ABSTRACT
The RNase activity of the envelope glycoprotein Erns of the pestivirus bovine viral diarrhea virus (BVDV) is required to block type I interferon (IFN) synthesis induced by single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA) in bovine cells. Due to the presence of an unusual membrane anchor at its C terminus, a significant portion of Erns is also secreted. In addition, a binding site for cell surface glycosaminoglycans is located within the C-terminal region of Erns. Here, we show that the activity of soluble Erns as an IFN antagonist is not restricted to bovine cells. Extracellularly applied Erns protein bound to cell surface glycosaminoglycans and was internalized into the cells within 1 h of incubation by an energy-dependent mechanism that could be blocked by inhibitors of clathrin-dependent endocytosis. Erns mutants that lacked the C-terminal membrane anchor retained RNase activity but lost most of their intracellular activity as an IFN antagonist. Surprisingly, once taken up into the cells, Erns remained active and blocked dsRNA-induced IFN synthesis for several days. Thus, we propose that Erns acts as an enzymatically active decoy receptor that degrades extracellularly added viral RNA mainly in endolysosomal compartments that might otherwise activate intracellular pattern recognition receptors (PRRs) in order to maintain a state of innate immunotolerance.
IMPORTANCE The pestiviral RNase Erns was previously shown to inhibit viral ssRNA- and dsRNA-induced interferon (IFN) synthesis. However, the localization of Erns at or inside the cells, its species specificity, and its mechanism of interaction with cell membranes in order to block the host's innate immune response are still largely unknown. Here, we provide strong evidence that the pestiviral RNase Erns is taken up within minutes by clathrin-mediated endocytosis and that this uptake is mostly dependent on the glycosaminoglycan binding site located within the C-terminal end of the protein. Remarkably, the inhibitory activity of Erns remains for several days, indicating the very potent and prolonged effect of a viral IFN antagonist. This novel mechanism of an enzymatically active decoy receptor that degrades a major viral pathogen-associated molecular pattern (PAMP) might be required to efficiently maintain innate and, thus, also adaptive immunotolerance, and it might well be relevant beyond the bovine species.
INTRODUCTION
Bovine viral diarrhea virus (BVDV) is a pathogen of cattle that is spread worldwide. Together with the classical swine fever virus (CSFV) and border disease virus (BDV), this positive-sense, single-stranded RNA (ssRNA) virus belongs to the genus Pestivirus of the family Flaviviridae (1). BVDV infections are either transient or persistent. Persistent infections may occur when the fetus is infected by a noncytopathogenic (ncp) biotype of virus early in its development (2, 3). The persistent virus elicits immunotolerance that is specific to the infecting strain. In contrast to other genera of the family Flaviviridae family, like the hepaciviruses, pestiviruses express two unique proteins to block type I interferon (alpha/beta interferon [IFN-α/β]) induction, i.e., the N-terminal protease Npro and the structural glycoprotein Erns. Both proteins are required to establish persistent infections (4). The nonstructural protein Npro targets the transcription factor IRF3 for proteasomal degradation (5), thus antagonizing interferon induction, e.g., by double-stranded RNA (dsRNA), in virus-infected cells (6, 7). Erns harbors an RNase active domain belonging to the T2 RNase superfamily (8), and this enzymatic activity is essential for its ability to block the induction of IFN-α/β (9–11). Together with viral glycoproteins E1 and E2, Erns forms the envelope of the virus, but a significant portion of the Erns protein is also secreted into the extracellular space (8). Attachment of Erns to cell membranes is mediated by an amphipathic helix that acts as an unusual membrane anchor at the C terminus that embeds the protein in plane into cell membranes (12, 13), which might explain its dual function as an envelope glycoprotein and a secreted RNase. The cell tropism of pestiviruses has been attributed to E2, which binds to its receptor, CD46 (14–16), followed by cellular uptake by clathrin-mediated endocytosis (17–19). In contrast, Erns may bind to a different receptor (20), but this might not be required for virus particles to infect their host cells, as E1- and E2-pseudotyped viruses are sufficient to mediate cell entry (21). Although binding of Erns to glycosaminoglycans and immobilized heparin has been shown, the possibility that a cell- or species-specific receptor existed could not be excluded. Thus, binding of Erns was saturable to fetal bovine epithelial or porcine PK15 cells, indicative of receptor-mediated attachment, but not to porcine SK6, hamster BHK-21, or insect Sf21 cells (20). On the basis of the broad pH optimum of its RNase activity (22) and the ability to cleave dsRNA only at low pH values, it was proposed that Erns might be active mainly in endolysosomal compartments (23). However, the latter restriction was recently extended, as dsRNA is also cleaved at neutral pH (11). Previous experiments showed that extracellularly added Erns blocks IFN induction by ss- and dsRNA in bovine cells and that Erns could be removed just prior to the addition of dsRNA, which suggested the possibility of an intracellular activity of this viral RNase (10, 11). Nevertheless, the location of Erns at or inside a cell is still unknown, and its exact role in the evasion of the innate immune system remains elusive so far.
Here we provide evidence that soluble Erns protein enters cells within minutes in an energy-dependent fashion via clathrin-dependent endocytosis and then remains active for several days. The activity of the protein was observed not only in bovine cells but also in caprine, ovine, canine, and human cells. Heparin effectively competes with the binding of Erns to extracellular glycosaminoglycans, and thus, cell-bound Erns could be removed by washing with heparin, which impeded its ability to block the interferon expression induced by dsRNA. Erns protein lacking the C-terminal membrane anchor retained RNase activity but had a strongly reduced activity in blocking dsRNA-induced IFN synthesis.
MATERIALS AND METHODS
Reagents.
Cell culture media were purchased from Seromed (Biochrom, Munich, Germany). Fetal calf serum (FCS) confirmed to be free from BVDV and from antibodies to BVDV was from Sigma (Buchs, Switzerland) or Oxoid GmbH (Wesel, Germany).
Chlorpromazine (CPZ), monodansylcadaverine (MDC), methyl-β-cyclodextrin (MβCD), heparin sodium salt from porcine intestinal mucosa, chondroitin sulfate A sodium salt from bovine trachea, and the synthetic dsRNA poly(I·C) were purchased from Sigma, whereas 5-(N-ethyl-N-isopropyl)amiloride (EIPA) was from Enzo Life Sciences (Lausen, Switzerland).
Cells.
Bovine turbinate (BT) cells, goat synovial membrane (GSM) cells, and lamb synovial membrane (LSM) cells were prepared at the Institute of Veterinary Virology from bovine fetuses or animals obtained from a local abattoir and were maintained in Earle's minimal essential medium (MEM) supplemented with 15% FCS (2% during experiments), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere. Human umbilical vein endothelial cells (HUVECs) were kindly provided by Stefano Di Santo (University Hospital Inselspital, Bern, Switzerland) and cultured using a standard protocol (24). Canine keratinocytes, provided by Philippe Plattet (Division of Experimental Clinical Research, University of Bern, Bern, Switzerland), were maintained as described in reference 25.
Western blotting.
Cells, cultured in 24-well plates, were washed with phosphate-buffered saline (PBS) and directly lysed in the wells with 30 μl M-PER mammalian protein extraction reagent (Pierce, Socochim SA, Lausanne, Switzerland) containing complete protease inhibitor cocktail (Roche Diagnostics, Rotkreuz, Switzerland). Protein separation was performed on 10% SDS-polyacrylamide gels (Bio-Rad, Reinach, Switzerland), and proteins were electroblotted on nitrocellulose membranes (Amersham Biosciences, GE Healthcare, Glattbrugg, Switzerland) in a Mini Trans-Blot cell (Bio-Rad). Membranes were blocked in PBS containing 5% low-fat dry milk and 0.1% Tween 20 before probing for the expression of the Mx protein using a mouse monoclonal antibody against human MxA (kindly provided by Jovan Pavlovic, Institute of Medical Virology, University of Zurich, Zurich, Switzerland) collected from hybridoma supernatants (diluted 1:10). β-Actin, detected with a mouse anti-β-actin antibody (diluted 1:50,000; Sigma), served as loading control for the individual lanes. Peroxidase-labeled donkey anti-mouse antibody (Jackson ImmunoResearch Laboratories, Milan Analytica AG, Rheinfelden, Switzerland) was used as a secondary antibody (diluted 1:5,000), and proteins were visualized using WesternBright enhanced chemiluminescence horseradish peroxidase substrate (Witec AG, Lucerne, Switzerland) according to the manufacturer's protocol. Signal intensities were quantified using the advanced image data analyzer software AIDA (Raytest, Wetzikon, Switzerland).
Assays for Erns import.
BT cells were seeded in 24-well plates at a density of 7.5 × 104 cells/well. The confluent cell monolayer was preincubated with the various inhibitors, as indicated in the appropriate figures, for 30 min prior to the addition of 2.5 μg/ml Erns, still in the presence of the inhibitors, for 60 min in 250 μl of cell culture medium. Cells were then washed twice with MEM containing 0.1 mg/ml heparin and once with MEM only. Control samples were washed three times with MEM. Thereafter, 2 μg/ml poly(I·C) was incubated with the cells for 18 to 20 h before collecting them for Western blot analysis.
Expression of Erns variants.
Wild-type (wt) Erns and the RNase-inactive mutant H30F were expressed in MDBK Tet-On cells using a tetracycline-inducible expression plasmid as described previously (10). Supernatants were collected and concentrated using Vivaspin 20 10,000-molecular weight cutoff (MWCO) filter tubes (Milian AG, Wohlen, Switzerland) before use.
The Erns mutant lacking the C terminus was expressed in 293T/17 cells (kindly provided by Philippe Plattet) that had been transfected with pCI-Erns or empty vectors (pCI mammalian expression vector; Promega, Dübendorf, Switzerland) by use of the Fugene HD transfection reagent (Roche Diagnostics) and a 3:1 ratio of transfection reagent to cDNA. The supernatant was harvested after 3 days, followed by concentration with Vivaspin 6 30,000-MWCO filter tubes. The concentration of Erns in the preparations was quantified by a commercially available enzyme-linked immunosorbent assay (ELISA; IDEXX BVDV Ag/Serum Plus; IDEXX Switzerland AG, Bern-Liebefeld, Switzerland) as described previously (10).
RNase activity assay.
A total of 250 ng of in vitro-transcribed 300-bp dsRNA fragments from the 5′ untranslated region (UTR) of BVDV strain Ncp7 (prepared as described in reference 11) was incubated for 1 h at 37°C with the supernatant of Erns-expressing cells in the presence of 40 U RNasin (Promega), to block unspecific RNase activity (11, 23), in 100 mM Tris-acetate buffer at pH 6.5. Digested RNA was diluted in 2× RNA loading dye solution (Fermentas, Fisher Scientific, Wohlen, Switzerland) and separated on a 1% agarose gel for 25 min at 100 V. Gels were stained with ethidium bromide, and visualization and image capture were done with a U:Genius gel imaging system (Syngene, Biolabo Scientific Instruments SA, Châtel-St-Denis, Switzerland).
RESULTS
Erns is active in nonbovine cells.
The activity of extracellularly supplied BVDV Erns protein against the synthetic dsRNA poly(I·C) in bovine cells has been established previously (10). To investigate whether this activity is restricted to bovine cells and to provide evidence that Erns does not require a species-specific receptor for its activity, we used cells from a variety of host species. Only cells that were able to express Mx protein, a widely used, sensitive, and reliable marker for the presence of IFN-α/β (26), upon stimulation by extracellularly added poly(I·C) were used to test the effect of Erns. Caprine GSM and ovine LSM cells, canine keratinocytes, and HUVECs of human origin all showed robust Mx protein expression in response to poly(I·C). In these cell types, Erns dose-dependently inhibited dsRNA-induced Mx synthesis (Fig. 1). Whereas GSM cells (Fig. 1A), LSM cells (Fig. 1B), and canine keratinocytes (Fig. 1C) showed full inhibition of Mx expression at a similar ratio of Erns to poly(I·C), HUVECs were less responsive to the inhibitory effect of Erns but still maintained a dose-dependent inhibition of the Mx signal (Fig. 1D). Mutant Erns (Erns H30F), which represents the most appropriate negative control exclusively lacking the RNase activity of the wt protein (10), was tested at the highest concentration applied with wt Erns and did not inhibit dsRNA-induced Mx synthesis in any of the cell types tested.
FIG 1.
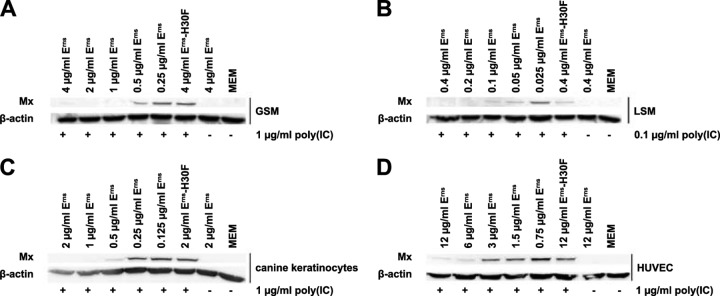
Extracellularly added Erns inhibits Mx induction by poly(I·C) in nonbovine cells. Confluent GSM cells (A), LSM cells (B), canine keratinocytes (C), and HUVECs (D) were stimulated with poly(I·C) for 20 h in the presence or absence of Erns or the RNase-inactive mutant Erns H30F at various concentrations, as indicated. Cell culture medium (MEM) and Erns applied at the highest concentrations used, but in the absence of poly(I·C), served as negative controls. Cytosolic extracts were assayed for Mx and β-actin expression by Western blotting. Typical results out of three independent experiments are shown.
Erns enters the cells within minutes and remains active for several days.
As the Erns-containing supernatant could be removed just prior to the addition of dsRNA (10; unpublished observation), we investigated whether Erns was actively taken up by the cells and how long its inhibition of IFN synthesis induced by dsRNA continued.
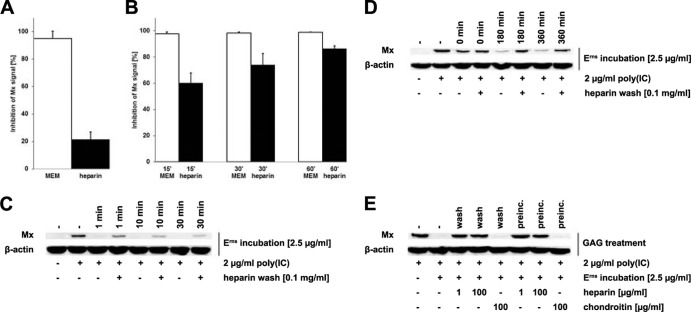
Notably, the ability of Erns to block Mx induction after poly(I·C) stimulation remained intact for up to 3 days in BT cells after the cells were incubated for 1 h in the presence of wt Erns but not mutant H30F with inactive RNase (Fig. 2). The Erns protein was therefore firmly associated with the cells, which allowed us to temporally separate the incubation of the cells with Erns from the one with poly(I·C) for further investigation. To assess whether Erns remains on the cell surface or is taken up, we washed the cells with soluble heparin, which can compete for Erns binding to the cell surface glycosaminoglycans (27). The activity of Erns that might remain cell associated after the wash procedure was assayed by determination of its ability to block Mx induction upon subsequent addition of extracellular dsRNA (Fig. 3). Erns lost most of its activity when the viral RNase was preincubated with soluble heparin, which indicates that heparin effectively prevents Erns from binding to the cells (Fig. 3A). Similarly, washing the cells with heparin after they were preincubated with Erns reduced the ability of Erns to block the Mx synthesis induced by poly(I·C) in a time-dependent manner. Thus, washing the cells with heparin after 15 min of incubation with Erns was able to reduce its activity to only about half of its maximal activity, and after 1 h, heparin was only marginally able to prevent the IFN-antagonistic properties of Erns (Fig. 3B). Further reduction of the time interval between the addition of Erns and washing with heparin showed that already at 1 min after Erns addition, heparin was not able to completely remove Erns from the cell surface (Fig. 3C). As the variability of the effect within the first 10 min of incubation was rather high (not shown), a quantitative assessment of the Western blot was not feasible. In contrast, heparin was still able to remove cell-bound Erns when the latter was incubated for up to 6 h with the cells at 4°C instead of 37°C (Fig. 3D), revealing the involvement of an energy-dependent mechanism of uptake of extracellular Erns protein into the cells. The notion of a washing effect by heparin was challenged by the fact that the Erns RNase activity was decreased in the presence of high concentrations of heparin in in vitro experiments (data not shown). The reduction of the RNase activity was about 100 times lower in the presence of chondroitin sulfate, another glycosaminoglycan that, however, does not bind to Erns (27). To verify that the inhibitory effect observed with heparin is based on its ability to wash away cell surface-bound Erns and not inhibition of Erns RNase activity, we either preincubated Erns or washed Erns-treated cells with heparin or chondroitin sulfate at concentrations that revealed the same inhibition of Erns in the in vitro RNase assays. No reduction of the Erns activity was observed when the protein was preincubated or washed with 100 μg/ml chondroitin sulfate, while even at 1 μg/ml, heparin retained the full activity required to interfere with the action of Erns (Fig. 3E). Accordingly, at this low concentration, which is 100-fold lower than that used in the standard assays, heparin did not inhibit or only marginally reduced the RNase activity of Erns in the in vitro RNase assays (not shown). These results clearly indicate that the effect of heparin on Erns-treated cells is indeed related to the removal of the Erns protein bound to the cell surface instead of the inhibition of its RNase activity.
FIG 2.
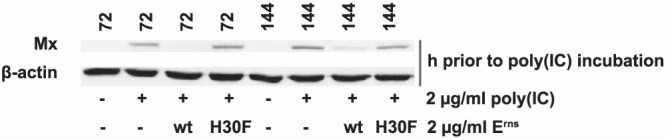
Erns remains active for at least 72 h. BT cells were incubated in the presence of wt Erns protein or the RNase-inactive mutant H30F for 1 h prior to washing the cells with MEM. Thereafter, cells were incubated for 3 days (72 h, as indicated above the lanes) or 6 days (144 h) in fresh medium. Samples incubated for 6 days were detached with trypsin-EDTA and passaged at a ratio of 1 to 2 in new flasks after 3 days. Subsequently, poly(I·C) stimulation was performed for 20 h. The samples were probed for Mx and β-actin protein expression by Western blotting as described in the legend to Fig. 1. A typical result out of three independent experiments is shown.
FIG 3.
Erns bound to the cell membrane can be washed off with soluble heparin. Erns was preincubated in wash medium (0.1 mg/ml heparin in MEM) for 30 min before incubation on BT cells for 30 min at 37°C (A). Alternatively, Erns was incubated with BT cells for 15 to 60 min (B) or 1 to 30 min (C), as indicated in the figure, in the absence of heparin prior to treatment with wash medium. Thereafter, BT cells were stimulated with 2 μg/ml poly(I·C) for 18 h at 37°C and cytosolic extracts were assayed as described in the legend to Fig. 1. The signal intensities of Mx synthesis were quantified relative to the levels of β-actin expression (A, B), with complete inhibition of Mx expression being set equal to 100% (mean ± SD, n = 3). Similarly, Erns was incubated for up to 6 h prior to washing, as described above for panels B and C, but at 4°C instead of 37°C (D). To compare the inhibitory activity of heparin and chondroitin, Erns was either preincubated at 4°C in the presence or absence of glycosaminoglycans (preinc.) or incubated on BT cells for 15 min at 4°C prior to washing with either heparin or chondroitin sulfate, followed by Western blotting as described in the legend to Fig. 1 (E). Typical results out of two (C) or three (D, E) independent experiments are shown.
Extracellularly added Erns enters the cells via clathrin-dependent endocytosis.
With the energy-dependent uptake of Erns into the cells having been established, we further investigated the specific mechanism of this uptake process. Chlorpromazine (CPZ), an inhibitor of the assembly of clathrin-coated pits (28), and monodansylcadaverine (MDC), an agent blocking the transglutaminase (29), were used to block clathrin-dependent endocytosis. Caveolin-dependent endocytosis was inhibited with methyl-β-cyclodextrin (MβCD), an agent that extracts cholesterol from membranes (30, 31). 5-(N-Ethyl-N-isopropyl)amiloride (EIPA), a specific inhibitor of the Na+/H+ antiporter, was used to block macropinocytosis (32).The inhibitory activities of the agents in BT cells were confirmed by fluorescence-activated cell sorter analysis (not shown) of Alexa 488-labeled transferrin, cholera toxin subunit B, and dextran, markers for clathrin- and caveolin-dependent endocytosis (33, 34) and for macropinocytosis (35), respectively. In our bovine cells, MβCD indeed inhibited caveolin-dependent endocytosis but, to a lesser degree, also inhibited the clathrin-dependent pathway, which is in accordance with reports that showed that it also perturbs clathrin-coated vesicles (36). All these endocytosis inhibitors showed only minimal cytotoxicity in BT cells at the concentrations used in the assays.
To test the effects of the inhibitors on the uptake of the viral RNase, the efficacy of inhibition of dsRNA-induced Mx synthesis by cell surface-bound Erns was assessed by determination of the ability of heparin to remove any residual Erns proteins prior to the addition of dsRNA. Thus, Erns preincubated on BT cells in the presence of CPZ and MDC for 1 h was partially washed away by heparin-containing medium, as verified by the loss of activity against Mx induction by poly(I·C). In the absence of any drugs, there was no or very little interference with Erns activity by heparin washes (Fig. 4A). No decrease in the activity of Erns was observed in the presence of MβCD and EIPA (Fig. 4B), indicating that Erns is basically taken up via clathrin-dependent endocytosis.
FIG 4.
Inhibitors of clathrin-dependent endocytosis interfere with Erns uptake. BT cells were preincubated in the presence of 25 μM CPZ or 0.2 mM MDC (A) and 10 mM MβCD or 20 μM EIPA (B) for 30 min. Thereafter, cells were supplemented with Erns and incubated in the presence of the inhibitors for another hour. Washing of the cells with soluble heparin or medium was performed prior to stimulation of the cells with poly(I·C) for 18 h, followed by analysis for Mx and β-actin expression by Western blotting. Typical results out of three independent experiments are shown.
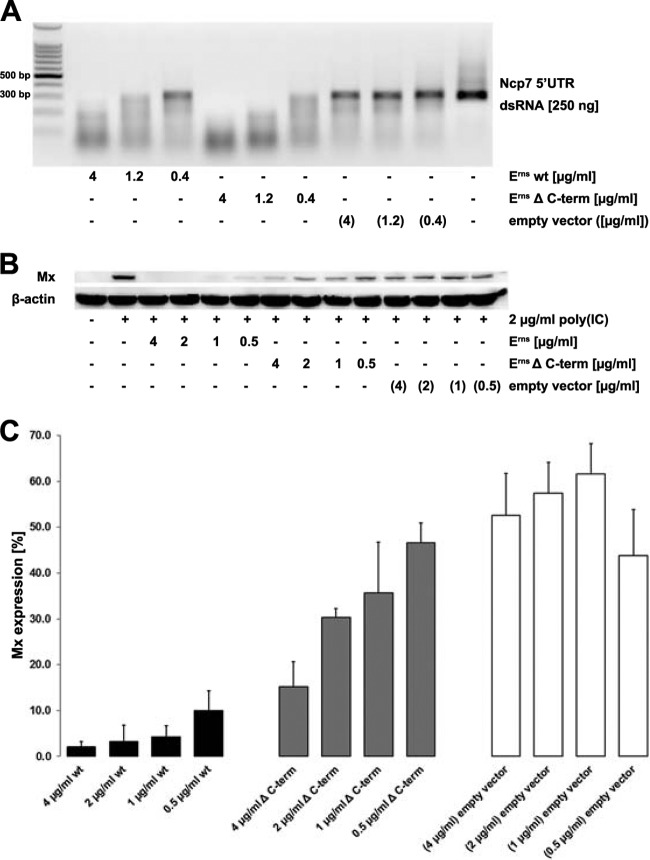
The IFN-antagonistic activity but not the RNase activity of Erns is strongly reduced in the absence of its C terminus.
The membrane association of Erns has been attributed to an unusual membrane anchor at the C terminus of the protein (12). An Erns mutant lacking the 37 C-terminal residues (Erns ΔC-term) was constructed and tested for its ability to degrade dsRNA and to block IFN induction in BT cells stimulated by poly(I·C). Dose-dependent degradation of 300-bp dsRNA fragments of BVDV was observed to occur at an even slightly better efficiency with Erns ΔC-term than with the wt enzyme (Fig. 5A), but the small difference might originate in the variability of the quantification of the protein concentration by ELISA rather than in a true difference in enzyme activity. The possibility of unspecific RNase activity in the supernatant of the cells expressing the Erns protein was excluded, as equal amounts of supernatant concentrated from cells expressing the empty vector showed no activity against the dsRNA (Fig. 5A). In contrast, 1 μg/ml wt Erns was sufficient to completely block Mx induction by poly(I·C), whereas 4 μg/ml of Erns ΔC-term was only partially able to do so (Fig. 5B). Quantitative analysis of these Western blots revealed that wt Erns is approximately 1 order of magnitude more efficient than Erns ΔC-term in inhibiting the IFN synthesis induced by extracellularly added dsRNA (Fig. 5C). Again, cells incubated with supernatant concentrated from cells expressing the empty vector only slightly reduced the expression of the Mx protein in response to poly(I·C), and this was possibly caused by the large amount of various serum components present in the concentrated cell culture supernatants (Fig. 5B and C).
FIG 5.
The activity for inhibition of IFN synthesis, but not the RNase activity, of Erns is reduced in the absence of its C terminus. (A) A 300-bp dsRNA fragment from the 5′ UTR of BVDV strain Ncp7 was incubated in 100 mM Tris-acetate buffer in the presence of wt Erns, Erns lacking the C terminus (Erns ΔC-term), or concentrated supernatant of cells transfected with the empty vector for 1 h at 37°C. RNA was separated on 1% agarose gels and visualized by UV light after staining with ethidium bromide. (B) Erns was incubated on BT cells for 30 min before the cells were washed and incubated with 2 μg/ml poly(I·C) for 18 h at 37°C. Cytosolic extracts were assayed for Mx as described in the legend to Fig. 1. Typical results out of three independent experiments are shown. (C) Three independent replicates performed as described for panel B were quantified for the efficiency of Erns inhibition of dsRNA-induced Mx expression by AIDA software, as described in the legend to Fig. 3, with the level of Mx expression induced by poly(I·C) alone being set equal to 100% (mean ± SD, n = 3).
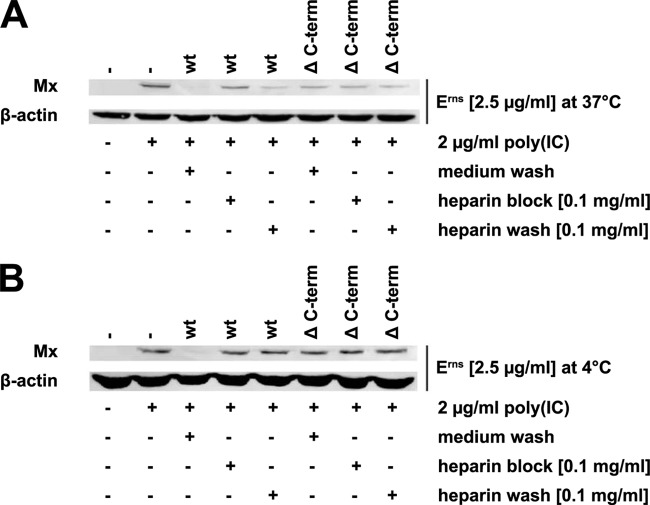
Finally, as it was reported that a strong heparin-binding site is located within the C-terminal domain (9), we monitored the effect of the heparin washes on the inhibition of Mx synthesis by the Erns ΔC-term mutant protein. In accordance with the findings shown in Fig. 5, the Erns ΔC-term mutant did not completely inhibit the Mx synthesis induced by poly(I·C) (Fig. 6A). However, incubation of Erns ΔC-term, but not wt Erns, in the presence or absence of heparin for 15 min or washing with heparin-containing medium thereafter at 37°C had no discernible effect on the partial inhibition of dsRNA-induced IFN synthesis (Fig. 6A). Moreover, binding of Erns ΔC-term to the cell surface was very weak and, thus, could be washed away at 4°C with cell culture medium even in the absence of heparin (Fig. 6B).
FIG 6.
Erns ΔC-term is not affected by heparin treatment at 37°C and is unable to bind to the cell surface at 4°C. Erns was either preincubated in the presence of 0.1 mg/ml heparin for 15 min (heparin block) or left untreated before incubation on BT cells at 37°C (A) or 4°C (B) for 30 min. Unattached protein was removed by washes with medium (medium wash) or with medium containing 0.1 mg/ml heparin (heparin wash). Incubation of the cells in the presence of poly(I·C) at 37°C for 20 h was followed by extraction of the cytosol to evaluate Mx and β-actin expression by Western blotting. Typical results out of three independent experiments are shown.
DISCUSSION
Pestiviruses express the three envelope glycoproteins Erns, E1, and E2, with the first one being the most unusual, as it is the only viral structural protein to possess RNase activity (37). In addition, Erns is also secreted from infected cells and was shown to cleave ss- and dsRNA and, thus, to antagonize the IFN synthesis induced by extracellularly added viral RNA (10, 11, 23). However, details of the mechanism of this innate immune evasion are not yet known. Here, we show that soluble Erns is quickly taken up by cells (within approximately 1 h after addition) and remains active to inhibit dsRNA-induced IFN synthesis for several days. Uptake appears to be energy dependent, involves elements of clathrin-mediated endocytosis, and is much less efficient when Erns lacks its C-terminal membrane anchor. These data provide strong evidence that Erns is mainly active intracellularly, e.g., in endolysosomal compartments, to inhibit the effect of extracellularly added viral RNA that might trigger the host's pattern recognition receptors (PRRs) and activate the innate immune response. However, the precise intracellular location where Erns resides and where it is active at degrading ss- and dsRNA remain to be determined.
The Erns expressed in bovine cells was also able to efficiently block IFN induction by poly(I·C) in nonbovine cells, i.e., in cells of caprine, ovine, canine, and human origin (Fig. 1). This is in accordance with reports demonstrating that Erns, mostly expressed in insect cells, is able to bind to the surface of a large variety of cell types from different species (20, 38). This also confirms that Erns, in contrast to the envelope glycoprotein E2 (14), does not attach to the cells via a specific receptor, which would be expected to be more species specific. Thus, the activity of Erns was similar in GSM and LSM cells and canine keratinocytes, all of which were from species that were shown to be at least partially susceptible to BVDV infection (39–42). In contrast, human cells are reported to be resistant to pestivirus infections, but Erns was nevertheless able to inhibit dsRNA-induced Mx expression in HUVECs, though at concentrations somewhat higher than those used for the other cell types. The reason for this difference is not known but might be related to different numbers of Erns binding sites (whether saturable or not) on the various cell types (20). In addition, further investigations are required to establish a possible link of cell permissibility for BVDV infection and the activity of Erns.
The activity of the Erns protein was surprisingly robust over time, maintaining its full activity even after 72 h (Fig. 2). Notably, even passaging of BT cells that were treated with Erns for 1 h, but not the inactive mutant H30F, at 3 days posttreatment and further incubation in fresh medium for another 3 days only slightly reduced the inhibition of poly(I·C)-induced Mx synthesis. In contrast, Erns-treated MDBK cells lost the capacity to inhibit dsRNA-stimulated IFN expression within 5 days even without passaging (not shown). This strongly indicates that a certain amount of Erns must reside inside a cell at a given time point in order to be able to act as IFN antagonist, as MDBK cells proliferate much faster than BT cells, and over time, the viral RNase becomes thereby much more diluted in MDBK cells than in BT cells. This also suggests that the viral RNase must be present over the whole time period in order to block dsRNA-induced IFN synthesis rather than induce a state of unresponsiveness in the cell by an unknown mechanism. The former is not an implausible possibility, as Erns is a very stable protein that displays RNase activity even at high salt concentrations or in 7 M urea (22, 43; unpublished observation). Accordingly, spiking of Erns-treated BT cells with naive cells dose-dependently reduced the block in Mx synthesis, and this blocking activity could not be transferred via the supernatant (not shown), further indicating that Erns mainly resides inside the cells. Notwithstanding its long-lasting effect, the uptake of Erns was rather rapid, with maximal activity being reached after 30 to 60 min of incubation (Fig. 3). The protein was not internalized at 4°C, even when the incubation time was extended to 6 h, as shown by the strong washing effect of the heparin solution. For that reason, Erns is clearly taken up by an energy-dependent pathway.
The uptake of Erns was efficiently inhibited by inhibitors of clathrin-mediated endocytosis, CPZ and MDC, but not by inhibitors of caveolin-dependent endocytosis and macropinocytosis, MβCD and EIPA, respectively (Fig. 4), further confirming the energy dependence of the uptake mechanism. Interestingly, CPZ and MβCD were shown to completely inhibit BVDV infections (18, 44), but only CPZ was able to inhibit Erns uptake in our experiments. However, unlike CPZ, MβCD was also able to partially inhibit infection with bovine herpesvirus 1 (BHV-1 [18]), and even though MβCD is widely used to block caveolin-dependent endocytosis (31, 45, 46), it might also perturb the formation of clathrin-coated endocytic vesicles (36). CPZ inhibited Erns activity more efficiently than MDC, which might be based on its ability to bind to glycosaminoglycans, thereby competing for binding sites on the cell membrane (47), in addition to its activity as an inhibitor of clathrin-mediated endocytosis. Despite the limitations on the specificity of endocytosis inhibitors (48, 49), clathrin-dependent endocytosis is the most likely pathway for Erns cell entry. The insensitivity to MβCD might indicate that the uptake of the Erns protein differs from the uptake of the complete virion after binding of E2 to its receptor, CD46 (15). The latter is in accordance with the report that E2, but not Erns, is able to prevent the cell-to-cell spread of CSFV (20). Nevertheless, the use of a similar uptake mechanism for Erns and virus particles might well be beneficial for the virus, as any genomic viral RNA inadvertently released from virions or defective particles (50) into endolysosomal compartments might be degraded by free Erns present at the same location or even by viral RNase originating from the virion prior to the activation of intracellular PRRs.
Removal of the C-terminal membrane anchor of Erns strongly reduced its IFN-inhibitory activity without inhibiting its RNase activity (Fig. 5). Thus, the intracellular activity of Erns ΔC-term was reduced by about 1 order of magnitude compared to that of the wt enzyme. Notably, the remaining activity as an IFN antagonist in cell culture could not be blocked by heparin treatment at 4°C and at 37°C (Fig. 6), indicating that the heparin-binding site that was reported to be present within the C-terminal membrane anchor (9) is important for cell attachment prior to internalization. It remains to be established whether other positively charged regions on the surface of the Erns protein (9, 51) account for residual binding to and uptake into the cells. Remarkably, C-terminal peptides of Erns were also reported to translocate across the cell membrane within minutes and to be targeted to, among other locations, the nucleoli, with peptides encompassing the heparin-binding site being the most efficient ones (38). The peptides were able to carry active enzymes, including full-length Erns, into the cells, but this could not be reproduced even at high concentrations of Erns (20; unpublished observation). In addition, penetration of the C-terminal peptides was reported to occur not only at 37°C but also at 4°C, indicating an energy-independent translocation, which is in contrast to what we observed in this study using full-length and C-terminally truncated Erns (Fig. 3D and 6, respectively). The reasons for these discrepancies are not known, but an independent confirmation of the cell-penetrating properties of the C-terminal peptide fragments of Erns is currently lacking.
The PRRs that are activated by extracellularly added dsRNA, such as poly(I·C), in various cell types are not known in every case. Knockdown of scavenger receptors that act as extracellular receptors for dsRNA affected activation of Toll-like receptor 3 (TLR-3) in endolysosomes and the cytosolic RIG-I-like receptors (RLRs) RIG-I and Mda-5 in a dsRNA length-dependent manner (52). However, the mechanism of how poly(I·C) escapes the endosomal compartment after clathrin-mediated endocytosis to activate the cytosolic RLRs is not yet known (52). As the pestiviral Erns is remarkably stable, i.e., the RNase activity is not affected by temperatures of up to 60°C, pH values from 7 to as low as 4.5, or the presence of dithiothreitol (22, 37, 53), it can be envisioned that Erns is able to degrade the RNA substrates in the extracellular space as much as in endolysosomal compartments prior to their possible escape to the cytosol, thus effectively preventing the activation of cytosolic and endolysosomal PRRs. Accordingly, intracellular Erns of CSFV was shown to potently inhibit TLR-7-dependent IFN-α expression in plasmacytoid dendritic cells induced by cell-cell interaction with cells infected with RNA viruses, such as CSFV, foot-and-mouth disease virus (FMDV), or transmissible gastroenteritis virus (TGEV) (54). As the cell types employed in our study probably express TLR-3 rather than TLR-7 (55–57) and as Erns is an effective ss- and dsRNase (11), degradation of viral ss- and dsRNA by Erns might be able to effectively prevent the activation of TLR-3 and TLR-7/8, depending on the cell type analyzed.
Taken together, we show that extracellularly added Erns has to enter the target cells in order to efficiently block the IFN synthesis induced by ss- and dsRNA. Quantification by ELISA of Erns added to the cells and removed after 1 h of incubation indicated that, within the limits of quantification by this method, all of the Erns was removed again, and thus, only a few molecules entered the cells. This further suggests that the enzymatic function of this protein, rather than a stoichiometric interaction with the viral RNA, may be responsible. The fate of the internalized Erns protein is as yet unknown, but localization in an endolysosomal compartment appears to be likely. Immunofluorescent-antibody localization of Erns after extracellular addition was not successful, possibly because the method is not sensitive enough to detect the few molecules that entered the cells, whereas the same antibody brightly stained the viral RNase in BVDV-infected cells.
As a nonstructural protein, BVDV Npro efficiently blocks IFN synthesis, e.g., that induced by replication intermediates, in infected cells. However, it is well established that not all host cells are infected with BVDV even in persistently infected animals. Genomic viral RNA replicating in the cytosol or being protected by the viral envelope is usually not accessible to the Erns that is located extracellularly or intracellularly in endolysosomal compartments. However, any viral RNA that reaches these functionally extracellular compartments, either in a free form that is resistant to digestion by serum RNases (11) or in infected cells or cell fragments, might be a potential danger signal for uninfected cells that have an intact IFN signaling pathway. Therefore, we propose that the main function of Erns as a viral RNase is to act as an enzymatically active decoy receptor that avoids the recognition of viral ss- and dsRNA by intracellular pattern recognition receptors and their subsequent activation of the innate immune response in uninfected cells. As a result, BVDV is able to establish persistent infection and to maintain the strain-specific B- and T-cell tolerance by perpetuating an innate immunotolerance while concurrently avoiding the detrimental effects of the systemic expression of type I IFN (for reviews, see references 26 and 58).
ACKNOWLEDGMENTS
We appreciate the generosity of Till Rümenapf (Institute of Virology, University of Veterinary Medicine, Vienna, Austria), Stefano Di Santo (University Hospital Inselspital, Bern, Switzerland), Jovan Pavlovic (Institute of Medical Virology, University of Zurich, Zurich, Switzerland), and Philippe Plattet (Division of Experimental Clinical Research, University of Bern, Bern, Switzerland) for providing MDBK Tet-On cells expressing wt Ncp7 Erns, the HUVEC line, the Mx antibodies, and both HEK 293T/17 cells and canine keratinocytes, respectively. We thank Ernst Peterhans for critically reading the manuscript and Ruth Parham for linguistic improvements.
Footnotes
Published ahead of print 16 April 2014
REFERENCES
- 1.Lindenbach BD, Thiel HJ, Rice CM. 2007. Flaviviridae: the viruses and their replication, p 1101–1152 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott William & Wilkins, Philadelphia, PA [Google Scholar]
- 2.McClurkin AW, Littledike ET, Cutlip RC, Frank GH, Coria MF, Bolin SR. 1984. Production of cattle immunotolerant to bovine viral diarrhea virus. Can. J. Comp. Med. 48:156–161 [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlie J, Clarke MC, Howard CJ. 1984. Experimental production of fatal mucosal disease in cattle. Vet. Rec. 114:535–536. 10.1136/vr.114.22.535 [DOI] [PubMed] [Google Scholar]
- 4.Meyers G, Ege A, Fetzer C, von Freyburg M, Elbers K, Carr V, Prentice H, Charleston B, Schürmann EM. 2007. Bovine viral diarrhea virus: prevention of persistent fetal infection by a combination of two mutations affecting Erns RNase and Npro protease. J. Virol. 81:3327–3338. 10.1128/JVI.02372-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton L, Moganeradj K, Zhang G, Chen YH, Randall RE, McCauley JW, Goodbourn S. 2006. The NPro product of bovine viral diarrhea virus inhibits DNA binding by interferon regulatory factor 3 and targets it for proteasomal degradation. J. Virol. 80:11723–11732. 10.1128/JVI.01145-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil LHVG, Ansari IH, Vassilev V, Liang DL, Lai VCH, Zhong WD, Hong Z, Dubovi EJ, Donis RO. 2006. The amino-terminal domain of bovine viral diarrhea virus Npro protein is necessary for alpha/beta interferon antagonism. J. Virol. 80:900–911. 10.1128/JVI.80.2.900-911.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruggli N, Bird BH, Liu L, Bauhofer O, Tratschin JD, Hofmann MA. 2005. Npro of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-α/β induction. Virology 340:265–276. 10.1016/j.virol.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 8.Rümenapf T, Unger G, Strauss JH, Thiel HJ. 1993. Processing of the envelope glycoproteins of pestiviruses. J. Virol. 67:3288–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal M, McCauley JW. 2002. Identification of the glycosaminoglycan-binding site on the glycoprotein Erns of bovine viral diarrhoea virus by site-directed mutagenesis. J. Gen. Virol. 83:2153–2159 [DOI] [PubMed] [Google Scholar]
- 10.Magkouras I, Mätzener P, Rümenapf T, Peterhans E, Schweizer M. 2008. RNase-dependent inhibition of extracellular, but not intracellular, dsRNA-induced interferon synthesis by Erns of pestiviruses. J. Gen. Virol. 89:2501–2506. 10.1099/vir.0.2008/003749-0 [DOI] [PubMed] [Google Scholar]
- 11.Mätzener P, Magkouras I, Rümenapf T, Peterhans E, Schweizer M. 2009. The viral RNase Erns prevents IFN type-I triggering by pestiviral single- and double-stranded RNAs. Virus Res. 140:15–23. 10.1016/j.virusres.2008.10.015 [DOI] [PubMed] [Google Scholar]
- 12.Fetzer C, Tews BA, Meyers G. 2005. The carboxy-terminal sequence of the pestivirus glycoprotein Erns represents an unusual type of membrane anchor. J. Virol. 79:11901–11913. 10.1128/JVI.79.18.11901-11913.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tews BA, Meyers G. 2007. The pestivirus glycoprotein Erns is anchored in plane in the membrane via an amphipathic helix. J. Biol. Chem. 282:32730–32741. 10.1074/jbc.M706803200 [DOI] [PubMed] [Google Scholar]
- 14.Liang DL, Sainz IF, Ansari IH, Gil LHVG, Vassilev V, Donis RO. 2003. The envelope glycoprotein E2 is a determinant of cell culture tropism in ruminant pestiviruses. J. Gen. Virol. 84:1269–1274. 10.1099/vir.0.18557-0 [DOI] [PubMed] [Google Scholar]
- 15.Maurer K, Krey T, Moennig V, Thiel HR, Rümenapf T. 2004. CD46 is a cellular receptor for bovine viral diarrhea virus. J. Virol. 78:1792–1799. 10.1128/JVI.78.4.1792-1799.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krey T, Himmelreich A, Heimann M, Menge C, Thiel HJ, Maurer K, Rümenapf T. 2006. Function of bovine CD46 as a cellular receptor for bovine viral diarrhea virus is determined by complement control protein 1. J. Virol. 80:3912–3922. 10.1128/JVI.80.8.3912-3922.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grummer B, Grotha S, Greiser-Wilke I. 2004. Bovine viral diarrhoea virus is internalized by clathrin-dependent receptor-mediated endocytosis. J. Vet. Med. B Infect. Dis. Vet. Public Health 51:427–432. 10.1111/j.1439-0450.2004.00798.x [DOI] [PubMed] [Google Scholar]
- 18.Krey T, Thiel HJ, Rümenapf T. 2005. Acid-resistant bovine pestivirus requires activation for pH-triggered fusion during entry. J. Virol. 79:4191–4200. 10.1128/JVI.79.7.4191-4200.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecot S, Belouzard S, Dubuisson J, Rouille Y. 2005. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 79:10826–10829. 10.1128/JVI.79.16.10826-10829.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulst MM, Moormann RJM. 1997. Inhibition of pestivirus infection in cell culture by envelope proteins Erns and E2 of classical swine fever virus: Erns and E2 interact with different receptors. J. Gen. Virol. 78:2779–2787 [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Nie YC, Wang PG, Ding MX, Deng HK. 2004. Characterization of classical swine fever virus entry by using pseudotyped viruses: E1 and E2 are sufficient to mediate viral entry. Virology 330:332–341. 10.1016/j.virol.2004.09.023 [DOI] [PubMed] [Google Scholar]
- 22.Windisch JM, Schneider R, Stark R, Weiland E, Meyers G, Thiel HJ. 1996. RNase of classical swine fever virus: biochemical characterization and inhibition by virus-neutralizing monoclonal antibodies. J. Virol. 70:352–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iqbal M, Poole E, Goodbourn S, McCauley JW. 2004. Role for bovine viral diarrhea virus Erns glycoprotein in the control of activation of beta interferon by double-stranded RNA. J. Virol. 78:136–145. 10.1128/JVI.78.1.136-145.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaffe EA, Nachman RL, Becker CG, Minick CR. 1973. Culture of human endothelial cells derived from umbilical veins—identification by morphologic and immunological criteria. J. Clin. Invest. 52:2745–2756. 10.1172/JCI107470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivals JP, Plattet P, Currat-Zweifel C, Zurbriggen A, Wittek R. 2007. Adaptation of canine distemper virus to canine footpad keratinocytes modifies polymerase activity and fusogenicity through amino acid substitutions in the P/V/C and H proteins. Virology 359:6–18. 10.1016/j.virol.2006.07.054 [DOI] [PubMed] [Google Scholar]
- 26.Peterhans E, Schweizer M. 2013. BVDV: a pestivirus inducing tolerance of the innate immune response. Biologicals 41:39–51. 10.1016/j.biologicals.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 27.Iqbal M, Flick-Smith H, McCauley JW. 2000. Interactions of bovine viral diarrhoea virus glycoprotein Erns with cell surface glycosaminoglycans. J. Gen. Virol. 81:451–459 [DOI] [PubMed] [Google Scholar]
- 28.Wang LH, Rothberg KG, Anderson RGW. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107–1117. 10.1083/jcb.123.5.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitzki A, Willingham M, Pastan I. 1980. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc. Natl. Acad. Sci. U. S. A. 77:2706–2710. 10.1073/pnas.77.5.2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. 1989. Differential-effects of α-cyclodextrins, β-cyclodextrins and γ-cyclodextrins on human-erythrocytes. Eur. J. Biochem. 186:17–22. 10.1111/j.1432-1033.1989.tb15171.x [DOI] [PubMed] [Google Scholar]
- 31.Puri V, Watanabe R, Singh RD, Dominguez M, Brown JC, Wheatley CL, Marks DL, Pagano RE. 2001. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154:535–547. 10.1083/jcb.200102084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fretz M, Jin J, Conibere R, Penning NA, Al-Taei S, Storm G, Futaki S, Takeuchi T, Nakase I, Jones AT. 2006. Effects of Na+/H+ exchanger inhibitors on subcellular localisation of endocytic organelles and intracellular dynamics of protein transduction domains HIV-TAT peptide and octaarginine. J. Control. Release 116:247–254. 10.1016/j.jconrel.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 33.Hansen SH, Sandvig K, Vandeurs B. 1991. The preendosomal compartment comprises distinct coated and noncoated endocytic vesicle populations. J. Cell Biol. 113:731–741. 10.1083/jcb.113.4.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montesano R, Roth J, Robert A, Orci L. 1982. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature 296:651–653. 10.1038/296651a0 [DOI] [PubMed] [Google Scholar]
- 35.Oliver JM, Berlin RD, Davis BH. 1984. Use of horseradish-peroxidase and fluorescent dextrans to study fluid pinocytosis in leukocytes. Methods Enzymol. 108:336–347. 10.1016/S0076-6879(84)08100-3 [DOI] [PubMed] [Google Scholar]
- 36.Rodal SK, Skretting G, Garred Ø, Vilhardt F, van Deurs B, Sandvig K. 1999. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961–974. 10.1091/mbc.10.4.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider R, Unger G, Stark R, Schneider-Scherzer E, Thiel HJ. 1993. Identification of a structural glycoprotein of an RNA virus as a ribonuclease. Science 261:1169–1171. 10.1126/science.8356450 [DOI] [PubMed] [Google Scholar]
- 38.Langedijk JPM. 2002. Translocation activity of C-terminal domain of pestivirus Erns and ribotoxin L3 loop. J. Biol. Chem. 277:5308–5314. 10.1074/jbc.M104147200 [DOI] [PubMed] [Google Scholar]
- 39.Bachofen C, Vogt HR, Stalder H, Mathys T, Zanoni R, Hilbe M, Schweizer M, Peterhans E. 2013. Persistent infections after natural transmission of bovine viral diarrhea virus from cattle to goats and among goats. Vet. Res. 44:32. 10.1186/1297-9716-44-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dezengrini R, Weiblen R, Flores EF. 2006. Selection and characterization of canine, swine and rabbit cell lines resistant to bovine viral diarrhea virus. J. Virol. Methods 137:51–57. 10.1016/j.jviromet.2006.05.032 [DOI] [PubMed] [Google Scholar]
- 41.Harasawa R, Mizusawa H. 1995. Demonstration and genotyping of pestivirus RNA from mammalian-cell lines. Microbiol. Immunol. 39:979–985. 10.1111/j.1348-0421.1995.tb03301.x [DOI] [PubMed] [Google Scholar]
- 42.Meier P. 1998. Studies on the molecular evolution of bovine viral diarrhea virus (BVDV). Ph.D. thesis University of Bern, Bern, Switzerland [Google Scholar]
- 43.Hausmann Y, Roman-Sosa G, Thiel HJ, Rümenapf T. 2004. Classical swine fever virus glycoprotein Erns is an endoribonuclease with an unusual base specificity. J. Virol. 78:5507–5512. 10.1128/JVI.78.10.5507-5512.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathapati BS, Mishra N, Rajukumar K, Nema RK, Behera SP, Dubey SC. 2010. Entry of bovine viral diarrhea virus into ovine cells occurs through clathrin-dependent endocytosis and low pH-dependent fusion. In Vitro Cell. Dev. Biol. Anim. 46:403–407. 10.1007/s11626-009-9263-9 [DOI] [PubMed] [Google Scholar]
- 45.Yumoto R, Nishikawa H, Okamoto M, Katayama H, Nagai J, Takano M. 2006. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L946–L955. 10.1152/ajplung.00173.2005 [DOI] [PubMed] [Google Scholar]
- 46.Van Hamme E, Dewerchin HL, Cornelissen E, Verhasselt B, Nauwynck HJ. 2008. Clathrin- and caveolae-independent entry of feline infectious peritonitis virus in monocytes depends on dynamin. J. Gen. Virol. 89:2147–2156. 10.1099/vir.0.2008/001602-0 [DOI] [PubMed] [Google Scholar]
- 47.Blumenkrantz N, Asboe-Hansen G. 1974. Reaction of cationic groups of chlorpromazine with anionic macromolecules—complexes with DNA, RNA, hyaluronic-acid and heparin. Acta Pharmacol. Toxicol. (Copenh.) 34:27–32 [DOI] [PubMed] [Google Scholar]
- 48.Vercauteren D, Vandenbroucke RE, Jones AT, Rejman J, Demeester J, De Smedt SC, Sanders NN, Braeckmans K. 2010. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol. Ther. 18:561–569. 10.1038/mt.2009.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dutta D, Donaldson JG. 2012. Search for inhibitors of endocytosis. Cell. Logist. 2:203–208. 10.4161/cl.23967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kane M, Case LK, Wang C, Yurkovetskiy L, Dikiy S, Golovkina TV. 2011. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity 35:135–145. 10.1016/j.immuni.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krey T, Bontems F, Vonrhein C, Vaney MC, Bricogne G, Rümenapf T, Rey FA. 2012. Crystal structure of the pestivirus envelope glycoprotein Erns and mechanistic analysis of its ribonuclease activity. Structure 20:862–873. 10.1016/j.str.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 52.DeWitte-Orr SJ, Collins SE, Bauer CMT, Bowdish DM, Mossman KL. 2010. An accessory to the ‘trinity': SR-As are essential pathogen sensors of extracellular dsRNA, mediating entry and leading to subsequent type I IFN responses. PLoS Pathog. 6:e1000829. 10.1371/journal.ppat.1000829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hulst MM, Moormann RJM. 2001. Erns protein of pestiviruses. Methods Enzymol. 342:431–440. 10.1016/S0076-6879(01)42564-X [DOI] [PubMed] [Google Scholar]
- 54.Python S, Gerber M, Suter R, Ruggli N, Summerfield A. 2013. Efficient sensing of infected cells in absence of virus particles by plasmacytoid dendritic cells is blocked by the viral ribonuclease Erns. PLoS Pathog. 9:e1003412. 10.1371/journal.ppat.1003412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwasaki A, Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987–995. 10.1038/ni1112 [DOI] [PubMed] [Google Scholar]
- 56.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. 10.1016/j.cell.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 57.Barton GM. 2007. Viral recognition by Toll-like receptors. Semin. Immunol. 19:33–40. 10.1016/j.smim.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 58.Schweizer M, Peterhans E. 2014. Pestiviruses. Annu. Rev. Anim. Biosci. 2:141–163. 10.1146/annurev-animal-022513-114209 [DOI] [PubMed] [Google Scholar]