macrophage infiltrates are also prominent features of several viral arthritides and are well described for alphaviral arthropathies (1) including CHIKV disease in mice, monkeys, and humans (2, 10, 11). Treatments with anti-CCL2 antibody or bindarit, a new drug purported to target CCL2 production, were shown to be effective at ameliorating rheumatic disease in CHIKV and Ross River virus mouse models (12, 13). As CCL2 generally appears to have no direct antiviral activity (1, 13–15), targeting of this chemokine and/or its receptor appears attractive for the treatment of viral arthritides.
The inflammation biology of CCR2/CCL2 has been found to be complicated. Collagen-induced arthritis in CCR2−/− mice is more severe, with increased macrophage and neutrophil infiltrates (16, 17), whereas in other arthritis models, inhibition of CCL2 ameliorated disease (18, 19). CCR2 deficiency was associated with reduced monocyte/macrophage infiltration in some settings (20–22) but not in others (16, 23). More neutrophil infiltrates were seen in CCR2−/− mice in certain settings (16, 23, 24) and were unchanged in some (25) and less in others (26). For dengue and influenza virus infections of CCR2−/− mice, the viral load was unchanged, and the pathology was attenuated (14, 15). In contrast, cytomegalovirus and West Nile virus infections in these mice resulted in increased viral loads and pathology (27, 28).
Given the recent outbreak of CHIKV and the interest in using CCR2/CCL2 blockers for alphaviral arthritides and other diseases, we sought to determine the effect of CCR2 deficiency on CHIKV arthritis using a recently established adult mouse model that mimics many aspects of human disease (10).
MATERIALS AND METHODS
Ethics statement.
Animals were handled in accordance with National Health and Medical Research Council (Australia) guidelines. Animals were kept in a modern Optimice caging system (Centennial, CO, USA). All animal experimentation was approved by the QIMR Berghofer animal ethics committee.
Virus isolates and preparation.
The Reunion Island (LR2006-OPY1) CHIKV isolate was prepared as described previously (10). The virus preparations had undetectable endotoxin and mycoplasma contamination (29, 30). As CHIKV is a biosafety level 3 (BSL3) pathogen, all work with infectious virus was conducted in the BSL3 facility at QIMR Berghofer.
Mouse infection, arthritis, and antibody monitoring.
Female 6- to 10-week-old mice were inoculated subcutaneously (s.c.) into each hind foot with CHIKV (LR2006-OPY1) (viral dose of 104 50% cell culture infectivity dose in 40 μl medium), and tissue viral titers and viremia were determined as described previously (10). Arthritis was monitored by measuring the height and width of the metatarsal area of the hind feet using digital calipers. The data are presented as a group average of the percent increase in foot height by width for each foot compared with the same foot on day 0 (31). C57BL/6 mice were supplied by the Animal Resources Centre (Perth, Australia). CCR2−/− mice on a C57BL/6 background (20) were bred in-house at QIMR Berghofer. Antibody responses were determined by an isotope-specific enzyme-linked immunosorbent assay (ELISA) using inactivated whole CHIKV as an antigen, as described previously (32).
Quantitative real-time reverse transcription-PCR analyses.
RNA isolation from feet, cDNA preparation, and quantitative real-time PCR analyses were performed as described previously (10). Quantitative real-time reverse transcription-PCR (qRT-PCR) analyses were performed by using 1 μl (≈50 ng) of cDNA, 10 μl of SYBR green Super mix-UDG (Invitrogen), 1 μl of this kit's bovine serum albumin (BSA) solution, 6 μl H2O, and 1 μl of 10 μM forward and reverse primers. Each sample was analyzed in duplicate, and values were normalized to RPL13A mRNA levels (10). Primer sequences are shown in Table S1 in the supplemental material.
Histology and immunohistochemistry.
Hematoxylin and eosin (H&E), F4/80, and ApoTag staining (Millipore) were undertaken as described previously (10, 33). Anti-Ly6G staining was undertaken by using rat anti-mouse Ly6G (catalog number NMP-R14; Abcam, Cambridge, MA, USA) and was detected in the same way as F4/80. Leder (Naphthol AS-D chloroacetate; Sigma), Chromotrope2R (ProSciTech, Thuringowa, Australia), and Safranin O (BDH Laboratory Supplies, Poole, United Kingdom) staining were performed by using standard protocols. Stained whole foot sections were digitally scanned by using a Scan Scope XT digital slide scanner (Aperio, Vista, CA). Image analyses of whole foot sections in duplicate were undertaken by using Aperio ImageScope software (v10) and the Positive Pixel Count v9 algorithm using default “strong” settings.
MK0812 treatment.
MK0812 (Amandis Chemical, China) was dissolved in chloroform (5% in phosphate-buffered saline [PBS] [final]) and administered intraperitoneally (i.p.) at 30 mg/kg of body weight daily on days 1 to 6 postinfection. Control mice received 5% chloroform in PBS.
Microarray analyses.
Microarray analysis, identification of differentially expressed genes (DEGs), and ingenuity pathway analysis (IPA) were performed as described previously (33). DEGs were obtained at day 7 postinfection relative to day 0, separately for wild-type (WT) and CCR2−/− mice, using pooled RNA samples from feet of 4 to 6 mice for each time point and mouse strain. Genes with low expression levels in the microarray analysis (log2 expression, <7) for both days 0 and 7 were removed. Gene set enrichment analysis (GSEA) was undertaken as described previously (33), using publicly available microarray raw data files from studies of synovial biopsy specimens from healthy patients and patients with RA (GEO accession number GSE1919). Human genes were converted into mouse homologs by utilizing the NCBI Homolo-Gene annotation (available at http://www.ncbi.nlm.nih.gov/homologene).
Statistics.
Analysis was performed by using IBM SPSS Statistics (version 19). The t test was used if the difference in the variances was <4, skewness was >−2, and kurtosis was <2. Where the data were nonparametric and the difference in variances was <4, the Mann-Whitney U test was used, and if the difference in variances was >4, the Kolmogorov-Smirnov test was used.
Microarray data accession number.
The microarray data reported herein are available from the Gene Expression Omnibus (GEO) repository under accession number GSE56965.
RESULTS
Foot swelling in CHIKV-infected wild-type and CCR2−/− mice.
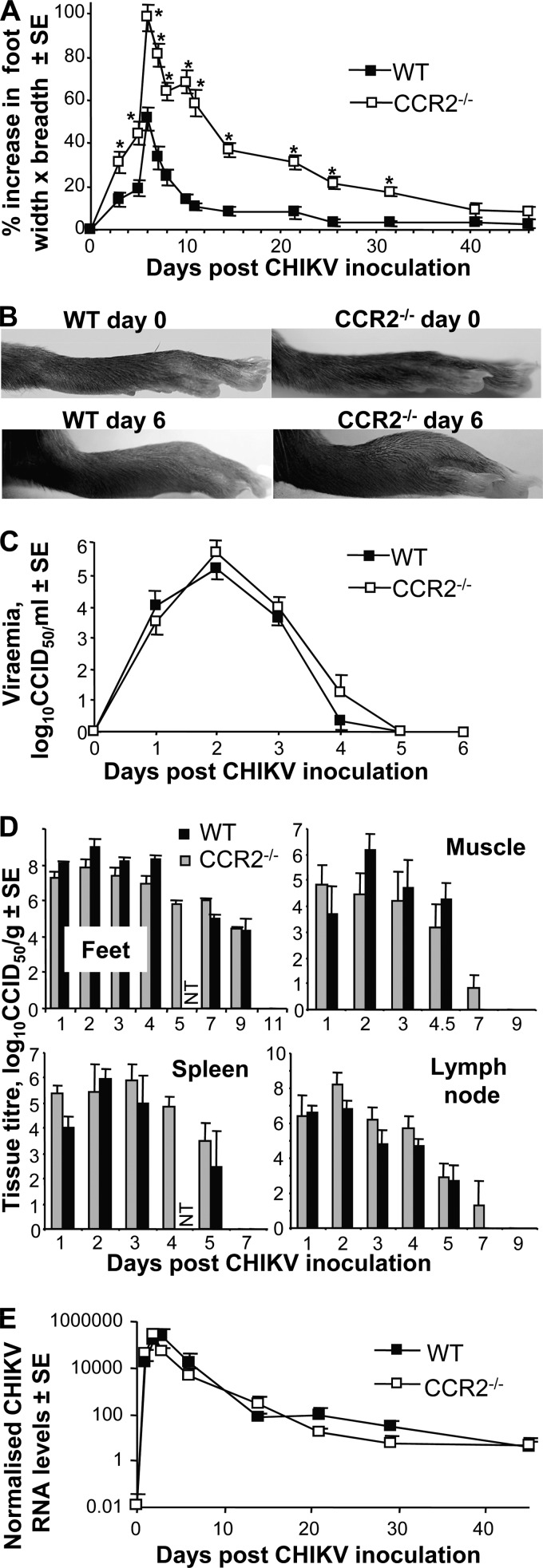
After CHIKV infection of adult WT mice, clearly discernible foot swelling is observed, which peaks on day 6/7 and largely resolves by days 10 to 14 (Fig. 1A), as reported previously (10). The same CHIKV infection of CCR2−/− mice resulted in nearly 2-fold-higher mean peak foot swelling (day 6) (Fig. 1A), with differences being clearly observable by eye (Fig. 1B). The swelling in CCR2−/− mice also resolved much more slowly, returning to normal after day 40 (Fig. 1A). The level of foot swelling in CCR2−/− mice was significantly higher than that in WT mice for all time points from days 3 to 31.5 (Fig. 1A). Calculations of the area under the curve showed that CCR2−/− mice experienced >4-fold more foot swelling than WT mice (not shown).
FIG 1.
Disease, virus replication, and RNA persistence. (A) Mean percent increase in foot swelling over time for wild-type (WT) (n = 16 feet; 8 mice) and CCR2−/− (n = 14 feet; 7 mice) mice. Asterisks indicate significant differences (P < 0.01) by the t test or Kolmogorov-Smirnov test; the choice of test at each time point was dependent on the skewness, kurtosis, and variance of the data being compared (see Materials and Methods). (A single experiment, representative of 3 repeat experiments, is shown.) (B) Images of WT and CCR2−/− feet on day 0 and day 6 (peak arthritis). (C) Peripheral blood viremia (n = 7 for CCR2−/− mice; n = 8 to 14 for WT mice). (D) Virus titers in the indicated tissues at the indicated times postinfection (n = 3 to 6 mice per time point) (NT, not tested). (E) qRT-PCR analyses of CHIKV RNA in feet. Feet were taken at the indicated times (n = 3 to 6), and the level of CHIKV positive-strand RNA was determined by using qRT-PCR analyses and primers specific for E1 (a major CHIKV structural protein); data were normalized to RPL13A mRNA levels.
Virus and viral RNA in WT and CCR2−/− mice.
There were no consistent and/or significant differences in (i) viremia (Fig. 1C); (ii) virus titers in feet, muscle, spleen, and lymph node (Fig. 1D); or (iii) viral RNA levels (Fig. 1E) between WT and CCR2−/− mice following CHIKV infection. CCR2 deficiency thus does not appear to have a significant effect on CHIKV replication or the persistence of CHIKV RNA (2). The increased and more protracted disease seen in CCR2−/− mice (Fig. 1A) was thus not due to increased viral replication or increased persistence of viral RNA in these mice.
Histology and immunohistochemistry of the cellular infiltrates in arthritic feet of WT and CCR2−/− mice.
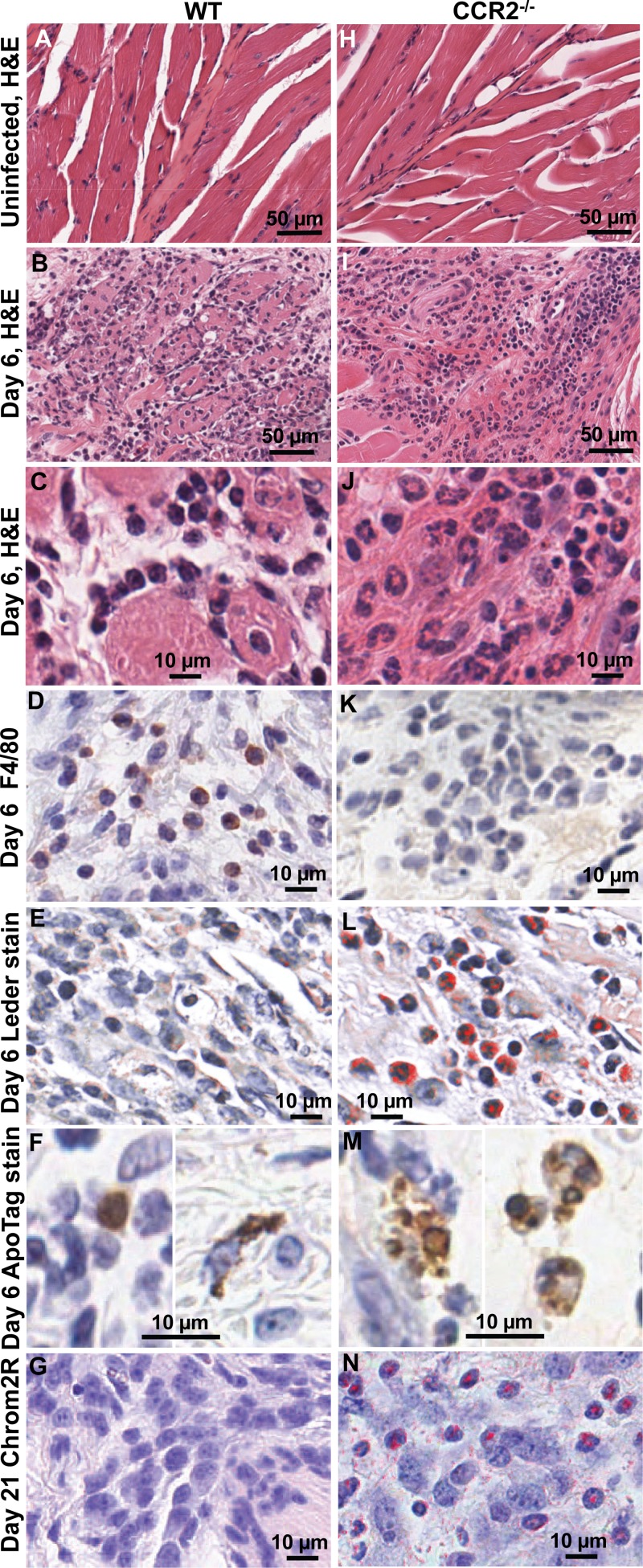
Histological examination of arthritic feet of WT mice showed a predominance of mononuclear cell infiltrates on day 6 (the day of peak arthritis), as described previously (10). This could be clearly seen by H&E staining of muscle (compare Fig. 2A with B and C). Staining of foot sections with F4/80 antibody (a monoclonal antibody recognizing mouse monocytes/macrophages) also showed a large number of infiltrating F4/80-positive (F4/80+) cells (Fig. 2D), as reported previously (10). Leder staining (Fig. 2E) showed that neutrophils were largely absent from infiltrates in day 6 feet of WT mice (Fig. 2E). ApoTag staining revealed some apoptosis, primarily in cells with monocytic and fibroblastic morphology (Fig. 2F). Chromotrope2R staining (Fig. 2F) showed that eosinophils were largely absent (Fig. 2G).
FIG 2.
Histology and immunohistochemistry of rheumatic lesions in CHIKV-infected WT and CCR2−/− mice. (A to G) WT mice. (H to N) CCR2−/− mice. (A and H) H&E staining of muscle from uninfected mice. (B and I) H&E staining of muscle at day 6 postinfection. (C and J) High magnification of H&E staining of muscle at day 6 postinfection. (D and K) F4/80 antibody (monocyte/macrophage) staining of connective tissue at day 6 postinfection. (E and L) Leder staining (neutrophils) of connective tissue at day 6 postinfection. (F and M) ApoTag staining (apoptotic cells) at day 6 postinfection (two images for panel F and three images for panel M). (G and N) Chromotrope2R staining (eosinophils) of connective tissue at day 21 postinfection.
The cellular infiltrates in the feet of CCR2−/− mice were quite distinct from those seen in WT mice. H&E staining showed a predominance of neutrophils (compare Fig. 2H with I and J) (many with hypersegmented nuclei), with few F4/80+ cells being detected in the infiltrates (Fig. 2K). Prodigious neutrophil infiltrates were also clearly seen by Leder staining (Fig. 2L). ApoTag staining was more widespread, with frequent staining of cytoplasmic debris (Fig. 2M, left), which is suggestive of secondary necrosis. ApoTag staining was often also present in cells with polymorphonuclear morphology (Fig. 2M, right). The latter finding suggests inflammatory neutrophils undergoing apoptosis, a well-described phenomenon in inflammation biology (34). Chromotrope2R staining also revealed that a large number of eosinophils were present in the arthritic infiltrates in CCR2−/− feet on day 21 postinfection (Fig. 2L).
Quantitation of histology and immunohistochemistry of cellular infiltrates in arthritic feet of WT and CCR2−/− mice.
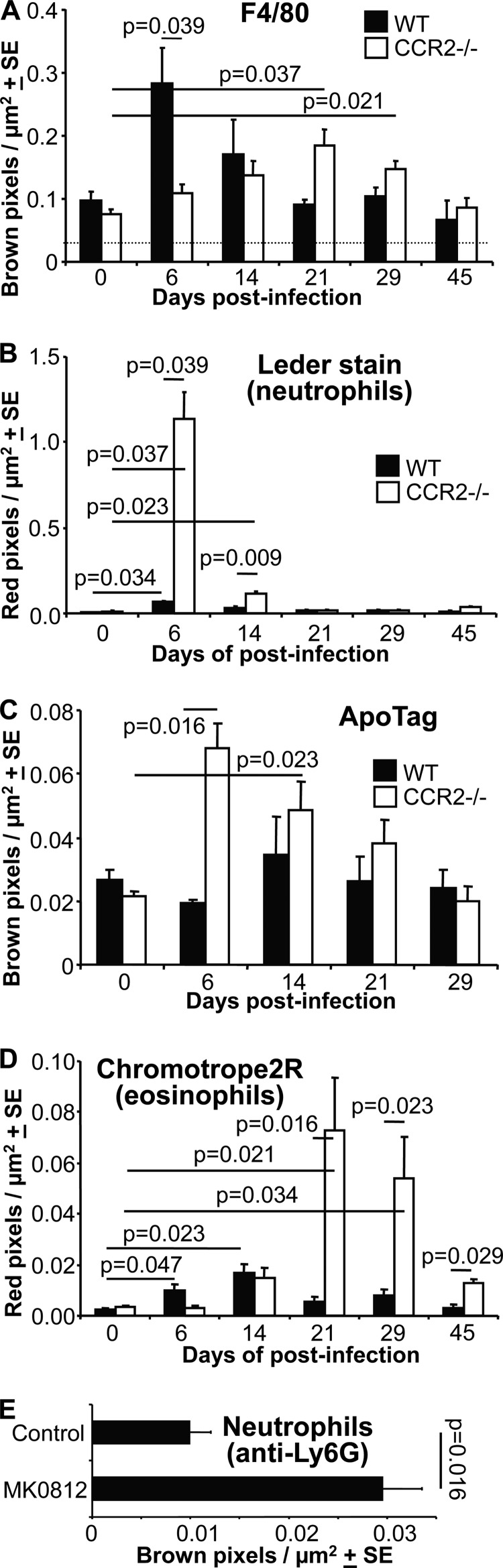
To quantitate the features described above, the indicated numbers of feet were processed and stained in parallel, and slides were digitally scanned (Aperio) and analyzed by using the positive pixel count algorithm (Fig. 3). F4/80 staining increased substantially in WT feet on day 6 postinfection (as reported previously [10]). This did not occur in CCR2−/− feet, with F4/80 staining being significantly higher in WT feet than in CCR2−/− feet at this time (Fig. 3A). These data support the widely expressed view that the prodigious monocyte/macrophage infiltrate seen in WT mice after infection with arthritogenic alphaviruses is dependent on CCL2/CCR2 expression (1, 2, 10–13, 35). We cannot formally exclude the possibility that the reduced monocyte/macrophage recruitment in CCR2−/− mice is due, at least in part, to the reduced levels of circulating monocytes in these animals (28). However, pixel counts of F4/80 staining of feet on day 6 postinfection after adoptive transfer (prior to infection) of WT CCR2+/+ splenocytes (3 × 107) into CCR2−/− mice gave a mean value and standard error (SE) of 0.172 ± 0.02 Brown pixels/μm2 (settings are described in the legend of Fig. 3A), whereas after adoptive transfer of CCR2−/− splenocytes into CCR2−/− mice, this value was 0.119 ± 0.006 Brown pixels/μm2 (i.e., very similar to that for CCR2−/− mice on day 6) (Fig. 3A and data not shown). The difference between the two adoptive transfer groups was significant (P = 0.015; n = 8 feet from 4 mice per group). Pixel counts of Ly6C staining of the same feet also indicated an ≈25% reduction in neutrophil infiltration in CCR2−/− mice given WT cells, but this only approached significance (P = 0.057) (data not shown).
FIG 3.
Histology quantitation. (A) Image analysis (strong brown) of F4/80 staining of whole foot sections from WT and CCR2−/− mice at the indicated times postinfection. The dotted line indicates the upper limit of nonspecific staining in control slides (n = 3 to 5 feet from different mice per time point and mouse strain; 2 sections per foot were analyzed). Statistics were determined by the Kolmogorov-Smirnov test. (B) Image analysis (strong red) of Leder staining of whole foot sections of WT and CCR2−/− mice at the indicated times postinfection (same numbers of feet as described above for panel A). Statistics were determined by Kolmogorov-Smirnov, Mann-Whitney U, or t tests. (C) Image analysis (strong brown) of ApoTag staining of whole foot sections of WT and CCR2−/− mice at the indicated times postinfection (same numbers of feet as described above for panel A). Statistics were determined by Kolmogorov-Smirnov tests. (D) Image analysis (strong red) of Chromotrope2R staining of whole foot sections of WT and CCR2−/− mice at the indicated times postinfection (same numbers of feet as described above for panel A). Statistics were determined by Kolmogorov-Smirnov or Mann-Whitney U tests. (E) WT mice were treated with the CCR2 antagonist MK0815 or diluent (control) after CHIKV infection, and feet were stained with anti-Ly6G (a neutrophil-specific marker), followed by image analysis. Statistics were determined by a Kolmogorov-Smirnov test (n = 4 to 6 feet per group).
F4/80 staining increased significantly in CCR2−/− feet on days 21 and 29 postinfection (Fig. 3A). Rather than being associated with monocytes/macrophages, these increases were likely associated with infiltrating eosinophils (see below), which also stain with F4/80 antibody (data not shown), as reported previously (36).
Leder staining was dramatically increased in CCR2−/− feet on day 6 postinfection and was still significantly elevated on day 14, consistent with the increase in the number of neutrophils seen by H&E staining (Fig. 3B). In WT mice, Leder staining was slightly increased on day 6 but was substantially lower than that for CCR2−/− feet on days 6 and 14 (Fig. 3B). Immunohistochemistry using a neutrophil-specific anti-Ly6G antibody and image analysis confirmed the stark and significant contrast in neutrophil infiltrates between WT and CCR2−/− feet on day 6 (data not shown). To investigate how important neutrophils were for the increased foot swelling seen in these mice (Fig. 1A), neutrophils were depleted by using an anti-Ly6G antibody (administered on days 4, 5, and 6 postinfection at 0.3 mg per day), with an ≈85% reduction in circulating neutrophil numbers being achieved on day 7. However, this led to slightly increased foot swelling (days 5 to 7), with widespread hemorrhage and edema being evident (data not shown). Neutrophil depletion in CCR2−/− mice post-CHIKV infection thus appears to promote a new pathology (31), complicating any analysis of the arthritogenic role of neutrophils by using this approach.
The level of ApoTag staining of apoptotic cells was significantly higher in CCR2−/− feet on day 6 and was also significantly elevated on day 14, whereas in WT mice, there was no significant difference between levels at any of the time points shown (33) (Fig. 3C). Chromotrope2R staining (eosinophils) was slightly increased on days 6 and 14 in WT mice but was substantially increased in CCR2−/− mice on days 21 and 29 (Fig. 3D).
In summary, the increased and prolonged arthritic disease seen in CCR2−/− mice was associated with a loss of infiltrating monocytes/macrophages and severe neutrophil infiltration followed by eosinophil infiltration, with more apoptotic cells also being seen during peak disease.
Treatment with a CCR2 antagonist.
To determine whether treatment with a CCR2 antagonist might have an effect on neutrophil infiltration similar to that seen in CCR2−/− mice, WT mice were treated with MK0812, a CCR2 antagonist tested (without clinical benefit) on rheumatoid arthritis patients (37). The drug has been shown to reduce peripheral blood monocyte frequency and monocyte recruitment to sites of inflammation in animal models (38, 39). On day 6 postinfection, a significant increase in Ly6G staining was seen in feet of WT mice treated with MK0812, compared with control treatment (Fig. 3E). This observation supports the view that a reduction in CCR2 signaling can lead to increased neutrophil infiltration following CHIKV infection.
Cartilage damage in CHIKV-infected CCR2−/− mice.
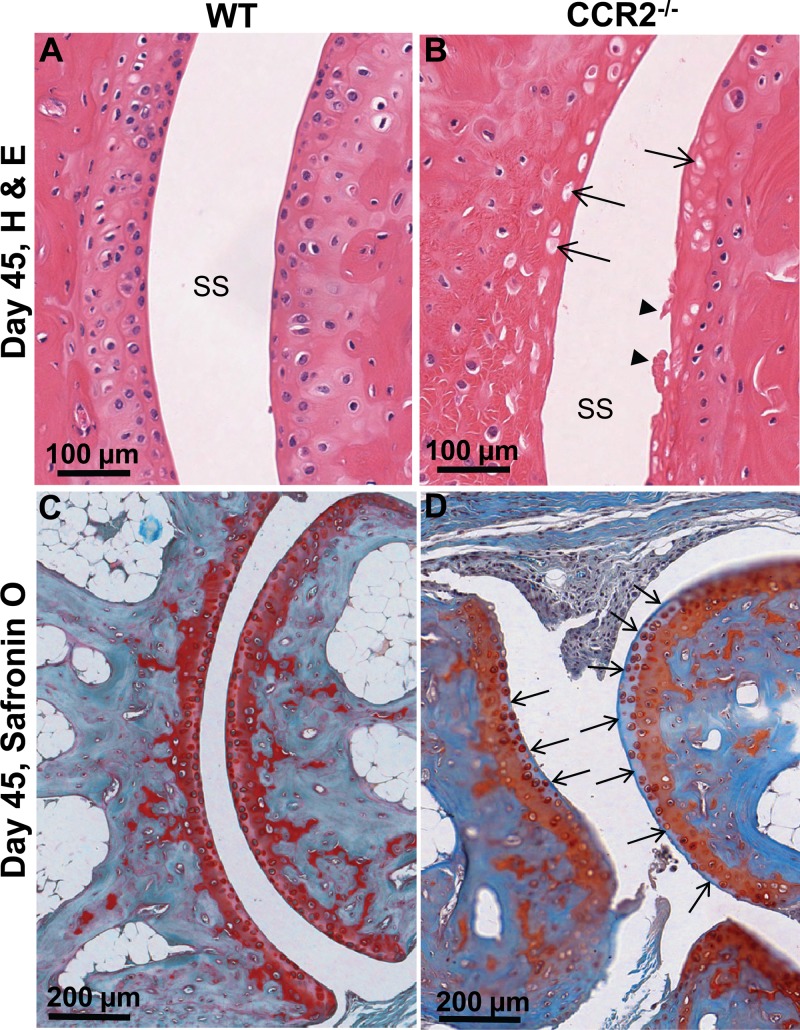
In contrast to most autoimmune arthritides, alphaviral arthritides (and most viral arthritides [1]) are generally not associated with joint damage (2). Joint damage was also not seen in CHIKV-infected WT mice in this study (6/6 feet examined) (Fig. 4A) or in previous studies (10, 31) or in CHIKV-infected monkeys (11). However, H&E staining of joints from CHIKV-infected CCR2−/− mice on day 45 postinfection often showed (in 6/6 feet examined) a loss of chondrocytes with empty lacunae in one or more foot joints (Fig. 4, arrows). Clear signs of cartilage damage at the articular surfaces (never seen in WT mice) was also occasionally observed in CCR2−/− mice (twice in 6 feet) (Fig. 4B, arrowhead). Loss of cartilage collagen (as revealed by Safranin O staining) was not observed in WT mice (Fig. 4C) but was clearly seen in at least 1, and occasionally 2, joints per foot in CCR2−/− mice (6 feet examined) (Fig. 4D, blue areas indicated by arrows). CCR2 deficiency thus increased the severity of CHIKV arthritis, with cartilage damage being clearly apparent.
FIG 4.
Cartilage damage in CCR2−/− feet. (A) H&E staining of joint from a WT mouse on day 45 postinfection. SS, synovial space. (B) H&E staining of a joint from a CCR2−/− mouse on day 45 postinfection. Arrows indicate empty chondrocytic lacunae within the hyaline cartilage. Arrowheads show cartilage damage at the articular surface. (C) Safranin O staining (cartilage) of a joint from a WT mouse on day 45 postinfection. (D) Safranin O staining (cartilage) of a joint from a CCR2−/− mouse on day 45 postinfection. Arrows show a loss of red collagen staining (replaced by blue staining) at the articular surfaces.
Antiviral Th1/Th2/Th17 responses in CHIKV-infected WT and CCR2−/− mice.
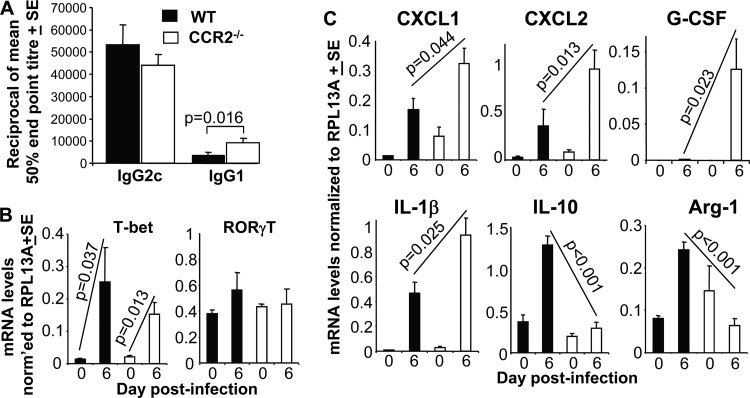
CHIKV infections induce a strongly Th1-biased immune response (10, 11), with CCR2−/− mice being reported to have impaired Th1 responses (20, 40, 41). However, analysis of anti-CHIKV IgG2c (Th1) and IgG1 (Th2) titers (32, 42) illustrated that the dominant IgG2c responses were not significantly different in CCR2−/− mice (Fig. 5A). Anti-CHIKV-specific IgG1 levels were, however, significantly elevated in CCR2−/− mice (Fig. 5A), perhaps consistent with the increased eosinophilia seen in these mice (Fig. 3D). Serum gamma interferon (IFN-γ) levels (measured as described previously [10]) and neutralizing antibody levels (measured as described previously [32]) were not significantly different between WT and CCR2−/− mice (data not shown).
FIG 5.
Immune and inflammatory mediator responses. (A) CHIKV-specific IgG2c and IgG1 titers at 7 weeks postinfection as determined by ELISA. Statistics were determined by a Mann-Whitney U test (n = 5 mice per group, assayed in duplicate). (B) qRT-PCR analysis of T-bet (Th1 T cell transcription factor) and RORγT (Th17 transcription factor) at the indicated times postinfection. The key is the same as the one shown in panel A. Statistics were determined by Kolmogorov-Smirnov tests (n = 4 to 5 mice and feet per group and time point, assayed in duplicate). (C) qRT-PCR analysis of the indicated species at the indicated times postinfection. The key is the same as the one shown in panel A. Statistics were determined by Kolmogorov-Smirnov or t tests (n = 4 to 5 mice and feet per group and time point, assayed in duplicate).
qRT-PCR analysis of T-bet, a key transcription factor for Th1 cells (43), showed a clear increase in WT mice on day 6 (peak arthritis) (Fig. 5B), consistent with the arthritogenic role of CD4 T cells and IFN-γ reported previously (33). However, for CCR2−/− mice, T-bet mRNA induction and levels were not significantly different from those of WT mice (Fig. 5B), supporting the view that anti-CHIKV Th1 responses were not significantly impaired in CCR2−/− mice.
Expansion of Th17 cells was reported previously to be responsible for the increase in neutrophil infiltration and exacerbated arthritic disease seen in collagen-induced arthritis in CCR2−/− mice (17). However, no significant increases in RORγT mRNA levels were observed in either mouse strain on day 6 postinfection (Fig. 5B). RORγT is a key transcription factor for Th17 cells (44).
Changes in inflammatory mediators in arthritic feet of CCR2−/− mice.
Increased levels of neutrophil infiltrates in CCR2−/− mice have been associated with elevated levels of (i) the neutrophil-attracting chemokines CXCL1 (KC) and/or CXCL2 (macrophage inflammatory protein 2 alpha) (16, 23, 24) and/or (ii) granulocyte colony-stimulating factor (G-CSF) (22), a key growth factor for neutrophil development and recruitment involved in inflammatory arthritis (45). Interleukin-1β (IL-1β) can also promote neutrophil recruitment (46), and IL-10 can inhibit neutrophil recruitment (47). qRT-PCR analyses showed that significantly higher levels of CXCL1, CXCL2, G-CSF, and IL-1β and lower levels of IL-10 were present in arthritic feet of CCR2−/− mice (Fig. 5C), suggesting that multiple mediators were involved in the switch from the predominantly monocyte/macrophage infiltrates to neutrophil infiltrates. (Serum IL-10 levels were also elevated earlier in the course of infection in WT mice [data not shown].) qRT-PCR analysis also showed that Arg-1 expression (a marker of M2 macrophages), although upregulated in WT feet, as expected (48), was not upregulated in CCR2−/− feet (Fig. 5C). M2 macrophages are associated with resolution of inflammation (34).
Global gene expression changes induced by CHIKV infection.
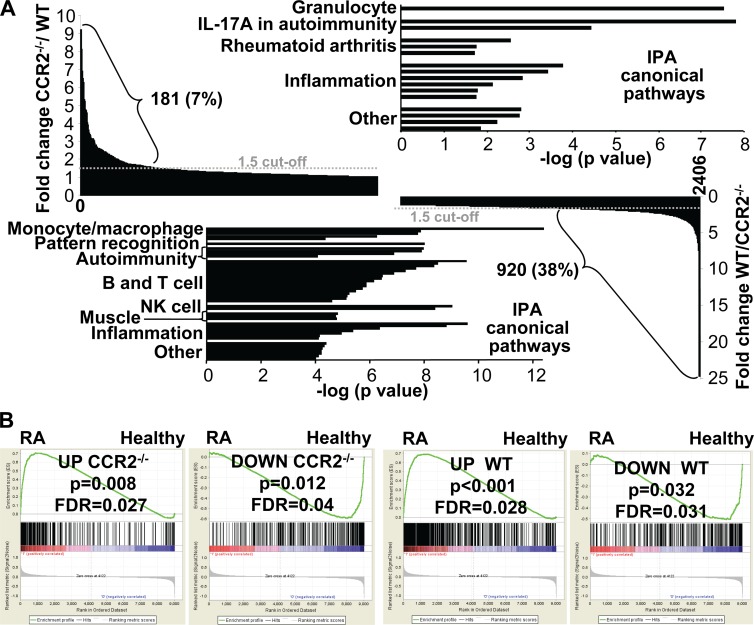
Microarray analysis was undertaken to determine what genes were up- and downregulated in arthritic feet of CHIKV-infected WT and CCR2−/− mice. The genes up- or downregulated on day 7 postinfection (1 day after peak arthritis to capture genes associated with resolution initiation) relative to day 0 were determined for both mouse strains. A total of 2,406 genes were up- or downregulated following CHIKV infection in WT and/or CCR2−/− mice. When the fold up- or downregulation of these differentially expressed genes (DEGs) was compared, 81% showed a <2-fold difference between WT and CCR2−/− mice, illustrating that most genes were similarly regulated in feet on day 7 postinfection in the two mouse strains. However, consistent with histological analyses, monocyte/macrophage-associated genes and pathways were more upregulated/significant in WT feet, and neutrophil-associated genes and pathways were more upregulated/significant in CCR2−/− feet (see Fig. S1 in the supplemental material). The CHIKV infection-associated induction of multiple chemokines and chemokine receptors was also different in the two mouse strains, with these differences again being consistent with the contrasting cellular infiltrates in WT and CCR2−/− mice (see Table S2 in the supplemental material).
To gain a better understanding of the differences in infection-associated gene regulation between CCR2−/− and WT mice, the differences in fold changes in gene expression between the two strains for all 2,406 DEGs were expressed as a ratio. For 181 genes (7.5% of all DEGs), the ratio of the fold change in CCR2−/− feet to the fold change in WT feet was >1.5 (Fig. 6, top left). For 920 genes (38% of all DEGs), the ratio of the fold change in WT feet to the fold change in CCR2−/− feet was >1.5. The former 181 genes thus represent genes that were more induced in CCR2−/− mice than in WT mice, and the latter 920 genes represent genes that were less induced in CCR2−/− mice than in WT mice. IPA of the 181 genes more induced in CCR2−/− mice revealed canonical pathways associated with granulocytes, IL-17A in autoimmunity, RA, and inflammation (Fig. 6, top; see also Table S3 in the supplemental material). Although autoimmune pathways were identified (consistent with the worse pathology), CCR2−/− mice did not develop antinuclear antibodies (data not shown). IPA of the 920 genes less induced in CCR2−/− feet revealed canonical pathways associated with monocyte/macrophage, pattern recognition, autoimmunity, B and T cell (mostly Th cell), NK cell, muscle, and inflammation pathways (Fig. 6, bottom; see also Table S4 in the supplemental material). Many of these pathways are consistent with the above-mentioned changes in infiltrates and inflammation. The identification of muscle pathways likely reflects increased muscle damage (33) in CCR2−/− feet. The identification of NK, T, and B cell pathways in this gene set also suggests reduced recruitment of these cells (10) into infected feet of CCR2−/− mice; T cells can also express CCR2 (49).
FIG 6.
Microarray analysis of arthritic feet from WT and CCR2−/− mice. (A) Genes differentially expressed on day 7 postinfection (compared to day 0) in WT and CCR2−/− feet were determined by microarray analysis. A total of 2,406 differentially expressed genes were up- or downregulated in WT and/or CCR2−/− feet (primary data available upon request). For 181 of these genes, the ratio of the fold change in CCR2−/− mice to the fold change in WT mice was >1.5 (top left) (for 603 DEGs, this ratio was >1). For 920 of these genes, the ratio of the fold change in WT mice to the fold change in CCR2−/− mice was >1.5 (bottom right) (for 1,794 genes, this ratio was >1, and for 9 genes, this ratio was 1). IPAs of the 181 genes more induced in CCR2−/− feet (top right) and the 920 genes less induced in CCR2−/− feet (bottom left) are shown, with pathways grouped into themes. Pathway details are shown in Tables S3 and S4 in the supplemental material. (B) Comparison of gene signatures of chikungunya virus (CHIKV) arthritis in WT and CCR2−/− mice with a gene signature from RA patients. Gene set enrichment analysis showed statistically significant enrichment of upregulated (UP) genes (163 genes in CCR2−/− mice and 341 genes in WT mice in “core enrichment”; overlap, 150) and downregulated (DOWN) genes (82 genes in CCR2−/− mice and 76 genes in WT mice in “core enrichment”) from CHIKV arthritis in the up- and downregulated genes, respectively, identified in synovial biopsy specimens from RA patients.
The same 181- and 920-gene sets were subjected to ingenuity upstream regulator analysis. STAT1 and -3 were identified as upstream regulators in both gene sets (see Tables S5 and S6 in the supplemental material), consistent with STAT1 and -3 regulating a distinct set of genes in monocytes/macrophages and neutrophils (50). Several muscle-associated upstream regulators were identified in the 181-gene set (with negative z-scores) and in the 920-gene set (with positive z-scores), again indicating increased muscle damage in CCR2−/− feet. NF-κB pathways were prominent upstream regulators identified in the 181-gene set (see Table S5 in the supplemental material), consistent with the NF-κB-dependent expression of the multiple chemokines and proinflammatory mediators seen in this gene set. Perhaps surprising (given no change in viral loads [Fig. 1C to E]) was the identification of STAT1, IRF1, IRF3, and IRF7 as upstream regulators in the 920 genes less induced in CCR2−/− mice (see Table S6 in the supplemental material). Reduced STAT1 signaling is perhaps consistent with the slightly lower IFN-α/β receptor and IFN-γ expression levels in CCR2−/− feet. Reduced interferon signaling is also supported by the lower overall fold upregulation of interferon-associated genes in CCR2−/− feet (see Fig. S2 in the supplemental material).
Comparison of gene expression profiles of feet from CHIKV-infected CCR2−/− and WT mice and of synovial biopsy specimens from human RA patients.
The DEGs identified for CCR2−/− mice and the DEGs identified for WT mice (both comparing day 7 with day 0) were each separately compared with a publicly available gene set from a microarray study of RA patients (33). Gene set enrichment analysis (GSEA) software was used to determine whether the rank-ordered list of genes in the RA study (i.e., genes ranked by their fold changes in expression levels in RA patients relative to those in healthy individuals) was enriched in the DEGs identified in CCR2−/− and WT mice. Nominal P values and false discovery rates (FDRs) are provided for comparisons of both up- and downregulated genes (Fig. 6B). In all cases, there was significant enrichment, suggesting that the high degree of similarity between the gene signatures of RA and CHIKV arthritis in WT mice (as reported previously [33]) was largely retained for CCR2−/− mice. (Previous analyses [33] used a consensus signature from 2 CHIKV isolates, whereas the data in Fig. 6B represent an analysis of the Reunion Island isolate alone to allow comparison with data from CCR2−/− mice.)
DISCUSSION
This paper describes the first analysis of viral arthritis in CCR2−/− mice and illustrates that a deficiency in this gene results in more severe, prolonged, and erosive arthritis. The prodigious monocyte/macrophage infiltrate seen in WT mice was almost entirely replaced by a neutrophil infiltrate in CCR2−/− mice, with this switch being associated with the dysregulation of a range of inflammatory and anti-inflammatory pathways. Recruitment of CCR2+ monocytes during CHIKV arthritis, although likely contributing to acute inflammatory disease (1, 2), thus also protects against even worse neutrophil-mediated pathology.
CXCL1, CXCL2, G-CSF, and IL-1β levels were increased in arthritic feet of CCR2−/− mice, whereas IL-10 levels were decreased, suggesting that multiple mediators were involved in promoting neutrophil recruitment (16, 22–24, 45–47). The reduction in IL-10 levels and the lack of Arg-1 induction also suggest paucities of (CCR2+-derived) M2 and resolving macrophages and their anti-inflammatory activities in CCR2−/− mice. This contention is supported by array data that show less upregulation of a number of M2 and resolving macrophage markers in CCR2−/− feet (e.g., transforming growth factor β [TGF-β], Ym1, and Msr1) (see Fig. S3 in the supplemental material). M2 macrophages have high efferocytosis activity, with efferocytosis being a key driver of inflammation resolution (34). The increase in ApoTag staining in CCR2−/− mice also suggests that efferocytosis is compromised in these mice, with CCL2 having previously been shown to promote efferocytosis (51). Evidence for secondary necrosis was also present in CCR2−/− feet (Fig. 2M) and usually occurs when efferocytosis is compromised. Secondary necrosis itself promotes inflammation by releasing a number of soluble inflammatory mediators (52).
The eosinophil infiltration that follows neutrophil infiltration in CCR2−/− mice may be promoted by the tissue damage induced by neutrophils and/or secondary necrosis and may help downregulate the inflammatory response in these animals (53). Eosinophilic arthritis has been reported previously and is usually associated with a benign course of disease (54).
Exactly how CCR2+ monocytes recruited during CHIKV infection, and/or the macrophages that develop from them (and/or other CCR2+ cells), might prevent excessive neutrophil infiltration and pathology in WT mice remains unclear. IL-10 may be involved (47) but is unlikely to be the only player, with foot swelling and neutrophil infiltration being only slightly elevated (the latter not significantly) in IL-10−/− mice (data not shown). Treatment of CCR2−/− mice with anakinra (an IL-1 receptor antagonist) had no significant effect on peak foot swelling (data not shown). Anti-inflammatory macrophages might suppress neutrophil recruitment by, inter alia, secreting (i) a series of proresolving lipid mediators (55) and/or (ii) proteases that cleave neutrophil-attracting chemokines (56). The reduction in interferon signaling seen in CCR2−/− mice may also be involved, as the loss of IFN-α/β receptor signaling has been shown to promote the production of neutrophil-recruiting cytokines/chemokines in a number of infections (57, 58). Treg cells may be involved, as (i) the depletion of CCR2+ Treg cells has been shown to exacerbate collagen-induced arthritis (49), (ii) anti-inflammatory macrophages can promote Treg expansion via TGF-β and IL-10 (56, 59), (iii) T cells are important in driving CHIKV arthritis (33), and (iv) Th cell pathways were less prominent in CCR2−/− mice (Fig. 6), with the microarray analysis being unable to differentiate between Th and Treg cells. The increased IgG1 responses in CCR2−/− mice also support the view that T cell activities were altered in CHIKV-infected CCR2−/− mice. Further work is needed before the mechanisms and cells involved and their relative importance are fully elucidated, with other cell types (e.g., myeloid-derived suppressor cells [56]) potentially also being involved. What does emerge from these studies is that in CHIKV arthritis (and perhaps other viral arthritides [1]), inflammation-recruited CCR2+ monocytes/macrophages appear to be important for limiting neutrophil recruitment into infected and inflamed joint tissues. This contrasts with observations in other arthritis models, where these cells promoted neutrophil recruitment (60).
Treatment of WT mice with clodronate (1 day prior to CHIKV infection) to deplete monocytes/macrophages was previously shown to reduce foot swelling on day 6/7 after CHIKV infection (10), data which would seem at odds with the observations presented here. However, the recent recognition that CD4 T cells and IFN-γ play important roles in driving arthritis in this CHIKV mouse model (33), and the ability of clodronate treatment to suppress Th1 responses (61), might suggest that the observation mentioned above (10) has more to do with reduced Th1 responses than reduced monocyte/macrophage infiltration (62). (Th1 responses were not significantly affected in CHIKV-infected CCR2−/− mice.) Clodronate treatment of WT mice also extended viremia (10), whereas viremia was not significantly different in CCR2−/− and WT mice. Clodronate treatment was recently shown to affect germinal centers (63), with compromised antibody responses perhaps explaining the extended viremia (10).
The reduction in interferon signaling in CCR2−/− mice was surprising, as IFN-α/β receptor-, STAT1-, IRF3-, and IRF7-associated signaling (but not IFN-γ [33]) are important for antiviral activity against CHIKV (31), and viral titers and RNA levels were unchanged in CCR2−/− mice (Fig. 1C to E). Macrophages are infected with CHIKV, whereas neutrophils are generally not, so viral RNA-mediated IRF3 and IRF7 stimulation via the cytoplasmic IPS-1 pathway (31) would not occur in neutrophils. In contrast to monocytes/macrophages, neutrophil Toll-like receptor 3 (TLR3) expression levels are also low in both humans (64) and mice (http://www.immgen.org/databrowser/index.html), with TLR3 signaling (upstream of IRF3 and -7) known to be active during CHIKV infection (31). Conceivably, the reduced interferon signaling thus simply reflects fewer CCR2+ monocytes/macrophages being infected and responding to infection in CCR2−/− feet, with infection of these cells making a relatively minor contribution to the overall viral load (Fig. 1C to E). One might speculate that resident tissue macrophages (whose recruitment is CCR2 independent) may be a more important target of CHIKV infection and/or persistence (11, 48).
The increase in IL-17 pathway activation in feet of CHIKV-infected CCR2−/− mice did not appear to be due to increased levels of Th17 cells (as was reported previously for collagen-induced arthritis [17]). Recently, immune complexes present during acute arthritis were shown to induce IL-17 production by neutrophils (65). Similar levels of neutralizing antibody responses were raised against CHIKV in WT and CCR2−/− mice (data not shown), so immune complexes are likely to be present in CCR2−/− mice and may thus be responsible for triggering IL-17 production by infiltrating neutrophils.
The widespread dysregulation of inflammatory processes observed in CHIKV-infected CCR2−/− mice suggests that care might be warranted when considering therapeutic agents that target CCR2/CCL2 for treatment of alphaviral arthritides (13) (and perhaps other arthritides [23]). The association of (i) the neutrophil-recruiting chemokines IL-8 (homolog of murine CXCL1) and CCL3, the levels of which were elevated in CCR2−/− mice (see Table S2 in the supplemental material), and (ii) IL-6, the level of which was higher in the serum of CCR2−/− mice than in the serum of WT mice postinfection (data not shown), with chronic human CHIKV arthropathy (66) perhaps adds to these concerns. Complications might also arise if patients who are being treated with such agents (for other conditions) become infected with an arthritogenic alphavirus.
Supplementary Material
ACKNOWLEDGMENTS
We thank Clay Winterford and his team (Histotechnology Facility) and our animal house staff (QIMR Berghofer) for their excellent support.
This work was funded by a project grant from the National Health and Medical Research Council, Australia, and the Australian Infectious Diseases Research Centre. Yee Suan Poo received a University of Queensland international scholarship and a University of Queensland research scholarship. Biosafety level 3 equipment was funded by the Queensland Tropical Health Alliance, an initiative of the Queensland government.
Footnotes
Published ahead of print 2 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.03364-13.
REFERENCES
- 1.Suhrbier A, Mahalingam S. 2009. The immunobiology of viral arthritides. Pharmacol. Ther. 124:301–308. 10.1016/j.pharmthera.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 2.Suhrbier A, Jaffar-Bandjee MC, Gasque P. 2012. Arthritogenic alphaviruses—an overview. Nat. Rev. Rheumatol. 8:420–429. 10.1038/nrrheum.2012.64 [DOI] [PubMed] [Google Scholar]
- 3.Suhrbier A, La Linn M. 2004. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr. Opin. Rheumatol. 16:374–379. 10.1097/01.bor.0000130537.76808.26 [DOI] [PubMed] [Google Scholar]
- 4.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. 2012. Chikungunya: a re-emerging virus. Lancet 379:662–671. 10.1016/S0140-6736(11)60281-X [DOI] [PubMed] [Google Scholar]
- 5.Tsetsarkin KA, Chen R, Sherman MB, Weaver SC. 2011. Chikungunya virus: evolution and genetic determinants of emergence. Curr. Opin. Virol. 1:310–317. 10.1016/j.coviro.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subasinghe NL, Lanter J, Markotan T, Opas E, McKenney S, Crysler C, Hou C, O'Neill J, Johnson D, Sui Z. 2013. A novel series of N-(azetidin-3-yl)-2-(heteroarylamino)acetamide CCR2 antagonists. Bioorg. Med. Chem. Lett. 23:1063–1069. 10.1016/j.bmcl.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 7.Panee J. 2012. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine 60:1–12. 10.1016/j.cyto.2012.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N, Vastolo V, Navas L, Garrone B, Mangano G, Biondi G, Guglielmotti A. 2012. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin. Exp. Metastasis 29:585–601. 10.1007/s10585-012-9473-5 [DOI] [PubMed] [Google Scholar]
- 9.Lebre MC, Vergunst CE, Choi IY, Aarrass S, Oliveira AS, Wyant T, Horuk R, Reedquist KA, Tak PP. 2011. Why CCR2 and CCR5 blockade failed and why CCR1 blockade might still be effective in the treatment of rheumatoid arthritis. PLoS One 6:e21772. 10.1371/journal.pone.0021772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner J, Anraku I, Le TT, Larcher T, Major L, Roques P, Schroder WA, Higgs S, Suhrbier A. 2010. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 84:8021–8032. 10.1128/JVI.02603-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, Le Grand R, Roques P. 2010. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Invest. 120:894–906. 10.1172/JCI40104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rulli NE, Guglielmotti A, Mangano G, Rolph MS, Apicella C, Zaid A, Suhrbier A, Mahalingam S. 2009. Amelioration of alphavirus-induced arthritis and myositis in a mouse model by treatment with bindarit, an inhibitor of monocyte chemotactic proteins. Arthritis Rheum. 60:2513–2523. 10.1002/art.24682 [DOI] [PubMed] [Google Scholar]
- 13.Rulli NE, Rolph MS, Srikiatkhachorn A, Anantapreecha S, Guglielmotti A, Mahalingam S. 2011. Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J. Infect. Dis. 204:1026–1030. 10.1093/infdis/jir470 [DOI] [PubMed] [Google Scholar]
- 14.Guabiraba R, Marques RE, Besnard AG, Fagundes CT, Souza DG, Ryffel B, Teixeira MM. 2010. Role of the chemokine receptors CCR1, CCR2 and CCR4 in the pathogenesis of experimental dengue infection in mice. PLoS One 5:e15680. 10.1371/journal.pone.0015680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin KL, Sweeney S, Kang BD, Ramsburg E, Gunn MD. 2011. CCR2-antagonist prophylaxis reduces pulmonary immune pathology and markedly improves survival during influenza infection. J. Immunol. 186:508–515. 10.4049/jimmunol.1001002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinones MP, Ahuja SK, Jimenez F, Schaefer J, Garavito E, Rao A, Chenaux G, Reddick RL, Kuziel WA, Ahuja SS. 2004. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J. Clin. Invest. 113:856–866. 10.1172/JCI20126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rampersad RR, Tarrant TK, Vallanat CT, Quintero-Matthews T, Weeks MF, Esserman DA, Clark J, Di Padova F, Patel DD, Fong AM, Liu P. 2011. Enhanced Th17-cell responses render CCR2-deficient mice more susceptible for autoimmune arthritis. PLoS One 6:e25833. 10.1371/journal.pone.0025833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong JH, Ratkay LG, Waterfield JD, Clark-Lewis I. 1997. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J. Exp. Med. 186:131–137. 10.1084/jem.186.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahrara S, Proudfoot AE, Park CC, Volin MV, Haines GK, Woods JM, Aikens CH, Handel TM, Pope RM. 2008. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J. Immunol. 180:3447–3456 http://www.jimmunol.org/content/180/5/3447.long [DOI] [PubMed] [Google Scholar]
- 20.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV, Jr, Broxmeyer HE, Charo IF. 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 100:2552–2561. 10.1172/JCI119798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popivanova BK, Kostadinova FI, Furuichi K, Shamekh MM, Kondo T, Wada T, Egashira K, Mukaida N. 2009. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Res. 69:7884–7892. 10.1158/0008-5472.CAN-09-1451 [DOI] [PubMed] [Google Scholar]
- 22.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. 2008. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111:5457–5466. 10.1182/blood-2008-01-136895 [DOI] [PubMed] [Google Scholar]
- 23.Fujii H, Baba T, Yamagishi M, Kawano M, Mukaida M. 2012. The role of a chemokine receptor, CCR2, in suppressing the development of arthritis in IL-1 receptor antagonist-deficient mice. Inflamm. Regen. 32:124–131. 10.2492/inflammregen.32.124 [DOI] [Google Scholar]
- 24.Montgomery RR, Booth CJ, Wang X, Blaho VA, Malawista SE, Brown CR. 2007. Recruitment of macrophages and polymorphonuclear leukocytes in Lyme carditis. Infect. Immun. 75:613–620. 10.1128/IAI.00685-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunay IR, Fuchs A, Sibley LD. 2010. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect. Immun. 78:1564–1570. 10.1128/IAI.00472-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. 2011. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am. J. Respir. Crit. Care Med. 183:234–242. 10.1164/rccm.201003-0416OC [DOI] [PubMed] [Google Scholar]
- 27.Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. 2005. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J. Immunol. 174:1549–1556 http://www.jimmunol.org/content/174/3/1549.long [DOI] [PubMed] [Google Scholar]
- 28.Lim JK, Obara CJ, Rivollier A, Pletnev AG, Kelsall BL, Murphy PM. 2011. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J. Immunol. 186:471–478. 10.4049/jimmunol.1003003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson BJ, Le TT, Dobbin CA, Banovic T, Howard CB, Flores FML, Vanags D, Naylor DJ, Hill GR, Suhrbier A. 2005. Heat shock protein 10 inhibits lipopolysaccharide-induced inflammatory mediator production. J. Biol. Chem. 280:4037–4047. 10.1074/jbc.M411569200 [DOI] [PubMed] [Google Scholar]
- 30.La Linn M, Bellett AJ, Parsons PG, Suhrbier A. 1995. Complete removal of mycoplasma from viral preparations using solvent extraction. J. Virol. Methods 52:51–54. 10.1016/0166-0934(94)00136-5 [DOI] [PubMed] [Google Scholar]
- 31.Rudd PA, Wilson J, Gardner J, Larcher T, Babarit C, Le TT, Anraku I, Kumagai Y, Loo YM, Gale M, Jr, Akira S, Khromykh AA, Suhrbier A. 2012. Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock. J. Virol. 86:9888–9898. 10.1128/JVI.00956-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Suhrbier A, Penn-Nicholson A, Woraratanadharm J, Gardner J, Luo M, Le TT, Anraku I, Sakalian M, Einfeld D, Dong JY. 2011. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine 29:2803–2809. 10.1016/j.vaccine.2011.01.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakaya HI, Gardner J, Poo YS, Major L, Pulendran B, Suhrbier A. 2012. Gene profiling of Chikungunya virus arthritis in a mouse model reveals significant overlap with rheumatoid arthritis. Arthritis Rheum. 64:3553–3563. 10.1002/art.34631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ariel A, Serhan CN. 2012. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front. Immunol. 3:4. 10.3389/fimmu.2012.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lidbury BA, Rulli NE, Suhrbier A, Smith PN, McColl SR, Cunningham AL, Tarkowski A, van Rooijen N, Fraser RJ, Mahalingam S. 2008. Macrophage-derived proinflammatory factors contribute to the development of arthritis and myositis after infection with an arthrogenic alphavirus. J. Infect. Dis. 197:1585–1593. 10.1086/587841 [DOI] [PubMed] [Google Scholar]
- 36.McGarry MP, Stewart CC. 1991. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J. Leukoc. Biol. 50:471–478 [DOI] [PubMed] [Google Scholar]
- 37.Zhao Q. 2010. Dual targeting of CCR2 and CCR5: therapeutic potential for immunologic and cardiovascular diseases. J. Leukoc. Biol. 88:41–55. 10.1189/jlb.1009671 [DOI] [PubMed] [Google Scholar]
- 38.Min SH, Wang Y, Gonsiorek W, Anilkumar G, Kozlowski J, Lundell D, Fine JS, Grant EP. 2010. Pharmacological targeting reveals distinct roles for CXCR2/CXCR1 and CCR2 in a mouse model of arthritis. Biochem. Biophys. Res. Commun. 391:1080–1086. 10.1016/j.bbrc.2009.12.025 [DOI] [PubMed] [Google Scholar]
- 39.Wisniewski T, Bayne E, Flanagan J, Shao Q, Wnek R, Matheravidathu S, Fischer P, Forrest MJ, Peterson L, Song X, Yang L, Demartino JA, Struthers M. 2010. Assessment of chemokine receptor function on monocytes in whole blood: in vitro and ex vivo evaluations of a CCR2 antagonist. J. Immunol. Methods 352:101–110. 10.1016/j.jim.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 40.Iijima N, Mattei LM, Iwasaki A. 2011. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl. Acad. Sci. U. S. A. 108:284–289. 10.1073/pnas.1005201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato N, Ahuja SK, Quinones M, Kostecki V, Reddick RL, Melby PC, Kuziel WA, Ahuja SS. 2000. CC chemokine receptor (CCR)2 is required for Langerhans cell migration and localization of T helper cell type 1 (Th1)-inducing dendritic cells. Absence of CCR2 shifts the Leishmania major-resistant phenotype to a susceptible state dominated by Th2 cytokines, B cell outgrowth, and sustained neutrophilic inflammation. J. Exp. Med. 192:205–218. 10.1084/jem.192.2.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schroder WA, Le TT, Major L, Street S, Gardner J, Lambley E, Markey K, MacDonald KP, Fish RJ, Thomas R, Suhrbier A. 2010. A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity. J. Immunol. 184:2663–2670. 10.4049/jimmunol.0902187 [DOI] [PubMed] [Google Scholar]
- 43.Lipscomb MW, Chen L, Taylor JL, Goldbach C, Watkins SC, Kalinski P, Butterfield LH, Wesa AK, Storkus WJ. 2009. Ectopic T-bet expression licenses dendritic cells for IL-12-independent priming of type 1 T cells in vitro. J. Immunol. 183:7250–7258. 10.4049/jimmunol.0901477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. 2011. The Th17 immune response is controlled by the Rel-RORgamma-RORgamma T transcriptional axis. J. Exp. Med. 208:2321–2333. 10.1084/jem.20110462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF, James WG, Metcalf D, Campbell IK, Wicks IP. 2008. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood 112:5193–5201. 10.1182/blood-2008-02-139535 [DOI] [PubMed] [Google Scholar]
- 46.Sadik CD, Kim ND, Iwakura Y, Luster AD. 2012. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcgammaR signaling. Proc. Natl. Acad. Sci. U. S. A. 109:E3177–E3185. 10.1073/pnas.1213797109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. 1999. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J. Immunol. 162:1685–1691 [PubMed] [Google Scholar]
- 48.Stoermer KA, Burrack A, Oko L, Montgomery SA, Borst LB, Gill RG, Morrison TE. 2012. Genetic ablation of arginase 1 in macrophages and neutrophils enhances clearance of an arthritogenic alphavirus. J. Immunol. 189:4047–4059. 10.4049/jimmunol.1201240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bruhl H, Cihak J, Schneider MA, Plachy J, Rupp T, Wenzel I, Shakarami M, Milz S, Ellwart JW, Stangassinger M, Schlondorff D, Mack M. 2004. Dual role of CCR2 during initiation and progression of collagen-induced arthritis: evidence for regulatory activity of CCR2+ T cells. J. Immunol. 172:890–898 http://www.jimmunol.org/content/172/2/890.long [DOI] [PubMed] [Google Scholar]
- 50.Hankey PA. 2009. Regulation of hematopoietic cell development and function by Stat3. Front. Biosci. 14:5273–5290. 10.2741/3597 [DOI] [PubMed] [Google Scholar]
- 51.Tanaka T, Terada M, Ariyoshi K, Morimoto K. 2010. Monocyte chemoattractant protein-1/CC chemokine ligand 2 enhances apoptotic cell removal by macrophages through Rac1 activation. Biochem. Biophys. Res. Commun. 399:677–682. 10.1016/j.bbrc.2010.07.141 [DOI] [PubMed] [Google Scholar]
- 52.Miyake Y, Yamasaki S. 2012. Sensing necrotic cells. Adv. Exp. Med. Biol. 738:144–152. 10.1007/978-1-4614-1680-7_9 [DOI] [PubMed] [Google Scholar]
- 53.Lotfi R, Herzog GI, DeMarco RA, Beer-Stolz D, Lee JJ, Rubartelli A, Schrezenmeier H, Lotze MT. 2009. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J. Immunol. 183:5023–5031. 10.4049/jimmunol.0900504 [DOI] [PubMed] [Google Scholar]
- 54.Vazquez-Trinanes C, Sopena B, Gonzalez-Gonzalez L, Diaz R, Rivera A, Freire M, Martinez-Vazquez C. 2013. Synovial fluid eosinophilia: a case series with a long follow-up and literature review. Rheumatology (Oxford) 52:346–351. 10.1093/rheumatology/kes236 [DOI] [PubMed] [Google Scholar]
- 55.Serhan CN. 2010. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177:1576–1591. 10.2353/ajpath.2010.100322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortega-Gomez A, Perretti M, Soehnlein O. 2013. Resolution of inflammation: an integrated view. EMBO Mol. Med. 5:661–674. 10.1002/emmm.201202382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brzoza-Lewis KL, Hoth JJ, Hiltbold EM. 2012. Type I interferon signaling regulates the composition of inflammatory infiltrates upon infection with Listeria monocytogenes. Cell. Immunol. 273:41–51. 10.1016/j.cellimm.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xin L, Vargas-Inchaustegui DA, Raimer SS, Kelly BC, Hu J, Zhu L, Sun J, Soong L. 2010. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J. Immunol. 184:7047–7056. 10.4049/jimmunol.0903273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.London A, Benhar I, Mattapallil MJ, Mack M, Caspi RR, Schwartz M. 2013. Functional macrophage heterogeneity in a mouse model of autoimmune central nervous system pathology. J. Immunol. 190:3570–3578. 10.4049/jimmunol.1202076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang B, Zinselmeyer BH, Runnels HA, LaBranche TP, Morton PA, Kreisel D, Mack M, Nickerson-Nutter C, Allen PM, Miller MJ. 2012. In vivo imaging implicates CCR2(+) monocytes as regulators of neutrophil recruitment during arthritis. Cell. Immunol. 278:103–112. 10.1016/j.cellimm.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brewer JM, Richmond J, Alexander J. 1994. The demonstration of an essential role for macrophages in the in vivo generation of IgG2a antibodies. Clin. Exp. Immunol. 97:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cote CH, Bouchard P, van Rooijen N, Marsolais D, Duchesne E. 2013. Monocyte depletion increases local proliferation of macrophage subsets after skeletal muscle injury. BMC Musculoskelet. Disord. 14:359. 10.1186/1471-2474-14-359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nikbakht N, Shen S, Manser T. 2013. Cutting edge: macrophages are required for localization of antigen-activated B cells to the follicular perimeter and the subsequent germinal center response. J. Immunol. 190:4923–4927. 10.4049/jimmunol.1300350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas CJ, Schroder K. 2013. Pattern recognition receptor function in neutrophils. Trends Immunol. 34:317–328. 10.1016/j.it.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 65.Katayama M, Ohmura K, Yukawa N, Terao C, Hashimoto M, Yoshifuji H, Kawabata D, Fujii T, Iwakura Y, Mimori T. 2013. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One 8:e62231. 10.1371/journal.pone.0062231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaaitanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, Ghosal SR, Muthumani K, Vijayachari P. 2011. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 24:265–271. 10.1089/vim.2010.0123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.