Abstract
Myeloid-derived suppressor cells (MDSC) are immature myeloid cells with immunosuppressive function. Compared to the level in healthy controls (HC), no elevation of MDSC in chronic hepatitis C (cHEP-C) patients was found, and there was no difference in MDSC based on genotype or viral load (P > 0.25). Moreover, MDSC of cHEP-C patients inhibited CD8 T cell function as efficiently as MDSC of HC did. Since we detected neither quantitative nor qualitative differences in MDSC of cHEP-C patients relative to those of HC, we postulate that MDSC in peripheral blood are most likely not significant regarding immune dysfunction in cHEP-C.
TEXT
Human myeloid-derived suppressor cells (MDSC) represent a heterogeneous population of immature myeloid cells with immunosuppressive function. They are divided by phenotype into at least two subsets: MDSC of the granulocytic type (G-MDSC) are identified as CD11b+ CD14− CD33int CD15+ or CD66+ (where “int” represents “intermediate”), and MDSC of the monocytic type (M-MDSC) are described as CD11b+ CD14+ CD33+ HLA-DR−/low (1). The best defining feature of MDSC, however, is their suppressive action on, e.g., T cells (1). Under various pathological conditions, increased MDSC levels are reported to occur in the peripheral blood and various tissues. Elevated human MDSC levels are described mostly for a variety of malignant tumors (e.g., hepatocellular carcinoma [HCC], non-small cell lung carcinoma, and melanoma) (2). However, accumulating data show that MDSC play a role in nonmalignant diseases as well. Recently, we, as well as other researchers, have described the significance of MDSC in the peripheral blood of patients with chronic progressive HIV-1 infection (cHIV-1) and were able to demonstrate the suppressive effect of MDSC on HIV-specific CD8 T cells (3, 4). Chronic hepatitis C (cHEP-C) is another chronic viral disease with proven impaired T cell responses and immune exhaustion (5). We therefore hypothesized that MDSC also play a role in the development of T cell exhaustion in this clinical setting.
For this purpose, we studied 40 individuals with cHEP-C for G-MDSC and M-MDSC frequencies in the peripheral blood and determined the suppressive effects of these cells in vitro in comparison to those in healthy controls (HC). Clinical data for the study subjects are shown in Table 1. The study was approved by the Institutional Review Board of the Ludwig-Maximilians-Universität, Munich, Germany, and we obtained written informed consent from all study subjects. The control groups consisted of 23 healthy volunteers as negative controls (i.e., HC) and 44 HIV-1 (cHIV-1)-infected, untreated patients as positive controls for G-MDSC (cHIV-1 data derived from our previous project [3]). Both control groups were matched for age. Phenotypic analysis of MDSC was performed by flow cytometry as described previously (3). Gating strategies were according to reference 3 for G-MDSC and reference 6 for M-MDSC (see Fig. S1 in the supplemental material).
TABLE 1.
Clinical data for study subjectsa
| Identification no. | Sex | HCV genotype | Viral load (IU/ml) | AST (U/liter) | ALT (U/liter) |
|---|---|---|---|---|---|
| H01 | m | 1 | 2,200,000 | 102 | 229 |
| H02 | f | 1 | 1,760,000 | 39 | 75 |
| H03 | f | 1 | 1,830,000 | 18 | 20 |
| H04 | m | 1 | 4,082,000 | 42 | 51 |
| H05 | f | 1 | 150,000 | 323 | 448 |
| H06 | m | 1 | 600,000 | 30 | 34 |
| H07 | f | 1 | 350,000 | 39 | 30 |
| H08 | f | 1 | 250,000 | 38 | 68 |
| H09 | f | 1 | 1,100,000 | 43 | 63 |
| H10 | m | 1 | 510,000 | 40 | 44 |
| H11 | m | 1 | 730,000 | 95 | 121 |
| H12 | m | 1 | 3,700,000 | 74 | 119 |
| H13 | f | 1 | 430,000 | 89 | 70 |
| H14 | m | 1 | 140,000 | 120 | 116 |
| H15 | f | 1 | 8,950,000 | 42 | 45 |
| H16 | m | 1 | 860,000 | 36 | 45 |
| H17 | f | 1 | 1,000,000 | 49 | 52 |
| H18 | f | 1 | 1 000,000 | 31 | 40 |
| H19 | f | 1 | 110,000 | 29 | 47 |
| H20 | m | 1 | 5,050,000 | 39 | 40 |
| H21 | m | 1 | 120,000 | 168 | 181 |
| H22 | m | 1 | 520,000 | 182 | 266 |
| H23 | m | 1 | 1,700,000 | 36 | 51 |
| H24 | m | 1 | 240,000 | 58 | 104 |
| H25 | f | 1 | 2,200,000 | 43 | 11 |
| H26 | m | 2 | 1,100,000 | 65 | 113 |
| H27 | m | 2 | 20,000,000 | 28 | 24 |
| H28 | m | 2 | 43,000 | 36 | 53 |
| H29 | f | 2 | 640,000 | 23 | 27 |
| H30 | m | 2 | 200,000 | 25 | 34 |
| H31 | f | 3 | 3,000 | 22 | 39 |
| H32 | f | 3 | 670,000 | 32 | 29 |
| H33 | m | 3 | 32,000 | 41 | 26 |
| H34 | m | 3 | 590,000 | 77 | 194 |
| H35 | f | 3 | 80,000 | 20 | 21 |
| H36 | m | 3 | 980,000 | 378 | 100 |
| H37 | f | 3 | 9,000,000 | 45 | 36 |
| H38 | m | 3 | 40,000 | 46 | 35 |
| H39 | m | 3 | 320,000 | 95 | 67 |
| H40 | m | 4 | 2,700,000 | 57 | 98 |
f, female (n = 17); m, male (n = 23). Normal ranges for AST and ALT were <35 U/liter for females and <50 U/liter for males. Boldface indicates liver enzyme levels above the normal range.
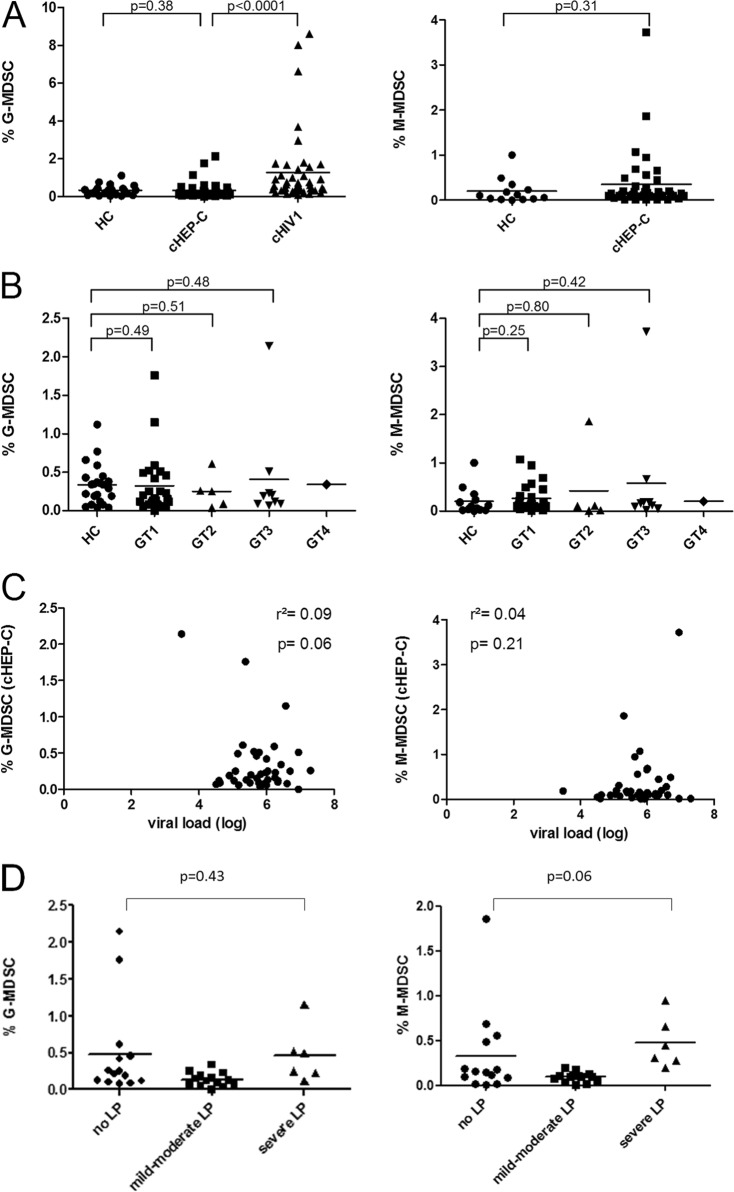
Examining the percentage of MDSC among freshly isolated peripheral blood mononuclear cells (PBMC) in 40 cHEP-C patients, we did not find a significant elevation of G-MDSC (CD11b+ CD14− CD15+ CD33+) or M-MDSC (CD11b+ CD14+ HLA-DRlow/−) compared to the levels in HC (P values of 0.38 and 0.31, respectively). In contrast, G-MDSC of cHIV-1 patients were significantly elevated compared to the levels in cHEP-C patients (P < 0.0001) (Fig. 1A). In addition, we did not find significant differences when stratifying into cHEP-C virus genotypes, neither between genotypes nor relative to HC. Correlations between G-MDSC and M-MDSC and viral load (r2 values of 0.09 [P = 0.06] and 0.04 [P = 0.21] for G-MDSC and M-MDSC, respectively) (Fig. 1C) or liver enzymes (r2 ≤ 0.02, P ≥ 0.34; data not shown) were not significant. Single subjects with elevated G-MDSC levels did not match subjects with elevated M-MDSC levels.
FIG 1.
MDSC in the peripheral blood of 40 chronically HCV-infected patients (cHEP-C patients). (A) Percentages of G-MDSC and M-MDSC of cHEP-C patients compared to those of healthy controls (HC) and patients with chronic HIV-1 infection (cHIV-1). G-MDSC levels of HIV patients were significantly elevated (P < 0.0001), whereas G-MDSC as well as M-MDSC levels of cHEP-C patients showed no difference relative to those of HC (P values of 0.38 and 0.31, respectively [Mann-Whitney test]). (B) Subdividing the patients by the particular HCV genotypes (GT) 1 to 4 did not yield significant differences relative to MDSC of HC, either (for GT1, G-MDSC P = 0.49 and M-MDSC P = 0.25; for GT2, G-MDSC P = 0.51 and M-MDSC P = 0.80; for GT3, G-MDSC P = 0.48 and M-MDSC P = 0.42 [Mann-Whitney test]). (C) G-MDSC- and M-MDSC-levels of cHEP-C did not correlate with individual viral load (for G-MDSC, r2 = 0.09 and P = 0.06; for M-MDSC, r2 = 0.04 and P = 0.21 [linear regression]). (D) There was no significant difference of MDSC frequencies between different stages of liver pathology (LP) measured by ultrasound (no liver pathology versus severe liver pathology for G-MDSC, P = 0.43, and for M-MDSC, P = 0.06 [Mann-Whitney test]).
Ultrasound data were obtained for 34 of the 40 study subjects. On the basis of the ultrasound results, we divided the patients into three groups: those with no liver pathology (n = 14), mild to moderate liver pathology (n = 14), and severe liver pathology (i.e., advanced fibrosis or cirrhosis; n = 6). However, there was still no statistically significant difference between G-MDSC or M-MDSC frequencies in patients with no liver damage and patients with liver damage (e.g., for patients with no liver pathology compared to patients with severe liver pathology, there were P values of 0.43 for G-MDSC and 0.06 for M-MDSC) (Fig. 1D).
Currently, MDSC in human diseases represent a highly studied but also controversial field of research. While no data for G-MDSC in cHEP-C exist so far, there are three studies concerning M-MDSC in peripheral blood and cHEP-C (6–8). Two of them reported increased M-MDSC frequencies in cHEP-C patients compared to HC levels, and one of the two found a positive correlation between M-MDSC levels and viral load (7, 8). However, both studies were small (n = 5 and 14, respectively) and either gave no data on clinical parameters or included mainly subjects with cHEP-C virus genotype 2. For our study, we were able to include only five subjects with genotype 2. However, four of them had very low M-MDSC levels (Fig. 1B). Our data are in concordance with very limited data by Hoechst et al., who found elevated levels of M-MDSC in patients with HCC but not in subjects with cHEP-C without HCC (6). One crucial point in studying human MDSC is certainly the methodology used. Based on a comparison of fresh and frozen samples, we are convinced that MDSC should be studied on freshly isolated cells. In addition, it has been clearly shown that freezing of PBMC influences MDSC frequency and functional properties (9, 10). Both studies reporting elevated M-MDSC frequencies in cHEP-C used frozen PBMC, which may explain the differences in the results.
As we did not find quantitative differences between MDSC of cHEP-C patients and HC, we postulated that MDSC isolated from these two groups differ in function. For this assessment, we used magnetic-bead-isolated G-MDSC. We decided on this subtype as our group has solid data on the suppressive activity of G-MDSC in HIV infection. In analogy to references 11 and 12, we used CD66b as the marker for isolation of G-MDSC. PBMC were stained with the EasySep human whole blood CD66b positive selection kit (Stemcell Technologies, France) according to the manufacturer's protocol. CD66b+ cells were positively selected in the magnet (purity, ≥60%). The supernatant out of the magnet (MDSC-depleted PBMC [PBMC-MDSC]) was used as a control in functionality assays (containing ≤0.05% G-MDSC) (see Fig. S2 in the supplemental material). In our coincubation experiments, allogeneic PBMC of healthy controls (i.e., targets) were stained with carboxyfluorescein succinimidyl ester (CFSE) as described previously (3). They were then stimulated with phytohemagglutinin (PHA) (1.25 μg/ml) and incubated alone or with bead-isolated MDSC or PBMC-MDSC (ratio = 2:1). Monensin (Golgi-Stop; BD) was added for the last 16 h of incubation. After 72 h, cells were stained with anti-CD8–peridinin chlorophyll protein (PerCP) externally and with anti-gamma interferon–allophycocyanin (APC) internally. Readouts for functionality of CD8 T cells were proliferation and gamma interferon production of target cells. Assays were done in parallel for G-MDSC of hepatitis C virus (HCV) patients and of healthy controls as effectors. For the proliferation assays (and gamma interferon production assays), we isolated G-MDSC of 4 different cHEP-C patients and performed 7 independent assays. We isolated G-MDSC of 3 different healthy controls and performed 5 independent assays.
As shown in Fig. 2, G-MDSC of cHEP-C patients and HC—again isolated from fresh PBMC—were equally able to suppress proliferative capacity and also gamma interferon production of CD8 T cells of the same healthy controls (i.e., targets) significantly relative to the levels obtained by incubation with cHEP-C/HC MDSC-depleted PBMC. The latter did not significantly alter proliferation compared to the positive control for which no additional cells, but PHA, were added. Another feature of MDSC with suppressive capacity is the increased expression of IL-4Rα (11, 13). However, we did not find significant differences between IL-4Rα expression levels on G-MDSC in the peripheral blood of cHEP-C patients and HC (P = 0.87; data not shown).
FIG 2.
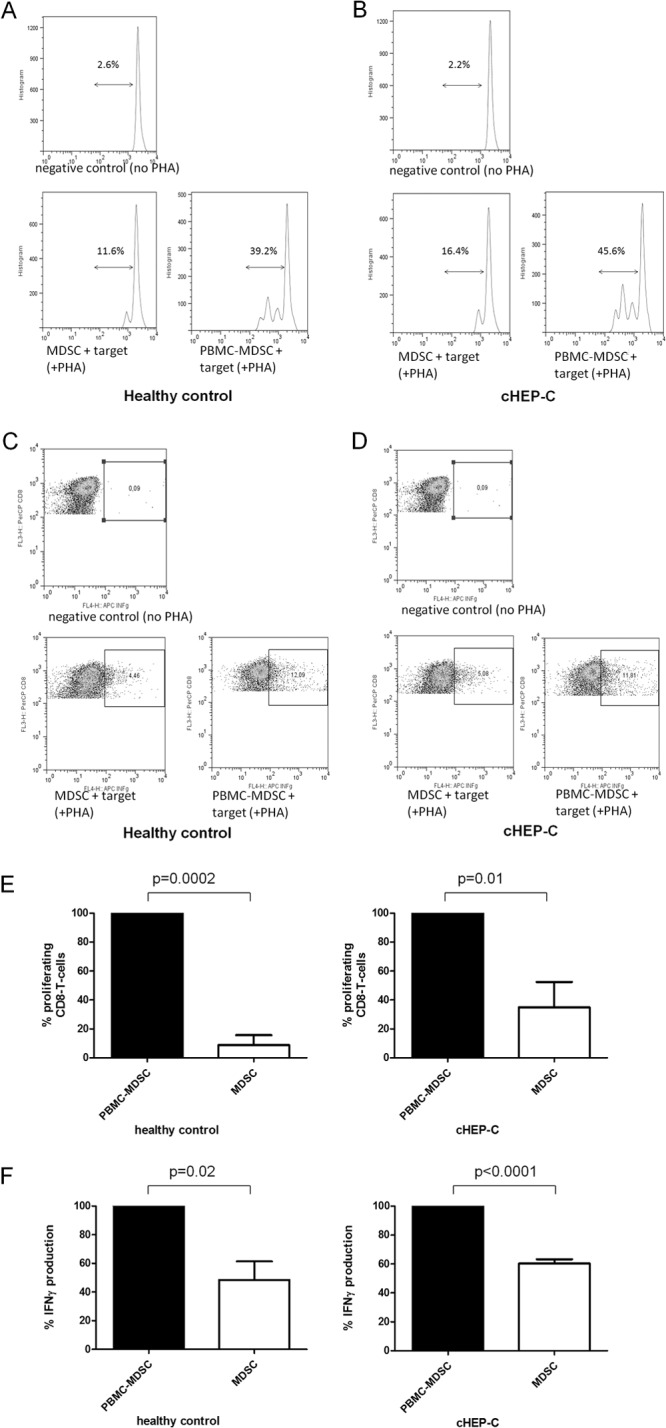
Suppressive function of G-MDSC of cHEP-C and healthy controls on CD8 T cells. (A and B) Representative histograms of proliferation assays as described below. (C and D) Representative dot plots of gamma interferon (INFg) production assays as described below. FL3-H, CD8-PerCP; FL4-H, gamma interferon-APC. (E) Proliferation of PHA-stimulated CD8 T cells of healthy controls as targets incubated with MDSC-depleted PBMC (PBMC-MDSC) or bead-isolated MDSC of healthy controls (left; P = 0.0002) and of cHEP-C patients (right; P = 0.01) (paired t test). Proliferation with MDSC-depleted PBMC was set as 100% and the proliferation with MDSC was calculated as a percentage thereof. (F) Gamma interferon (IFNγ) production of PHA-stimulated CD8 T cells of healthy controls as targets incubated with PBMC-MDSC or bead-isolated MDSC of healthy controls (left; P = 0.02) and of cHEP-C patients (right; P < 0.0001) (paired t test). Similarly, gamma interferon production with MDSC-depleted PBMC was set as 100% and gamma interferon production with MDSC was calculated as a percentage thereof.
Interestingly, in our study, G-MDSC isolated from healthy donors inhibited CD8 T cell function significantly. The conclusion would be that G-MDSC found in the peripheral blood have suppressive properties no matter what type of patient and the important parameter is the frequency of these cells. Supporting this hypothesis, all studies reporting MDSC found elevated MDSC frequencies in study subjects compared to those of healthy controls (2). Not much is known about MDSC in healthy subjects to date. Recently, it has been described that G-MDSC levels increase with age in healthy individuals (14) and that HLA-DR− CD14+ MDSC populations isolated from healthy donors can inhibit proliferation of autologous CD4 T cells (9). However, future studies are required in order to evaluate this in more detail.
This result is in clear contrast to the case for chronic HIV infection (3, 4). HIV infection affects the lymphoid tissue of the whole body, whereas cHEP-C is an infection which affects the liver in particular, and MDSC accumulation could be limited to the liver site. Future studies should therefore aim to study MDSC in liver tissue.
We conclude that neither G-MDSC nor M-MDSC in PBMC of cHEP-C patients show quantitative differences to those from HC. In addition, G-MDSC of cHEP-C are functionally active but not different from G-MDSC of HC. We therefore postulate that MDSC in the peripheral blood are most likely not significant regarding immune dysfunction in cHEP-C.
Supplementary Material
ACKNOWLEDGMENTS
We thank all study participants and the dedicated clinical staff at the hospital.
This work was supported by the Deutsche Forschungsgemeinschaft (German Research Association grant DR 424/3-1 to R.D.), the Friedrich-Baur-Stiftung (grant number 36/09 to R.D.), and BayImmuNet (grant F2–F5121.7.1.1/8/1 to R.D.).
Footnotes
Published ahead of print 16 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00113-14.
REFERENCES
- 1.Greten TF, Manns MP, Korangy F. 2011. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11:802–807. 10.1016/j.intimp.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khaled YS, Ammori BJ, Elkord E. 2013. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol. Cell Biol. 91:493–502. 10.1038/icb.2013.29 [DOI] [PubMed] [Google Scholar]
- 3.Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, Lechner A, Bourquin C, Draenert R. 2012. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS 26:F31–F37. 10.1097/QAD.0b013e328354b43f [DOI] [PubMed] [Google Scholar]
- 4.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, Liu Y, Yan D, Hu F, Guo P, Chen X, Chen L, Zhang H, Tang X, Zhou J. 2013. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J. Virol. 87:1477–1490. 10.1128/JVI.01759-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larrubia JR, Benito-Martinez S, Miquel J, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. 2009. Costimulatory molecule programmed death-1 in the cytotoxic response during chronic hepatitis C. World J. Gastroenterol. 15:5129–5140. 10.3748/wjg.15.5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. 2008. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135:234–243. 10.1053/j.gastro.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 7.Tacke RS, Lee HC, Goh C, Courtney J, Polyak SJ, Rosen HR, Hahn YS. 2012. Myeloid suppressor cells induced by hepatitis C virus suppress T cell responses through the production of reactive oxygen species. Hepatology 55:343–353. 10.1002/hep.24700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai W, Qin A, Guo P, Yan D, Hu F, Yang Q, Xu M, Fu Y, Zhou J, Tang X. 2013. Clinical significance and functional studies of myeloid-derived suppressor cells in chronic hepatitis C patients. J. Clin. Immunol. 33:798–808. 10.1007/s10875-012-9861-2 [DOI] [PubMed] [Google Scholar]
- 9.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. 2012. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J. Immunol. Methods 381:14–22. 10.1016/j.jim.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trellakis S, Bruderek K, Hutte J, Elian M, Hoffmann TK, Lang S, Brandau S. 2013. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun. 19:328–36. 10.1177/1753425912463618 [DOI] [PubMed] [Google Scholar]
- 11.Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, Schafer I, Wecker I, Neri D, Wirth A, Mays L, Zundel S, Fuchs J, Handgretinger R, Stern M, Hogardt M, Doring G, Riethmuller J, Kormann M, Hartl D. 2013. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J. Immunol. 190:1276–1284. 10.4049/jimmunol.1202144 [DOI] [PubMed] [Google Scholar]
- 12.Rieber N, Gille C, Kostlin N, Schafer I, Spring B, Ost M, Spieles H, Kugel HA, Pfeiffer M, Heininger V, Alkhaled M, Hector A, Mays L, Kormann M, Zundel S, Fuchs J, Handgretinger R, Poets CF, Hartl D. 2013. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin. Exp. Immunol. 174:45–52. 10.1111/cei.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P. 2009. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J. Immunol. 182:6562–6568. 10.4049/jimmunol.0803831 [DOI] [PubMed] [Google Scholar]
- 14.Verschoor CP, Johnstone J, Millar J, Dorrington MG, Habibagahi M, Lelic A, Loeb M, Bramson JL, Bowdish DM. 2013. Blood CD33(+)HLA-DR(-) myeloid-derived suppressor cells are increased with age and a history of cancer. J. Leukoc. Biol. 93:633–637. 10.1189/jlb.0912461 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.