ABSTRACT
Virus-specific CD8+ T cells provide classical adaptive immunity by responding to cognate peptide antigen, but they may also act in an “innate” capacity by responding directly to cytokine stimulation. Here, we examined regulation of these distinct T cell functions by anti-inflammatory cytokines (interleukin-4 [IL-4], IL-10, and transforming growth factor β [TGF-β]). Innate gamma interferon (IFN-γ) production by CD8+ T cells following exposure to IL-12 plus IL-18, IL-12 plus tumor necrosis factor alpha (TNF-α), or IL-12 plus IL-15 was inhibited by exposure to anti-inflammatory cytokines either before or shortly after stimulation. However, inhibition was not universal, as other activation parameters, including upregulation of CD25 and CD69, remained largely unaltered. In contrast, peptide-specific T cell responses were resistant to inhibition by anti-inflammatory cytokines. This was not due to downregulation of cytokine receptor expression or an inability to signal through cytokine receptors since phosphorylation of STAT proteins remained intact. These results highlight key differences in cytokine-mediated regulation of innate and adaptive T cell functions, which may help balance effective antiviral immune responses while reducing T cell-mediated immunopathology.
IMPORTANCE This study demonstrates key differences between the regulation of “innate” and “adaptive” CD8+ T cell functions following activation by innate cytokines or viral peptide. Innate production of IFN-γ by CD8+ T cells following exposure to IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15 was inhibited by exposure to anti-inflammatory cytokines (IL-4, IL-10, and TGF-β). However, inhibition was not universal, as other activation parameters, including upregulation of CD25 and CD69, remained largely unaltered. In contrast, peptide-specific T cell responses were resistant to inhibition by anti-inflammatory cytokines. This distinct regulation of innate and adaptive T cell functions may serve to reduce T cell-mediated immunopathology while still allowing for effective antiviral responses at a site of infection.
INTRODUCTION
CD8+ T cells play a critical role in the control and clearance of many viral infections through the release of antiviral cytokines and lysis of infected cells. During the course of acute viral infection, antigen-specific T cells monitor their local microenvironment and in addition to responding to cognate antigen through the T cell receptor (TCR), antigen-experienced effector and memory CD8+ T cells can function in a non-antigen-specific, “innate” capacity by responding directly to cytokines (1–5). This allows virus-specific CD8+ T cells to act as “sentinels” and respond to subsequent, unrelated infections, even when their specific cognate antigen may not be present. In this manner, “bystander activation” of CD8+ T cells can play a role in the early control of bacterial infections and confer innate protection (2, 6, 7). However, nonspecific cytokine-induced T cell activation may also contribute to immunopathology. For example, endotoxic shock associated with Gram-negative bacterial infection is exacerbated by a cytokine storm that includes gamma interferon (IFN-γ)-mediated pathology due to innate activation of NK cells and CD8+ T cells (4, 8). This highlights the critical importance of regulating CD8+ T cell activation.
Lymphocytic choriomeningitis virus (LCMV) infection of mice is a well-established model for studying CD8+ T cell responses (9–11) and provides an ideal system to examine innate and adaptive CD8+ T cell functions (5, 10, 12). Virus-specific T cells are readily identified using peptide-major histocompatibility complex (MHC) tetramer reagents, making it possible to monitor the responses of T cells with defined antigenic specificity at various stages of infection. Although interleukin-12 (IL-12) and IL-18 are the prototypical CD8+ T cell activating cytokines that elicit IFN-γ production, programmed proliferation, and enhanced antiviral activity (12), a wide array of inflammatory cytokine combinations are capable of modulating CD8+ T cell function in a synergistic manner (4, 13–16). The interplay between inflammatory and anti-inflammatory cytokines on various CD8+ T cell functions is poorly understood. In previous studies examining the effects of >1,800 cytokine combinations on LCMV-specific CD8+ T cell activation, we identified several cytokines that could effectively reduce innate IFN-γ production, including IL-4, IL-10, and transforming growth factor β (TGF-β) (13). These are prototypical anti-inflammatory cytokines, but their direct effects on CD8+ T cells are not fully defined and appear to be context dependent (13, 17–20). Moreover, IL-10 and TGF-β have been implicated in mediating T cell dysfunction during chronic LCMV infection (21–25). However, the ways in which these anti-inflammatory cytokines may act in concert to regulate the innate and adaptive functions of virus-specific CD8+ T cells are not fully understood.
Here, we have examined the abilities of IL-4, IL-10, and TGF-β to modify cytokine-mediated or peptide-mediated activation of virus-specific CD8+ T cells to determine whether the control of these distinct “innate” and “adaptive” T cell functions are differentially regulated. Interestingly, anti-inflammatory cytokines did not block all T cell functions but instead resulted in preferential downregulation of the secreted protein, IFN-γ, while allowing upregulation of CD25 and CD69 on the T cell surface to occur unabated in response to innate activating cytokines. In contrast to the robust inhibition of innate cytokine-mediated IFN-γ production by virus-specific CD8+ T cells, anti-inflammatory cytokines had no direct effect on adaptive CD8+ T cell functions in response to peptide stimulation through the TCR. Together, these data show that activation of virus-specific CD8+ T cells in response to innate cytokine stimuli or viral peptide antigen is independently regulated in a sophisticated and highly controlled manner that may provide optimal innate/adaptive T cell-mediated immunity for the host while minimizing immunopathology.
MATERIALS AND METHODS
Mice and viral infections.
BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were infected at 6 to 12 weeks of age via intraperitoneal injection of 2 × 105 PFU LCMV-Armstrong, and T cell responses were analyzed at either 8 days postinfection (acute) or >60 days postinfection (immune/memory). Spleens were pressed through 70-μm-pore nylon filters to create single-cell suspensions and depleted of red blood cells by NH4Cl lysis prior to direct ex vivo stimulation. All animal experimental procedures were reviewed and approved by the Oregon Health and Science University Institutional Animal Care and Use Committee.
Reagents.
Recombinant murine cytokines were purchased from R&D Systems (Minneapolis, MN), and high-pressure liquid chromatography (HPLC)-purified (>95% pure) LCMV nucleoprotein 118-126 (NP118) peptide was obtained from Alpha Diagnostics (San Antonio, TX). NP118 tetramers (H-2Ld) were provided by the NIH Tetramer Core Facility (Atlanta, GA). Anti-CD8α (53-6.7), anti-IFN-γ (XMG1.2), anti-tumor necrosis factor alpha (anti-TNF-α) (MP6-XT22), anti-CD11a (2D7), anti-phospho-STAT3 (4/P-STAT3), anti-phospho-STAT6 (J71-773.58.11) were purchased from BD Pharmingen. Anti-CD25 (PC61) was purchased from BioLegend (San Diego, CA), and anti-CD69 (H1.2F3) was purchased from eBioscience (San Diego, CA). Antibodies to IL-12 receptor β2 (IL-12Rβ2), IL-15Rα, IL-18Rα, and tumor necrosis factor receptor (TNFR1) were purchased from R&D Systems (Minneapolis, MN). Aqua cell viability stain and carboxyfluorescein diacetate succinimidyl ester (CFSE) were purchased from Invitrogen (Carlsbad, CA).
Stimulations and staining.
Cells were stimulated with cytokines (10 ng/ml) or NP118 peptide (1 × 10−7 M) in RPMI supplemented with 10% fetal bovine serum (FBS), 20 mM HEPES, penicillin-streptomycin, and l-glutamine at 37°C in 6% CO2. To assess cytokine production during stimulation, brefeldin A (2 μg/ml) (Sigma-Aldrich, St. Louis, MO) was added during the final hour of incubation. Cells were stained overnight at 4°C with Aqua (to identify live cells) and antibodies to cell surface markers in phosphate-buffered saline (PBS) plus 1% fetal calf serum (FCS) with 0.1 mg/ml mouse IgG (Sigma-Aldrich) and 1 μg/ml anti-CD16/32 (2.4G2, Fc block). Cells were washed, fixed with 2% formaldehyde in PBS, permeabilized with Permwash (0.1% saponin [Sigma-Aldrich], 0.1% NaN3 [Sigma-Aldrich], 2% FBS in PBS), and stained for intracellular cytokines for 1 h. Analysis of STAT phosphorylation was performed using a previously described sequential staining technique (26, 27). Briefly, cells were fixed in 2% formaldehyde, stained for surface markers, permeabilized in 100% ice-cold methanol, washed, and incubated with anti-phospho-STAT antibodies. NP118 tetramer staining was not feasible after fixation (data not shown), but virus-specific T cells were identified by gating on CD11ahi CD8+ T cells. Data were acquired on an LSR Fortessa flow cytometer (BD, San Jose, CA) and analyzed using FlowJo software (Treestar, Ashland, OR).
MACS purification.
CD8+ T cell purification was performed by magnetically activated cell sorting (MACS) in accordance with the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Briefly, single-cell suspensions were incubated with anti-CD8α microbeads in MACS buffer (PBS, 0.5% FBS, 2 mM EDTA) at 1 × 108 cells/ml at 4°C for 15 min, washed with MACS buffer, and applied to a magnetic column (LS column; Miltenyi Biotec). The column was washed, and the retained CD8+ cells were eluted with MACS buffer. Flow cytometric analysis of sorted cells indicated >95% purity.
RNA isolation, cDNA synthesis, and quantitative real-time PCR.
Total RNA was isolated using the Isol-RNA lysis reagent (5 PRIME, Gaithersburg MD) method and reverse transcribed using the High-capacity cDNA reverse transcription kit (Life Technologies, Carlsbad, CA). Gene expression was measured by quantitative real-time PCR using Maxima Probe/ROX quantitative PCR (qPCR) master mix (2×) (Fermentas, Glen Burnie, MD) and TaqMan gene expression assays (Life Technologies) using a StepOnePlus instrument (Life Technologies). Duplicate reactions of IFN-γ (Mm01168134_m1) and β-2-microglobulin (Mm01168134_m1) were performed for each cDNA template analyzed. Duplicate cycle threshold (CT) values were analyzed using the 2−ΔΔCT method. The values were first normalized to the endogenous reference gene (coding for β-2-microglobulin) and are presented as relative change in comparison to the “medium-only” sample in relative quantification (RQ) units.
CTL assays.
Flow cytometry-based cytotoxic T lymphocyte (CTL) assays were performed as previously described (12). A20 target cells were labeled with 4 μM CFSE (CFSEhigh) or 1 μM CFSE (CFSElow) for 7 min at room temperature and subsequently washed to remove excess CFSE. CFSElow cells were coated with NP118 peptide (10−7 M) for 1 h at 37°C, washed to remove unbound peptide, and combined with uncoated CFSEhigh cells at a 1:1 ratio. Splenocytes were isolated from LCMV-infected BALB/c mice at 8 days postinfection, and an aliquot of CD8+ T cells was stained with NP118 tetramers to determine effector-to-target (E:T) cell ratios. Target cells were combined with effector cells at specified E:T ratios and incubated at 37°C in 6% CO2 for 5 h. Cells were then washed with PBS plus 1% FCS, incubated for 1 h at 4°C with Aqua (Invitrogen, Carlsbad, CA) to allow for determination of target cell viability, and washed again prior to analysis. Data were acquired on an LSR Fortessa flow cytometer (BD, San Jose, CA) and analyzed using FlowJo software (Treestar, Ashland, OR). Peptide-specific killing of target cells at each E:T ratio was calculated by determining the percentage of dead (Aquahigh) CFSElow cells after subtracting “background” cell death, which was measured as the percentage of Aquahigh CFSElow target cells that were observed when no effector cells were added to the culture. Nonspecific lysis was defined by the percentage of Aquahigh CFSEhigh cells at each E:T ratio after subtraction of the percentage of Aquahigh CFSElow target cells observed in the absence of effector T cells.
Statistical analysis.
A two-tailed Student's t test was used to evaluate the statistical significance of differences between groups. A value of P < 0.05 was considered significant.
RESULTS
Innate activation of virus-specific CD8+ T cells by inflammatory cytokines.
IL-12 is an innate inflammatory cytokine that is elicited in response to a number of microbial infections (3, 4, 28), and although it is only weakly stimulatory on its own (13), it synergizes with several other cytokines to elicit IFN-γ production by virus-specific CD8+ T cells (13). To further characterize cytokine-mediated effector (i.e., day 8 postinfection) and memory (i.e., >60 days postinfection) CD8+ T cell activation, splenocytes from LCMV-infected mice were tested for IFN-γ production by intracellular cytokine staining for up to 24 h (Fig. 1). Virus-specific CD8+ T cells were identified using MHC class I tetramers loaded with LCMV nucleoprotein (NP) peptide 118-126 (NP118), which is the immunodominant epitope that comprises ∼90% of the CD8+ T cell response to LCMV in BALB/c mice (29, 30).
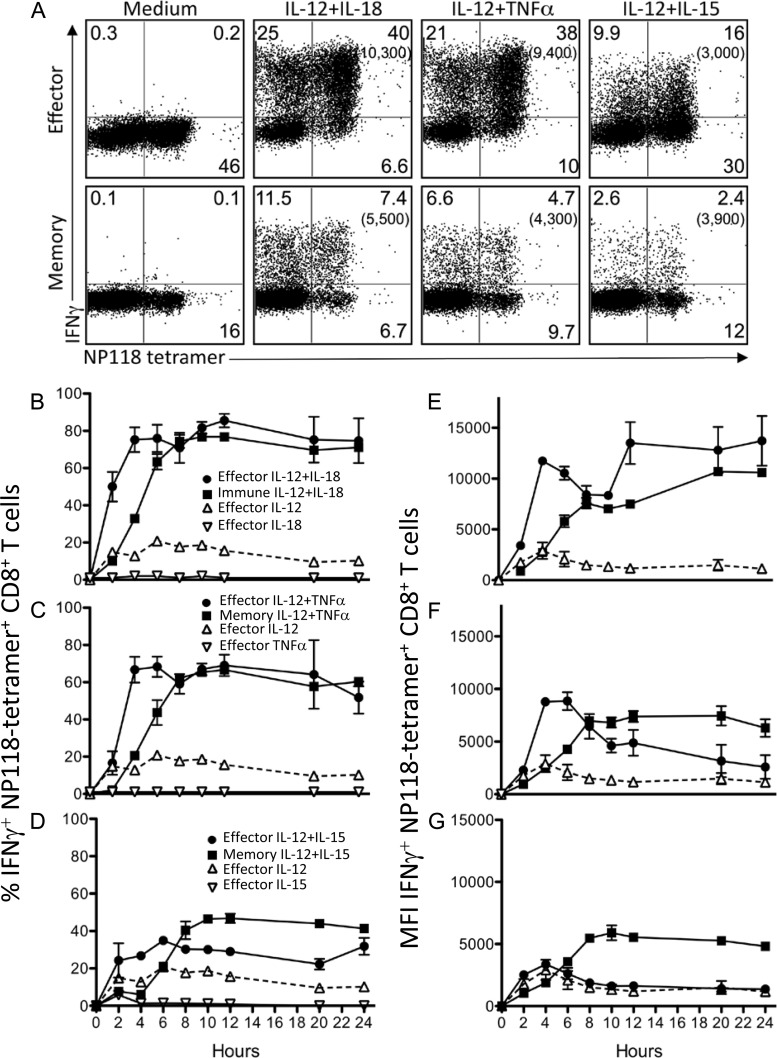
FIG 1.
Innate cytokine-induced IFN-γ production by virus-specific CD8+ T cells. Splenocytes from BALB/c mice at 8 days (effector) or >60 days (memory) after LCMV infection were stimulated with the indicated cytokines (10 ng/ml each) directly ex vivo for up to 24 h. Brefeldin A was added to cultures for the final hour of incubation to allow for visualization of cytokine production by intracellular cytokine staining at each time point. (A) Innate IFN-γ production by CD8+ effector T cells following stimulation with IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15 for 6 h. Numbers in parentheses represent the mean fluorescence intensity (MFI) of IFN-γ+ NP118 tetramer+ CD8+ T cells. The percentage of NP118 tetramer+ CD8+ T cells producing IFN-γ was monitored for 24 h after stimulation with IL-12 plus IL-18 (B), IL-12 plus TNF-α (C), or IL-12 plus IL-15 (D). Responses to individual cytokines (IL-12, IL-15, IL-18, and TNF-α) in immune mice were <5% IFN-γ+ CD8+ T cells at all time points tested. The MFI of IFN-γ expression by NP118 tetramer+ CD8+ T cells was measured for 24 h after stimulation with IL-12 plus IL-18 (E), IL-12 plus TNF-α (F), or IL-12 plus IL-15 (G). Numbers show the average ± standard deviation (SD) from 4 to 6 mice.
Virus-specific CD8+ T cells were activated by IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15, and both effector and memory cells displayed variable levels of IFN-γ production after 6 h of stimulation with these cytokine combinations (Fig. 1A). IL-12 plus IL-18 elicited the strongest response, with 80% of virus-specific effector T cells and 53% of virus-specific memory T cells producing IFN-γ (mean fluorescence intensities [MFIs], 10,300 and 5,500, respectively) at this time point. IL-12 plus TNF-α was also a potent cytokine pair, as 79% of virus-specific effector T cells (MFI, 9,400) and 33% of memory T cells (MFI, 4,300) produced IFN-γ in response to this cytokine combination. The combination of IL-12 and IL-15 was a relatively weaker stimulus, eliciting IFN-γ production from 34% of effector T cells (MFI, 3,000) and 17% of memory T cells (MFI, 3,900) after 6 h of exposure. These data demonstrate three cytokine combinations of various potencies, all of which activate at least a subpopulation of virus-specific effector and memory CD8+ T cells in the absence of their cognate antigen.
In prior studies, we found that effector T cells expressed IFN-γ more rapidly than memory T cells following stimulation with IL-12 plus IL-18 (31), but it was unclear if this was unique to the combination of IL-12 plus IL-18 or common to other stimulatory cytokine combinations. Effector and memory CD8+ T cells were rapidly activated following exposure to the indicated combinations of cytokines (Fig. 1). IL-12 plus IL-18 was the most potent cytokine combination, eliciting IFN-γ production by approximately 80% of NP118 tetramer-positive (NP118 tetramer+) CD8+ T cells (Fig. 1B), with a maximum IFN-γ MFI of 13,700 (effector) or 10,700 (memory), (Fig. 1E). Effector T cells reached maximum IFN-γ production in as little as 4 to 6 h after cytokine exposure, while memory T cells (>60 days postinfection) were slower to respond to innate stimulation, requiring up to 8 h to reach peak IFN-γ production (31). IFN-γ production by both T cell populations was sustained for at least 24 h in the continued presence of stimulation. As expected, stimulation occurred in a potently synergistic manner, with individual cytokines having limited ability to elicit IFN-γ production on their own. Stimulation of virus-specific CD8+ T cells with IL-12 plus TNF-α led to synergistic activation kinetics that were similar to those observed with IL-12 plus IL-18 treatment with 60 to 70% of both effector and memory T cells producing IFN-γ in response to this cytokine combination (Fig. 1C), reaching a maximum IFN-γ MFI of approximately 8,000 (Fig. 1F). IL-12 plus IL-15 was the weakest combination of stimulatory cytokines, but it still showed the capacity to synergistically trigger IFN-γ production by 30 to 40% of virus-specific CD8+ T cells (Fig. 1D). Similar to IL-12 plus IL-18 or IL-12 plus TNF-α stimulation, effector T cells upregulated IFN-γ production somewhat more rapidly than memory T cells. However, at the peak of the cytokine-induced IFN-γ response, nearly half (47%) of the memory T cells were IFN-γ+, whereas only about one-third of effector T cells (34%) became IFN-γ+ in response to this cytokine pair (Fig. 1D). Memory T cells also reached a higher MFI (peak MFI, 5,500) than effector cells (peak MFI, ∼3,400), (Fig. 1G).
Innate IFN-γ production is inhibited by brief prior exposure to anti-inflammatory cytokines.
IL-4, IL-10, and TGF-β are widely recognized as anti-inflammatory cytokines that can dampen inflammatory responses (32, 33), and here we compared the abilities of these cytokines (individually or in combination) to directly inhibit innate functions of virus-specific NP118 tetramer+ CD8+ T cells. We began by exposing T cells to combinations of IL-4, IL-10, and TGF-β for 2 h prior to activation by innate inflammatory cytokines, including IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15, for an additional 6 h. Individual anti-inflammatory cytokines had only modest effects, with pretreatment with IL-4 or TGF-β reducing effector T cell responses from 80% IFN-γ+ to ∼66% IFN-γ+ (P < 0.05), whereas IL-10 had little to no effect on IFN-γ production (Fig. 2A). The paired combinations of IL-4 plus IL-10, IL-4 plus TGF-β, and IL-10 plus TGF-β were more effective, reducing the effector T cell responses to 48%, 50%, and 62% IFN-γ+, respectively (P < 0.01 versus no pretreatment). A similar trend was observed in NP118-specific memory T cells, with IL-4 plus IL-10 being the most effective inhibitory cytokine pair, reducing IFN-γ production from 60% IFN-γ+ to 21% IFN-γ+ (P < 0.01). When all three anti-inflammatory cytokines were used together, the percentages of NP118-specific T cells producing IFN-γ in response to IL-12 plus IL-18 were reduced to 40% and 14% in effector and memory T cells, respectively (P < 0.01). To determine the relative resistance/susceptibility to cytokine-mediated inhibition of effector and memory T cells, the results were normalized to the maximum IFN-γ response observed in the absence of anti-inflammatory cytokines (Fig. 2A, right panel).
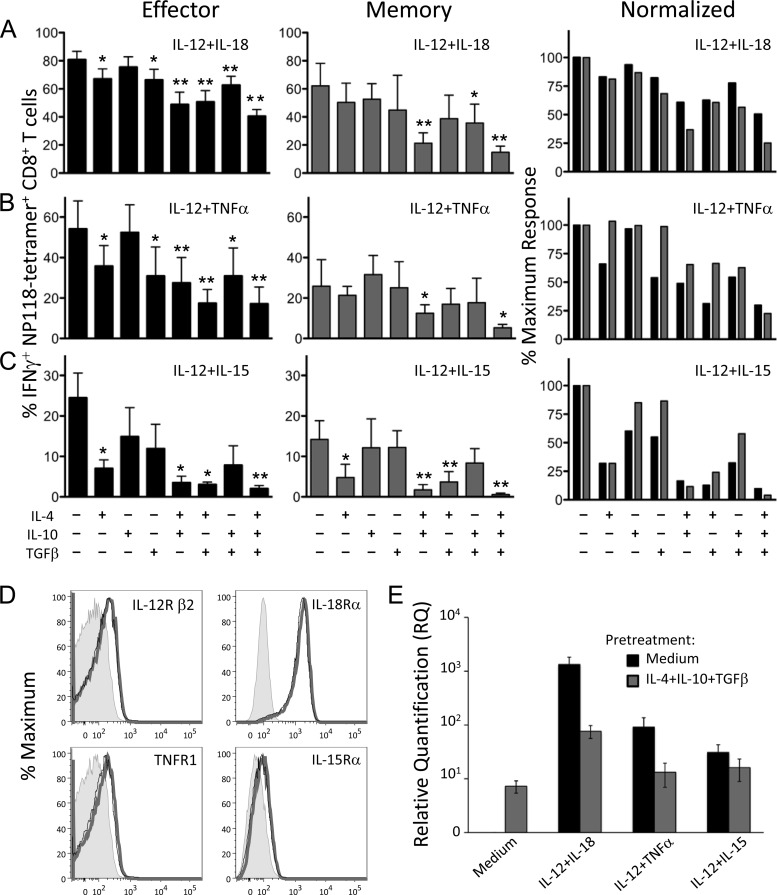
FIG 2.
Exposure to anti-inflammatory cytokines inhibits innate IFN-γ production by CD8+ T cells. Splenocytes from BALB/c mice at 8 days (effector) or >60 days (memory) after LCMV infection were treated directly ex vivo with combinations of IL-4, IL-10, and TGF-β (10 ng/ml each) for 2 h, followed by stimulation with IL-12 plus IL-18 (A), IL-12 plus TNF-α (B), or IL-12 plus IL-15 (C) at 10 ng/ml for 6 h. Brefeldin A was added to cultures for the final hour of incubation, and cytokine production was assessed by intracellular cytokine staining and flow cytometry. Numbers represent the percentage of NP118 tetramer+ CD8+ T cells producing IFN-γ and are the average ± SD from 4 to 6 mice. IFN-γ responses of T cells pretreated with inhibitory cytokines were compared to those of cells pretreated with medium alone using an unpaired two-tailed Student's t test. Inhibitory cytokine combinations that significantly reduced IFN-γ production are marked with asterisks: *, P < 0.05; **, P < 0.01. For normalization, IFN-γ responses from day 8 (black bars) or immune responses (gray bars) are graphed as a percentage of the maximum response to proinflammatory cytokine stimulation with no IL-4, IL-10, or TGF-β added. (D) Cytokine receptor expression on virus-specific CD8+ T cells. Splenocytes from BALB/c mice at 8 days post-LCMV infection were treated with IL-4 plus IL-10 plus TGF-β or medium alone for 2 h at 37°C. Levels of IL-12Rβ2, IL-18Rα, TNFR1, and IL-15Rα on NP118 tetramer+ CD8+ T cells were examined by flow cytometry. Gray-shaded histograms represent unstained controls. Compared to cells treated with medium alone (thick gray line), cells treated with IL-4 plus IL-10 plus TGF-β (thin black line) showed no significant difference in expression levels of IL-12Rβ2 (P = 0.48), IL-18Rα (P = 0.52), TNFR1 (P = 0.55), or IL-15Rα (P = 0.2), and the histograms were nearly superimposable. Data show representative histograms from 3 mice. (E) Anti-inflammatory cytokines reduce IFN-γ transcription in response to subsequent stimulation. MACS-purified splenic CD8+ T cells (>95% pure) from BALB/c mice at 8 days post-LCMV infection were pretreated with medium or IL-4 plus IL-10 plus TGF-β (10 ng/ml each) for 2 h at 37°C and then treated for 6 h with the indicated cytokine pairs (10 ng/ml each). RNA was isolated, and levels of IFN-γ transcript were assessed by quantitative RT-PCR. Two spleens were pooled for each sample, and the results are the average ± SD from three individual samples.
IL-12 plus TNF-α provided an intermediate level of inflammatory T cell activation (Fig. 2B), and both IL-4 and TGF-β were effective individual inhibitors of effector T cell responses to this cytokine pair, dropping the percentage of IFN-γ+ NP118 tetramer+ CD8+ T cells by nearly half (to 35% and 30% IFN-γ+, respectively), whereas IL-10 again had no measurable effect. For memory T cells, the three individual anti-inflammatory cytokines had no significant impact on IFN-γ production. Pairwise combinations of IL-4, IL-10, and TGF-β did not lead to substantially more inhibition than IL-4 by itself, but the triple combination of IL-4, IL-10, and TGF-β resulted in significantly reduced IFN-γ production from 54% IFN-γ+ to 17% IFN-γ+ NP118 tetramer+ CD8+ effector T cells and from 26% to 5% IFN-γ+ memory T cells (P < 0.05).
Of the three stimulatory cytokine combinations examined, IL-12 plus IL-15 elicited the least IFN-γ production from virus-specific CD8+ T cells but was also the most sharply reduced by anti-inflammatory cytokine exposure in both effector and memory cells (Fig. 2C). IL-4 was the most inhibitory individual cytokine, reducing IFN-γ+ NP118-specific CD8+ T cells from 23% to 9% IFN-γ+ in effector T cells and from 15% to 4% IFN-γ+ in memory T cells. IL-10 or TGF-β showed a small but insignificant trend toward reduced IFN-γ production, but the triple combination of IL-4, IL-10, and TGF-β reduced T cell responses to only 2% IFN-γ+ effector T cells and to <1% IFN-γ+ memory T cells, representing a >90% reduction in IFN-γ expression compared to IL-12 plus IL-15 alone (P < 0.05). Collectively, these data demonstrate that effector and memory CD8+ T cells respond similarly to combinations of stimulatory or inhibitory cytokines.
Despite the ability of IL-4 plus IL-10 plus TGF-β to dramatically reduce innate IFN-γ production, we found that these anti-inflammatory cytokines had no effect on expression levels of IL-12Rβ2, IL-18Rα, TNFR1, or IL-15Rα on CD8+ effector T cells (Fig. 2D), indicating that inhibition is likely occurring further downstream in the cellular activation pathway. Such regulation may allow for fine-tuning of immune responses by continuing to allow inflammatory signals into the cell while specifically reducing the production of cytokines, such as IFN-γ, which may contribute to immunopathology. Pretreatment with IL-4 plus IL-10 plus TGF-β resulted in a dramatic reduction in IFN-γ transcript levels in MACS-purified CD8+ T cells in response to IL-12 plus IL-18 (95% inhibition) or IL-12 plus TNF-α (86% inhibition) compared to pretreatment with medium alone (Fig. 2E). Inhibition of the response to IL-12 plus IL-15 was less pronounced (48% inhibition); however, this cytokine pair is not as effective at eliciting strong IFN-γ production in purified CD8+ T cell cultures (13). This indicates that the regulatory effects of these anti-inflammatory cytokines on IFN-γ production begin at the transcriptional level.
Differential regulation of secreted versus membrane-bound immunomodulatory proteins.
IFN-γ production is only one outcome of T cell activation, and virus-specific CD8+ T cells also upregulate several surface immunomodulatory proteins in response to cytokine stimulation. To determine whether other activation parameters are modulated by anti-inflammatory cytokines, we monitored the expression of CD25, which is important for cellular proliferation and homeostasis (34, 35), and CD69, a surface glycoprotein that is one of the earliest activation markers to be upregulated and alters lymphocyte migration (36, 37). LCMV-specific CD8+ T cells were cultured in medium or with IL-4 plus IL-10 plus TGF-β for 2 h prior to stimulation with IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15 (Fig. 3). Preexposure to IL-4, IL-10, and TGF-β led to a reduction in both the percentage of virus-specific CD8+ T cells producing IFN-γ and the amount of IFN-γ being produced per cell (as measured by mean fluorescence intensity [MFI]) (Fig. 3A). For example, following treatment with IL-12 plus IL-18, 77% of NP118-specific CD8+ T cells produce IFN-γ (MFI, 17,000). However, if the cells were first exposed to IL-4 plus IL-10 plus TGF-β, then only 49% of NP118-specific CD8+ T cells produced IFN-γ (MFI, 8,000). The response to IL-12 plus TNF-α was reduced from 59% IFN-γ+ (MFI, 13,000) to 26% IFN-γ+ (MFI, 3,300), and the response to IL-12 plus IL-15 was reduced from 37% IFN-γ+ (MFI, 5,800) to 6% IFN-γ+ (MFI, 1,300) by anti-inflammatory cytokine exposure. These results show that not only is the number of IFN-γ-producing T cells reduced, but the amount of IFN-γ expression is also greatly decreased.
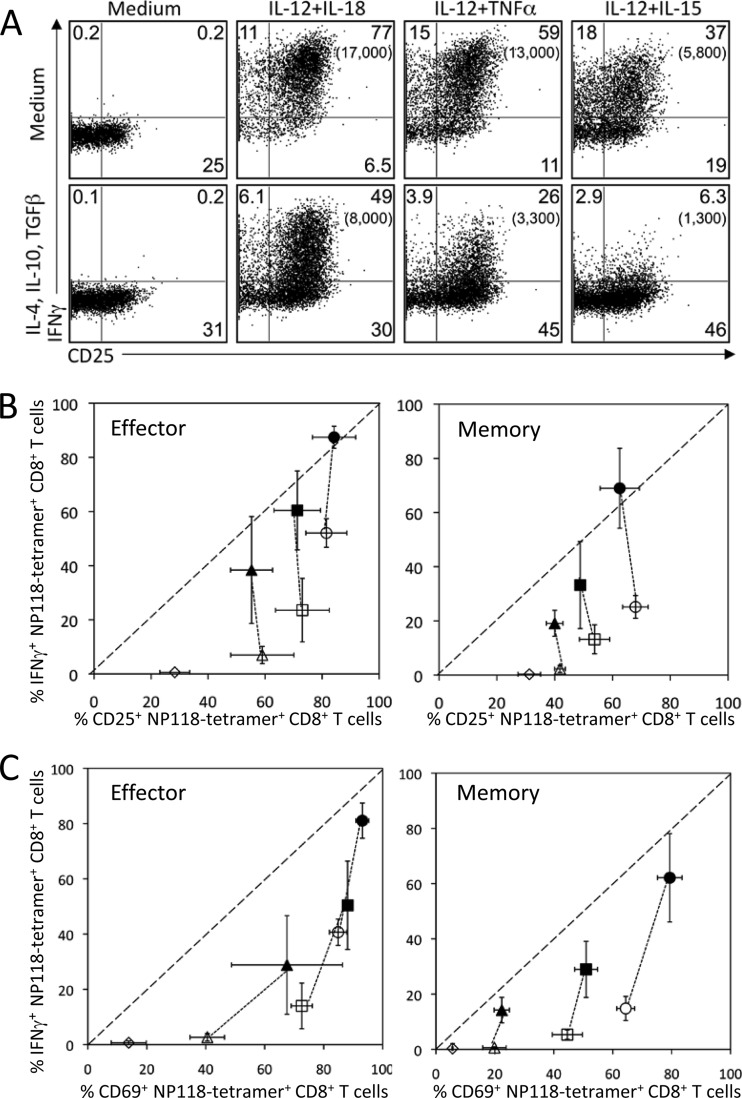
FIG 3.
Differential regulation of secreted protein and surface membrane-bound immunomodulatory protein expression following cytokine exposure. At 8 days (effector) or >60 days (memory) after LCMV infection, T cells were pretreated with medium or IL-4, IL-10, and TGF-β (10 ng/ml each) for 2 h, followed by stimulation with IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15 (10 ng/ml) for 6 h. (A) Innate upregulation of CD25 expression and IFN-γ production by virus-specific CD8+ effector T cells. The numbers in each quadrant represent the percentage of NP118 tetramer+ CD8+ T cells expressing IFN-γ and/or CD25, while the numbers in parentheses represent the MFI of IFN-γ+ T cells. Data are representative of 4 mice from 2 independent experiments. (B and C) Surface marker upregulation and IFN-γ production in response to proinflammatory cytokine stimulation following pretreatment with medium only (solid symbols) or medium containing inhibitory cytokines (open symbols). Each data point represents the percentage of NP18-tetramer+ CD8+ T cells that expressed IFN-γ and CD25 (B) or IFN-γ and CD69 (C) following treatment with IL-12 plus IL-18 (circles), IL-12 plus TNF-α (squares), IL-12 plus IL-15 (triangles), or medium alone (diamonds). Data represent the average ± SD from 4 mice per group from 2 independent experiments.
Despite the sharp reduction in the IFN-γ response, CD25 upregulation by CD8+ effector T cells following incubation with IL-12 plus IL-15, IL-12 plus IL-18, or IL-12 plus TNF-α remained relatively stable regardless of prior history of exposure to anti-inflammatory cytokines (Fig. 3A and B). Memory T cells showed similar results, with sharply reduced IFN-γ expression but largely unaltered CD25 expression (Fig. 3B). CD69 expression was not inhibited as dramatically as IFN-γ production but was almost as resistant as CD25 in terms of modification by anti-inflammatory cytokines (Fig. 3C). Collectively, these data demonstrate that the inhibitory effects of IL-4, IL-10, and TGF-β were not all encompassing but instead resulted in differential regulation of surface immunomodulatory proteins (CD25 and CD69) and secreted antiviral proteins such as IFN-γ, indicating a unique hierarchy of inhibition among different CD8+ T cell activation parameters.
Inhibition of innate CD8+ T cell activation occurs within a limited time frame.
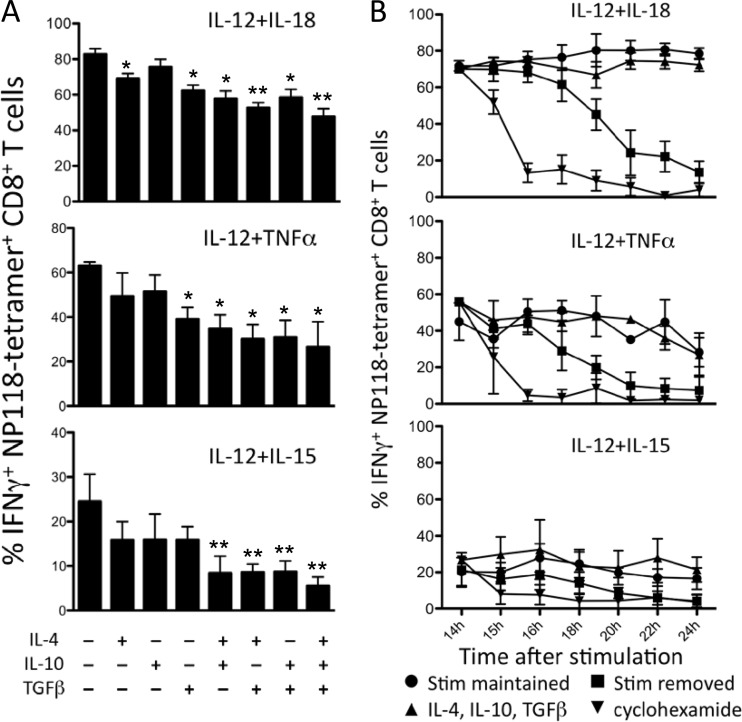
The data in Fig. 2 and 3 show that prior exposure to anti-inflammatory cytokines can limit T cell-mediated IFN-γ production following subsequent innate activation by IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15. However, virus-specific CD8+ T cells in vivo may first encounter proinflammatory cytokines before receiving inhibitory/anti-inflammatory cytokine signals. We therefore determined whether IL-4, IL-10, and TGF-β could reduce IFN-γ production by T cells after induction of the IFN-γ response had been initiated (Fig. 4). Effector T cells were stimulated directly ex vivo for a total of 6 h (Fig. 4A) or 24 h (Fig. 4B) with IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15. In Fig. 4A, combinations of IL-4, IL-10, and TGF-β were added to the cultures at 2 h after incubation with the indicated innate cytokine combinations, and IFN-γ production was monitored at the 6-h time point. Even though CD8+ T cell activation had already begun, IFN-γ production could still be substantially reduced by the addition of anti-inflammatory cytokines. The strongest inhibition was observed when all three anti-inflammatory cytokines were added simultaneously. In contrast, if CD8+ T cells were exposed to anti-inflammatory cytokines at 14 h after initial innate cytokine stimulation, then there was virtually no effect on the continued IFN-γ production (Fig. 4B). As expected, addition of the protein synthesis inhibitor cycloheximide quickly abrogated IFN-γ production, as did withdrawal of the stimulatory cytokines as previously described (31). However, the addition of IL-4 plus IL-10 plus TGF-β had no impact on the IFN-γ response to IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15. Similar results were observed when memory CD8+ T cells were examined (data not shown). Taken together, these data demonstrate that anti-inflammatory cytokines can inhibit the early stages of IFN-γ production, but there is a finite window of opportunity during which regulation of these innate CD8+ T cell functions can occur.
FIG 4.
Anti-inflammatory cytokines have a limited therapeutic window to inhibit innate IFN-γ production. (A) CD8+ T cells from mice at 8 days post-LCMV infection were stimulated (stim) directly ex vivo for 6 h with IL-12 plus IL-18, IL-12 plus TNF, or IL-12 plus IL-15. Combinations of IL-4, IL-10, and TGF-β (10 ng/ml each) were added 2 h after stimulation began. IFN-γ responses were compared to the maximum response in the absence of anti-inflammatory cytokines using an unpaired two-tailed Student's t test. Inhibitory cytokine combinations that significantly reduced IFN-γ production are marked with asterisks: *, P < 0.05; **, P < 0.01. (B) Splenocytes from mice at 8 days post-LCMV infection were stimulated directly ex vivo with the indicated cytokines for 14 h. Activated T cells were then either maintained in the indicated stimulatory cytokines, treated with anti-inflammatory cytokines (IL-4, IL-10, and TGF-β at 10 ng/ml each), treated with cycloheximide (100 μg/ml), or washed and placed in medium without cytokines. Brefeldin A was added to cultures for the final hour of incubation, and IFN-γ production at each time point was assessed by intracellular cytokine staining and flow cytometry. The numbers represent the percentage of NP118 tetramer+ CD8+ T cells producing IFN-γ and are the average ± SD from 4 to 6 mice.
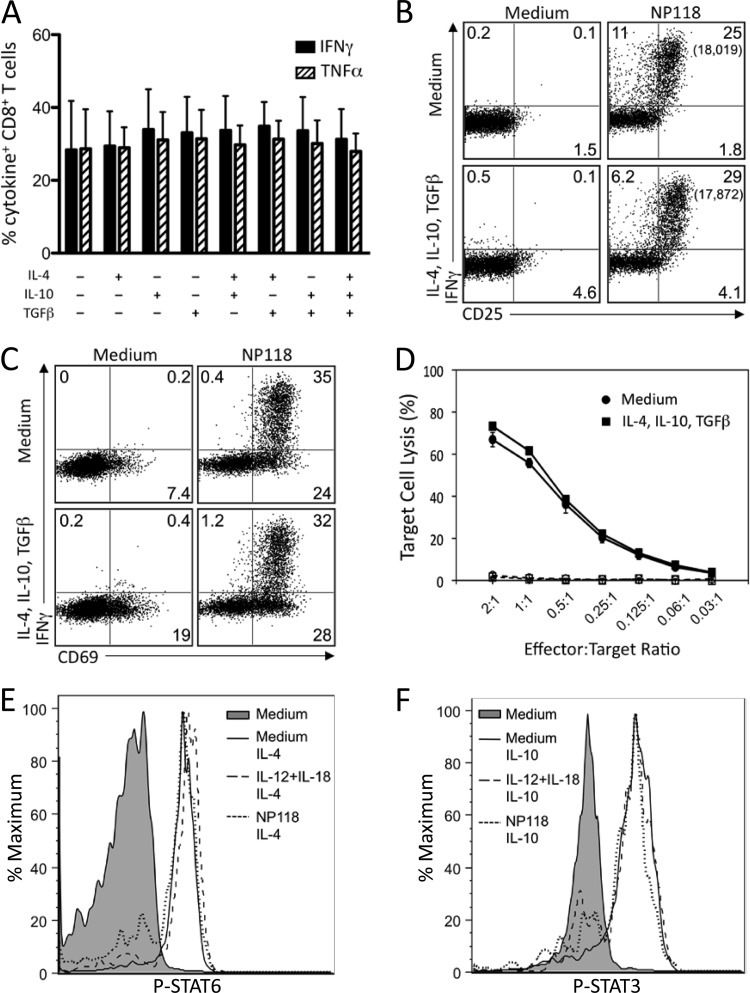
Peptide-specific CD8+ T cell responses are not directly inhibited by IL-4, IL-10, or TGF-β.
To determine the potential impact of anti-inflammatory cytokines on peptide-specific T cell activation, we incubated virus-specific CD8+ T cells in medium alone or in medium containing the combination of IL-4 plus IL-10 plus TGF-β for 2 h prior to stimulation with NP118 peptide (Fig. 5). In stark contrast to the inhibition of innate cytokine-mediated T cell activation (Fig. 2), these anti-inflammatory cytokines had no measurable effect on the frequency of IFN-γ+ or TNF-α+ CD8+ T cells after 6 h of peptide stimulation (Fig. 5A). Moreover, the amount of IFN-γ produced on a per cell basis in response to NP118 peptide stimulation (i.e., cytokine MFI) remained unaltered, regardless of exposure to anti-inflammatory cytokines (Fig. 5B) (data not shown). Interestingly, a reduction in IFN-γ transcript levels was observed in response to NP118 peptide stimulation following pretreatment with anti-inflammatory cytokines; however, this did not correlate with reduced IFN-γ protein expression after 6 h of stimulation, indicating that mRNA levels may not necessarily predict protein levels at this early time point (data not shown). Expression of CD25 (Fig. 5B) and CD69 (Fig. 5C) was rapidly upregulated following peptide stimulation, regardless of whether the cells had been cultured with or without IL-4, IL-10, and TGF-β.
FIG 5.
Anti-inflammatory cytokines do not inhibit peptide-specific CD8+ T cell responses. Splenocytes from BALB/c mice at 8 days post-LCMV infection were treated directly ex vivo with the indicated combinations of IL-4, IL-10, and TGF-β (10 ng/ml each) for 2 h, followed by stimulation with NP118 peptide (1 × 10−7 M) for 6 h. (A) Peptide-specific IFN-γ and TNF-α production by virus-specific CD8+ T cells. Numbers represent the percentage of NP118 tetramer+ CD8+ T cells producing IFN-γ or TNF-α and are the average ± SD from 4 mice. (B and C) Surface marker upregulation in response to peptide stimulation with or without pretreatment with anti-inflammatory cytokines. Numbers in each quadrant represent the percentage of CD8+ T cells expressing IFN-γ and/or CD25 (B) or CD69 (C), while numbers in parentheses represent the MFI of IFN-γ+ T cells. Data are representative of 4 mice from 2 independent experiments. (D) At 8 days following infection with LCMV, T cells were incubated in medium alone or medium containing IL-4, IL-10, and TGF-β for 2 h prior to incubation with NP118-coated (solid lines) or uncoated (dashed lines) target cells for 5 h. Results are representative of 2 independent experiments. (E) At 8 days post-LCMV infection, splenocytes were stimulated with IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15 (10 ng/ml each) or NP118 peptide directly ex vivo. After 2 h, IL-4 (E) or IL-10 (F) was added for 20 min, cells were immediately fixed and permeabilized, and phosphorylation of STAT3 (E) and STAT6 (F) was assessed. Results are representative of 4 mice and 2 independent experiments.
Strong peptide-specific cytolytic activity is a hallmark of CD8+ T cell effector function, and we determined whether CTL responses might be inhibited by exposure to anti-inflammatory cytokines. Virus-specific CD8+ T cells were cultured in medium alone or in medium containing IL-4 plus IL-10 plus TGF-β for 2 h prior to incubation with peptide-coated target cells at the indicated effector-to-target (E:T) ratios (Fig. 5D). Similar to peptide-specific cytokine responses, the anti-inflammatory cytokines had no measurable impact on peptide-specific cytolytic activity. This resistance to inhibition of peptide-specific responses was observed in both effector T cells (Fig. 5) and memory T cells (data not shown) and highlights an important and fundamental difference in the regulation of innate and adaptive CD8+ T cell functions.
T cell resistance to IL-4 and IL-10 occurs despite efficient signaling through the JAK/STAT pathway.
There are many ways in which a T cell might lose reactivity to cytokine-mediated regulation, including downregulation of cytokine receptors from the cell surface or loss of signaling through those receptors. To examine this question in more detail, we measured phosphorylation of STAT3 and STAT6 in CD8+ T cells that had been previously activated by innate cytokines or peptide. CD8+ T cells cultured in medium alone did not express phosphorylated STAT6 (Fig. 5E). However, following direct exposure to IL-4, STAT6 was rapidly phosphorylated. Likewise, CD8+ T cells previously exposed to IL-12 plus IL-18 (which are susceptible to IL-4-mediated inhibition) (Fig. 4) also showed phosphorylation of STAT6 following exposure to IL-4. Peptide-stimulated CD8+ T cells were resistant to IL-4-mediated inhibition, but surprisingly, these cells still phosphorylated STAT6 following exposure to IL-4, indicating that IL-4 receptor signaling was still intact, despite the ability of peptide-stimulated T cells to resist IL-4-mediated downregulation of effector function. Similar results were observed with IL-10-mediated phosphorylation of STAT3 (Fig. 5F). This indicates that cytokine-resistant virus-specific CD8+ T cells are able to override the downregulatory signals initiated by IL-4 and IL-10 through their respective receptors, even though these anti-inflammatory cytokines initiate effective signal transduction and STAT phosphorylation.
DISCUSSION
Virus-specific CD8+ T cells are able to respond to innate cytokines as well as their cognate antigen, which allows for activation of T cells during infection with pathogens that are unrelated to their antigen specificity. While this ability diversifies the contributions of virus-specific CD8+ T cells to host immunity, it must be carefully regulated to avoid immunopathology. In this study, we examined the regulation of cytokine-mediated or peptide-specific activation of virus-specific CD8+ T cells by anti-inflammatory cytokines and show that these two aspects of T cell functionality are differentially regulated by IL-4, IL-10, and TGF-β. This provides a unique and previously unrecognized mechanism for fine-tuning CD8+ T cell responses by the local microenvironment, which is likely to be essential for balancing effective host immune responses while attempting to avoid severe immunopathology.
Interestingly, although IFN-γ production was almost completely abrogated by anti-inflammatory cytokines, CD25 and CD69 upregulation remained largely intact. This shows that inhibition of innate activation of virus-specific CD8+ T cells by IL-4 plus IL-10 plus TGF-β is not global in nature, but instead appears to target selected T cell activation outcomes. Upregulation of CD69 inhibits lymphocyte migration, allowing T cells to remain at a site of infection (37, 38). Increased expression of the high-affinity IL-2 receptor CD25 enhances the ability of T cells to respond to the proliferative and activation signals provided by IL-2 and is important in programmed proliferation and rapid expansion of antigen-specific T cells (12). Controlling IFN-γ secretion while allowing these other activation events to proceed could serve to reduce immunopathology, while enabling CD8+ T cells to better respond to signals such as IL-2, which is likely to be generated near a site of active infection. Importantly, the timing of exposure to proinflammatory or anti-inflammatory cytokine signals was critical in determining innate T cell outcomes. Although pretreatment with anti-inflammatory cytokines allowed them to exert the most substantial inhibitory effects on T cell activation, the addition of IL-4 plus IL-10 plus TGF-β 2 h after IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15 was still able to reduce IFN-γ production. However, there is a limit to this window of opportunity for inhibition, since exposure to anti-inflammatory cytokines at 14 h after initial stimulation had no measurable effect on IFN-γ production. This has implications for the treatment of acute inflammation or sepsis with anti-inflammatory cytokines since treatment at the early stages of inflammation would be essential to achieve maximum benefit.
Signaling through the T cell receptor and through cytokine receptors results in the activation of a group of shared and distinct transcription factors that regulate T cell activation in a combinatorial fashion (39–42). Because of the commonalities of TCR and cytokine-induced transcription factors (e.g., NF-κB activation), stimulations of T cells with peptide or cytokines share some matched outcomes (e.g., IFN-γ production). However, differences between the precise combinations of signaling cascades and transcription factors that are activated by innate and antigenic signals also result in distinct cellular outcomes following exposure to these stimuli and offer the opportunity for differential regulation. Previous studies have demonstrated that several differences in virus-specific CD8+ T cell responses exist following exposure to either cognate antigen or innate cytokines. For example, stimulation of LCMV-specific CD8+ T cells with viral peptide leads to production of IFN-γ, TNF-α, and IL-2, whereas TNF-α and IL-2 are not produced following exposure to IL-12 plus IL-18, despite robust IFN-γ production (31). The data presented herein demonstrate that suppression of innate and adaptive T cell functions is differentially regulated as well. While cytokine-mediated IFN-γ production was highly susceptible to inhibition by IL-4 plus IL-10 plus TGF-β, peptide-specific effector functions (e.g., cytokine production, CTL activity, etc.) were resistant to cytokine-mediated inhibition. Despite these disparate cellular outcomes, cells that had been exposed to either activating cytokines or peptide remained similarly capable of initiating signaling in response to anti-inflammatory cytokines. We examined STAT phosphorylation in response to anti-inflammatory cytokines after pretreatment with either viral peptide (NP118) or stimulatory cytokines (IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15) and found that activation of both STAT3 and STAT6 remained intact, regardless of pretreatment. This suggests that the mechanism for overriding anti-inflammatory signals in peptide-stimulated T cells exists downstream of the initial signaling events leading to STAT phosphorylation. This result is not unexpected, as the differential regulation of IFN-γ and surface molecules such as CD25 and CD69 (Fig. 3) also suggests a downstream mechanism to fine-tune cellular responses, since the effects of inhibitory cytokines are not all-encompassing. In vivo, this differential regulation of innate and adaptive CD8+ T cell functions may serve to limit unwanted inflammation while still allowing potent peptide-specific responses at a site of infection to function at full potential.
Collectively, the data described here highlight the intriguing differential regulation of anti-inflammatory cytokines on CD8+ T cells and demonstrate that the initiation of innate, “bystander” activation of virus-specific CD8+ T cells is more susceptible to this form of immune regulation than antigen-specific T cell activation. Further studies are needed to elucidate the precise mechanisms that contribute to these distinct cellular outcomes, particularly when cells are receiving multiple stimulatory and inhibitory signals at a site of infection, and also to determine whether they might be exploited for therapeutic purposes. In vivo, these fundamental differences in innate and adaptive CD8+ T cell functions likely allow for finely tuned cellular activation that is regulated at least in part, by the local microenvironment.
ACKNOWLEDGMENTS
This work was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), grants U01 AI082196 and R01 AI054458 (to M.K.S.) and Oregon National Primate Research Center grant 8P51 OD011092-53 (to M.K.S.).
H-2Ld NP118 tetramers were prepared by the NIH Tetramer Core Facility (Atlanta, GA).
Footnotes
Published ahead of print 16 April 2014
REFERENCES
- 1.Berg RE, Cordes CJ, Forman J. 2002. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur. J. Immunol. 32:2807–2816. [DOI] [PubMed] [Google Scholar]
- 2.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097–1105. 10.4049/jimmunol.166.2.1097 [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146. 10.1038/nri1001 [DOI] [PubMed] [Google Scholar]
- 4.Raue HP, Brien JD, Hammarlund E, Slifka MK. 2004. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 173:6873–6881. 10.4049/jimmunol.173.11.6873 [DOI] [PubMed] [Google Scholar]
- 5.Ingram JT, Yi JS, Zajac AJ. 2011. Exhausted CD8 T cells downregulate the IL-18 receptor and become unresponsive to inflammatory cytokines and bacterial co-infections. PLoS Pathog. 7:e1002273. 10.1371/journal.ppat.1002273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. 2013. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 3:701–708. 10.1016/j.celrep.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg RE, Crossley E, Murray S, Forman J. 2003. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 198:1583–1593. 10.1084/jem.20031051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen KB, Biron CA. 1999. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J. Immunol. 162:5238–5246 [PubMed] [Google Scholar]
- 9.Whitton JL, Slifka MK, Liu F, Nussbaum AK, Whitmire JK. 2004. The regulation and maturation of antiviral immune responses. Adv. Virus Res. 63:181–238. 10.1016/S0065-3527(04)63003-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanolkar A, Fuller MJ, Zajac AJ. 2002. T cell responses to viral infections: lessons from lymphocytic choriomeningitis virus. Immunol. Res. 26:309–321. 10.1385/IR:26:1-3:309 [DOI] [PubMed] [Google Scholar]
- 11.Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MB. 1980. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv. Immunol. 30:275–331. 10.1016/S0065-2776(08)60197-2 [DOI] [PubMed] [Google Scholar]
- 12.Raue HP, Beadling C, Haun J, Slifka MK. 2013. Cytokine-mediated programmed proliferation of virus-specific CD8(+) memory T cells. Immunity 38:131–139. 10.1016/j.immuni.2012.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman BE, Hammarlund E, Raue HP, Slifka MK. 2012. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl. Acad. Sci. U. S. A. 109:9971–9976. 10.1073/pnas.1203543109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beadling C, Slifka MK. 2006. Regulation of innate and adaptive immune responses by the related cytokines IL-12, IL-23, and IL-27. Arch. Immunol. Ther. Exp. (Warsz.) 54:15–24. 10.1007/s00005-006-0002-6 [DOI] [PubMed] [Google Scholar]
- 15.Ahn HJ, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. 1997. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J. Immunol. 159:2125–2131 [PubMed] [Google Scholar]
- 16.Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. 1996. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 26:1647–1651. 10.1002/eji.1830260736 [DOI] [PubMed] [Google Scholar]
- 17.Sad S, Li L, Mosmann TR. 1997. Cytokine-deficient CD8+ Tc1 cells induced by IL-4: retained inflammation and perforin and Fas cytotoxicity but compromised long term killing of tumor cells. J. Immunol. 159:606–613 [PubMed] [Google Scholar]
- 18.Sad S, Mosmann TR. 1995. Interleukin (IL) 4, in the absence of antigen stimulation, induces an anergy-like state in differentiated CD8+ TC1 cells: loss of IL-2 synthesis and autonomous proliferation but retention of cytotoxicity and synthesis of other cytokines. J. Exp. Med. 182:1505–1515. 10.1084/jem.182.5.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliver JA, Stolberg VR, Chensue SW, King PD. 2012. IL-4 acts as a potent stimulator of IFN-gamma expression in CD8+ T cells through STAT6-dependent and independent induction of Eomesodermin and T-bet. Cytokine 57:191–199. 10.1016/j.cyto.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groux H, Bigler M, de Vries JE, Roncarolo MG. 1998. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J. Immunol. 160:3188–3193 [PubMed] [Google Scholar]
- 21.Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. 2008. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc. Natl. Acad. Sci. U. S. A. 105:20428–20433. 10.1073/pnas.0811139106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12:1301–1309. 10.1038/nm1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 203:2461–2472. 10.1084/jem.20061462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prochazkova J, Pokorna K, Holan V. 2012. IL-12 inhibits the TGF-beta-dependent T cell developmental programs and skews the TGF-beta-induced differentiation into a Th1-like direction. Immunobiology 217:74–82. 10.1016/j.imbio.2011.07.032 [DOI] [PubMed] [Google Scholar]
- 25.Blackburn SD, Wherry EJ. 2007. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 15:143–146. 10.1016/j.tim.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 26.Schulz KR, Danna EA, Krutzik PO, Nolan GP. 2012. Single-cell phospho-protein analysis by flow cytometry. Curr. Protoc. Immunol. Chapter 8:Unit 8.17. 10.1002/0471142735.im0817s78 [DOI] [PubMed] [Google Scholar]
- 27.Krutzik PO, Clutter MR, Nolan GP. 2005. Coordinate analysis of murine immune cell surface markers and intracellular phosphoproteins by flow cytometry. J. Immunol. 175:2357–2365. 10.4049/jimmunol.175.4.2357 [DOI] [PubMed] [Google Scholar]
- 28.Janeway CA, Jr, Medzhitov R. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197–216. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Whitton JL, Slifka MK. 2004. The rapidity with which virus-specific CD8+ T cells initiate IFN-gamma synthesis increases markedly over the course of infection and correlates with immunodominance. J. Immunol. 173:456–462. 10.4049/jimmunol.173.1.456 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez F, Slifka MK, Harkins S, Whitton JL. 2001. Two overlapping subdominant epitopes identified by DNA immunization induce protective CD8+ T-cell populations with differing cytolytic activities. J. Virol. 75:7399–7409. 10.1128/JVI.75.16.7399-7409.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beadling C, Slifka MK. 2005. Differential regulation of virus-specific T-cell effector functions following activation by peptide or innate cytokines. Blood 105:1179–1186. 10.1182/blood-2004-07-2833 [DOI] [PubMed] [Google Scholar]
- 32.Hanlon AM, Jang S, Salgame P. 2002. Signaling from cytokine receptors that affect Th1 responses. Front. Biosci. 7:d1247–d1254. 10.2741/hanlon [DOI] [PubMed] [Google Scholar]
- 33.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683–765. 10.1146/annurev.immunol.19.1.683 [DOI] [PubMed] [Google Scholar]
- 34.Smith KA. 1988. Interleukin-2: inception, impact, and implications. Science 240:1169–1176. 10.1126/science.3131876 [DOI] [PubMed] [Google Scholar]
- 35.Letourneau S, Krieg C, Pantaleo G, Boyman O. 2009. IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J. Allergy Clin. Immunol. 123:758–762. 10.1016/j.jaci.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 36.Ziegler SF, Ramsdell F, Alderson MR. 1994. The activation antigen CD69. Stem Cells 12:456–465. 10.1002/stem.5530120502 [DOI] [PubMed] [Google Scholar]
- 37.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544. 10.1038/nature04606 [DOI] [PubMed] [Google Scholar]
- 38.Feng C, Woodside KJ, Vance BA, El-Khoury D, Canelles M, Lee J, Gress R, Fowlkes BJ, Shores EW, Love PE. 2002. A potential role for CD69 in thymocyte emigration. Int. Immunol. 14:535–544. 10.1093/intimm/dxf020 [DOI] [PubMed] [Google Scholar]
- 39.Carroll HP, Paunovic V, Gadina M. 2008. Signalling, inflammation and arthritis: crossed signals: the role of interleukin-15 and -18 in autoimmunity. Rheumatology (Oxford) 47:1269–1277. 10.1093/rheumatology/ken257 [DOI] [PubMed] [Google Scholar]
- 40.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. 2004. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol. Rev. 202:139–156. 10.1111/j.0105-2896.2004.00211.x [DOI] [PubMed] [Google Scholar]
- 41.Smith-Garvin JE, Koretzky GA, Jordan MS. 2009. T cell activation. Annu. Rev. Immunol. 27:591–619. 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdeil G, Chaix J, Schmitt-Verhulst AM, Auphan-Anezin N. 2006. Temporal cross-talk between TCR and STAT signals for CD8 T cell effector differentiation. Eur. J. Immunol. 36:3090–3100. 10.1002/eji.200636347 [DOI] [PubMed] [Google Scholar]