ABSTRACT
Chronic human immunodeficiency virus type 1 (HIV-1) infection is associated with induction of T-cell coinhibitory pathways. However, the mechanisms by which HIV-1 induces upregulation of coinhibitory molecules remain to be fully elucidated. The aim of the present study was to determine whether and how HIV-1 Tat protein, an immunosuppressive viral factor, induces the PD-1/PD-L1 coinhibitory pathway on human dendritic cells (DCs). We found that treatment of DCs with whole HIV-1 Tat protein significantly upregulated the level of expression of PD-L1. This PD-L1 upregulation was observed in monocyte-derived dendritic cells (MoDCs) obtained from either uninfected or HIV-1-infected patients as well as in primary myeloid DCs from HIV-negative donors. In contrast, no effect on the expression of PD-L2 or PD-1 molecules was detected. The induction of PD-L1 on MoDCs by HIV-1 Tat (i) occurred in dose- and time-dependent manners, (ii) was mediated by the N-terminal 1–45 fragment of Tat, (iii) did not require direct cell-cell contact but appeared rather to be mediated by soluble factor(s), (iv) was abrogated following neutralization of tumor necrosis factor alpha (TNF-α) or blocking of Toll-like receptor 4 (TLR4), (v) was absent in TLR4-knockoout (KO) mice but could be restored following incubation with Tat-conditioned medium from wild-type DCs, (vi) impaired the capacity of MoDCs to functionally stimulate T cells, and (vii) was not reversed functionally following PD-1/PD-L1 pathway blockade, suggesting the implication of other Tat-mediated coinhibitory pathways. Our results demonstrate that HIV-1 Tat protein upregulates PD-L1 expression on MoDCs through TNF-α- and TLR4-mediated mechanisms, functionally compromising the ability of DCs to stimulate T cells. The findings offer a novel potential molecular target for the development of an anti-HIV-1 treatment.
IMPORTANCE The objective of this study was to investigate the effect of human immunodeficiency virus type 1 (HIV-1) Tat on the PD-1/PD-L1 coinhibitory pathway on human monocyte-derived dendritic cells (MoDCs). We found that treatment of MoDCs from either healthy or HIV-1-infected patients with HIV-1 Tat protein stimulated the expression of PD-L1. We demonstrate that this stimulation was mediated through an indirect mechanism, involving tumor necrosis factor alpha (TNF-α) and Toll-like receptor 4 (TLR4) pathways, and resulted in compromised ability of Tat-treated MoDCs to functionally stimulate T-cell proliferation.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) infection is characterized by a multitude of complex interactions between the virus and its host immune system (1). Starting from the acute phase, HIV-1 infection establishes a peak of virus replication, followed by a severe and rapid depletion of CD4+ T cells in the lymphoid tissues (2). In addition to CD4+ T cells, HIV-1 also targets and infects monocytes, macrophages, and, to a lesser level, dendritic cells (DCs), leading to the weakening of the host's immune responses to infection. DCs, the main antigen-presenting cells (APC), play key roles in both innate and adaptive immune responses (3–5). Interactions between HIV-1 and the DCs lead to immune activation starting from the acute phase of infection (6, 7). This immune activation, which persists throughout the chronic phase of infection, is associated with gradual depletion of circulating CD4+ T cells and increased exhaustion of T cells associated with a high set point of viral replication (8–11). This persists despite an increase in T-cell turnover (12), a decrease in plasmacytoid DC (pDC) (13) and myeloid DC (mDC) numbers (14), and increased production of proinflammatory cytokines and chemokines (15, 16). Consequently, this leads inevitably to a further weakening of the immune system, a situation that facilitates HIV-1 replication and persistence and leads to fast progression to AIDS (8–11).
HIV-1 infection is also associated with upregulation of the PD-1/PD-L1 immunosuppressive pathway, but the viral factors and mechanisms by which HIV-1 may induce upregulation of these coinhibitory molecules on DCs remain to be fully elucidated.
PD-L1 and PD-L2 ligands share several domains, characteristic of the B7 immunoglobulin family (17, 18). Several pathogens that lead to chronic or persistent infections, including lymphocytic choriomeningitis virus (LCMV) (19), simian immunodeficiency virus (SIV) (20), HIV-1 (21–23), hepatitis B virus (HBV) (24), human T-cell leukemia (HTLV) (22), hepatitis C virus (HCV) (25), and herpes simplex virus (HSV) (26, 27), have been reported to induce the PD-1/PD-L1 coinhibitory pathway as an immune evasion mechanism, often associated with the functional exhaustion (i.e., dysfunction) of virus-specific CD4+ and CD8+ T cells (28).
Blockade of PD-1/PD-L1 interaction has been shown, both in vitro and in vivo, to reverse the exhaustion and restore the function of T cells (29–32). Although HIV-1 has been reported to use many T-cell coinhibitory pathways, including PD-1/PD-L1, to evade control by the immune system, the viral factors by which HIV-1 induces upregulation of many T-cell coinhibitory molecules remain to be fully elucidated.
It has been reported that, among the viral factors, Tat protein interferes with the normal function of the immune system (33–43). HIV-1 Tat is a protein of 14 kDa, composed of 86 to 104 amino acids (aa), that is produced early after HIV-1 infection (41). At the structural level, Tat protein is structured in several domains, including the N-terminal region of aa 1 to 47 which contains the activation domain and the basic region of aa 49 to 57, which is essential for Tat internalization, nuclear localization, and RNA binding at the long terminal repeat (LTR)-TAR region (41). In addition to its essential role in the viral cycle, Tat protein is found at nanomolar (nM) levels in the sera of HIV-1-infected patients (44–46). However, it can reasonably be assumed that this quantification of Tat protein is underestimated, given the amount of Tat already adsorbed on the surface of cell membranes via heparan sulfates (47) and because this concentration is much larger near the lymphoid organs and in the vicinity of infected cells than in the sera (48).
Tat protein participates in the pathogenesis of HIV-1 infection by its capacity to interact with different cell types (48). It is secreted by infected cells and can act on neighboring immune cells whether they are infected or not (34, 49). In addition to its direct role in viral promoter trans activation of transcription, Tat also contributes indirectly to the spread of HIV-1 through an increase of the rate of CCR5 and CXCR4 cell surface expressions (50, 51) and through the activation of quiescent CD4+ T cells, which in turn are used by the virus as new targets to enhance HIV-1 replication (52). Tat has also been found to induce neurotoxicity in the central nervous system (53–55) and apoptosis in CD4+ T cells (45, 56, 57).
While some of the above-given effects were mediated following intracellular uptake of Tat, others were mediated by the extracellular interaction of Tat with specific cellular receptors (48). Different domains of Tat can interact with specific membrane receptors, including the CD26 receptor (58), the CXCR4 chemokine receptor (46), the L-type calcium channel (59), integrin αvβ3 and α5β1 of DCs (60), membrane lipids (55), and the Flk-1/KDR receptor (61). In addition to these potential Tat receptors, we have recently demonstrated that Tat protein is able to recruit the Toll-like receptor 4 (TLR4) pathway, following its interaction with high affinity with TLR4-MD2 complex, to activate the production of proinflammatory tumor necrosis factor alpha (TNF-α) and anti-inflammatory interleukin 10 (IL-10) cytokines (62).
The aims of the study presented here were (i) to explore the potential effect of HIV-1 Tat protein on the upregulation of PD-1, PD-L1, and PD-L2 coinhibitory molecules on the surface of MoDCs and (ii) to understand the underlying molecular mechanisms by which HIV-1 Tat would affect the phenotypic and functional expression of these coinhibitory molecules. The results show that the HIV-1 Tat protein specifically induced the upregulation of PD-L1 (also known as B7-H1) on MoDCs through an indirect mechanism involving TNF-α and TLR4 pathways.
MATERIALS AND METHODS
Recombinant and synthetic proteins.
HIV-1 Tat recombinant protein (aa 1 to 86) from the HIV-1 Lai strain was obtained from the Agence Nationale de la Recherche sur le SIDA (ANRS; Paris, France). HIV-1 Tat protein from the SF2 strain (Tat aa 1 to 101) or N-terminal Tat fragments (Tat aa 1 to 45) fused to glutathione S-transferase (GST) were produced and purified in our laboratory as previously described (33). Chemically synthesized Tat (aa 1 to 86) protein from the HIV-1 Lai strain and the oxidized form of Tat were produced as described by Vives et al. (63). The level of endotoxin in all these recombinant proteins was assessed using the Limulus amebocyte lysate assay (Bio-Sepra, Villeneuve la Garenne, France) and was shown to be below 0.3 EU/μg, the limit of detection of this test.
Chemical products.
Lipopolysaccharide (LPS), from Escherichia coli serotype R515, was purchased from Alexis Biochemicals. Pam3CSK4 (TLR1/2 ligand) was purchased from Invitrogen.
Recombinant cytokines and antibodies.
Recombinant human cytokines TNF-α, IL-10, IL-6, and gamma interferon (IFN-γ) were purchased from eBioscience. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 were purchased from HumanZyme. Recombinant murine GM-CSF was purchased from Tebu Bio. Mouse monoclonal anti-human TNF-α (TNF-D), anti-TLR4/MD2 (HTA 125), and mouse IgG isotype control antibodies were purchased from eBioscience. Anti-Tat antibodies were obtained from ANRS. Polyclonal goat anti-human IFN-γ and monoclonal mouse anti-human IL-10 (clone number 25209) were purchased from R&D Systems. For cell surface labeling, fluorochrome-conjugated antibodies anti-CD1a-fluorescein isothiocyanate (FITC) (HI149), anti-CD14-phycoerythrin (PE) (HCD14), anti-CD80-FITC (2D10), anti-CD83-FITC (HB15e), anti-CD86-PE (IT2.2), anti-HLA-DR-FITC (L243), anti-mouse CD86-PE (PO3), and isotype control were purchased from BioLegend. Anti-human CD1c-FITC (AD5-8E7) was purchased from Miltenyi Biotech. Anti-PD-1-APC (eBioJ105), anti-PD-L1-APC (MIH1), anti-PD-L2-PE (MIH18), anti-mouse PD-L1-PE (MIH5), and isotype controls were purchased from eBioscience.
Generation of monocyte-derived DCs.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy blood donors (from Etablissement Français du Sang [EFS], Toulouse) or from blood samples of HIV-1-infected patients who were under effective antiretroviral treatment (from the Department of Infectious Diseases of Toulouse University Hospital) by centrifugation on Ficoll-Paque density gradient (GE Healthcare). Monocytes were isolated by adherence to tissue culture plastic on 6-well plates (Becton Dickinson) after 1 h at 37°C and 5% CO2. Nonadherent cells were removed, and adherent cells were washed three times with PBS. This adherent population was CD14+ (>94%) when analyzed by flow cytometry. To allow their differentiation into monocyte-derived DCs (MoDCs), cells were cultured in RPMI medium (Invitrogen) supplemented with 10% fetal calf serum (FCS) (Invitrogen), 100 IU/ml penicillin, 100 μg/ml streptomycin, 10 ng/ml GM-CSF, and 10 ng/ml IL-4. Alternatively, monocytes were isolated by positive selection using a CD14+ isolation kit (Myltenyi Biotec). After 5 days of culture, loosely adherent cells were recovered by gentle pipetting and used as immature DCs in our experiments. Over 90% of cells had the standard phenotype of immature DCs: CD1a+ CD14− CD80+ CD86+ CD83− HLA-DR+.
Isolation of primary human myeloid DCs.
Primary myeloid DCs were isolated from human PBMCs by negative selection, using the myeloid dendritic cell isolation kit, according to the manufacturer's instructions (Miltenyi Biotec). The isolated DCs were characterized by the expression of a CD1c (BDCA-1) marker. The purity of the isolated CD1c+ DCs was >95%, as determined by flow cytometry.
Generation of bone marrow-derived DCs.
The C57BL/6 mice were obtained from Charles River Laboratory. TLR4-knockout (KO) mice with a C57BL/6 genetic background were kindly provided by the laboratory of B. Ryffel (INEM, UMR7355 CNRS—University of Orleans, France). Myeloid cells were extracted from the bone marrow of mouse femur by being washed with 5 ml of complete RPMI 1640, using a syringe. After homogenization, filtration, and centrifugation (5 min at 20°C and 1,200 rpm), the cell suspension was recovered in 10 ml of complete RPMI 1640 medium. To stimulate the differentiation into DCs, myeloid cells were cultured in 100- by 20-mm petri dishes (Dominique Dutscher) at 2 × 106 cells in 10 ml of complete RPMI 1640 medium in the presence of 50 ng/ml of recombinant murine GM-CSF. The medium was renewed after 3 and 6 days of culture. Bone marrow-derived DCs (BMDCs) were recovered in the supernatant after 9 days of culture and used as immature cells.
Treatment of DCs.
Immature MoDCs or BMDCs were resuspended in RPMI complete medium and distributed in 6-, 12-, or 24-well plates at a density of 1 × 106 cells/ml and then treated for 24 h or for the indicated time. Then, cells were recovered and analyzed for the expression of surface molecules by flow cytometry. Cell culture supernatants were collected and kept frozen until cytokine quantification.
To obtain Tat-conditioned medium, MoDCs or BMDCs were treated with Tat for 1 h, washed three times with PBS to remove soluble Tat, and then cultured for a further 24 h. After this time, the cell supernatant was recovered, centrifuged for 10 min at 1,200 rpm, and used as Tat-conditioned medium to stimulate autologous cells.
In the transwell experiment, untreated MoDCs were cultured in 6-well plates (Becton, Dickinson). In the upper chamber of the 1-μm transwell insert, we added autologous MoDCs previously incubated with Tat for 1 h and washed three times with PBS. Cells were kept in coculture for a further 24 h before they were separately analyzed for the expression of surface molecules by flow cytometry.
In the direct coculture experiment, MoDCs previously treated with Tat for 1 h and washed were mixed with autologous Tat-untreated carboxyfluorescein succinimidyl ester (CFSE)-labeled MoDCs. After 24 h of incubation, MoDCs were recovered and the phenotype was analyzed in CFSE-labeled and CFSE-unlabeled cells using different windows, gating on a FACSCalibur (Becton, Dickinson).
Phenotype analysis by flow cytometry.
For flow cytometry analysis, MoDCs were first washed once with PBS and 5 mM EDTA and then once with PBS and 5% FCS. Cells were then incubated cold for 30 min with fluorochrome-conjugated antibodies. After 2 washes with PBS-5% FCS, cells were recovered in PBS-0.01% azide before analysis on a FACSCalibur.
Cytokine quantifications.
Quantification of TNF-α, IL-10, IL-6, IFN-α1, IL-12p70, and IFN-γ in cell supernatants was performed with a specific ELISA kit from eBioscience. Briefly, the first monoclonal antibody used for capture was incubated overnight at 4°C in 96 wells (Nunc). After three washes with PBS containing 0.05% Tween 20, pH 7.4 (wash buffer), plates were saturated by adding 250 μl of a protein solution (diluent assay) for 1 h at room temperature. After three washes, culture supernatants (100 μl/well) were added and incubated for 2 h at room temperature. Plates were then washed three times and incubated for 1 h at room temperature with a biotinylated anticytokine antibody. After five washes, the bound biotinylated antibody was detected by an additional 30 min of incubation with streptavidin peroxidase. After seven washes, plates were incubated with the enzyme substrate (3,3′,5,5′-tetramethylbenzidine [TMB]). The reaction was stopped by adding 50 μl of H2SO4 (2N) to each well. Absorbance was read at 450 nm with a wavelength correction at 570 nm. Cytokines were quantified from a standard curve generated by using various concentrations of recombinant protein of each cytokine. The limit of detection of each cytokine was 4 pg/ml for TNF-α, 2 pg/ml for IL-10, 2 pg/ml for IL-6, 15 pg/ml for IFN-α, 4 pg/ml for IFN-γ, and 4 pg/ml for IL-12p70.
Effect of PD-L1 upregulation on T-cell proliferation.
Peripheral blood lymphocytes (PBLs) were isolated as a nonadherent cell, or CD14-negative untouched cell, fraction from PBMCs. After labeling with CFSE (2 μM) using the CellTrace CFSE proliferation kit (Invitrogen), labeled PBLs were cocultured with autologous MoDCs, previously treated with synthetic Tat (50 nM) or GST-recombinant proteins (100 nM) in the presence or absence of anti-PD-L1 antibodies (MIH1) at 10 μg/ml. As a control, the same isotype IgGs were used. MoDCs (2 × 105 cells) and PBLs (4 × 105 cells) were cocultured in the presence of a suboptimal concentration (10 ng/ml) of anti-CD3 (OK3) monoclonal antibody in a final volume of 200 μl complete medium in round-bottomed 96-well plates. After 5 days of coculture, cells were harvested and CD3-positive cells were labeled firstly with a mouse anti-CD3 antibody (OK3) and secondly with an anti-mouse IgG2a Alexa Fluor 633 antibody (Invitrogen). Proliferation was then analyzed by flow cytometry in CD3+ cells.
Statistical analyses.
Data for each assay were compared by Mann-Whitney and Student's t statistical tests using Graph Pad Prism 5 software. Data are expressed as the means ± standard deviations. Results were considered to be statistically significant when P values were ≤0.05.
RESULTS
HIV-1 Tat protein induced phenotypic maturation of monocyte-derived dendritic cells.
HIV-1 infection is associated with an increase of immunosuppressive factors such as IL-10 (64), PD-1/PD-L1 T-cell coinhibitory pathway (65), and IDO (66), which in turn leads to establishment of an immunosuppressive state. Since HIV-1 Tat protein, used in either recombinant or synthetic form, has been (i) portrayed as an immunosuppressive factor by several reports, including ours (33, 40, 67–70), and (ii) shown to affect DC maturation (37, 71), it was of interest to determine whether HIV-1 Tat would also lead to induction of the maturation marker (CD83) or costimulatory (CD80 and CD86) and coinhibitory (PD-1/PD-L1/PD-L2) molecules on DCs.
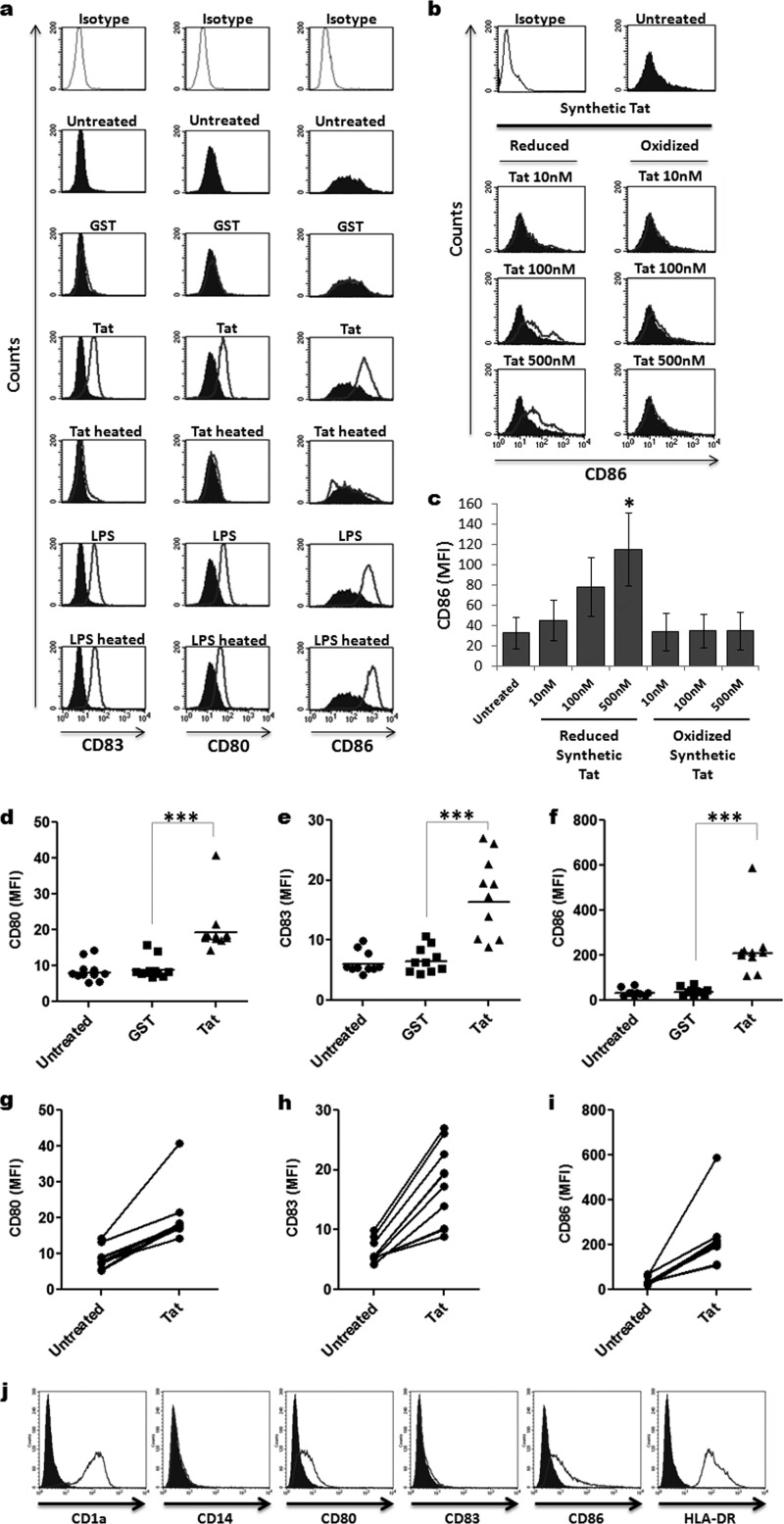
To this end, using flow cytometry (fluorescence-activated cell sorting [FACS]), we first monitored the expression of CD80 and CD86 costimulatory molecules and CD83 maturation marker (here designated maturation markers) on MoDCs following 24 h of incubation with either recombinant or chemically synthetic forms of HIV-1 Tat protein. We showed that monocyte-derived dendritic cells expressed the expected immature DC markers CD1a+, CD14−, CD80low, CD86low, CD83−, and HLA-DR+ (Fig. 1). As shown in Fig. 1a to c, incubation of immature MoDCs with HIV-1 Tat protein resulted in the acquisition of a phenotype characteristic of mature DCs, as determined by a significant increase in the level of expression of DC maturation markers (CD83, CD80, and CD86) compared to the relatively low levels expressed on untreated immature MoDCs (Fig. 1a). As a negative control, MoDCs treated similarly with the same amount of a recombinant GST protein did not show any significant increase in the level of expression of maturation markers (Fig. 1a to c). Moreover, we showed that synthetic reduced Tat protein, but not its oxidized form, stimulated CD86 expression in a dose-dependent manner (Fig. 1b and c). As expected, MoDCs treated with LPS (positive control) showed a significant increase in the expression level of all maturation markers (Fig. 1a).
FIG 1.
HIV-1 Tat upregulates maturation markers on MoDCs. (a) Immature MoDCs were treated for 24 h with 50 nM recombinant GST-Tat 1–101 protein (Tat) or an equal amount of GST protein alone (GST). Untreated and LPS-treated (100 ng/ml) MoDCs were used as negative and positive controls, respectively. Tat and LPS, heat-inactivated for 20 min at 95°C, were also included as negative controls (Tat heated and LPS heated, respectively). After 24 h of treatment, MoDCs were harvested and analyzed for CD80, CD83, and CD86 cell surface expression by flow cytometry. The gray lines on the top 3 histograms represent labeling with the IgG isotype control. The filled histograms correspond to the phenotypes of untreated MoDCs, and the unfilled histograms represent the effect of the indicated treatment on the expression of CD83, CD80, and CD86 maturation markers. (b and c) MoDCs were treated with increasing amounts of chemically synthesized Tat protein (10 nM, 100 nM, or 500 nM) in either reduced or oxidized forms. Twenty-four hours later, CD86 cell surface expression was determined by flow cytometry. One representative experiment and a graphical representation of three independent experiments with statistical analysis are depicted in panels b and c, respectively. (d to f) Mean fluorescence intensity (MFI) of CD80, CD83, and CD86 expression on MoDCs derived from 10 different donors analyzed after 24 h of incubation in the absence or in the presence of 50 nM GST (GST) or GST-Tat 1–101 (Tat). (g to i) Increase of the MFI of CD80, CD83, and CD86 on Tat-treated compared to untreated MoDCs of each donor. The results are representative of at least 3 independent experiments. Data were compared by Mann-Whitney and Student's t statistical tests, and the results were considered to be statistically significant when P values were ≤0.05. Asterisks represent P values: *, P < 0.05; *** P < 0.001. (j) Characterization of monocyte-derived dendritic cell phenotype. Monocytes were differentiated into DCs during 5 days of culture in the presence of GM-CSF and IL-4. Differentiation was checked by monitoring the specific MoDC surface markers, including CD1a+, CD14−, CD80+, CD86+, and HLA-DR+ by flow cytometry. The immature status of MoDCs was verified by the weak expression of surface markers CD83, CD80, and CD86.
To demonstrate the specificity of Tat action, we showed that treatment with previously heated Tat protein completely abrogated its capacity to induce MoDC phenotypic maturation, while treatment with heated LPS had no effect on its capacity to activate MoDC maturation (Fig. 1a). Cumulative data obtained from 10 independent experiments indicated that Tat induced a significant and reproducible upregulation of CD80, CD83, and CD86 maturation markers in MoDCs obtained from 10 different donors. A significant increase of the mean fluorescence intensity (MFI) was observed for MoDCs of all donors after 24 h of treatment with Tat (P < 0.001; Fig. 1d to i).
All together, these results indicate that HIV-1 Tat induced phenotypic maturation of MoDCs by stimulating cell surface expression of CD83, CD80, and CD86 maturation markers.
HIV-1 Tat protein induced expression of the PD-L1 coinhibitory molecule on DCs.
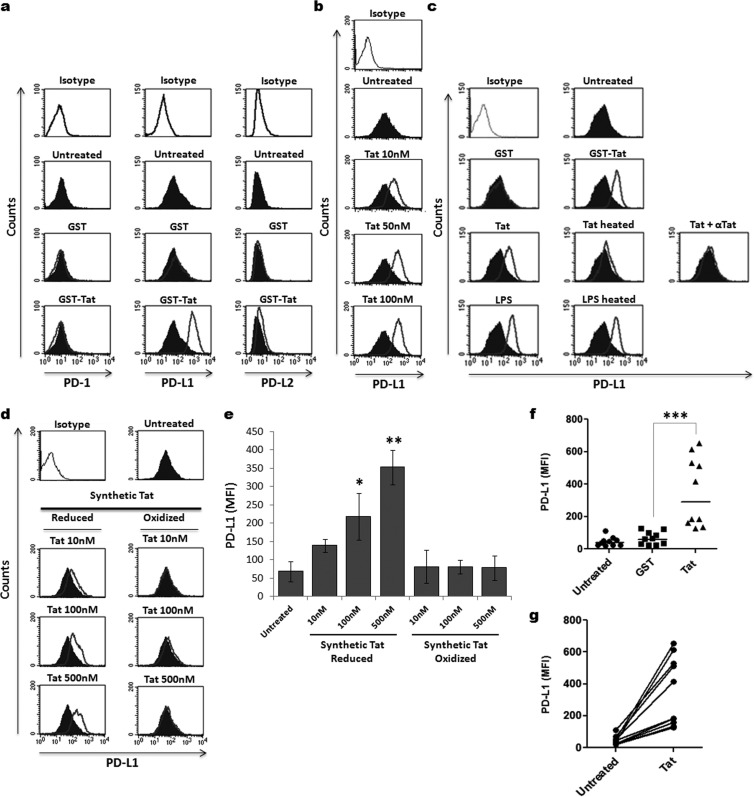
We next determined the effect of HIV-1 Tat protein on the expression of coinhibitory molecules, including PD-1, PD-L1, and PD-L2, on MoDCs. To this end, MoDCs were treated for 24 h with HIV-1 Tat protein, and the levels of the PD-1, PD-L1, and PD-L2 molecules expressed on the cell surface were determined by FACS, as described above. At steady state, immature MoDCs expressed a significant level of PD-L1 molecules on the cell surface compared to the levels of PD-1 and PD-L2 molecules, which were relatively low (Fig. 2a, Untreated). Incubation of immature MoDCs with GST-Tat led to a strong upregulation of PD-L1 on the cell surface (Fig. 2a, GST-Tat). In contrast, no effect on the expression of PD-L2 and PD-1 molecules on MoDCs was detected following treatment in the same conditions by GST-Tat protein (Fig. 2a, GST-Tat). No significant change in PD-L1 expression was detected on MoDCs treated in the same conditions by GST alone as a negative control (Fig. 2a and c, GST). In addition, we showed that synthetic reduced Tat protein, but not its oxidized form, modulated PD-L1 upregulation (Fig. 2d and e). Considering these results, we focused our study on the modulation of the PD-L1 coinhibitory molecule by Tat protein.
FIG 2.
HIV-1 Tat induced PD-L1 expression on MoDCs. (a) The effect of HIV-1 Tat protein on the expression of PD-L1 on MoDCs was analyzed by flow cytometry. MoDCs were treated for 24 h with 50 nM either recombinant GST-Tat 1–101 protein (GST-Tat) or GST alone (GST). Untreated cells were used as negative controls (Untreated). After 24 h, the levels of PD-1, PD-L1, and PD-L2 expression were determined by flow cytometry. (b) Dose-response effect of Tat. MoDCs were treated for 24 h with increasing amounts of recombinant GST-Tat 1–101 protein (10 nM, 50 nM, or 100 nM). The level of expression of PD-L1 was determined by flow cytometry. (c) Specificity of Tat action. MoDCs were treated for 24 h with 10 nM 1–86 Tat protein (Tat) or with 50 nM GST-Tat 1–101 protein (GST-Tat). Untreated cells were used as a negative control (Untreated). LPS-stimulated cells (100 ng/ml) were used as a positive control (LPS). To evaluate the specificity of the Tat effect on PD-L1 expression, additional controls were included: stimulation with 50 nM GST alone (GST), Tat preincubated for 30 min at 37°C with 3 μg/ml of anti-Tat antibodies (Tat + αTat), Tat heat inactivated for 20 min at 95°C (Tat heated), and LPS heat inactivated in the same conditions (LPS heated). (d) MoDCs were treated with increasing amounts of chemically synthesized Tat protein (10 to 500 nM) in either reduced or oxidized forms. Twenty-four hours later, PD-L1 cell surface expression was determined by flow cytometry. One representative experiment and graphical representation of three independent experiments with statistical analysis are depicted in panels d and e, respectively. (f) Mean fluorescence intensity (MFI) of PD-L1 on MoDCs derived from 10 different donors analyzed after 24 h of incubation in the absence or in the presence of 50 nM GST (GST) or GST-Tat 1–101 (Tat). (g) Increase of the MFI of PD-L1 on Tat-treated compared to untreated MoDCs of each donor. The results are representative of at least 3 independent experiments. Data were compared by Mann-Whitney and Student's t statistical tests, and the results were considered to be statistically significant when P values were ≤0.05. Asterisks represent P values: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We found that (i) in escalating-dose experiments, recombinant or synthetic Tat proteins stimulated PD-L1 expression in a dose-dependent manner (Fig. 2b, d, and e), (ii) heat treatment of Tat protein totally abolished its effect on PD-L1 expression on MoDCs, while the same treatment on LPS had no effect on its capacity to upregulate PD-L1 expression (Fig. 2c, middle), (iii) the effect of Tat protein on the expression of PD-L1 on MoDCs was totally abrogated in the presence of anti-Tat antibodies (Fig. 2c, right), (iv) SH-reduced chemically synthetic Tat protein, but not its oxidized form, stimulated PD-L1 upregulation on MoDCs, suggesting the importance of at least one SH-free group in this biological activity of Tat (Fig. 2d and e), (vi) PD-L1 upregulation was induced by Tat proteins from both the Lai and SF2 strains, indicating that the effect on PD-L1 was conserved at least in these two HIV-1 isolates (Fig. 2a, c, and d).
To determine whether the effect of HIV-1 Tat on the expression of PD-L1 can be generalized, we tested the effect of Tat protein on MoDCs derived from 10 different HIV-1-seronegative donors of various ages and both genders. As shown in Fig. 2f and g, the stimulation of PD-L1 expression induced by Tat protein was detected on MoDCs from each donor regardless of age or gender. Although the level of expression of PD-L1 varied among donors, a significant increase of the MFI was observed for all donors, with a mean ranging from 46 in untreated MoDCs to 352 in Tat-treated MoDCs (P < 0.001; Fig. 2f and g).
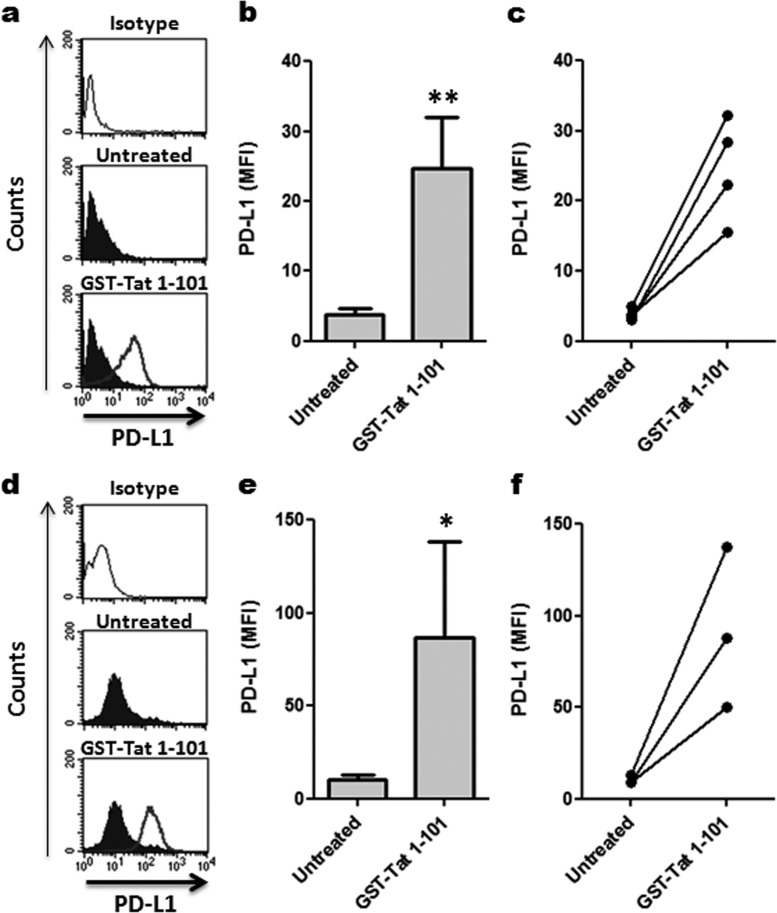
To address the effect of Tat protein on PD-L1 expression in a more physiological setting, we explored its effect on primary human myeloid DCs. To this ends, myeloid DCs were purified by negative selection from the PBMCs of four healthy donors. Analysis by flow cytometry of isolated cells confirmed their positivity for the CD1c marker. Interestingly, the treatment of primary myeloid DCs with Tat protein led to a significant upregulation of PD-L1 expression on DCs from each of the four donors (P < 0.01; Fig. 3a to c). This upregulation was observed for myeloid DCs of each donor starting 24 h after treatment with Tat (Fig. 3c) and was statistically significant (P < 0.01; Fig. 3b). Furthermore, we also explored this phenomenon in a pathological setting using DCs isolated from HIV-1-infected patients. However, for practical considerations, due to the rarity of peripheral blood myeloid DCs in HIV-1-infected patients, as an alternative, we tested the effect of Tat on MoDCs derived from three HIV-1-infected patients that were under effective antiretroviral treatments. The results confirmed that Tat continued to significantly stimulate PD-L1 expression even on MoDCs from HIV-1-infected patients (Fig. 3d and f; P < 0.05).
FIG 3.
Tat upregulates PD-L1 expression on DCs from HIV-1-infected and uninfected patients. (a to c) Effect of Tat on PD-L1 expression in primary myeloid DCs. Myeloid DCs from healthy donors were treated for 24 h with 100 nM GST-Tat 1–101 and analyzed by flow cytometry for PD-L1 expression in CD1c-positive cells. The data show one representative experiment (a), a graphical representation of four independent experiments with statistical analysis (b), and the increase of the MFI of PD-L1 on Tat-treated compared to untreated DCs of each donor (c). (d to f) Effect of Tat on PD-L1 expression in MoDCs derived from HIV-1-infected patients. MoDCs from HIV-1-infected patients were treated as described above and analyzed by flow cytometry for PD-L1 expression by gating on CD1a-positive cells. The data show one representative experiment (d), graphical representation of three independent experiments with statistical analysis (e), and the increase of PD-L1 expression on Tat-treated compared to untreated MoDCs of each HIV-1-infected donor (f). The results are representative of at least 3 independent experiments. Data of all the figures were compared by Student's t statistical tests, and the results were considered to be statistically significant when P values were ≤0.05. Asterisks represent P values: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
HIV-1 Tat protein induces PD-L1 expression on MoDCs through its N-terminal fragment.
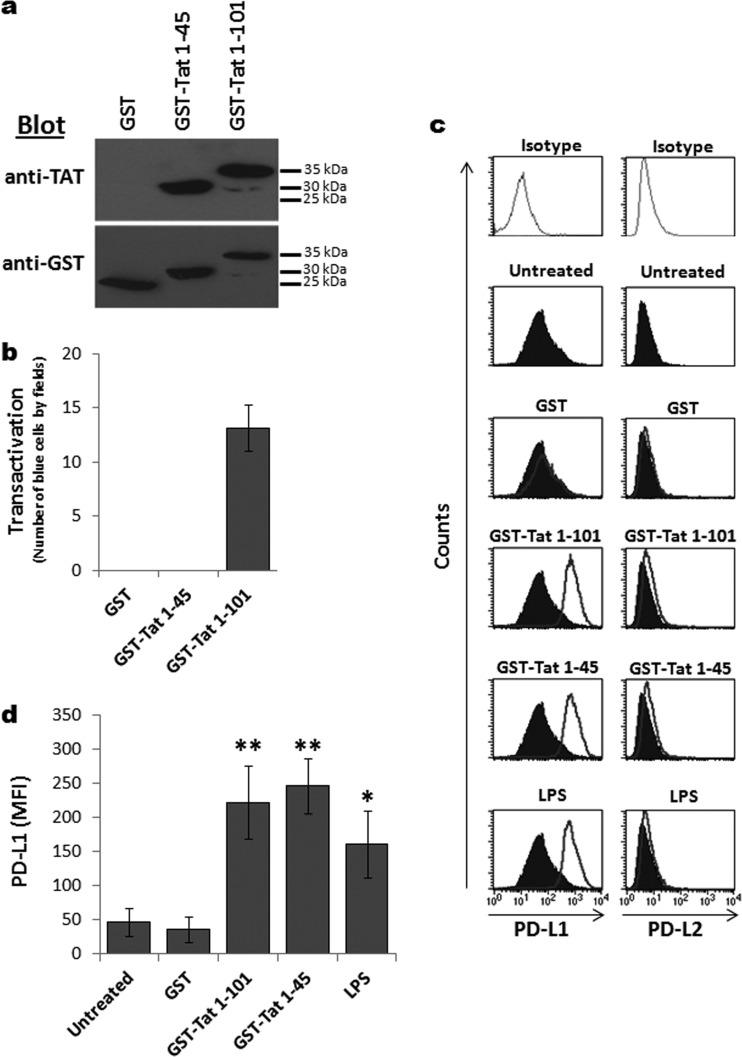
In order to evaluate the mechanism by which HIV-1 Tat stimulates PD-L1 expression on MoDCs, in addition to the whole GST-Tat 1–101 protein, we produced a truncated GST N-terminal fragment of Tat that was unable to trans activate HIV-1 LTR: the N-terminal 1–45 fragment. As expected, the small truncated GST N-terminal 1–45 fragment (i) migrated faster on the SDS-PAGE gel than the whole GST-Tat 1–101 protein (Fig. 4a) and (ii) was detected by antibodies specific either to Tat protein or to the GST-tagged region. In contrast, the GST-tagged region was detected by anti-GST antibodies but not by anti-Tat protein antibodies (Fig. 4a). As expected, unlike the whole GST-Tat 1–101 protein, the truncated GST N-terminal 1–45 fragment was unable to stimulate trans activation of the HIV-1 LTR, as assessed by its incapacity to activate the gene of β-galactosidase (β-Gal) under the control of the HIV-1 LTR (Fig. 4b).
FIG 4.
HIV-1 Tat protein induces PD-L1 expression on MoDCs via the N-terminal 1–45 fragment. (a) Equal amounts of GST, GST-Tat 1–45, and GST-Tat 1–101 recombinant proteins were analyzed by SDS-PAGE electrophoresis and immunoblotting using a monoclonal anti-Tat antibody targeting the N-terminal epitope (amino acids 1 to 15) or anti-GST antibodies. In panel b, the three recombinant proteins were tested for trans activation activity. HeLa cells stably transfected with a plasmid encoding the β-galactosidase protein under the control of the LTR promoter of HIV-1 were incubated for 24 h with 1 μM GST, GST-Tat 1–101, and GST-Tat 1–45 proteins. The HeLa cells were then washed with PBS, fixed with PBS-0.5% glutaraldehyde, and incubated for an additional 24 h with X-Gal as β-galactosidase substrate (0.4 mg/ml X-Gal, 5 mM potassium ferrocyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2). The number of blue-dyed cells corresponding to trans-activated cells was counted under an optical microscope (at a ×400 magnification). The results are represented as numbers of blue cells per field. (c and d) MoDCs were treated for 24 h with 100 nM of either the full-length GST-Tat 1–101 protein or the truncated form, GST-Tat 1–45. Untreated and LPS-stimulated cells (100 ng/ml) were used as negative and positive controls, respectively. After 24 h, the expression of PD-L1 and PD-L2 was analyzed by flow cytometry. (c) Corresponds to one representative experiment; (d) corresponds to graphical representation of three independent experiments with statistical analysis. The results are representative of at least 3 independent experiments. Asterisks represent P values: *, P < 0.05; **, P < 0.01.
At a functional level, we found that like the whole GST-Tat 1–101 protein, the truncated GST N-terminal 1–45 fragment was also able to stimulate the upregulation of PD-L1, but not PD-L2, on MoDCs, as detected by FACS (Fig. 4c and d).
All together, these results suggest that the observed effect of HIV-1 Tat on PD-L1 upregulation on MoDCs can be mapped to the N-terminal 1–45 fragment of Tat protein. The results also indicate that the effect of HIV-1 Tat on PD-L1 upregulation on MoDCs does not require uptake of the protein, since the 49–57 basic domain, which is essential for Tat protein uptake, nuclear localization, and Tat-TAR interaction, is not represented in the N-terminal 1–45 fragment. We therefore advanced two alternative hypotheses in which Tat protein stimulated the upregulation of PD-L1 on MoDCs either (i) directly following interaction of Tat protein with MoDCs or (ii) indirectly, through induction of a secondary factor.
Tat upregulates PD-L1 expression on MoDCs through an indirect mechanism.
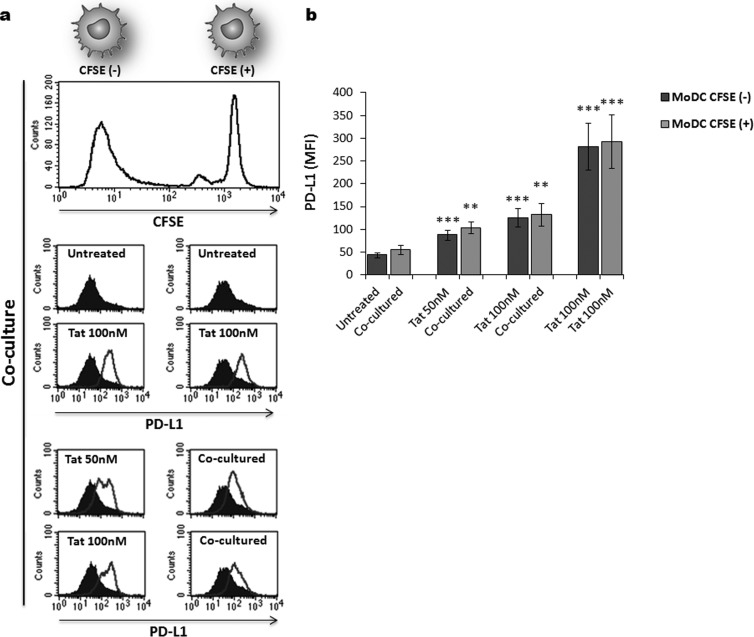
To assess whether the effect of Tat protein required direct interaction between Tat and MoDCs or acted indirectly through a secondary factor, we conducted the following coculture experiment. MoDCs were treated with Tat for 1 h, washed three times with PBS, and then cocultured, at a 1:1 ratio, with autologous Tat-untreated MoDCs. To identify Tat-treated and -untreated MoDCs from the same culture, only Tat-untreated MoDCs were prelabeled with CFSE (1 μM). After 24 h of coculture, the level of PD-L1 expression was determined on CFSE+ and CFSE− MoDCs. After the flow plots were gated on CFSE+ and CFSE− MoDCs, the flow cytometry analysis showed that preincubation with Tat protein induced PD-L1 upregulation on both Tat-treated CFSE− and untreated CFSE+ MoDCs in a dose-dependent manner (Fig. 5a and b). As a control, we showed that CFSE+ and CFSE− MoDCs expressed similar basal levels of PD-L1 in the absence of Tat stimulation (Fig. 5a and b). In addition, we showed that CFSE labeling had no effects on cell viability, as demonstrated by the capacity of Tat to continue to upregulate PD-L1 in a manner similar to that in non-CFSE-labeled MoDCs (Fig. 5a and b). This result suggests that Tat protein acts on PD-L1 modulation by an indirect mechanism through induction of a secondary factor, which could be soluble or membrane associated.
FIG 5.
Tat upregulates PD-L1 expression on MoDCs through an indirect mechanism. MoDCs were treated with increasing amounts of Tat protein (50 nM, 100 nM) for 1 h and then washed once with PBS to remove unbound Tat protein. Tat-treated MoDCs were then cocultured with autologous Tat-untreated MoDCs at a 1:1 ratio to allow cell-cell contact. To discriminate between the untreated and Tat-treated MoDCs in the coculture, untreated cells were prelabeled with 1 μM CFSE [CFSE(+)], whereas Tat-treated cells were kept unlabeled [CFSE(−)]. (a) Corresponds to one representative experiment. The upper histogram shows CFSE+ and CFSE− MoDC coculture. After 24 h of coculture, the level of PD-L1 was determined by flow cytometry by gating on CFSE-positive cells (left histograms) and CFSE-negative cells (right histograms). The filled histograms correspond to the phenotypes of untreated MoDCs, and the unfilled histograms represent PD-L1 expression after the indicated treatment. (b) Corresponds to graphical representation of three independent experiments with statistical analysis. The results are representative of at least 3 independent experiments. Asterisks represent P values: **, P < 0.01; ***, P < 0.001.
The induction of PD-L1 expression on MoDCs by HIV-1 Tat protein does not require direct cell-cell contact but is rather mediated by soluble factor(s).
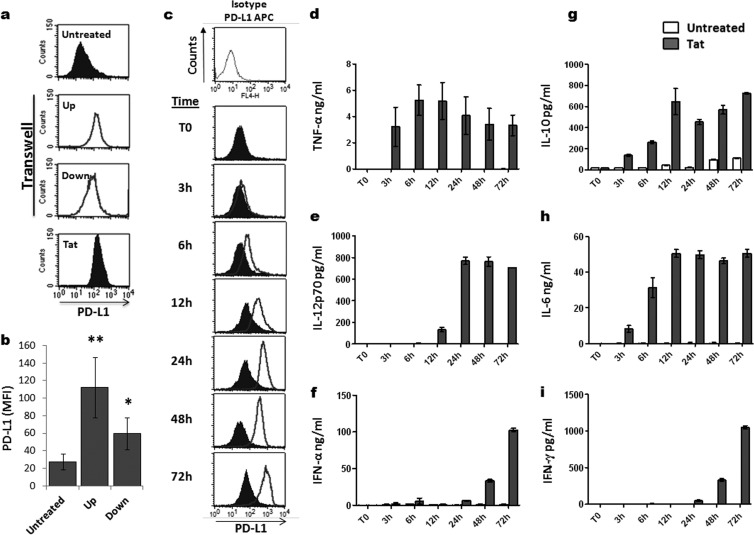
To understand the mode of action of Tat (via soluble or membrane-associated factors), we tested whether PD-L1 upregulation on MoDCs can occur without direct cell-cell contact through a soluble factor that might be released by Tat-treated MoDCs. To this end, this experiment was done in two steps: (i) following 1 h of treatment of MoDCs with Tat, the cells were washed to eliminate any residual soluble Tat protein; (ii) the Tat-treated MoDCs and untreated MoDCs were then cultured in two different compartments separated by a microporous membrane that allowed soluble factors to diffuse between the upper and lower compartments (known as the transwell coculture system). After an additional 24 h of coculture, MoDCs from each compartment were harvested and the level of PD-L1 expression on their cell surface was determined using FACS. Interestingly, in addition to the expected PD-L1 upregulation on Tat-treated MoDCs from the upper chamber, a significant upregulation of PD-L1 molecules was also detected on the MoDCs from the lower chamber, which had never been in direct contact with Tat protein (Fig. 6a and b). In agreement with the latter data, we also showed that Tat-conditioned medium obtained from the supernatants of MoDCs previously treated with Tat protein for 24 h was sufficient to induce the upregulation of PD-L1 on immature MoDCs in the absence of direct Tat treatment (Fig. 7a and b). These results indicate that the effect of Tat protein on PD-L1expression is independent of Tat-cell or cell-cell contacts and suggests that the effect is mediated indirectly through yet-to-be-determined soluble factors or cytokines.
FIG 6.
Role of soluble factors on the induction of PD-L1 expression by HIV-1 Tat. (a and b) Tat-treated MoDCs (Tat) and autologous untreated MoDCs (Untreated) were cocultured for 24 h in two different compartments separated by a 1-μm microporous membrane to avoid cell-cell contact. MoDCs that had been pretreated for 1 h with 100 nM Tat protein and washed three times with sterile PBS, to remove any residual Tat protein, were placed in the upper chamber of the transwell (Up). The untreated MoDCs were placed in the bottom chamber of the transwell (Down). Untreated MoDCs alone (Untreated) and MoDCs directly treated for 24 h with 100 nM Tat protein (Tat) were used as negative and positive controls, respectively. Following a 24-h coculture, the level of expression of PD-L1 was determined by flow cytometry in each compartment. (b) A graphical representation of three independent experiments showing PD-L1 MFI ± standard deviation with statistical analysis. Asterisks represent P values: *, P < 0.05; **, P < 0.01. (c) MoDCs were stimulated with 100 nM HIV-1 Tat protein for various times. As a negative control, MoDCs were kept untreated (filled histograms). After the indicated time (0 h, 3 h, 6 h, 12 h, 24 h, 48 h, 72 h), the level of PD-L1 expression was determined by flow cytometry, as described above. Filled histograms indicate the basal level of PD-L1 expression on untreated MoDCs, and the unfilled histograms represent the level of PD-L1 expression following treatment with the GST-Tat 1–101 protein. Data are representative of three independent experiments. (d to i) Cell supernatants were harvested at the indicated time (0 h, 3 h, 6 h, 12 h, 24 h, 48 h, 72 h) in untreated MoDCs (white bars) and in GST-Tat 1–101-treated MoDCs (black bars). The levels of TNF-α, IL-12p70, IL-6, IFN-α, IL-10, and IFN-γ cytokines were determined by ELISA, as detailed in Materials and Methods. The results are representative of at least 3 independent experiments. Data are the means from triplicates wells ± standard deviations.
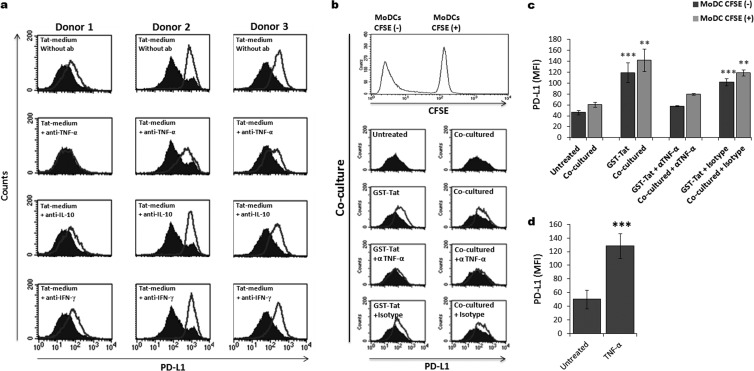
FIG 7.
Tat upregulates PD-L1 expression on MoDCs through a TNF-α-dependent mechanism. (a) MoDCs from three different donors were incubated in Tat-conditioned (unfilled histograms) or unconditioned (filled histograms) medium. In a parallel experiment, to determine the implication of TNF-α, IL-10, and IFN-γ cytokines in the PD-L1 upregulation induced by Tat protein, those cytokines were first selectively neutralized in Tat-conditioned medium by preincubation for 90 min with 20 μg/ml of anticytokine-specific antibodies (+anti-TNF-α, +anti-IL-10, and +anti-IFN-γ). After 24 h of coculture, the level of PD-L1 expression was determined by flow cytometry. The data show the results from three independent experiments. (b) MoDCs were treated by Tat protein for 1 h, washed, and cocultured with autologous untreated MoDCs at a 1:1 ratio as detailed in the Fig. 5 legend. To determine the implication of TNF-α, similar experiments were performed in the presence of 20 μg/ml of anti-TNF-α-specific antibody (+anti-TNF-α) or the corresponding isotype Ig (+Isotype). After 24 h of coculture, the level of PD-L1 expression was determined by flow cytometry on both CFSE positive (+) and CFSE negative (−) MoDCs. (c) Corresponds to graphical representation of three independent experiments with statistical analysis. The results are representative of at least 3 independent experiments. Asterisks represent P values: **, P < 0.01; ***, P < 0.001. (d) MoDCs from three different healthy blood donors were treated with recombinant human TNF-α (50 ng/ml) or kept untreated. After 24 h, the expression of PD-L1 was analyzed by flow cytometry. The figure shows graphical representation of three independent experiments with statistical analysis. Asterisks represent P values: ***, P < 0.001.
Therefore, in the next key kinetic experiment, we determined whether and when PD-L1 optimal expression on MoDCs coincided with production of the major Tat-induced cytokines, including TNF-α, IL-10, IL-12, IL-6, IFN-α, and IFN-γ cytokines. This strategy allowed the cytokines that are produced with later kinetics than PD-L1 expression to be excluded, as those cytokines may not be involved in the mechanisms of PD-L1 upregulation. As shown in (Fig. 6c to i), MoDCs that were directly treated with Tat protein exhibited PD-L1 upregulation in a time-dependent manner, as was also observed for cytokine induction. Specifically, upregulation of PD-L1 resumed 6 h after treatment of MoDCs with Tat protein (Fig. 6c). Maximum upregulation of PD-L1 was reached around 24 h poststimulation and was followed by a plateau of expression (Fig. 6c). Among the panel of six Tat-induced cytokines (Fig. 6d to i), we tested only TNF-α, IL-10, and IL-6, cytokines that were produced before 6 h (i.e., before PD-L1 expression was resumed following Tat stimulation; Fig. 6c).
These results suggest that TNF-α, IL-10, and IL-6 cytokines may be involved in a mechanism that leads to upregulation of PD-L1 on MoDCs following Tat treatment. However, because exogenous recombinant IL-6 failed to upregulate PD-L1 expression on MoDCs (data not shown), the following experiments were focused on the potential mechanism by which TNF-α and IL-10 might be involved in PD-L1 expression.
HIV-1 Tat protein induces PD-L1 expression on MoDCs through a TNF-α-mediated mechanism.
To determine whether TNF-α and IL-10 were involved in PD-L1 upregulation, each cytokine was selectively depleted from Tat-conditioned medium by incubation with specific neutralizing antibodies. Interestingly, when Tat-untreated MoDCs were stimulated using Tat-conditioned medium and in the presence of anti-TNF-α-neutralizing antibodies, PD-L1 upregulation was totally abolished and reversed to a basal level (Fig. 7a). In contrast, neutralization of IL-10 did not reverse PD-L1 upregulation on MoDCs (Fig. 7a). As a negative control, neutralization of IFN-γ did not affect PD-L1 upregulation (Fig. 7a). The crucial role of Tat-induced TNF-α was also demonstrated in the coculture experiments using CFSE-labeled MoDCs. In this assay, the addition of anti-TNF-α antibodies, in contrast to the corresponding isotype, strongly inhibited PD-L1 upregulation on both Tat-treated CFSE− and Tat-untreated CFSE+ MoDCs (Fig. 7b and c). In line with these results, we showed that treatment with exogenous recombinant TNF-α induced similar PD-L1 upregulation on MoDCs (Fig. 7d).
All together, these results indicate that the upregulation of PD-L1 molecules induced by Tat protein on MoDCs occurs essentially through a TNF-α-mediated mechanism.
The induction of PD-L1 expression on MoDCs by HIV-1 Tat protein involves the TLR4 pathway.
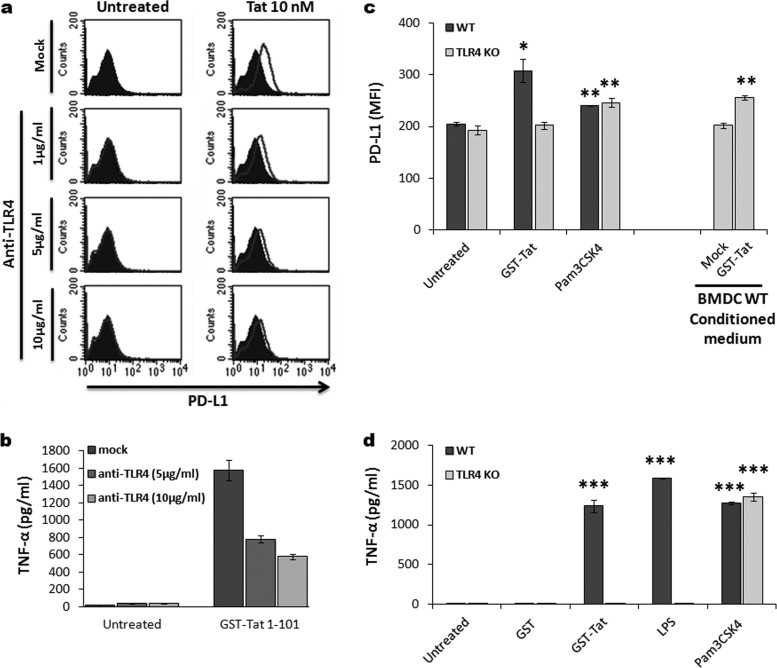
Since we had recently found that HIV-1 Tat protein (i) mediates TNF-α production in a TLR4-dependent manner (62) and (ii) interacts physically and with high affinity with TLR4-MD2 (62), we next explored the potential association between Tat-TLR4 interaction, TNF-α production, and PD-L1 upregulation, both in human MoDCs and in bone marrow-derived DCs (BMDCs) from either wild-type (wt) or TLR4-KO mice. We found that stimulation of MoDCs by Tat in the presence of increasing amounts of anti-TLR4 antibodies significantly inhibited Tat-induced PD-L1 upregulation (Fig. 8a). As in human MoDCs, Tat protein induced upregulation of PD-L1 expression in BMDCs from wt mice (Fig. 8c). However, HIV-1 Tat protein had no effect on the BMDCs obtained from TLR4-KO mice, indicating the involvement of the TLR4 pathway (Fig. 8c). As a control, treatment with pam3CSK4 (a TLR2 ligand) induced PD-L1 upregulation in a similar manner in BMDCs from wt or TLR4-KO mice (Fig. 8c). In line with the previous data implicating Tat-induced TNF-α (Fig. 7), we showed a direct association between Tat-induced TNF-α and PD-L1 upregulation (Fig. 8b and d). We demonstrated that PD-L1 upregulation of human MoDCs, in the presence of anti-TLR4 antibodies, or BMDCs from wt or TLR4-KO mice by Tat were strictly associated with the production of TNF-α (Fig. 8b and d). More interestingly, culturing BMDCs from TLR4-KO mice with supernatant from Tat-conditioned medium of BMDCs wt mice led to the upregulation of PD-L1 (Fig. 8c), thus giving an additional argument for the Tat-induced TNF-α in the PD-L1 upregulation on the surface of DCs.
FIG 8.
Role of Tat-TLR4 interaction in PD-L1 upregulation. (a) MoDCs were treated with 10 nM Tat protein or kept untreated without (Mock) or with increasing amount of anti-TLR4 antibodies (1 μg/ml, 5 μg/ml, 10 μg/ml). After 24 h, the level of expression of PD-L1 was determined by flow cytometry. Flow plots show one representative result from three independent experiments. (b) The level of TNF-α present in the supernatants of Tat-treated and -untreated MoDCs with or without the anti-TLR4-blocking MAb was determined by ELISA. Data are the means of triplicates wells ± standard deviations. (c) BMDCs derived from either wt (dark-gray bars) or TLR4-KO mice (light-gray bars) were treated for 24 h with 100 nM GST-Tat protein, 1 μg/ml TLR1/2 ligand (Pam3CSK4), and 1 μg/ml LPS as a positive control. Tat-conditioned and unconditioned medium from wt BMDCs was also included as an internal control. After 24 h, the level of PD-L1 expression was determined by flow cytometry. Histograms show cumulative data obtained from three independent experiments with statistical analysis. Asterisks represent P values: *, P < 0.05; **, P < 0.01. (d) The level of TNF-α present at 24 h in the supernatants of each group of BMDCs treated, determined by ELISA, is indicated: untreated; GST, 100 nM; GST-Tat 1–101, 100 nM; LPS, 1 μg/ml; Pam3CSK4, 1 μg/ml. The results are representative of at least 3 independent experiments. Data are the means of triplicates wells ± standard deviations.
HIV-1 Tat protein-mediated PD-L1 upregulation on MoDCs functionally compromises MoDC ability to stimulate T cells.
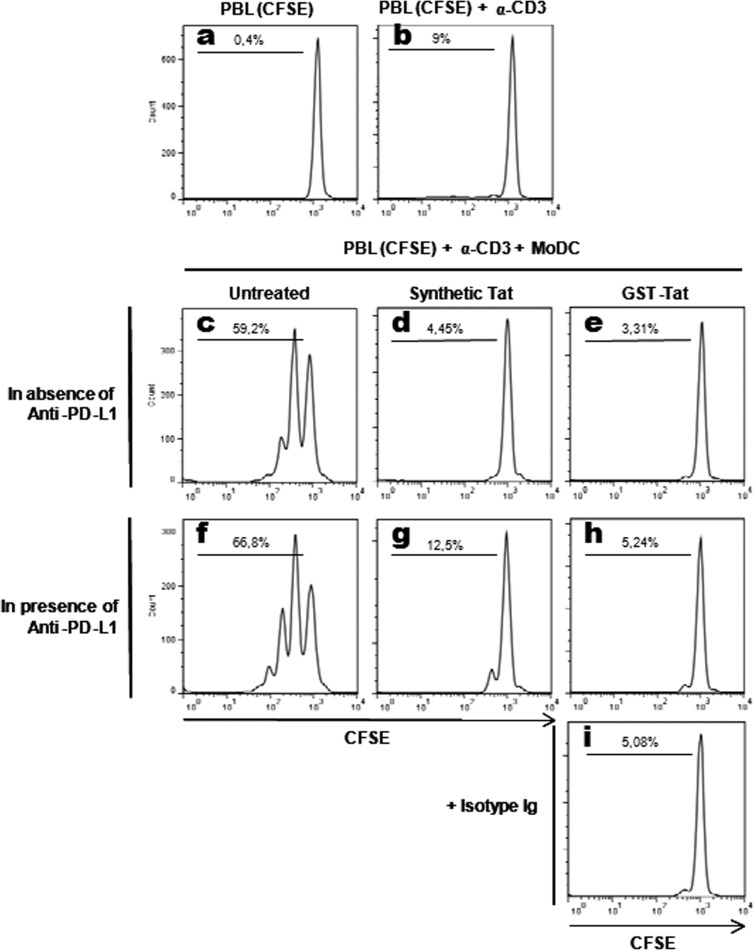
Coculture of untreated MoDCs with CFSE-labeled autologous T cells in the presence of a suboptimal amount of anti-CD3 antibodies (10 ng/ml) led to a significant T-cell proliferation, as shown in Fig. 8c (59.2% of proliferating T cells). In contrast, pretreatment of MoDCs with synthetic or recombinant HIV-1 Tat protein strongly inhibited the capacity of Tat-treated MoDCs to activate T-cell proliferation. This result suggests that activation of the PD-L1/PD-1 T-cell coinhibitory pathway following HIV-1 Tat treatment may have compromised the functional ability of Tat-treated MoDCs to stimulate T cells (Fig. 9d and e). This was supported by the fact that, following treatment with HIV-1 Tat protein, the functional inhibition of T-cell proliferation was closely correlated with the upregulation of the PD-L1 coinhibitory molecule seen on MoDCs (Fig. 2a to c). As controls, (i) no significant T-cell proliferation (less than 1%) was observed in the absence of MoDCs and anti-CD3 antibodies (Fig. 9a), and (ii) only a basal proliferation (about 10%) was observed when T cells were stimulated by anti-CD3 in the absence of MoDCs (Fig. 9b).
FIG 9.
Effect of HIV-1 Tat protein on the ability of MoDCs to induce T-cell proliferation. Peripheral blood lymphocytes (PBLs) were labeled with CFSE (2 μM) and kept in culture for 5 days alone (a), in the presence of a suboptimal concentration (10 ng/ml) of anti-CD3 (b), or cocultured with autologous MoDCs (previously treated for 48 h, as indicated) in the presence of anti-CD3 (c to i). MoDCs were kept untreated (c, f) or treated with 50 nM synthetic oxidized Tat (d, g) or 100 nM recombinant GST-Tat (e, h) protein. Coculture of MoDCs (2 × 105 cells) and PBLs (4 × 105 cells) was performed in the absence (c to e) or presence (f to h) of anti-PD-L1 antibodies at 10 μg/ml or an equal amount of isotype antibodies (i). After 5 days of coculture, cells were harvested and CD3-positive cells were labeled firstly with a mouse anti-CD3 antibody and then with an anti-mouse IgG2a Alexa Fluor 633 antibody. Proliferation was then analyzed by flow cytometry in CD3+ cells. The results are representative of at least 3 independent experiments.
In order to ascertain the involvement of the PD-1/PD-L1 pathway in the observed inhibition of T-cell proliferation following HIV-1 Tat treatment, we tested the blocking effect of anti-PD-L1 antibodies (10 μg/ml) (Fig. 9f to h). Unexpectedly, we found that the anti-PD-L1 blockade was unable to significantly restore the capacity of Tat-treated-MoDCs to activate T-cell proliferation (Fig. 9g to h). The absence of restoration of the capacity of MoDCs to activate T-cell proliferation, despite the interference with the PD-1/PD-L1 T-cell coinhibitory pathway, suggests the implication of other yet-to-be-determined T-cell coinhibitory pathways induced by HIV-1 Tat, such as TIM-3/Gal-9, BTLA/HVEM, and/or 2B4/CD48.
DISCUSSION
In the present study, we showed that treatment with HIV-1 Tat protein of primary myeloid DCs isolated from healthy donors or MoDCs obtained from either HIV-1-infected patients or uninfected healthy donors significantly upregulated the level of expression of PD-L1 ligand on these cells. This resulted in compromised ability of Tat-treated MoDCs to functionally stimulate T-cell proliferation. In contrast, no effect on the expression of PD-L2 ligand or PD-1 receptors was detected. The induction of PD-L1 expression on MoDCs by HIV-1 Tat protein (i) occurred in dose- and time-dependent manners, (ii) was activated by the N-terminal 1–45 fragment of Tat, (iii) was mediated by Tat-induced soluble factor(s) and thus did not require direct cell-cell contact, (iv) was totally or partially abrogated by neutralizing anti-TNF-α or blocking anti-TLR4 antibodies, respectively, (v) was absent in TLR4-KO mice but could be restored following incubation with Tat-conditioned medium from wt DCs, (vi) strongly impaired the capacity of MoDCs to stimulate T-cell proliferation, and (vii) was not reversed functionally by antibodies that blocked the PD-1/PD-L1 pathway, suggesting the implication of other Tat-mediated coinhibitory pathways. To the best of our knowledge, this is the first report showing that HIV-1 Tat protein, an immunosuppressive viral factor, modulates the PD-1/PD-L1 T-cell coinhibitory pathway of antigen-presenting cells (i.e., DCs) by hijacking the TLR4 pathway.
These findings, involving Tat/TLR4/TNF-α pathways, suggest a novel immune evasion mechanism whereby the HIV-1 Tat may promote functional exhaustion (i.e., dysfunction) of HIV-specific CD4+ and CD8+ T cells in chronically infected individuals, resulting in increased virus reactivation, increased virus load, and/or fast progression toward AIDS. An understanding, at molecular and cellular levels, of the underlying mechanisms of action may offer new potential molecular targets for the development of anti-HIV-1 treatment.
In agreement with the data reported by Fanales-Belasio and coworkers (37, 71), we found that Tat protein was able to induce the maturation of MoDCs as shown by the expression of the costimulatory molecules CD80 and CD86 and the phenotypic marker CD83. Because Tat-MoDC maturation can be blocked in the presence of anti-TNF-α and anti-TLR4 antibodies (data not shown), on the one hand, and can be stimulated by the N-terminal 1–45 fragment of Tat, on the other hand, our data suggest that MoDC maturation is primed by Tat action at the cell membrane level rather than following its uptake (37). Interestingly, HTLV-1 Tax protein, the equivalent of Tat protein in HIV-1, also induced MoDC maturation (72). Curiously, the activating effect of Tat protein seems to be counterbalanced by the action of Vpr, another HIV-1 regulatory protein, which has an antagonist effect on the maturation of DCs (73). In contrast to our study, Izmailova and coworkers, using recombinant adenovirus Tat and HIV-1 infectious particles as a source of Tat, did not observe any effect of Tat on MoDC maturation or on the production of TNF-α, IL-1, IL-6, and IL-12, proinflammatory cytokines that are associated with DC maturation (74). This apparent discrepancy may be related to the absence of a sufficient amount of Tat protein following adenovirus transduction and HIV-1 infection of immature DCs. In the latter study (74), although Tat mRNA was shown, Tat expression at the protein level was never checked.
Using a Tat-deleted mutant encoding only the N-terminal 1–45 region, we showed that this N-terminal Tat fragment continued to upregulate PD-L1 on MoDCs in a manner similar to that of the whole Tat protein. However, because the N-terminal 1–45 fragment does not have the capacity to trans activate HIV-1 LTR, we conclude that the upregulation of PD-L1 mediated by Tat is independent of the two crucial basic and cysteine domains essential for Tat trans activation activity. This conclusion is also in agreement with the inability of this N-terminal 1–45 fragment to be taken up by cells, because it lacks the 49–57 basic domain necessary for the cellular uptake of Tat protein. All together, these results rule out the possibility that Tat-mediated upregulation of PD-L1 on MoDCs occurred through an intracellular mechanism. The hypothesis of a direct action of Tat on the cell surface was further excluded, because Tat-conditioned medium was sufficient to upregulate PD-L1 on MoDCs, clearly showing that physical contact between Tat and MoDCs is not necessary for PD-L1 upregulation. To double-check the indirect hypothesis, we explored the potential implication of Tat-induced cytokines in the upregulation of PD-L1 on MoDCs in a kinetic study. Among the potential cytokines released in MoDC supernatant 6 h after Tat treatment, only TNF-α was able to induce PD-L1 upregulation on MoDCs. The indirect effect of this cytokine was ascertained by the capacity of anti-TNF-α antibodies to interfere with PD-L1 upregulation induced by Tat protein. The underlying mechanisms of how TNF-α contributed to PD-L1 upregulation mediated by Tat are beyond the scope of the present study and will be the subject of a future report.
In HIV-1-infected fast progressors, an increase of PD-L1 has been observed on MoDCs. This increase seems to be directly associated with an increase in the viral load, a decrease of CD4+ T-cell counts, and a fast progression to AIDS (23). In contrast, a nonsignificant upregulation of PD-L1 has been observed on MoDCs from HIV-1 nonprogressors (23). These observations were also confirmed in the SIV/macaque model in which an increase of PD-L1 on plasmacytoid and myeloid DCs and of PD-1 on CD4+ and CD8+ T cells was associated with higher loads and fast progression to disease (75). More interestingly, it has been shown that PD-L1- and PD-1-positive cells colocalized within the same sites of infection, suggesting a functional role of PD-1–PD-L1 interaction in the physiopathology of SIV infection (75). A subsequent in vivo study has shown that treatment of SIV-infected macaques with anti-PD-1-blocking antibodies was associated with (i) an expansion of anti-SIV CD8+ T cells, (ii) an enhancement of antibodies against SIV envelope glycoproteins, (iii) a reduction of plasmatic SIV viral load, and (iv) longer survival (32). In a more recent study, the same group reported that the PD-1 blockade, even during chronic infection, was also accompanied by a functional repair of the gastrointestinal tract barrier, as shown by the diminution of LPS in the plasma, and a reduction of hyperimmune activation, as shown by the diminution of the expression of type I IFN-stimulated genes (ISGs) (30).
The present study extends the above-described findings by showing that HIV-1 Tat protein was behind the upregulation of the PD-L1 molecule on MoDCs. However, the finding that the PD-1/PD-L1 T-cell coinhibitory pathway is affected by HIV-1 Tat does not rule out the possibility that other coinhibitory pathways are affected by Tat immunosuppressive viral protein. This is supported by our results showing that blockade of the PD-1/PD-L1 pathway was not sufficient to reverse the T-cell stimulation function of Tat-treated DC. The absence of restoration of the capacity of MoDCs to activate T-cell proliferation, despite the interference with the PD-1/PD-L1 coinhibitory pathway, suggests an implication of other yet-to-be-determined T-cell coinhibitory pathways, such as TIM-3/Gal-9, BTLA/HVEM, and/or 2B4/CD48 (76, 77), or other Tat-induced immunosuppressive factors that remain to be clearly identified. Accordingly, the effect of Tat on the potential modulation of other T-cell coinhibitory pathways and other immunosuppressive factors is currently being evaluated in our laboratory. Our group has previously shown that HIV-1 Tat protein is also able to stimulate the expression of IL-10 (33, 68) and IDO (78), two factors known for their highly immunosuppressive activities.
The effect of the blockade of the PD-1/PD-L1 pathway in the course of HIV-1 infection has also been confirmed in HIV-1-infected humanized BALB/c-Rag2−/− γc−/− (Rag-hum) mice (79). This model was first validated by showing that infection of humanized Rag-hum mice with the R5 tropic HIV-1-BaL isolate led to an increase of viral load in the plasma, accompanied by CD4+ T-cell depletion and an enhancement of PD-1 expression on T cells (79). As expected, treatment of HIV-1-infected humanized mice with anti-PD-L1 antibodies significantly restored T-cell function, elevated CD4+ T-cell count, and resulted in a plasmatic reduction of viral load (79). To the best of our knowledge, neither anti-PD-1 nor anti-PD-L1 treatment has ever been tested in clinical trials in HIV-1-infected human patients. Nevertheless, a treatment with anti-PD-1-blocking antibodies (lambrolizumab) has recently been tested in phases II and III in human patients with advanced melanoma (80). The results were promising, with significant tumor regression and only minor secondary effects (80). This obviously points to the possibility of a new treatment in the HIV setting.
In summary, to the best of our knowledge, the results presented in this study show, for the first time, that treatment of human MoDCs with HIV-1 Tat protein significantly upregulates the level of expression of PD-L1 ligand, which results in compromised ability of these professional APCs to functionally stimulate T cells. This effect is activated by the N-terminal Tat 1–45 domain and appears to be mediated by a yet-to-be-determined TNF-α- and TLR4-dependent mechanism. While HIV-1 Tat upregulated PD-L1 and strongly impaired the capacity of MoDCs to stimulate T-cell proliferation, this effect was not functionally reversed by antibodies blocking the PD-1/PD-L1 pathway. This suggests the implication of other Tat-mediated coinhibitory pathways, including TIM-3/Gal-9, CTLA4/B7-1/2, BTLA/HVEM, 2B4/CD48, CD160/HVEM, and LAG3/MHCII.
Since functional CD4+ and CD8+ T cells appear to be important effectors of HIV-1 protective immunity, the present findings offer new potential molecular targets for the development of anti-HIV-1 treatment that would reverse the apparent dysfunction (exhaustion) of HIV-specific T cells during chronic infection. A combination of blockade of the PD-1/PD-L1 T-cell coinhibitory pathway in combination with therapeutic vaccination, including oxidized Tat protein as the immunogen, may hold great therapeutic promise.
ACKNOWLEDGMENTS
This work was supported by grants from SIDACTION and ANRS to E.B., by INSERM, CNRS, and MRT, and by Public Health Service NIH grants EY14900 and EY019896 to L.B.; R.P. was supported by an MRT fellowship.
We thank B. Ryffel from the University of Orleans for providing the TLR4-KO mice used in this study, the staff of flow cytometry imaging at INSERM U1043, J. Auriol and S. Ethuin from UMR 5547 CNRS for animal housing, and M. Cazabat and M. Requena for their technical help. We thank N. Jabrane for helpful comments on the last version of the manuscript.
Footnotes
Published ahead of print 2 April 2014
REFERENCES
- 1.Luban J. 2012. Innate immune sensing of HIV-1 by dendritic cells. Cell Host Microbe 12:408–418. 10.1016/j.chom.2012.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swanstrom R, Coffin J. 2012. HIV-1 pathogenesis: the virus. Cold Spring Harb. Perspect. Med. 2:a007443. 10.1101/cshperspect.a007443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambotin M, Raghuraman S, Stoll-Keller F, Baumert TF, Barth H. 2010. A look behind closed doors: interaction of persistent viruses with dendritic cells. Nat. Rev. Microbiol. 8:350–360. 10.1038/nrmicro2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Woltman AM, Janssen HL, Boonstra A. 2009. Modulation of dendritic cell function by persistent viruses. J. Leukoc. Biol. 85:205–214. 10.1189/jlb.0408241 [DOI] [PubMed] [Google Scholar]
- 5.Reis e Sousa C. 2006. Dendritic cells in a mature age. Nat. Rev. Immunol. 6:476–483. 10.1038/nri1845 [DOI] [PubMed] [Google Scholar]
- 6.Miller E, Bhardwaj N. 2013. Dendritic cell dysregulation during HIV-1 infection. Immunol. Rev. 254:170–189. 10.1111/imr.12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wijewardana V, Kristoff J, Xu C, Ma D, Haret-Richter G, Stock JL, Policicchio BB, Mobley AD, Nusbaum R, Aamer H, Trichel A, Ribeiro RM, Apetrei C, Pandrea I. 2013. Kinetics of myeloid dendritic cell trafficking and activation: impact on progressive, nonprogressive and controlled SIV infections. PLoS Pathog. 9:e1003600. 10.1371/journal.ppat.1003600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appay V, Sauce D. 2008. Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 214:231–241. 10.1002/path.2276 [DOI] [PubMed] [Google Scholar]
- 9.Ipp H, Zemlin A. 2013. The paradox of the immune response in HIV infection: when inflammation becomes harmful. Clin. Chim. Acta 416:96–99. 10.1016/j.cca.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 10.Khaitan A, Unutmaz D. 2011. Revisiting immune exhaustion during HIV infection. Curr. HIV/AIDS Rep. 8:4–11. 10.1007/s11904-010-0066-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sauce D, Elbim C, Appay V. 2013. Monitoring cellular immune markers in HIV infection: from activation to exhaustion. Curr. Opin. HIV AIDS 8:125–131. 10.1097/COH.0b013e32835d08a9 [DOI] [PubMed] [Google Scholar]
- 12.Herbeuval JP, Shearer GM. 2007. HIV-1 immunopathogenesis: how good interferon turns bad. Clin. Immunol. 123:121–128. 10.1016/j.clim.2006.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. 2011. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J. Exp. Med. 208:2367–2374. 10.1084/jem.20110654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijewardana V, Soloff AC, Liu X, Brown KN, Barratt-Boyes SM. 2010. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 6:e1001235. 10.1371/journal.ppat.1001235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsikis PD, Mueller YM, Villinger F. 2011. The cytokine network of acute HIV infection: a promising target for vaccines and therapy to reduce viral set-point? PLoS Pathog. 7:e1002055. 10.1371/journal.ppat.1002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keating SM, Jacobs ES, Norris PJ. 2012. Soluble mediators of inflammation in HIV and their implications for therapeutics and vaccine development. Cytokine Growth Factor Rev. 23:193–206. 10.1016/j.cytogfr.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman GJ. 2008. Structures of PD-1 with its ligands: sideways and dancing cheek to cheek. Proc. Natl. Acad. Sci. U. S. A. 105:10275–10276. 10.1073/pnas.0805459105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. 2006. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 203:2223–2227. 10.1084/jem.20061800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, Veazey RS. 2010. Increased B7–H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J. Immunol. 185:7340–7348. 10.4049/jimmunol.1001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, Reilly CS, Silvestri G, Haase AT. 2008. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J. Immunol. 180:6798–6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zhang Z, Zhang S, Fu J, Yao J, Jiao Y, Wu H, Wang FS. 2008. B7–H1 up-regulation impairs myeloid DC and correlates with disease progression in chronic HIV-1 infection. Eur. J. Immunol. 38:3226–3236. 10.1002/eji.200838285 [DOI] [PubMed] [Google Scholar]
- 24.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. 2007. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215–4225. 10.1128/JVI.02844-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen T, Chen X, Chen Y, Xu Q, Lu F, Liu S. 2010. Increased PD-L1 expression and PD-L1/CD86 ratio on dendritic cells were associated with impaired dendritic cells function in HCV infection. J. Med. Virol. 82:1152–1159. 10.1002/jmv.21809 [DOI] [PubMed] [Google Scholar]
- 26.Chentoufi AA, Dervillez X, Dasgupta G, Nguyen C, Kabbara KW, Jiang X, Nesburn AB, Wechsler SL, BenMohamed L. 2012. The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells. Viral Immunol. 25:204–215. 10.1089/vim.2011.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, BenMohamed L. 2011. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J. Virol. 85:9127–9138. 10.1128/JVI.00587-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Channappanavar R, Twardy BS, Suvas S. 2012. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One 7:e39757. 10.1371/journal.pone.0039757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 30.Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, Amara RR. 2012. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J. Clin. Invest. 122:1712–1716. 10.1172/JCI60612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202. 10.1038/nm1482 [DOI] [PubMed] [Google Scholar]
- 32.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. 2009. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458:206–210. 10.1038/nature07662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badou A, Bennasser Y, Moreau M, Leclerc C, Benkirane M, Bahraoui E. 2000. Tat protein of human immunodeficiency virus type 1 induces interleukin-10 in human peripheral blood monocytes: implication of protein kinase C-dependent pathway. J. Virol. 74:10551–10562. 10.1128/JVI.74.22.10551-10562.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buonaguro L, Barillari G, Chang HK, Bohan CA, Kao V, Morgan R, Gallo RC, Ensoli B. 1992. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J. Virol. 66:7159–7167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buscemi L, Ramonet D, Geiger JD. 2007. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol. Dis. 26:661–670. 10.1016/j.nbd.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Contreras X, Bennasser Y, Bahraoui E. 2004. IL-10 production induced by HIV-1 Tat stimulation of human monocytes is dependent on the activation of PKC beta(II) and delta isozymes. Microbes Infect. 6:1182–1190. 10.1016/j.micinf.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 37.Fanales-Belasio E, Moretti S, Nappi F, Barillari G, Micheletti F, Cafaro A, Ensoli B. 2002. Native HIV-1 Tat protein targets monocyte-derived dendritic cells and enhances their maturation, function, and antigen-specific T cell responses. J. Immunol. 168:197–206 [DOI] [PubMed] [Google Scholar]
- 38.Gandhi N, Saiyed Z, Thangavel S, Rodriguez J, Rao KV, Nair MP. 2009. Differential effects of HIV type 1 clade B and clade C Tat protein on expression of proinflammatory and antiinflammatory cytokines by primary monocytes. AIDS Res. Hum. Retroviruses 25:691–699. 10.1089/aid.2008.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gee K, Angel JB, Ma W, Mishra S, Gajanayaka N, Parato K, Kumar A. 2006. Intracellular HIV-Tat expression induces IL-10 synthesis by the CREB-1 transcription factor through Ser133 phosphorylation and its regulation by the ERK1/2 MAPK in human monocytic cells. J. Biol. Chem. 281:31647–31658. 10.1074/jbc.M512109200 [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Boppana R, Mishra GC, Saha B, Mitra D. 2008. HIV-1 Tat suppresses gp120-specific T cell response in IL-10-dependent manner. J. Immunol. 180:79–88 [DOI] [PubMed] [Google Scholar]
- 41.Johri MK, Mishra R, Chhatbar C, Unni SK, Singh SK. 2011. Tits and bits of HIV Tat protein. Expert Opin. Biol. Ther. 11:269–283. 10.1517/14712598.2011.546339 [DOI] [PubMed] [Google Scholar]
- 42.Leghmari K, Bennasser Y, Tkaczuk J, Bahraoui E. 2008. HIV-1 Tat protein induces IL-10 production by an alternative TNF-alpha-independent pathway in monocytes: role of PKC-delta and p38 MAP kinase. Cell Immunol. 253:45–53. 10.1016/j.cellimm.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 43.Rubartelli A, Poggi A, Sitia R, Zocchi MR. 1998. HIV-I Tat: a polypeptide for all seasons. Immunol. Today 19:543–545. 10.1016/S0167-5699(98)01351-6 [DOI] [PubMed] [Google Scholar]
- 44.Goldstein G. 1996. HIV-1 Tat protein as a potential AIDS vaccine. Nat. Med. 2:960–964. 10.1038/nm0996-960 [DOI] [PubMed] [Google Scholar]
- 45.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497–500. 10.1038/375497a0 [DOI] [PubMed] [Google Scholar]
- 46.Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. 2000. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl. Acad. Sci. U. S. A. 97:11466–11471. 10.1073/pnas.97.21.11466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyagi M, Rusnati M, Presta M, Giacca M. 2001. Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276:3254–3261. 10.1074/jbc.M006701200 [DOI] [PubMed] [Google Scholar]
- 48.Noonan D, Albini A. 2000. From the outside in: extracellular activities of HIV Tat. Adv. Pharmacol. 48:229–250. 10.1016/S1054-3589(00)48008-7 [DOI] [PubMed] [Google Scholar]
- 49.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell GR, Loret EP, Spector SA. 2010. HIV-1 clade B Tat, but not clade C Tat, increases X4 HIV-1 entry into resting but not activated CD4+ T cells. J. Biol. Chem. 285:1681–1691. 10.1074/jbc.M109.049957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng L, Yang YD, Lu GC, Salvato MS. 2005. Extracellular HIV Tat and Tat cysteine rich peptide increase CCR5 expression in monocytes. J. Zhejiang Univ. Sci. B 6:668–672. 10.1631/jzus.2005.B0668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li CJ, Ueda Y, Shi B, Borodyansky L, Huang L, Li YZ, Pardee AB. 1997. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 94:8116–8120. 10.1073/pnas.94.15.8116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King JE, Eugenin EA, Buckner CM, Berman JW. 2006. HIV Tat and neurotoxicity. Microbes Infect. 8:1347–1357. 10.1016/j.micinf.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 54.Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. 1996. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J. Virol. 70:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabatier JM, Vives E, Mabrouk K, Benjouad A, Rochat H, Duval A, Hue B, Bahraoui E. 1991. Evidence for neurotoxic activity of Tat from human immunodeficiency virus type 1. J. Virol. 65:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell GR, Pasquier E, Watkins J, Bourgarel-Rey V, Peyrot V, Esquieu D, Barbier P, de Mareuil J, Braguer D, Kaleebu P, Yirrell DL, Loret EP. 2004. The glutamine-rich region of the HIV-1 Tat protein is involved in T-cell apoptosis. J. Biol. Chem. 279:48197–48204. 10.1074/jbc.M406195200 [DOI] [PubMed] [Google Scholar]
- 57.Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429–431. 10.1126/science.7716549 [DOI] [PubMed] [Google Scholar]
- 58.Gutheil WG, Subramanyam M, Flentke GR, Sanford DG, Munoz E, Huber BT, Bachovchin WW. 1994. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc. Natl. Acad. Sci. U. S. A. 91:6594–6598. 10.1073/pnas.91.14.6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Contreras X, Bennasser Y, Chazal N, Moreau M, Leclerc C, Tkaczuk J, Bahraoui E. 2005. Human immunodeficiency virus type 1 Tat protein induces an intracellular calcium increase in human monocytes that requires DHP receptors: involvement in TNF-alpha production. Virology 332:316–328. 10.1016/j.virol.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 60.Zocchi MR, Poggi A, Rubartelli A. 1997. The RGD-containing domain of exogenous HIV-1 Tat inhibits the engulfment of apoptotic bodies by dendritic cells. AIDS 11:1227–1235. 10.1097/00002030-199710000-00005 [DOI] [PubMed] [Google Scholar]
- 61.Albini A, Soldi R, Giunciuglio D, Giraudo E, Benelli R, Primo L, Noonan D, Salio M, Camussi G, Rockl W, Bussolino F. 1996. The angiogenesis induced by HIV-1 Tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2:1371–1375. 10.1038/nm1296-1371 [DOI] [PubMed] [Google Scholar]
- 62.Haij NB, Leghmari K, Planes R, Thieblemont N, Bahraoui E. 2013. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-alpha and IL-10. Retrovirology 10:123. 10.1186/1742-4690-10-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vives E, Charneau P, van Rietschoten J, Rochat H, Bahraoui E. 1994. Effects of the Tat basic domain on human immunodeficiency virus type 1 transactivation, using chemically synthesized Tat protein and Tat peptides. J. Virol. 68:3343–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, Porichis F, Le Gall S, Waring MT, Moss K, Jessen H, Pereyra F, Kavanagh DG, Walker BD, Kaufmann DE. 2009. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114:346–356. 10.1182/blood-2008-12-191296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang XC, Fan YS, Zhou ZQ, Li HQ, Zhang Z, Zhang T, Wang L, Yang XP, Mao L, Xie RH, Lei SY, Wu H, Wang FS. 2010. Expression of B7–H1 on peripheral myeloid dendritic cells in patients with HIV infection and its correlation with diseases progression. Zhonghua Yi Xue Za Zhi 90:588–592 (In Chinese.) [PubMed] [Google Scholar]
- 66.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM. 2010. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci. Transl. Med. 2:32ra36. 10.1126/scitranslmed.3000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennasser Y, Badou A, Tkaczuk J, Bahraoui E. 2002. Signaling pathways triggered by HIV-1 Tat in human monocytes to induce TNF-alpha. Virology 303:174–180. 10.1006/viro.2002.1676 [DOI] [PubMed] [Google Scholar]
- 68.Leghmari K, Bennasser Y, Bahraoui E. 2008. HIV-1 Tat protein induces IL-10 production in monocytes by classical and alternative NF-kappaB pathways. Eur. J. Cell Biol. 87:947–962. 10.1016/j.ejcb.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 69.Samikkannu T, Rao KV, Gandhi N, Saxena SK, Nair MP. 2010. Human immunodeficiency virus type 1 clade B and C Tat differentially induce indoleamine 2,3-dioxygenase and serotonin in immature dendritic cells: implications for neuroAIDS. J. Neurovirol. 16:255–263. 10.3109/13550284.2010.497809 [DOI] [PubMed] [Google Scholar]
- 70.Samikkannu T, Saiyed ZM, Rao KV, Babu DK, Rodriguez JW, Papuashvili MN, Nair MP. 2009. Differential regulation of indoleamine-2,3-dioxygenase (IDO) by HIV type 1 clade B and C Tat protein. AIDS Res. Hum. Retroviruses 25:329–335. 10.1089/aid.2008.0225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fanales-Belasio E, Moretti S, Fiorelli V, Tripiciano A, Pavone Cossut MR, Scoglio A, Collacchi B, Nappi F, Macchia I, Bellino S, Francavilla V, Caputo A, Barillari G, Magnani M, Laguardia ME, Cafaro A, Titti F, Monini P, Ensoli F, Ensoli B. 2009. HIV-1 Tat addresses dendritic cells to induce a predominant Th1-type adaptive immune response that appears prevalent in the asymptomatic stage of infection. J. Immunol. 182:2888–2897. 10.4049/jimmunol.0711406 [DOI] [PubMed] [Google Scholar]
- 72.Jain P, Ahuja J, Khan ZK, Shimizu S, Meucci O, Jennings SR, Wigdahl B. 2007. Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type 1. J. Leukoc. Biol. 82:44–56. 10.1189/jlb.1006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Majumder B, Janket ML, Schafer EA, Schaubert K, Huang XL, Kan-Mitchell J, Rinaldo CR, Jr, Ayyavoo V. 2005. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J. Virol. 79:7990–8003. 10.1128/JVI.79.13.7990-8003.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Izmailova E, Bertley FM, Huang Q, Makori N, Miller CJ, Young RA, Aldovini A. 2003. HIV-1 Tat reprograms immature dendritic cells to express chemoattractants for activated T cells and macrophages. Nat. Med. 9:191–197. 10.1038/nm822 [DOI] [PubMed] [Google Scholar]
- 75.Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, Veazey RS. 2010. Increased B7–H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J. Immunol. 185:7340–7348. 10.4049/jimmunol.1001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsson M, Shankar EM, Che KF, Saeidi A, Ellegard R, Barathan M, Velu V, Kamarulzaman A. 2013. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirology 10:31. 10.1186/1742-4690-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shankar EM, Che KF, Messmer D, Lifson JD, Larsson M. 2011. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T-cells following priming by HIV-1 pulsed dendritic cells. Mol. Med. 17:229–240. 10.2119/molmed.2010.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Planes R, Bahraoui E. 2013. HIV-1 Tat protein induces the production of IDO in human monocyte derived-dendritic cells through a direct mechanism: effect on T cells proliferation. PLoS One 8:e74551. 10.1371/journal.pone.0074551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer BE, Neff CP, Lecureux J, Ehler A, Dsouza M, Remling-Mulder L, Korman AJ, Fontenot AP, Akkina R. 2013. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J. Immunol. 190:211–219. 10.4049/jimmunol.1201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. 2013. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369:134–144. 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]