Abstract
Transcriptional changes following varicella-zoster virus (VZV) infection of cultured human neurons derived from embryonic stem cells were compared to those in VZV-infected human foreskin fibroblasts. Transcription of 340 neuronal genes significantly altered by VZV infection included 223 transcript changes unique to neurons. Strikingly, genes inhibiting apoptosis were upregulated in neurons, while proapoptotic gene transcription was increased in fibroblasts. These data are a basis for discovery of differences in virus-host interactions between these VZV targets.
TEXT
The alphaherpesvirus varicella-zoster virus (VZV) radically modifies the transcription of cellular genes upon infection (1, 2). However, while the transcriptional changes seen in lytic VZV infection of several cell types have been studied and partly delineated (1–4), changes in neurons have not been studied. Neurons host the VZV latent state after a primary infection, from which virus may reactivate after decades to cause herpes zoster. The strict human specificity of VZV precludes transcriptome analysis in animal neuron model systems. However, recently introduced in vitro human neuron systems, including neurons made from human embryonic stem cells (hESC), now permit comparison of cellular transcriptome changes following VZV infection to those in nonneuronal cells.
Neurons generated from hESC line H9 and human perinatal foreskin fibroblasts (HFF) were prepared and grown as detailed previously (5). Neurons (minimum of 2 weeks of terminal differentiation) and fibroblasts at 95% confluence were infected with cell-free preparations of recombinant VZV (6) expressing green fluorescent protein (GFP) as a fusion to the ORF23 capsid protein (7) at 10,000 PFU per well of a 24-well plate. RNA was prepared from 80% to 100% GFP-positive infected cultures (Fig. 1A and B) before plaque formation in fibroblasts and development of obvious cytopathology in neurons. RNA was hybridized to Agilent Human GE 4x44K v2 microarray transcriptome microarrays (G2519F-026652 covering 34,127 transcripts) and analyzed using standard procedures. Two biological replicates and two technical replicates were performed. Select changes were confirmed with quantitative reverse transcription-PCR (qRT-PCR), using Sybr green in-house, as well as blindly by a commercial service (Syd Labs, CA, USA).
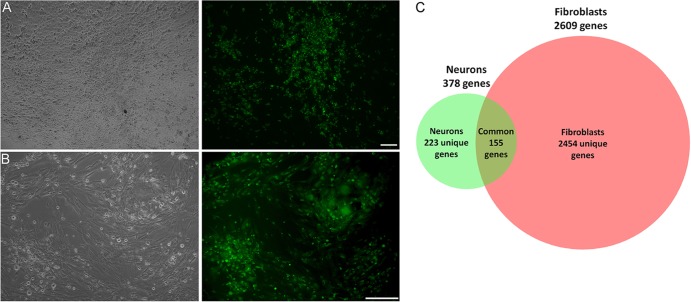
FIG 1.
(A and B) Infection of HFF and hESC-derived neurons with GFP-expressing VZV. VZV-infected hESC-derived neurons (A) and VZV-infected human foreskin fibroblasts (B) at time of harvest for RNA. Left panels, phase-contrast micrographs; right panels, fluorescence micrographs. Bars, 100 μm. (C) Venn diagram summarizing gene expression changes in neurons and fibroblasts following infection with VZV. The red circle represents the expression of 2,609 genes altered in infected fibroblasts compared to uninfected fibroblasts; 1,654 genes were upregulated and 955 genes were downregulated. Out of these genes, 2,474 genes were altered specifically in fibroblasts. The green circle represents the expression of 378 genes altered in infected neurons compared to uninfected neurons; 340 genes were upregulated and 38 genes were downregulated. Out of these, 223 genes were altered only in neurons.
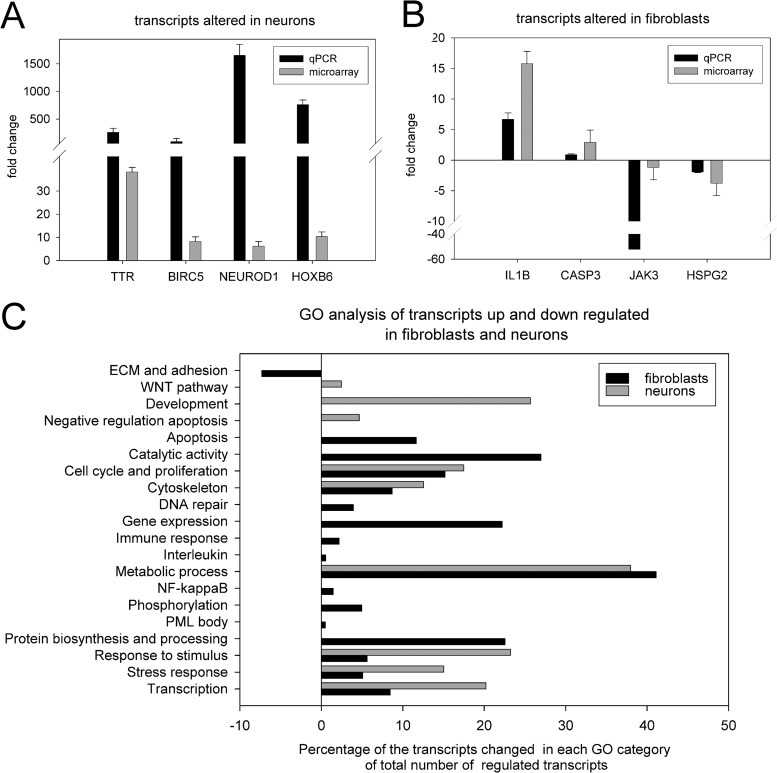
Using a cutoff of 2-fold changes with a significance of 0.05, the expression of 378 genes was modified in hESC-derived neurons, with 340 genes (90%) upregulated and only 38 transcripts (10%) downregulated by VZV infection (Fig. 1C). Similar skewing of transcription changes toward upregulation has been reported in neurons infected with other alphaherpesviruses (reviewed in reference 8). More significant changes in transcription followed VZV infection of HFF, with 2,609 significant changes (Fig. 1C), of which 1,654 (63%) showed upregulation and 955 (36%) showed downregulation. Many transcriptional changes were the same as those reported in array studies for fibroblasts detailed previously (1, 2). Of note is that 223 transcriptional changes were specific to neurons (Fig. 1C; see also Tables S1 to S3 in the supplemental material). Quantitative RT-PCR analyses of 8 select genes (4 with RNA from fibroblasts and 4 with RNA from neurons) all confirmed changes in expression identified by the microarray studies (Fig. 2A and B).
FIG 2.
(A and B) Confirmation of transcription changes by quantitative RT-PCR (qPCR) for four genes that were altered in neurons (A) and fibroblasts (B) as a result of VZV infection. (C) Gene ontogeny analysis of transcription changes in neurons and fibroblasts in response to VZV infection. All GO changes were significant with a Benjamini P value of <0.05. The bars indicate the genes changed as percentages of the total number of regulated genes in each GO category. Black bars depict fibroblast transcripts, and gray bars depict neuronal transcripts.
Gene ontology (GO) analysis using DAVID (Fig. 2C) revealed that transcription of genes in 17 functional categories was significantly increased in fibroblasts and those in only one category were downregulated. Transcriptional changes in neurons were in 9 functional categories that differed strikingly from those of fibroblasts. Importantly, neurons did not show the fibroblast-specific upregulation of groups classified with the innate immune responses, NF-κB-related genes, and response to stress and DNA repair clusters.
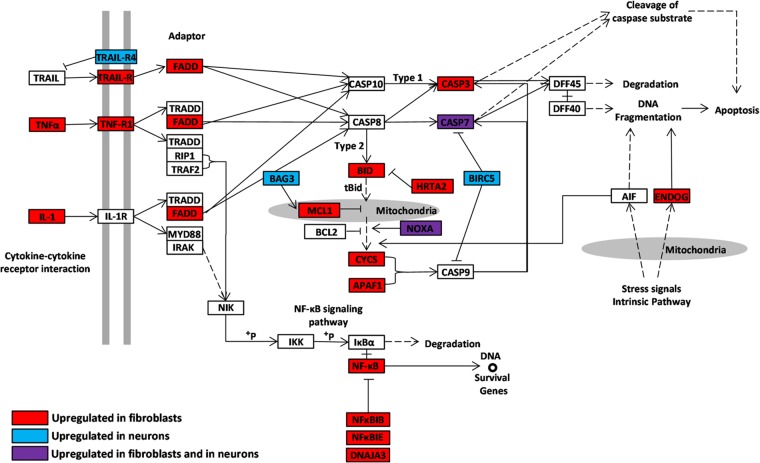
The most striking difference in transcriptional changes elicited by VZV infection between neurons and HFF was that of apoptosis-related genes (Fig. 3). In fibroblasts, a large cluster of 193 proapoptotic genes was upregulated by infection. Transcripts for both ligands and receptors involved in apoptotic pathways were elevated (i.e., tumor necrosis factor alpha [TNF-α], TNFR1, TRAIL-R, and interleukin-1 [IL-1]). In addition, some transcripts for genes downstream in the apoptosis pathway were elevated, including caspase-3, which plays a central role in execution of apoptosis. Several transcripts related to apoptosis were elevated (i.e., FADD, BID, CYCS, APAF-1, and ENDOG). Transcripts for the survival gene NF-κB subunit RelA were upregulated by VZV infection in fibroblasts, as well as transcripts for several NF-κB inhibitors (NF-κBIB, NF-κBIE, and DNAJA3). The NF-κB pathway in VZV-infected epidermal cells in SCID-Hu mice and in cultured fibroblasts has been examined in detail (2, 3). The interaction of VZV with apoptosis pathways in nonneuronal cells has been the target of several investigations focusing on the cellular signaling pathways involved, especially those relating to phosphatidylinositol 3-kinase (PI3K) and AKT (9, 10; also reviewed in reference 11).
FIG 3.
Distinct regulation of apoptosis pathway genes in neurons and fibroblasts in response to VZV infection. Pathway diagram modified from KEGG pathway database (www.genome.jp/kegg/). Red boxes, genes upregulated in fibroblasts; blue boxes, those upregulated in neurons; purple boxes, upregulated genes common to the two cell types.
In contrast to the transcriptional inductions of apoptosis-related genes in fibroblasts that we observed, no clusters of proapoptotic genes were upregulated in VZV-infected neurons. Instead, a cluster of 17 genes involved in antiapoptotic pathways was upregulated. Antiapoptotic genes upregulated in VZV-infected neurons include Trail-R4, BIRC5 (SURVIVIN), and BAG3. These changes reflect the observations that neurons productively infected with VZV do not undergo apoptosis in vitro (12, 13), as opposed to VZV-infected nonneuronal cells. The antiapoptotic effect of VZV infections on neurons is similar to changes seen in neurons latently or productively infected by herpes simplex virus 1 (HSV-1) (reviewed in reference 8). Increased transcription of BIRC5 was observed in VZV-infected embryonic lung fibroblasts (14); however, that study was performed at early stages of infection (24 h) when cells were not yet undergoing apoptosis. Transcripts for a few genes, such as CASP7 and NOXA, were elevated in response to VZV infection in both neurons and fibroblasts.
In addition to the differences in transcriptional regulation relating to apoptosis, striking differences were observed in several GO categories between fibroblasts and neurons. Transcripts related to the NF-κB pathway, PML bodies, and the immune response were upregulated in fibroblasts but not neurons. The lack of upregulation of immune response genes is intriguing, in light of our recent observation that VZV (and HSV)-infected human neurons are less able to exclude superinfecting virus (15). Upregulation of transcripts in the development and WNT pathways was observed for neurons but not for fibroblasts.
In summary, the transcriptome pattern for VZV-infected neurons is strikingly different from that reported for fibroblasts, T cells, and VZV-infected keratinocytes (1, 4). The transcription changes in neurons productively infected with VZV described here likely reflect changes following primary infection or reactivation, rather than the latent state normally established in varicella disease. The activation of antiapoptotic pathways in neurons infected with VZV is therefore consistent with what would be predicted to occur in vivo. Future development of a consistent model for VZV latency using hESC-derived neurons should allow evaluation of the transcriptome of neurons in this state and comparison to that observed for other alphaherpesviruses (8).
Nucleotide sequence accession number.
The raw data were deposited online in GEO (accession number GSE54385).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Israel Science Foundation grant 238/11 (R.S.G.); NIH grants NS082662 (with R.S.G.), NS064022, and EY08098; and funds from the Research to Prevent Blindness Inc. and The Eye & Ear Foundation of Pittsburgh (P.R.K.).
We are grateful for Aaron Javitt's efforts in this project. We also acknowledge technical and bioinformatics assistance from Michael Yee and Steven Harvey (University of Pittsburgh) and Chaim Wachtel (Bar-Ilan). Our sincere thanks as always to Chaya Morgenstern for expert technical and logistic support and our best wishes for a healthy and productive retirement.
ADDENDUM IN PROOF
Relevant to this article is a report by N. L. Baird et al. (J. Virol. 88:5877–5880, 2014, doi:10.1128/JVI.00476-14) in which the VZV (not the cellular) transcriptome was compared between infected fibroblasts and neurons. Differential expression of only 12 VZV genes was observed, and the authors concluded that the lack of cell death in neurons is likely not to be a consequence of differential transcription of viral genes.
Footnotes
Published ahead of print 16 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00500-14.
REFERENCES
- 1.Jones JO, Arvin AM. 2003. Microarray analysis of host cell gene transcription in response to varicella-zoster virus infection of human T cells and fibroblasts in vitro and SCIDhu skin xenografts in vivo. J. Virol. 77:1268–1280. 10.1128/JVI.77.2.1268-1280.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JO, Arvin AM. 2006. Inhibition of the NF-kappaB pathway by varicella-zoster virus in vitro and in human epidermal cells in vivo. J. Virol. 80:5113–5124. 10.1128/JVI.01956-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones JO, Arvin AM. 2005. Viral and cellular gene transcription in fibroblasts infected with small plaque mutants of varicella-zoster virus. Antiviral Res. 68:56–65. 10.1016/j.antiviral.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 4.Jones M, Dry IR, Frampton D, Singh M, Kanda RK, Yee MB, Kellam P, Hollinshead M, Kinchington PR, O'Toole EA, Breuer J. 2014. RNA-seq analysis of host and viral gene expression highlights interaction between varicella zoster virus and keratinocyte differentiation. PLoS Pathog. 10:e1003896. 10.1371/journal.ppat.1003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pomp O, Brokhman I, Ziegler L, Almog M, Korngreen A, Tavian M, Goldstein RS. 2008. PA6-induced human embryonic stem cell-derived neurospheres: a new source of human peripheral sensory neurons and neural crest cells. Brain Res. 1230:50–60. 10.1016/j.brainres.2008.07.029 [DOI] [PubMed] [Google Scholar]
- 6.Sloutskin A, Kinchington PR, Goldstein RS. 2013. Productive vs non-productive infection by cell-free varicella zoster virus of human neurons derived from embryonic stem cells is dependent upon infectious viral dose. Virology 443:285–293. 10.1016/j.virol.2013.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markus A, Grigoryan S, Sloutskin A, Yee MB, Zhu H, Yang IH, Thakor NV, Sarid R, Kinchington PR, Goldstein RS. 2011. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J. Virol. 85:6220–6233. 10.1128/JVI.02396-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szpara ML, Kobiler O, Enquist LW. 2010. A common neuronal response to alphaherpesvirus infection. J. Neuroimmune Pharmacol. 5:418–427. 10.1007/s11481-010-9212-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Li Q, Dowdell K, Fischer ER, Cohen JI. 2012. Varicella-zoster virus ORF12 protein triggers phosphorylation of ERK1/2 and inhibits apoptosis. J. Virol. 86:3143–3151. 10.1128/JVI.06923-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Cohen JI. 2014. Inhibition of Bim enhances replication of varicella-zoster virus and delays plaque formation in virus-infected cells. J. Virol. 88:1381–1388. 10.1128/JVI.01695-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James SF, Mahalingam R, Gilden D. 2012. Does apoptosis play a role in varicella zoster virus latency and reactivation? Viruses 4:1509–1514. 10.3390/v4091509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hood C, Cunningham AL, Slobedman B, Arvin AM, Sommer MH, Kinchington PR, Abendroth A. 2006. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J. Virol. 80:1025–1031. 10.1128/JVI.80.2.1025-1031.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hood C, Cunningham AL, Slobedman B, Boadle RA, Abendroth A. 2003. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J. Virol. 77:12852–12864. 10.1128/JVI.77.23.12852-12864.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen N, Che X, Rajamani J, Zerboni L, Sung P, Ptacek J, Arvin AM. 2012. Signal transducer and activator of transcription 3 (STAT3) and survivin induction by varicella-zoster virus promote replication and skin pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 109:600–605. 10.1073/pnas.1114232109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloutskin A, Yee MB, Kinchington PR, Goldstein RS. 2014. Varicella-zoster virus and herpes simplex virus 1 can infect and replicate in the same neurons whether co- or superinfected. J. Virol. 88:5079–5086. 10.1128/JVI.00252-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.