ABSTRACT
Robust activation of human immunodeficiency virus type 1 (HIV-1) gene expression occurs upon superinfection with Kaposi's sarcoma-associated herpesvirus (KSHV), a common AIDS-associated pathogen. Though the mechanisms underlying this phenotype remain unknown, several KSHV-encoded factors have been reported to stimulate HIV-1 long terminal repeat (LTR) activity. Here, we systematically evaluated the ability of KSHV tegument proteins to modulate the activation of an integrated HIV-1 LTR and revealed that the most potent individual activator is ORF45. ORF45 directs an increase in RNA polymerase II recruitment to the HIV-1 LTR, leading to enhanced transcriptional output. ORF45 is a robust activator of the p90 ribosomal S6 kinases (RSK), and we found that this activity is necessary but not sufficient to increase transcription from the LTR. Of the three widely expressed RSK isoforms, RSK2 appears to be selectively involved in LTR stimulation by both KSHV ORF45 and HIV-1 Tat. However, constitutively active RSK2 is unable to stimulate the LTR, suggesting that ORF45 may preferentially direct this kinase to a specific set of targets. Collectively, our findings reveal a novel transcriptional activation function for KSHV ORF45 and highlight the importance of RSK2 in shaping the transcriptional environment during infection.
IMPORTANCE Kaposi's sarcoma-associated herpesvirus (KSHV) is a prominent AIDS-associated pathogen. Previous studies have shown that infection of cells containing human immunodeficiency virus type 1 (HIV-1) with KSHV leads to potent stimulation of HIV-1 gene expression by activating the HIV-1 promoter, termed the long terminal repeat (LTR). Here, we compared the abilities of various KSHV proteins to activate gene expression from the HIV-1 LTR and found that KSHV ORF45 is the most potent activator. ORF45 is known to induce cell signaling through ribosomal S6 kinase (RSK) and enhance protein translation. However, we revealed that the activation of a specific isoform of RSK by ORF45 also leads to increased mRNA synthesis from the LTR by the host RNA polymerase. Collectively, our findings provide new insight into the interviral interactions between KSHV and HIV that may ultimately impact disease.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV) is a gamma-2 herpesvirus and is one of the seven known human cancer-causing viruses. In addition to several lymphoproliferative disorders, KSHV is the etiologic agent of Kaposi's sarcoma (KS), the most common AIDS-associated cancer (1–3). AIDS-associated KS is more clinically aggressive than KS that occurs in immunocompromised human immunodeficiency virus (HIV)-negative patients, and several studies have suggested that HIV plays a role in its pathogenesis (4). In particular, the HIV-1 Tat protein induces the expression of inflammatory cytokines that promote angiogenesis, as well as enhances KSHV infectivity for endothelial cells, and may thereby contribute to the highly aggressive nature of AIDS-associated KS (5–7). Furthermore, HIV infection or exogenous expression of Tat promotes KSHV reactivation in latently infected primary effusion lymphoma cells (8–10).
Some epidemiological studies have also found a positive correlation between KSHV coinfection and progression to AIDS, suggesting that KSHV may likewise influence the biology of HIV (11, 12). Indeed, KSHV infection of HIV-infected monocytic cell lines or peripheral blood mononuclear cells isolated from HIV-infected individuals induces HIV reactivation from a latent state (13). Although primarily lymphotropic, KSHV has been detected in a variety of cell types in vivo, including B cells, endothelial cells, and monocytes (14–17). Interestingly, there is accumulating evidence that KSHV can also infect T cells in vivo and human tonsillar CD4+ and CD8+ T cells, whether activated or resting, are susceptible to abortive KSHV infection ex vivo (18–21). Though it remains unclear whether KSHV can productively replicate in T cells, infection could nonetheless influence T-cell function via a variety of mechanisms. In particular, herpesviruses such as KSHV package a number of viral proteins that alter the cellular environment into their tegument, a region of the viral particle between the capsid and the envelope that is deposited directly into newly infected cells (22–24). The infection of T cells with KSHV may therefore have pathogenic relevance, particularly in HIV-infected patients.
While the molecular mechanisms by which KSHV activates HIV have yet to be elucidated, the activity of the HIV-1 long terminal repeat (LTR) is influenced by multiple KSHV gene products, including ORF45, replication and transcription activator (ORF50 or RTA), and latency-associated nuclear antigen (LANA) (25–27). Interestingly, all three KSHV proteins synergize with HIV-1 Tat to boost expression from the LTR, as well as activate a minimal LTR with the core promoter elements deleted. However, the relative contributions of these factors to LTR activation, as well as the mechanisms involved, remain unknown.
In the present study, we reveal that while multiple KSHV tegument proteins are capable of modulating LTR activity, the most robust activation is observed with ORF45. ORF45 has previously been shown to enhance translation through its ability to activate the cellular p90 ribosomal S6 kinase (RSK) (28, 29), and here we demonstrate that its expression also increases RNA polymerase II (RNAPII) recruitment to and transcription from an integrated HIV-1 LTR. Though not sufficient, the ability of ORF45 to bind and activate RSK is necessary for its LTR stimulation, indicating that its modulation of this kinase impacts multiple levels of gene expression. Using a series of dominant negative (DN) RSK isoforms, we show that RSK2 selectively impacts ORF45-induced transcriptional activation. ORF45 homologs in related gammaherpesviruses promote only weak RSK activation and fail to stimulate the HIV-1 LTR, demonstrating that this activity is specific to KSHV ORF45. Collectively, these findings provide mechanistic insight into how KSHV coinfection influences HIV-1 transcription, as well as identify a novel transcriptional activity for the KSHV ORF45 protein.
MATERIALS AND METHODS
Antibodies.
Anti-FLAG (clone M2; 1:5,000) and anti-β-tubulin (clone TUB 2.1; 1:3,000) antibodies were purchased from Sigma. All p90 RSK antibodies (p90RSK Antibody Sampler kit; 1:1,000) and the anti-histone H3 antibody (9715; 1:2,000) were purchased from Cell Signaling. RNAPII antibody (N20) was purchased from Santa Cruz Biotechnology. Anti-Strep antibody (StrepMAB-Classic; 1:1500) was purchased from IBA Life Sciences. Goat anti-rabbit and anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000) were purchased from Bio-Rad.
Cells, viruses, and plasmids.
HEK293T cells (American Type Culture Collection); iSLK cells, which are renal carcinoma cells that express a doxycycline-inducible RTA transgene (30, 31); iSLK-219 cells, which harbor recombinant KSHV.219 virus; and HeLa-based NH1 (32) and NH2 cells (33) were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) with 10% fetal calf serum (FBS; Invitrogen, HyClone). The NH1 cell line contains an integrated HIV-1 LTR-luciferase reporter construct. NH2 is a derivative of NH1 and harbors a constitutively expressed integrated Tat-hemagglutinin (HA) expression vector. The Jurkat-based cell line 1G5 containing an integrated HIV-1 LTR-luciferase reporter was maintained in RPMI 1640 (Invitrogen) with 10% FBS and 2 mM l-glutamine.
KSHV was reactivated from iSLK-219 cells by the addition of 1 μg/ml doxycycline to the medium for 5 days. For KSHV infection studies with NH1 and NH2 cells, supernatants from either doxycycline- or mock (distilled H2O [dH2O])-treated iSLK or iSLK-219 cells were filtered through 0.45-μm filters, supplemented with 8 μg/ml Polybrene, and placed on top of ∼70% confluent NH1 or NH2 cells. Cells were centrifuged for 2 h at 2,000 rpm at 25°C. Following spinfection, supernatants were replaced with DMEM containing 10% FBS. For experiments using clarified viral supernatants, iSLK-219 cells were reactivated as described above and the supernatants were split in two. One aliquot of virus was used for infection as described above, while KSHV virions were depleted from the second aliquot by ultracentrifugation at 100,000 × g for 1 h with a Beckman SW28 rotor as previously described (34). Heat inactivation of KSHV supernatants was carried out by incubation at 65°C for 1 h prior to infection.
cDNA expression constructs encoding known or predicted KSHV tegument proteins were cloned via reverse transcription (RT)-PCR from total RNA isolated from reactivated Trex BCBL-1 RTA cells. cDNA was cloned into pcDNA4/TO (Invitrogen) containing a C-terminal Strep tag (35). Epstein-Barr virus (EBV) FLAG-ORF45, KSHV FLAG-ORF45, and murine gammaherpesvirus 68 (MHV68) FLAG-ORF45 were cloned via RT-PCR from total RNA isolated from reactivated Akata cells, iSLK-219 cells, or MHV68-infected 3T3 cells, respectively. cDNA was cloned into pCDEF3. KSHV FLAG-tagged F66A mutant ORF45 was created by site-directed mutagenesis with PFU Ultra Polymerase. C- and N-terminal truncations of KSHV ORF45 were generated by PCR with KSHV FLAG-ORF45 as the template and cloned into pCDEF3. Wild-type (WT) and DN RSK expression constructs were generously provided by Warren J. Leonard of the National Heart, Lung, and Blood Institute (NIH) (36). pKH3-RSK2 and pKH3-RSK2–Y707A (constitutively active) were kindly provided by Deborah A. Lannigan at Vanderbilt University Medical Center (37).
HIV-1 LTR luciferase assays.
Luciferase assays were performed as previously described (33). Briefly, viral open reading frames (ORFs) or RSK plasmids were transfected into the NH1 and NH2 cell lines with Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instructions. At 48 h posttransfection, lysates were prepared from approximately equal number of cells and luciferase activity was determined with the Promega luciferase assay system. For luciferase assays with the Jurkat-based 1G5 cell line, 1 × 107 cells were electroporated in a Gene Pulser II (Bio-Rad Laboratories) at 250 V and 960 μF in 250 μl in a 0.4-cm cuvette with 5 μg of the plasmids indicated. Luciferase levels were determined at 24 h postelectroporation. All error bars represent standard deviations of the fold activation from three independent experiments.
Western blotting and immunoprecipitations.
Cells were lysed in NET-2 buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 3 mM MgCl2, 10% glycerol, 0.5% Nonidet P-40), and protein concentrations were determined by Bradford assay. Equivalent quantities of each sample were fractionated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and incubated with the appropriate antibodies. Western blot assays were developed with HRP-conjugated secondary antibodies and ECL reagents (Pierce).
For immunoprecipitations, whole-cell extracts prepared in NET-2 buffer were incubated overnight with either anti-FLAG or anti-HA antibody-conjugated agarose beads (Sigma). Beads were washed extensively with NET-2 buffer and then eluted with FLAG peptide (Sigma).
ChIP and qPCR.
Chromatin immunoprecipitation (ChIP) was performed essentially as described previously (38), except that a Covaris focused sonicator was used for chromatin shearing. Following reversal of cross-links, DNA was purified with a PCR purification spin column (Fermentas) and resuspended in 50 μl of dH2O; 1 to 2 μl of DNA was used for quantitative PCR (qPCR) with the DyNAmo ColorFlash SYBR green qPCR kit (Thermo Scientific) with primers located within either the HIV-1 LTR or luciferase. Signals obtained by qPCR were normalized to the input DNA.
For analysis of gene expression by RT-qPCR, total RNA was isolated with TRIzol (Invitrogen) in accordance with the manufacturer's instructions. cDNA was synthesized from 1 μg of RNA with random hexamers (Integrated DNA Technologies) and SuperScript II reverse transcriptase (Invitrogen). qPCR was performed as described above.
RESULTS
KSHV infection drives transcription from the HIV-1 LTR.
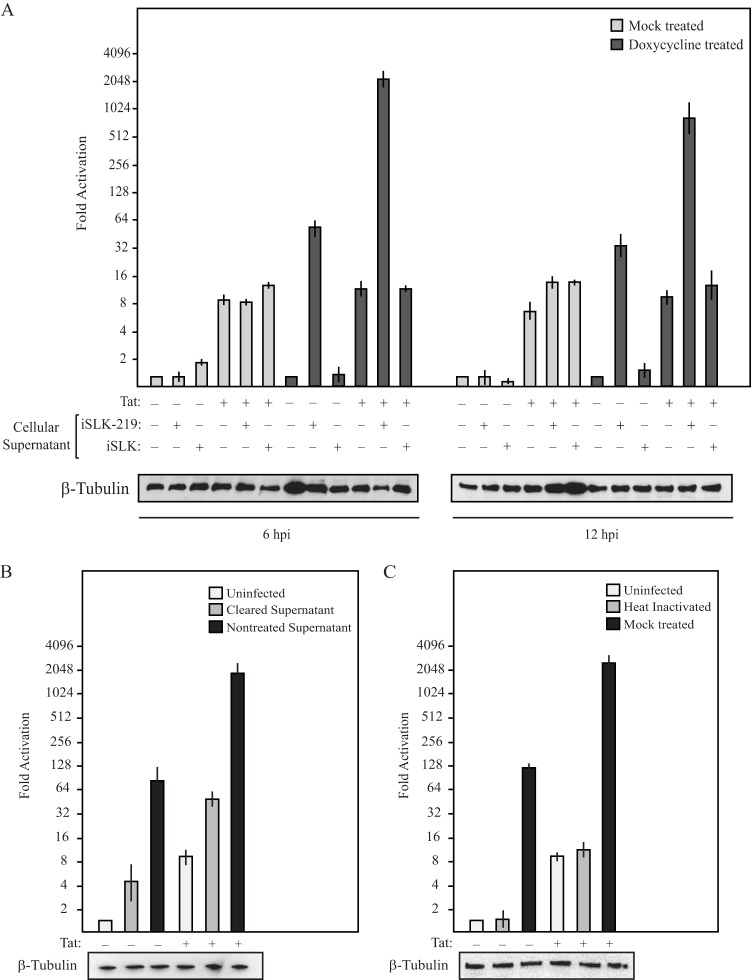
To explore the mechanisms underlying KSHV-induced activation of the HIV-1 LTR, we took advantage of two HeLa-based cell lines containing a stably integrated HIV-1 LTR-driven firefly luciferase reporter gene in either the absence (NH1) or the presence (NH2) of Tat (32, 33). This well-characterized system allowed us to monitor both basal (Tat-independent) and Tat-stimulated transcription from the integrated HIV-1 LTR. To verify that KSHV was able to modulate the activity of the HIV-1 LTR in this system, we incubated NH1 and NH2 cells with supernatants derived from a doxycycline-inducible KSHV producer cell line (iSLK-219) or a matched, KSHV-negative control line (iSLK). Luciferase levels were monitored at various time points postaddition of supernatants from the producer and control cells that had been either mock or doxycycline treated. As shown in Fig. 1A, NH1 and NH2 cells incubated with supernatants from reactivated KSHV-positive iSLK-219 cells resulted in extremely robust activation of the HIV-1 LTR compared to cells incubated with control supernatants. LTR activation reached a maximal level at 6 h postinfection, where KSHV infection caused 58- and 171-fold induction of basal and Tat-stimulated luciferase activity, respectively. Additionally, depletion of KSHV virions from the infectious supernatants by ultracentrifugation, as well as heat inactivation of viral supernatants, significantly reduced the ability of the supernatants to increase luciferase levels (Fig. 1B and C). It is possible that the moderate level of LTR activation from the KSHV-cleared supernatants is due to secreted factors produced during KSHV lytic infection or, alternatively, to a low level of residual viral particles remaining after ultracentrifugation. Nonetheless, these results indicate that KSHV infection of NH1 and NH2 cells can robustly stimulate the HIV-1 LTR.
FIG 1.
KSHV infection enhances the activity of the HIV-1 LTR. (A) Supernatants from doxycycline- or mock-treated KSHV producer iSLK-219 cells were used to infect NH1 and NH2 cells. As an additional control, NH1 and NH2 cells were treated with supernatants from similarly treated iSLK cells. Luciferase levels were determined at 6 and 12 h postinfection. Western blot analysis of β-tubulin served as a loading control. (B) NH1 and NH2 cells were mock treated or incubated with supernatants from reactivated iSLK-219 cells that were either left untreated or cleared of virus by ultracentrifugation. Luciferase levels were determined at 6 h postinfection. Western blot analysis of β-tubulin served as a loading control. (C) NH1 and NH2 cells were mock treated or incubated with viral supernatants that were heat inactivated or mock treated. Western blot analysis of β-tubulin served as a loading control.
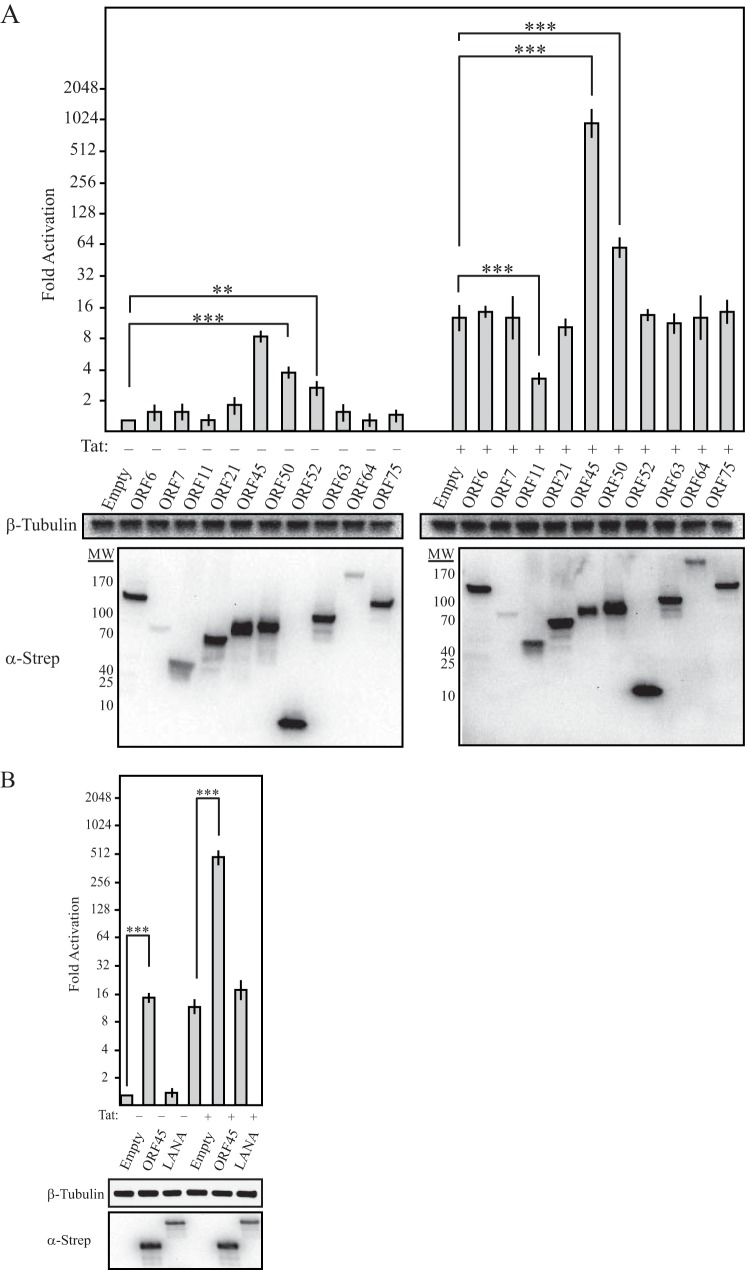
The fact that LTR activation occurred during the first 6 h postinfection suggested that one or more components of the KSHV tegument may drive this phenotype, as these factors are deposited directly into newly infected cells. In addition, while HeLa cells can be infected with KSHV, they are not permissive for lytic KSHV replication and thus presumably express a limited repertoire of lytic-cycle genes (39). Two KSHV tegument proteins, ORF45 and ORF50, were previously shown to enhance the activity of the HIV-1 LTR. However, there has been no systematic screening to measure the relative influence of KSHV tegument proteins on LTR activity, nor has their mechanism of LTR activation been determined. We therefore screened a collection of 10 C-terminally Strep-tagged KSHV ORFs representing established or predicted tegument proteins for the ability to activate either basal or Tat-activated transcription from the HIV-1 LTR (22). Western blot analysis confirmed the expression of all ORFs (Fig. 2A, bottom). Notably, ORF45 was the most robust LTR activator in this screen, increasing basal activation 10-fold and Tat transactivation 54-fold (Fig. 2A). We also detected ORF50 activity, as predicted, as well as ORF52 activity. However, ORF52 selectively affected basal transcription, as it failed to augment LTR activity in the presence of Tat. The screen also identified the uncharacterized protein ORF11 as a candidate inhibitor of Tat transactivation. Finally, we compared the activity of ORF45 and that of the KSHV major latency protein LANA, which was previously shown to enhance Tat-dependent activation of an HIV-1 LTR reporter plasmid. However, in our system, LANA failed to activate the HIV-1 LTR (Fig. 2B). It is possible that the effects of LANA on the LTR are cell type specific, though we also note that the previous study used a transfected reporter plasmid rather than a system in which the LTR was integrated into the host chromatin.
FIG 2.
Effects of KSHV tegument proteins on the HIV-1 LTR. (A and B) NH1 and NH2 cells were transfected with the plasmids indicated. At 48 h posttransfection, cell lysates were prepared and luciferase levels were determined. Expression of transfected ORFs was detected by anti-Strep Western blot analysis. Western blot analysis of β-tubulin served as a loading control. Statistical significance was determined by Student t test (**, P < 0.001; ***, P < 0.0001). MW, molecular weight in thousands.
Given that ORF45 was the most robust LTR activator in our screen, we decided to focus on this protein and its mechanism of action. To rule out any potential effect of the expression vector or epitope tag, ORF45 was cloned into a separate expression vector with an N-terminal FLAG tag. As expected, FLAG-ORF45 also produced robust stimulation of both basal and Tat-activated HIV-1 LTR activity, increasing luciferase levels 10- and 61.5-fold, respectively (Fig. 3A). A titration of ORF45 showed that LTR activation occurred in a dose-dependent manner, with clear basal activation detected with 200 ng of ORF45 plasmid and stimulation of Tat transactivation detected with as little as 50 ng of ORF45 plasmid (Fig. 3B).
FIG 3.
KSHV FLAG-ORF45 robustly activates the HIV-1 LTR. (A) KSHV FLAG-ORF45 was transfected into either NH1 or NH2 cells. At 48 h posttransfection, cell lysates were prepared and luciferase levels were determined. (B) Cells were transfected with the amounts of KSHV FLAG-ORF45 indicated. At 48 h posttransfection, cell lysates were prepared and luciferase levels were determined. Western blot analysis of β-tubulin served as a loading control.
KSHV ORF45 promotes transcriptional activation of the HIV-1 LTR.
The HIV-1 LTR is heavily regulated, particularly at the stage of transcription elongation, and has served as a model for understanding mechanisms of transcriptional control (40). Thus, we evaluated whether ORF45 was altering the transcriptional activity of the HIV-1 LTR. Luciferase assays are not a direct measure of transcriptional activity, as they represent a gene expression endpoint that is a composite of multiple upstream events, including transcription, RNA processing and stability, and translation. In addition, ORF45 has previously been shown to stimulate translation, and thus it was possible that ORF45 was simply augmenting the translation of preexisting luciferase mRNA (29). We therefore used RT-qPCR to measure levels of luciferase mRNA in cells transfected with either an empty vector or FLAG-ORF45. In support of a transcriptional effect, expression of ORF45 resulted in an ∼3-fold increase in mRNA levels for both basal and Tat-stimulated transcription (Fig. 4B).
FIG 4.
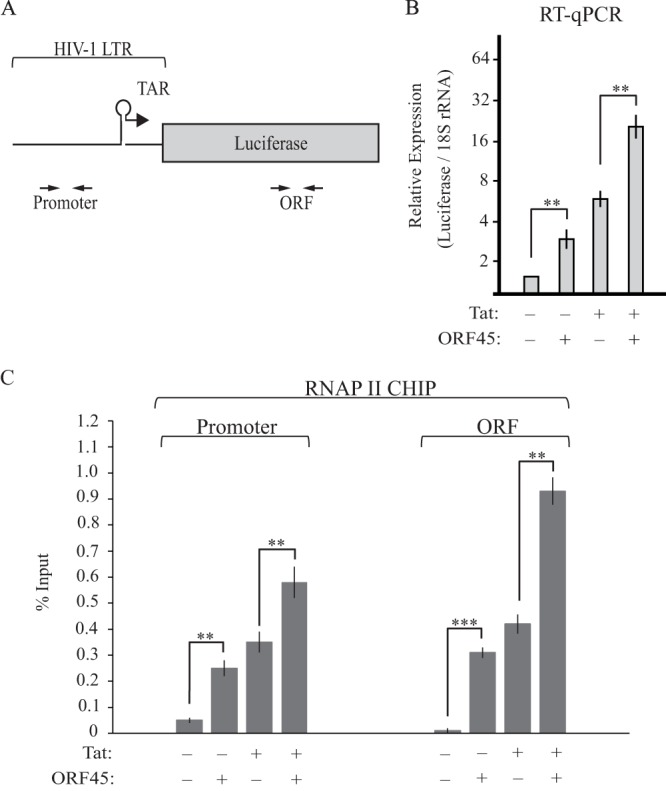
KSHV ORF45 transcriptionally activates the HIV-1 LTR. (A) Schematic representation of the integrated HIV-1 LTR reporter. The positions of the primers used for RT-qPCR and ChIP analysis are shown. (B) Total RNA from NH1 or NH2 cells transfected with either KSHV FLAG-ORF45 or the empty vector was subjected to RT-qPCR. Luciferase mRNA levels were normalized to 18S rRNA levels. (C) NH1 and NH2 cells were transfected with either the empty vector or KSHV FLAG-ORF45. At 48 h posttransfection, RNAPII ChIP analysis was performed. Immunoprecipitated DNA was detected by qPCR with primers located within the HIV-1 LTR or luciferase gene body (ORF). Statistical significance was determined by Student t test (**, P < 0.001; ***, P < 0.0001).
To evaluate the effect of ORF45 on LTR transcription directly, as opposed to alterations in RNA processing or stability, we next measured the amount of RNAPII on the HIV-1 LTR in either the absence or presence of ORF45 by ChIP (Fig. 4C). Expression of ORF45 resulted in an increase in RNAPII occupancy on both the HIV-1 LTR and within the luciferase ORF, confirming that it enhanced transcription (Fig. 4C). We were unable to detect ORF45 on the HIV-1 LTR or gene body by ChIP (data not shown), suggesting that it is not a direct transcriptional activator.
ORF45-induced RSK activation is necessary but not sufficient for activation of the HIV-1 LTR.
KSHV ORF45 is a potent activator of the p90 RSKs (28). To evaluate the role of RSK activation in ORF45-mediated induction of the HIV-1 LTR, we tested the activity of two mutant ORF45 proteins, a RSK activation-competent C-terminal truncation (amino acids 1 to 120) and an N-terminal truncation (amino acids 101 to 407) that is unable to activate RSK (Fig. 5A). Although the expression level of the C-terminal mutant protein was well below that of full-length ORF45, it nonetheless activated RSK with efficiency similar to that of the full-length protein (Fig. 5A). However, unlike full-length ORF45, neither mutant protein was able to stimulate the HIV-1 LTR, suggesting that RSK activation is not sufficient to mediate LTR induction (Fig. 5B). We next tested whether the ability to activate RSK is required for ORF45-induced LTR activation with the F66A mutant ORF45 protein. As previously reported (29), we found F66A mutant ORF45 to be defective in both RSK binding and subsequent activation (Fig. 5C and D). Additionally, it was unable to increase the activity of the HIV-1 LTR in luciferase assays (Fig. 5E) and failed to increase RNAPII occupancy on both the HIV-1 LTR and within the luciferase gene body (Fig. 5F). Thus, RSK activation by ORF45 is necessary but not sufficient to promote activation of the HIV-1 LTR.
FIG 5.
RSK activation is necessary but not sufficient to transcriptionally activate the HIV-1 LTR. (A) Cell lysates from HEK293T cells transfected with the plasmids indicated were analyzed by Western blotting with RSK phosphorylation-specific antibodies to pT359/S363, pS380, total RSK, and FLAG. (B) NH1 and NH2 cells were transfected with the plasmids indicated. Luciferase levels were determined at 48 h posttransfection. (C) Whole-cell extracts from HEK293T cells transfected with either WT or F66A mutant ORF45 were subjected to either anti-FLAG or anti-HA immunoprecipitation. Inputs and immunoprecipitated material were analyzed by Western blotting with antibodies against FLAG, RSK, and histone H3. (D) Lysates of HEK293T cells transfected with the plasmids indicated were analyzed by Western blotting with phosphorylation-specific antibodies to pT359/S363 or pS380, total RSK, and FLAG. (E) NH1 and NH2 cells were transfected with either WT or F66A mutant ORF45. At 48 h posttransfection, luciferase levels were determined. (F) NH1 and NH2 cells were transfected with either WT or F66A mutant ORF45. At 48 h posttransfection, RNAPII ChIP analysis was performed. Immunoprecipitated DNA was detected by qPCR with primers located within the HIV-1 LTR or luciferase gene body (ORF). (G) Jurkat 1G5 cells were transfected with the plasmids indicated, and luciferase levels were determined at 24 h posttransfection. Western blot analysis of β-tubulin served as a loading control. (H) RT-qPCR analysis of RNA extracted from panel G. Statistical significance was determined by Student t test (**, P < 0.001; ***, P < 0.0001).
To confirm that our findings were not limited to HeLa cells, we tested whether they could be recapitulated in T cells, which are more relevant for studying HIV infection. To this end, we used the 1G5 Jurkat T-cell model of HIV-1 transcription because it also contains an integrated luciferase gene under the transcriptional control of the HIV-1 LTR (41). Importantly, transfection of WT but not F66A mutant ORF45 was capable of enhancing both basal and Tat-dependent transactivation of the HIV-1 LTR in 1G5 Jurkat cells (Fig. 5G and H). Thus, ORF45 mediates RSK-dependent transcriptional activation in the HIV-1 LTR in both the HeLa and T-cell models.
ORF45-induced HIV-1 LTR activation is mediated in part by RSK2.
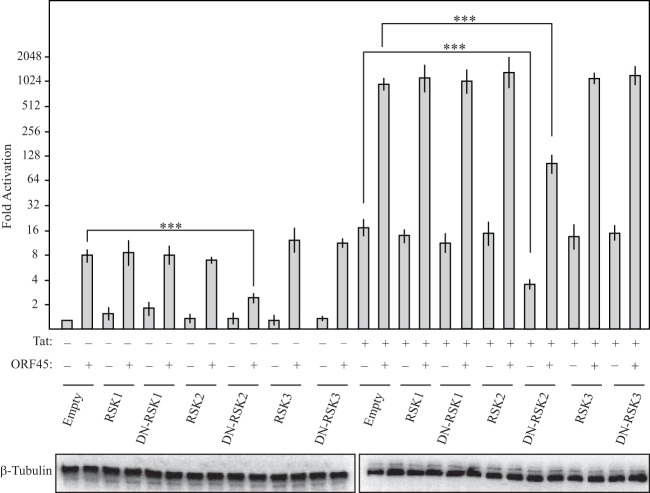
Mammals contain four RSK isoforms; RSK1 to -3 are expressed ubiquitously, while the expression of RSK4 is variable and tissue specific (42, 43). ORF45 has been shown to interact with RSK1 and RSK2 (28), suggesting that one or both of these isoforms might be required for LTR activation. We reasoned that if a particular RSK isoform were involved in ORF45-mediated induction of the LTR, then selective inhibition of that isoform should restrict LTR activation. We therefore took advantage of a series of well-defined dominant negative (DN) versions of constitutively expressed RSK1, RSK2, and RSK3 (36). Plasmids expressing WT or DN versions of the RSK proteins were transfected into NH1 and NH2 cells alone or together with FLAG-ORF45, and HIV-1 LTR activity was assessed 48 h later. Indeed, expression of DN RSK2 reduced ORF45-mediated induction of both basal and Tat-activated transcription by greater than 50%, whereas none of the WT or mutant RSKs impacted the degree of LTR activation. Interestingly, DN RSK2 also reduced the ability of Tat to transactivate the HIV-1 LTR in the absence of ORF45 (Fig. 6), which is notable because Tat has similarly been shown to bind activated RSK2 (44). These data suggest that RSK2 is the primary, and perhaps sole, RSK isoform involved in activation of the HIV-1 LTR.
FIG 6.
ORF45 mediates activation of the HIV-1 LTR through RSK2. NH1 and NH2 cells were transfected with either WT or DN RSK1, RSK2, or RSK3. At 48 h posttransfection, luciferase levels were determined. Western blot analysis of β-tubulin served as a loading control. Statistical significance was determined by Student t test (***, P < 0.0001).
An ORF45-RSK2 complex is required for transactivation of the HIV-1 LTR.
Given our observation that RSK2 was directly involved in ORF45-induced LTR activation, we considered whether the ORF45 C-terminal truncation described in Fig. 5 might be defective because of a selective failure to activate RSK2. This was a possibility because the phosphospecific antibodies cross-react with multiple RSK isoforms, and thus a change in isoform specificity would be missed. To test this hypothesis, we evaluated whether a constitutively active version of RSK2 promoted LTR activation. The C terminus of RSK2 contains an autoinhibitory domain; the Y707A mutation within this region results in a constitutively active kinase (37). Although expression of Y707A mutant RSK2 led to RSK activation as measured by Western blotting for phospho-RSK, it failed to enhance transcription from the HIV-1 LTR (Fig. 7A and B). These data confirmed that RSK2 activation, while necessary, is not sufficient to activate the LTR in the absence of ORF45. They further suggest that an ORF45-RSK2 complex may be required for HIV-1 LTR transactivation. Indeed, coexpression of F66A mutant ORF45, which cannot interact with RSK, with either WT or constitutively active RSK2 failed to activate the HIV-1 LTR (Fig. 7C). Taken in aggregate, our data suggest that through its interaction with RSK2, ORF45 directs a unique signaling outcome distinct from that induced by the constitutively active version of RSK2.
FIG 7.

An ORF45-RSK2 complex is necessary for activation of the HIV-1 LTR. (A) Western blot analysis of HEK293T cell extracts prepared from cells transfected with either WT or Y707A mutant RSK2. Lysates were prepared from serum-starved cells and analyzed by Western blotting with RSK phosphorylation-specific antibodies to pT359/S363 or pS380, and total RSK. (B) NH1 and NH2 cells were transfected with either WT or Y707A mutant RSK2. At 48 h posttransfection, luciferase levels were determined. Western blot analysis of β-tubulin served as a loading control. (C) NH1 and NH2 cells were transfected with F66A mutant ORF45 and either WT or Y707A mutant RSK2. At 48 h posttransfection, luciferase levels were determined. Western blot analysis of β-tubulin served as a loading control.
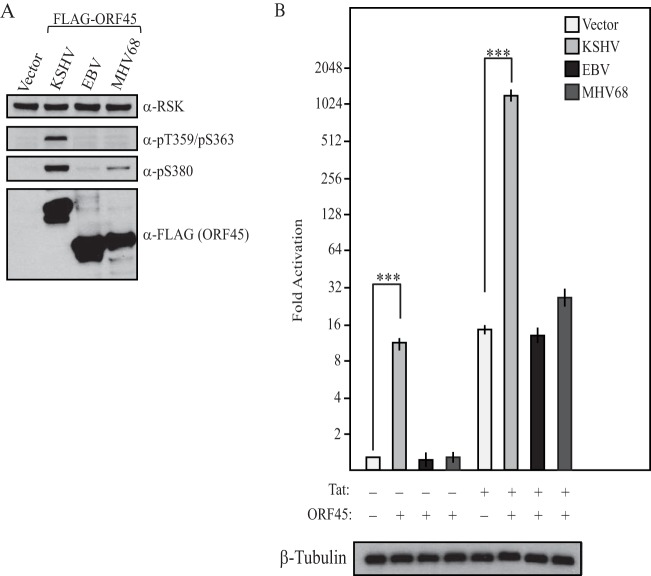
Related gammaherpesvirus ORF45s fail to activate the p90 RSKs and transcription from the HIV-1 LTR.
KSHV ORF45 homologues are absent from both the alpha- and betaherpesviruses. However, ORF45 homologs have been identified in numerous gammaherpesviruses, including EBV and MHV68, where they are 15 and 17% identical, respectively. Whether the ORF45 homologs are similarly capable of activating the p90 RSKs or the HIV-1 LTR is unknown (45, 46). We therefore compared these activities of the three gammaherpesvirus homologs from KSHV, EBV, and MHV68 (Fig. 8). Unlike KSHV ORF45, the EBV and MHV68 ORF45 homologs failed to robustly activate the p90 RSKs (Fig. 8A). Furthermore, neither EBV nor MHV68 ORF45 was able to activate the HIV-1 LTR, suggesting that its degree of RSK2 activation was insufficient and/or that its potential association with RSK does not direct the same signaling outcome as that of KSHV ORF45 (Fig. 8B). Thus, the ability to both robustly activate the p90 RSKs and enhance the transcriptional activity of the HIV-1 LTR is specific to KSHV ORF45.
FIG 8.
Activation of p90 RSKs and the HIV-1 LTR is specific to KSHV ORF45. (A) The empty vector, KSHV FLAG-ORF45, EBV FLAG-ORF45, or MHV68 FLAG-ORF45 was transfected into HEK293T cells. At 24 h posttransfection, the medium was replaced with serum-free medium and the cells were maintained for an additional 24 h. Lysates of serum-starved cells were prepared and analyzed by Western blotting with RSK phosphorylation-specific antibodies to pT359/S363 or pS380, total RSK, and FLAG. (B) The empty vector, KSHV FLAG-ORF45, EBV FLAG-ORF45, or MHV68 FLAG-ORF45 was transfected into NH1 or NH2 cells, and luciferase levels were determined at 48 h posttransfection. Western blot analysis of β-tubulin served as a loading control. Statistical significance was determined by Student t test (***, P < 0.0001).
DISCUSSION
KSHV stimulates HIV-1 reactivation in coinfection experiments, and several studies indicate that HIV-1 and KSHV may target similar populations of cells in vivo, suggesting that this interplay is biologically relevant (13, 14, 18–20). Here, we dissected the relative contributions of individual KSHV tegument proteins to HIV-1 LTR activation, revealing that multiple KSHV proteins are capable of modulating the activity of the HIV-1 LTR both positively (ORF45, ORF50, and ORF52) and negatively (ORF11). However, KSHV ORF45 was found to be the most potent individual modulator of the HIV-1 promoter. In addition to stimulating basal LTR activity, ORF45 robustly synergized with the HIV-1 transcription elongation factor Tat to enhance the activity of the LTR ∼1,000-fold in both HeLa cells and in Jurkat T cells. Thus, our findings uncover a novel transcriptional activation function for ORF45 that presumably complements its previously described ability to boost translation (29). Both outcomes rely on ORF45-induced activation of RSK, indicating that manipulation of this kinase upon KSHV infection impacts multiple stages of the gene expression cascade. Our results further suggest that ORF45 may direct activated RSK2 to a key subset of signaling targets that are perhaps distinct from those induced by other mitogenic stimuli. Revealing how viral proteins such as ORF45 impact RSK signaling is relevant for understanding mechanisms underlying both viral pathogenesis and a number of nonviral diseases linked to constitutive RSK signaling. For example, aberrant RSK activation contributes to multiple malignant conditions, including various breast and ovarian cancers and myeloma (47–50). Additionally, inactivating mutations in the RSK2-encoding gene are responsible for Coffin-Lowry syndrome, which is characterized by severe mental retardation and progressive skeletal deformations (51–53).
RSKs are a family of Ser/Thr kinases activated through the mitogen-activated protein kinase signal transduction pathway (54). The binding of ORF45 to RSK increases the association of extracellular signal-regulated kinase with RSK in a manner that shields these kinases against dephosphorylation, resulting in a high level of sustained RSK activity (55). Of the four mammalian RSK isoforms, ORF45 interacts with RSK1 and RSK2 (28). Our data indicate that RSK2 is specifically involved in the transcriptional induction of the HIV-1 LTR, suggesting that RSK1 may instead be used by ORF45 to manipulate separate cellular processes during infection. RSK2 targets multiple proteins with a diverse range of functions, including the modulation of gene expression. Prominent among its targets are transcription factors and coactivators, including ATF4, CBP, CREB, and c-fos, as well as several chromatin modification enzymes (54). However, our finding that constitutively active RSK2 Y707A fails to activate the HIV-1 LTR suggests that ORF45 may redirect RSK2 to novel substrates distinct from those triggered by other mitogenic stimuli. In this model, ORF45 would function as a RSK2 scaffolding protein and direct the RSK2-mediated phosphorylation of novel substrates or otherwise low-abundance RSK2 targets that are then capable of modulating HIV-1 transcription. Indeed, scaffolding proteins are a common theme in signal transduction pathways and serve as a mechanism to direct, or enhance, the signal toward a particular outcome (56). In this regard, KSHV is known to alter the substrate specificity of host-encoded cyclin-dependent kinases through KSHV-encoded v-cyclin (57–59).
ORF45 has previously been shown to enhance cellular gene expression by promoting the RSK-mediated phosphorylation of eukaryotic translation initiation factor 4B (eIF4B), which promotes 40S ribosomal subunit recruitment to mRNA and subsequently facilitates protein translation (29, 60). Although our data indicate that ORF45 promotes transcriptional activation of the LTR, translational enhancement may serve to increase the magnitude of LTR-driven gene expression. For instance, translational enhancement could contribute to the robust synergism between ORF45 and Tat, as the increase in luciferase mRNA is 20-fold yet luciferase levels increase ∼1,000-fold. That said, it is difficult to make a direct comparison of mRNA levels and translational output, as small increases in mRNA can result in large increases in protein levels. Thus, KSHV ORF45 is a two-pronged activator of gene expression that targets both transcriptional and translational regulatory pathways.
Interestingly, HIV-1 Tat also interacts with activated RSK2 in a manner important for its transactivation of the LTR (44). The fact that two independent LTR activators converge on this kinase highlights a possible central role for RSK2 in regulation of the transcriptional landscape during infection. It remains to be determined whether ORF45 and Tat require similar downstream targets of RSK2 to promote LTR activation. One possibility is that the robust synergism observed between ORF45 and Tat is a result of ORF45 increasing the cellular pool of activated RSK2 available to Tat. However, our observation that neither WT nor constitutively active RSK2 functions synergistically with Tat argues against this model and suggests that RSK2 levels are not limiting. An additional possibility is that RSK2 forms distinct complexes with Tat and ORF45 that function separately to enhance the activity of the HIV-1 LTR, perhaps each directing RSK2 to distinct targets. In line with this hypothesis, we were unable to detect an interaction between ORF45 and Tat (data not shown). Furthermore, ORF45 does not interact with CDK9, cyclinT1, or components of the superelongation complex (data not shown), suggesting that rather than directly targeting the 7SK small nuclear RNP, the ORF45-RSK2 complex targets other LTR-binding factors. Ultimately, quantitative phosphoproteomic analysis is required to address target specificity and should provide significant mechanistic insight into this process.
A number of small molecules are currently being explored as a potential means to force HIV reactivation in the “shock and kill” strategy of eliminating HIV reservoirs (61–64). Though this approach is promising, an important challenge is achieving a high reactivation frequency across diverse HIV integration sites and cell states. Agents with enhanced specificity, as well as those that increase the efficacy of current therapeutic strategies, would therefore be highly beneficial in this regard. An in-depth understanding of how ORF45 and Tat coordinate RSK2 and potentially other cellular factors to activate the HIV-1 LTR may enable the design of novel therapeutic peptides that mediate the selective activation of RSK2, potentially enhancing the activity of agents available to combat HIV.
ACKNOWLEDGMENTS
We thank Zoe Davis for constructing the plasmids expressing the KSHV tegument proteins, as well as all members of the Glaunsinger and Zhou labs for helpful discussions.
This research was supported by a Damon Runyon Cancer Research Foundation fellowship (DRG 2121-12) to J.K., as well as a California HIV/AIDS Research Program IDEA Award (ID13-B-529) and grants from the W. M. Keck Foundation (Young Scholars Award) and the Burroughs Wellcome Fund (Investigators in the Pathogenesis of Infectious Disease Award) to B.G., and by Public Health Service grants R01AI095057 and R01AI41757 from the National Institutes of Health to Q.Z.
Footnotes
Published ahead of print 9 April 2014
REFERENCES
- 1.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M-F, Clauvel J-P, Raphael M, Degos L, Sigaux F. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276–1280 [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186–1191. 10.1056/NEJM199505043321802 [DOI] [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869. 10.1126/science.7997879 [DOI] [PubMed] [Google Scholar]
- 4.Ganem D. 2010. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J. Clin. Invest. 120:939–949. 10.1172/JCI40567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki Y, Tosato G. 2004. HIV-1 Tat enhances Kaposi sarcoma-associated herpesvirus (KSHV) infectivity. Blood 104:810–814. 10.1182/blood-2003-07-2533 [DOI] [PubMed] [Google Scholar]
- 6.Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B. 1992. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J. Virol. 66:7159–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barillari G, Sgadari C, Palladino C, Gendelman R, Caputo A, Morris CB, Nair BC, Markham P, Nel A, Stürzl M, Ensoli B. 1999. Inflammatory cytokines synergize with the HIV-1 Tat protein to promote angiogenesis and Kaposi's sarcoma via induction of basic fibroblast growth factor and the alpha v beta 3 integrin. J. Immunol. 163:1929–1935 [PubMed] [Google Scholar]
- 8.Varthakavi V, Browning PJ, Spearman P. 1999. Human immunodeficiency virus replication in a primary effusion lymphoma cell line stimulates lytic-phase replication of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:10329–10338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merat R, Amara A, Lebbe C, de The H, Morel P, Saib A. 2002. HIV-1 infection of primary effusion lymphoma cell line triggers Kaposi's sarcoma-associated herpesvirus (KSHV) reactivation. Int. J. Cancer 97:791–795. 10.1002/ijc.10086 [DOI] [PubMed] [Google Scholar]
- 10.Harrington W, Jr, Sieczkowski L, Sosa C, Chan-a-Sue S, Cai JP, Cabral L, Wood C. 1997. Activation of HHV-8 by HIV-1 tat. Lancet 349:774–775. 10.1016/S0140-6736(05)60199-7 [DOI] [PubMed] [Google Scholar]
- 11.Suligoi B, Dorrucci M, Uccella I, Andreoni M, Rezza G, Italian Seroconversion S 2003. Effect of multiple herpesvirus infections on the progression of HIV disease in a cohort of HIV seroconverters. J. Med. Virol. 69:182–187. 10.1002/jmv.10281 [DOI] [PubMed] [Google Scholar]
- 12.Chandra A, Demirhan I, Massambu C, Pyakurel P, Kaaya E, Enbom M, Urassa W, Linde A, Heiden T, Biberfeld P, Doerr HW, Cinatl J, Loewer J, Chandra P. 2003. Cross-talk between human herpesvirus 8 and the transactivator protein in the pathogenesis of Kaposi's sarcoma in HIV-infected patients. Anticancer Res. 23:723–728 [PubMed] [Google Scholar]
- 13.Caselli E, Galvan M, Cassai E, Caruso A, Sighinolfi L, Di Luca D. 2005. Human herpesvirus 8 enhances human immunodeficiency virus replication in acutely infected cells and induces reactivation in latently infected cells. Blood 106:2790–2797. 10.1182/blood-2005-04-1390 [DOI] [PubMed] [Google Scholar]
- 14.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer NH, Tschachler E, Colombini S, Ensoli B, Stürzl M. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J. Virol. 71:7963–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boshoff C, Schulz TF, Kennedy MM, Graham AK, Fisher C, Thomas A, McGee JO, Weiss RA, O'Leary JJ. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274–1278. 10.1038/nm1295-1274 [DOI] [PubMed] [Google Scholar]
- 16.Sharp TV, Boshoff C. 2000. Kaposi's sarcoma-associated herpesvirus: from cell biology to pathogenesis. IUBMB Life 49:97–104. 10.1080/15216540050022395 [DOI] [PubMed] [Google Scholar]
- 17.Mesri EA, Cesarman E, Arvanitakis L, Rafii S, Moore MA, Posnett DN, Knowles DM, Asch AS. 1996. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J. Exp. Med. 183:2385–2390. 10.1084/jem.183.5.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myoung J, Ganem D. 2011. Infection of primary human tonsillar lymphoid cells by KSHV reveals frequent but abortive infection of T cells. Virology 413:1–11. 10.1016/j.virol.2010.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrington WJ, Jr, Bagasra O, Sosa CE, Bobroski LE, Baum M, Wen XL, Cabral L, Byrne GE, Pomerantz RJ, Wood C. 1996. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi's sarcoma patients. J. Infect. Dis. 174:1101–1105. 10.1093/infdis/174.5.1101 [DOI] [PubMed] [Google Scholar]
- 20.Sirianni MC, Vincenzi L, Topino S, Scala E, Angeloni A, Gonnella R, Uccini S, Faggioni A. 1997. Human herpesvirus 8 DNA sequences in CD8+ T cells. J. Infect. Dis. 176:541 (Letter.) 10.1086/514072 [DOI] [PubMed] [Google Scholar]
- 21.Boshoff C, Whitby D, Hatziioannou T, Fisher C, van der Walt J, Hatzakis A, Weiss R, Schulz T. 1995. Kaposi's-sarcoma-associated herpesvirus in HIV-negative Kaposi's sarcoma. Lancet 345:1043–1044. 10.1016/S0140-6736(95)90780-7 [DOI] [PubMed] [Google Scholar]
- 22.Sathish N, Wang X, Yuan Y. 2012. Tegument proteins of Kaposi's sarcoma-associated herpesvirus and related gamma-herpesviruses. Front. Microbiol. 3:98. 10.3389/fmicb.2012.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bechtel JT, Winant RC, Ganem D. 2005. Host and viral proteins in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:4952–4964. 10.1128/JVI.79.8.4952-4964.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu FX, Chong JM, Wu L, Yuan Y. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800–811. 10.1128/JVI.79.2.800-811.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang LM, Chao MF, Chen MY, Shih H, Chiang YP, Chuang CY, Lee CY. 2001. Reciprocal regulatory interaction between human herpesvirus 8 and human immunodeficiency virus type 1. J. Biol. Chem. 276:13427–13432. 10.1074/jbc.M011314200 [DOI] [PubMed] [Google Scholar]
- 26.Hyun TS, Subramanian C, Cotter MA, II, Thomas RA, Robertson ES. 2001. Latency-associated nuclear antigen encoded by Kaposi's sarcoma-associated herpesvirus interacts with Tat and activates the long terminal repeat of human immunodeficiency virus type 1 in human cells. J. Virol. 75:8761–8771. 10.1128/JVI.75.18.8761-8771.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caselli E, Menegazzi P, Bracci A, Galvan M, Cassai E, Di Luca D. 2001. Human herpesvirus-8 (Kaposi's sarcoma-associated herpesvirus) ORF50 interacts synergistically with the tat gene product in transactivating the human immunodeficiency virus type 1 LTR. J. Gen. Virol. 82:1965–1970 http://vir.sgmjournals.org/content/82/8/1965.long [DOI] [PubMed] [Google Scholar]
- 28.Kuang E, Tang Q, Maul GG, Zhu F. 2008. Activation of p90 ribosomal S6 kinase by ORF45 of Kaposi's sarcoma-associated herpesvirus and its role in viral lytic replication. J. Virol. 82:1838–1850. 10.1128/JVI.02119-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuang E, Fu B, Liang Q, Myoung J, Zhu F. 2011. Phosphorylation of eukaryotic translation initiation factor 4B (EIF4B) by open reading frame 45/p90 ribosomal S6 kinase (ORF45/RSK) signaling axis facilitates protein translation during Kaposi sarcoma-associated herpesvirus (KSHV) lytic replication. J. Biol. Chem. 286:41171–41182. 10.1074/jbc.M111.280982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myoung J, Ganem D. 2011. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. J. Virol. Methods 174:12–21. 10.1016/j.jviromet.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stürzl M, Gaus D, Dirks WG, Ganem D, Jochmann R. 2013. Kaposi's sarcoma-derived cell line SLK is not of endothelial origin, but is a contaminant from a known renal carcinoma cell line. Int. J. Cancer 132:1954–1958. 10.1002/ijc.27849 [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Zhu Q, Luo K, Zhou Q. 2001. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317–322. 10.1038/35104575 [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Guo J, Wu Y, Zhou Q. 2013. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 41:277–287. 10.1093/nar/gks976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang H, Wiedmer A, Yuan Y, Robertson E, Lieberman PM. 2011. Coordination of KSHV latent and lytic gene control by CTCF-cohesin mediated chromosome conformation. PLoS Pathog. 7:e1002140. 10.1371/journal.ppat.1002140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jäger S, Cimermancic P, Gulbahce N, Johnson JR, McGovern KE, Clarke SC, Shales M, Mercenne G, Pache L, Li K, Hernandez H, Jang GM, Roth SL, Akiva E, Marlett J, Stephens M, D'Orso I, Fernandes J, Fahey M, Mahon C, O'Donoghue AJ, Todorovic A, Morris JH, Maltby DA, Alber T, Cagney G, Bushman FD, Young JA, Chanda SK, Sundquist WI, Kortemme T, Hernandez RD, Craik CS, Burlingame A, Sali A, Frankel AD, Krogan NJ. 2012. Global landscape of HIV-human protein complexes. Nature 481:365–370. 10.1038/nature10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JX, Spolski R, Leonard WJ. 2008. Critical role for Rsk2 in T-lymphocyte activation. Blood 111:525–533. 10.1182/blood-2007-02-072207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poteet-Smith CE, Smith JA, Lannigan DA, Freed TA, Sturgill TW. 1999. Generation of constitutively active p90 ribosomal S6 kinase in vivo. Implications for the mitogen-activated protein kinase-activated protein kinase family. J. Biol. Chem. 274:22135–22138 [DOI] [PubMed] [Google Scholar]
- 38.Listerman I, Sapra AK, Neugebauer KM. 2006. Cotranscriptional coupling of splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. Nat. Struct. Mol. Biol. 13:815–822. 10.1038/nsmb1135 [DOI] [PubMed] [Google Scholar]
- 39.Bechtel JT, Liang Y, Hvidding J, Ganem D. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474–6481. 10.1128/JVI.77.11.6474-6481.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q, Yik JH. 2006. The Yin and Yang of P-TEFb regulation: implications for human immunodeficiency virus gene expression and global control of cell growth and differentiation. Microbiol. Mol. Biol. Rev. 70:646–659. 10.1128/MMBR.00011-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguilar-Cordova E, Chinen J, Donehower L, Lewis DE, Belmont JW. 1994. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res. Hum. Retroviruses 10:295–301. 10.1089/aid.1994.10.295 [DOI] [PubMed] [Google Scholar]
- 42.Zeniou M, Ding T, Trivier E, Hanauer A. 2002. Expression analysis of RSK gene family members: the RSK2 gene, mutated in Coffin-Lowry syndrome, is prominently expressed in brain structures essential for cognitive function and learning. Hum. Mol. Genet. 11:2929–2940. 10.1093/hmg/11.23.2929 [DOI] [PubMed] [Google Scholar]
- 43.Dümmler BA, Hauge C, Silber J, Yntema HG, Kruse LS, Kofoed B, Hemmings BA, Alessi DR, Frodin M. 2005. Functional characterization of human RSK4, a new 90-kDa ribosomal S6 kinase, reveals constitutive activation in most cell types. J. Biol. Chem. 280:13304–13314. 10.1074/jbc.M408194200 [DOI] [PubMed] [Google Scholar]
- 44.Hetzer C, Bisgrove D, Cohen MS, Pedal A, Kaehlcke K, Speyerer A, Bartscherer K, Taunton J, Ott M. 2007. Recruitment and activation of RSK2 by HIV-1 Tat. PLoS One 2:e151. 10.1371/journal.pone.0000151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu FX, Cusano T, Yuan Y. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5556–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia Q, Chernishof V, Bortz E, McHardy I, Wu TT, Liao HI, Sun R. 2005. Murine gammaherpesvirus 68 open reading frame 45 plays an essential role during the immediate-early phase of viral replication. J. Virol. 79:5129–5141. 10.1128/JVI.79.8.5129-5141.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang S, Dong S, Gu TL, Guo A, Cohen MS, Lonial S, Khoury HJ, Fabbro D, Gilliland DG, Bergsagel PL, Taunton J, Polakiewicz RD, Chen J. 2007. FGFR3 activates RSK2 to mediate hematopoietic transformation through tyrosine phosphorylation of RSK2 and activation of the MEK/ERK pathway. Cancer Cell 12:201–214. 10.1016/j.ccr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AE, Charnock FM, Beck S, Dunham I, Mungall AJ, Ganesan TS. 2007. RPS6KA2, a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene 26:683–700. 10.1038/sj.onc.1209827 [DOI] [PubMed] [Google Scholar]
- 49.Berns K, Hijmans EM, Mullenders J, Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M, Nijkamp W, Weigelt B, Agami R, Ge W, Cavet G, Linsley PS, Beijersbergen RL, Bernards R. 2004. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature 428:431–437. 10.1038/nature02371 [DOI] [PubMed] [Google Scholar]
- 50.Thakur A, Rahman KW, Wu J, Bollig A, Biliran H, Lin X, Nassar H, Grignon DJ, Sarkar FH, Liao JD. 2007. Aberrant expression of X-linked genes RbAp46, Rsk4, and Cldn2 in breast cancer. Mol. Cancer Res. 5:171–181. 10.1158/1541-7786.MCR-06-0071 [DOI] [PubMed] [Google Scholar]
- 51.Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I, Mandel JL, Sassone-Corsi P, Hanauer A. 1996. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature 384:567–570. 10.1038/384567a0 [DOI] [PubMed] [Google Scholar]
- 52.Jacquot S, Merienne K, De Cesare D, Pannetier S, Mandel JL, Sassone-Corsi P, Hanauer A. 1998. Mutation analysis of the RSK2 gene in Coffin-Lowry patients: extensive allelic heterogeneity and a high rate of de novo mutations. Am. J. Hum. Genet. 63:1631–1640. 10.1086/302153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanauer A, Young ID. 2002. Coffin-Lowry syndrome: clinical and molecular features. J. Med. Genet. 39:705–713. 10.1136/jmg.39.10.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anjum R, Blenis J. 2008. The RSK family of kinases: emerging roles in cellular signalling. Nat. Rev. Mol. Cell Biol. 9:747–758. 10.1038/nrm2509 [DOI] [PubMed] [Google Scholar]
- 55.Kuang E, Wu F, Zhu F. 2009. Mechanism of sustained activation of ribosomal S6 kinase (RSK) and ERK by Kaposi sarcoma-associated herpesvirus ORF45: multiprotein complexes retain active phosphorylated ERK and RSK and protect them from dephosphorylation. J. Biol. Chem. 284:13958–13968. 10.1074/jbc.M900025200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrison DK, Davis RJ. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19:91–118. 10.1146/annurev.cellbio.19.111401.091942 [DOI] [PubMed] [Google Scholar]
- 57.Godden-Kent D, Talbot SJ, Boshoff C, Chang Y, Moore P, Weiss RA, Mittnacht S. 1997. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 71:4193–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaldis P. 2005. The N-terminal peptide of the Kaposi's sarcoma-associated herpesvirus (KSHV)-cyclin determines substrate specificity. J. Biol. Chem. 280:11165–11174. 10.1074/jbc.M408887200 [DOI] [PubMed] [Google Scholar]
- 59.Li M, Lee H, Yoon DW, Albrecht JC, Fleckenstein B, Neipel F, Jung JU. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holz MK, Ballif BA, Gygi SP, Blenis J. 2005. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123:569–580. 10.1016/j.cell.2005.10.024 [DOI] [PubMed] [Google Scholar]
- 61.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482–485. 10.1038/nature11286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, Coffin JM, Bosch RJ, Margolis DM. 2005. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet 366:549–555. 10.1016/S0140-6736(05)67098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee C, Archin N, Michaels D, Belkina AC, Denis GV, Bradner J, Sebastiani P, Margolis DM, Montano M. 2012. BET bromodomain inhibition as a novel strategy for reactivation of HIV-1. J. Leukoc. Biol. 92:1147–1154. 10.1189/jlb.0312165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deeks SG. 2012. HIV: shock and kill. Nature 487:439–440. 10.1038/487439a [DOI] [PubMed] [Google Scholar]