ABSTRACT
Botryosphaeria dothidea is an important pathogenic fungus causing fruit rot, leaf and stem ring spots and dieback, stem canker, stem death or stool mortality, and decline of pear trees. Seven double-stranded RNAs (dsRNAs; dsRNAs 1 to 7 with sizes of 3,654, 2,773, 2,597, 2,574, 1,823, 1,623, and 511 bp, respectively) were identified in an isolate of B. dothidea exhibiting attenuated growth and virulence and a sectoring phenotype. Characterization of the dsRNAs revealed that they belong to two dsRNA mycoviruses. The four largest dsRNAs (dsRNAs 1 to 4) are the genomic components of a novel member of the family Chrysoviridae (tentatively designated Botryosphaeria dothidea chrysovirus 1 [BdCV1]), a view supported by the morphology of the virions and phylogenetic analysis of the putative RNA-dependent RNA polymerases (RdRps). Two other dsRNAs (dsRNAs 5 and 6) are the genomic components of a novel member of the family Partitiviridae (tentatively designated Botryosphaeria dothidea partitivirus 1 [BdPV1]), which is placed in a clade distinct from other established partitivirus genera on the basis of the phylogenetic analysis of its RdRp. The smallest dsRNA, dsRNA7, seems to be a noncoding satellite RNA of BdPV1 on the basis of the conservation of its terminal sequences in BdPV1 genomic segments and its cosegregation with BdPV1 after horizontal transmission. This is the first report of a chrysovirus and a partitivirus infecting B. dothidea and of a chrysovirus associated with the hypovirulence of a phytopathogenic fungus.
IMPORTANCE Our studies identified and characterized two novel mycoviruses, Botryosphaeria dothidea chrysovirus 1 (BdCV1) and Botryosphaeria dothidea partitivirus 1 (BdPV1), associated with the hypovirulence of an important fungus pathogenic to fruit trees. This is the first report of a chrysovirus and a partitivirus infecting B. dothidea and of a chrysovirus associated with the hypovirulence of a phytopathogenic fungus. BdCV1 appears to be a good candidate for the biological control of the serious disease induced by B. dothidea. Additionally, BdPV1 is placed in a clade distinct from the established genera. The BdCV1 capsid has two major structural proteins, and the capsid is distinct from that made up by a single polypeptide of the typical chrysoviruses. BdPV1 is the second partitivirus in which the putative capsid protein shares no significant identity with any mycovirus protein. A small accompanying dsRNA that is presumed to be a noncoding satellite RNA of BdPV1 is the first of its kind reported for a partitivirus.
INTRODUCTION
Mycoviruses, which are widespread in all major groups of plant-pathogenic fungi, are associated with latent infections of their hosts. Mycoviruses that debilitate the virulence of their phytopathogenic fungal hosts are valuable for the development of novel biocontrol strategies (1) and represent an important way to combat fungal diseases, as exemplified by the successful control of chestnut blight caused by virulent strains of Cryphonectria (Endothia) parasitica with hypovirulent strains of this pathogen from Europe (2–4). Recently, a growing number of this kind of mycoviruses has been reported (5): Rosellinia necatrix megabirnavirus 1 (RnMBV1) shows significant potential for the biological control of apple white root rot disease induced by Rosellinia necatrix, as does Sclerotinia sclerotiorum hypovirulence-associated DNA virus 1 (SsHADV-1) for the biological control of the diseases induced by Sclerotinia sclerotiorum (6, 7). Key to this purpose is to find mycoviruses that debilitate specific phytopathogenic fungi, because the natural host range of the former is limited to individuals within the same or closely related vegetative compatibility groups (1).
Pear is the third most important temperate fruit species, after grape and apple, and is widely cultivated on five continents, with major production taking place in China, the United States, Italy, Argentina, and Spain (8). Botryosphaeria dothidea (Moug.: Fr.) Cesati & De Notaris (anamorph, Fusicoccum aesculi Corda) is the causal agent of pear ring spot, an important disease characterized by ring spots on leaves and stems, fruit rot, dieback, stem canker, stem death or stool mortality, and decline. This fungus has a worldwide distribution and an extremely broad host range, being almost ubiquitous in endophytic communities of woody plants (9). The fungus causes damage to the host when the host is stressed by environmental conditions, competition, insect injury, or mechanical damage (10). At present, control of the disease is restricted to cultural and chemical approaches, with the long life span of woody plants adding further difficulties. Biocontrol measures may represent an important way to combat fungal diseases.
Apart from R. necatrix and Helicobasidium mompa, no other fungi attacking fruit trees have been reported to be infected by mycoviruses (6, 11–17). Here we report on a strain of B. dothidea (LW-1) isolated from a pear tree from Wuhan, China. On culture medium, LW-1 grows very slowly and with a sectoring phenotype, and on pear it is hypovirulent compared to the levels of virulence of other strains of B. dothidea. Seven double-stranded RNAs (dsRNAs) were detected in the mycelia of strain LW-1 but not in virulent strains, suggesting that strain LW-1 might be infected with one or more mycoviruses. Supporting this view, we have identified and characterized in strain LW-1 two novel mycoviruses, Botryosphaeria dothidea chrysovirus 1 (BdCV1) and Botryosphaeria dothidea partitivirus 1 (BdPV1), associated with the hypovirulence of this strain. Our results may provide a new approach for the biocontrol of pear ring spot disease.
MATERIALS AND METHODS
Fungal isolates and biological characterization.
Hypovirulent strain LW-1 and virulent strain HL-1 of Botryosphaeria dothidea were isolated from sandy pear trunks ( Pyrus pyrifolia Nakai cv. ‘Jinshuiyihao' and P. pyrifolia Nakai cv. ‘Hualiyihao', respectively) collected at the Fruit and Tea Research Institute, Agricultural Scientific Academy, Wuhan, Hubei Province, China. Strain LW-1 was further purified from a separately cultured single hyphal cell which was derived from mycelium protoplasts prepared as described in a previous report (18). Virus-free strain LW-1-9 was obtained from LW-1 by the hyphal tipping technique (19), with the absence of dsRNAs being assessed by agarose gel electrophoresis. Virulent strain JS-1 was isolated from a sandy pear trunk (P. pyrifolia Nakai cv. ‘Huanghua') collected in Nanjing, Jiangsu Province, China.
dsRNA extraction and purification.
For dsRNA extraction, the virulent strains were cultured on cellophane membranes on potato dextrose agar (PDA) plates for 4 to 5 days, and the hypovirulent strains with a low growth rate were cultured for 10 to 15 days. The mycelia were collected, ground to a fine powder in liquid nitrogen, and subjected to dsRNA extraction using a patented method developed in our labs (unpublished data). The dsRNA preparation was digested with DNase I and S1 nuclease (New England BioLabs), electrophoresed on a 1.2% agarose gel, and then visualized by staining with ethidium bromide. dsRNAs were separately excised and purified with a gel extraction kit (Qiagen), dissolved in diethyl pyrocarbonate-treated water, and kept at −70 °C until use.
cDNA synthesis and molecular cloning.
The cDNA sequence of genomic dsRNA was determined following a reported method (20), with some modification. Briefly, purified dsRNAs were subjected to cDNA synthesis using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega Corp., Madison, WI) with tagged random primer dN6 (5′-CGATCGATCATGATGCAATGCNNNNNN-3′). The cDNAs were amplified using the tagged oligonucleotide 5′-CGATCGATCATGATGCAATGC-3′ in combination with end filling with Taq (TaKaRa, Dalian, China). The amplified PCR products were cloned into the pMD18-T vector (TaKaRa, Dalian, China) and transformed into competent cells of Escherichia coli DH5α. Sequence gaps between clones were determined by reverse transcription-PCR (RT-PCR) using specific primers designed on the basis of the cDNA sequences obtained. The 5′- and 3′-terminal sequences of dsRNA were determined as previously described (21). Briefly, the 3′ terminus of each strand of dsRNA was ligated by use of the closed adaptor primer RACE-OLIGO with a phosphorylated (p) 5′ end and an NH2 3′ end [5′-p-GCATTGCATCATGATCGATCGAATTCTTTAGTGAGGGTTAATTGCC-(NH2)-3′] and T4 RNA ligase (New England BioLabs, Beijing, Ltd., China) at 16°C for 16 h, and the oligonucleotide-ligated dsRNA was reverse transcribed with M-MLV reverse transcriptase and 3 pmol of a primer complementary to the oligonucleotide used for the RNA ligation (primer oligo REV [5′-GGCAATTAACCCTCACTAAAG-3′]). The cDNA was amplified using another primer complementary to the RNA ligation oligonucleotide (primer O5RACE-2 [5′-TCACTAAAGAATTCGATCGATC-3′] or O5RACE-3 [5′-CGATCGATCATGATGCAATGC-3′]) and sequence-specific primers corresponding to the 5′- and 3′-terminal sequences of the dsRNA and also cloned as described above.
Sequencing was performed at the Nanjing Jinsirui Biotechnology Co., Ltd., China, and every nucleotide was determined by sequencing at least three independent overlapping clones in both orientations.
Virus purification from mycelia.
Approximately 30 g fresh mycelia was homogenized in a mixer with 200 ml of phosphate buffer (PB; 8.0 mM Na2HPO4, 2.0 mM NaH2PO4, pH 7.2) containing 10 mM MgCl2, 0.45% (wt/vol) sodium diethyldithiocarbamate trihydrate, and 10% (vol/vol) chloroform at room temperature and then cultured in PB at 28°C for 10 days. The homogenate was shaken at 150 rpm for 30 min at 10°C and centrifuged at 5,000 × g for 20 min. NaCl and polyethylene glycol 6000 were added to the resulting supernatant to final concentrations of 1% (wt/vol) and 8% (wt/vol), respectively, and the mixture was left at 4°C for 1 h and then centrifuged at 5,500 × g for 15 min at 4°C. The precipitate was resuspended in 10 mM PB and subsequently subjected to ultracentrifugation at 148,400 × g for 2 h (Optima LE-80K; Beckman Coulter, Inc.). The resultant sediment was resuspended in 10 mM PB and centrifuged in sucrose density gradients (100 to 500 mg/ml with intervals of 100 mg/ml) at 112,700 × g for 4 h. An aliquot of each fraction was mixed with an equivalent volume of chloroform, the mixture was briefly vortexed and centrifuged at 12,000 × g for 10 min, and the resulting supernatant was subjected to viral dsRNA precipitation with ethanol. The preparation was visualized by agarose gel electrophoresis after treatment with DNase I and S1 nuclease (New England BioLabs). The fractions containing viral dsRNAs were reultracentrifuged, and the pellets were resuspended in 200 μl 10 mM PB, stained with 2% (wt/vol) uranyl acetate, and observed with a transmission electron microscope (TEM; H7650; Hitachi).
SDS-PAGE and PMF analysis of viral proteins.
Proteins extracted from each fraction were analyzed by 12% SDS-PAGE with 25 mM Tris-glycine and 0.1% SDS. After electrophoresis, the gels were stained with Coomassie brilliant blue R-250 (Bio-Safe CBB; Bio-Rad). The protein bands on the gel were individually excised and subjected to peptide mass fingerprinting (PMF) analysis at Sangon Biotech (Shanghai) Co., Ltd., China, according to a previously reported method (22).
Horizontal transmission of hypovirulence traits.
The horizontal transmission of the hypovirulence traits of strain LW-1 was assessed according to a previous method (23). Strain LW-1 and LW-1-9 or HL-1 were dually cultured at 28°C for 7 days to allow the two colonies to contact each other in each dish (diameter, 9 cm); the dsRNA-containing hypovirulent strain LW-1 served as the donor, whereas virus-free strain LW-1-9 or HL-1 served as the recipient. After incubation of the contact cultures, mycelial agar plugs from the colony margin of strain LW-1-9 or HL-1 were placed on a fresh PDA plate, and three or four derived isolates were obtained from each recipient strain in the contact cultures. They were biologically characterized and their virulence was determined as described above in the section “Fungal isolates and biological characterization.” Parental strains LW-1, LW-1-9, and HL-1 were included as controls.
Growth rate and virulence assay.
Mycelial agar plugs (diameter, 5 mm) punched from the colony margin of a 5-day-old culture of each strain or isolate were placed on PDA in petri dishes (diameter, 9 cm) and incubated at 28°C in the dark for determination of the mycelial growth rate and for observation of the colony morphology in quadruplicate. The virulence of each strain was determined by inoculating detached fruits (in quadruplicate) or branches (in triplicate) of P. pyrifolia Nakai cv. ‘Hongxiangsu' according to a reported procedure, unless stated otherwise (1). Briefly, fruits or branches were inoculated with agar plugs of actively growing mycelia, placed in a Styrofoam chamber, and covered with a plastic membrane to keep a constant humid atmosphere (90% relative humidity) at between 25°C and 30°C. Noncolonized PDA discs were also inoculated and incubated in parallel as a control. The lesions developed from the inoculated samples were measured and photographed at 7 days postinoculation (dpi) for the inoculated fruits and 10 dpi for the inoculated branches.
Sequence analysis.
Sequence similarity searches were performed using the National Center for Biotechnology Information (NCBI) databases with the BLAST program. Multiple-sequence alignments of the nucleic and amino acid sequences were conducted using the MAFFT (version 6.85) program implemented at http://www.ebi.ac.uk/Tools/msa/mafft with default settings except for refinement with 10 iterations. The resulting data were shaded in GeneDoc software (24). Identity analyses were performed with the molecular evolutionary genetic analysis (MEGA; version 4) program (25). The phylogenetic trees for RNA-dependent RNA polymerase (RdRp) and capsid protein (CP) sequences were constructed as described by M. Nibert et al. (a taxonomic proposal for the family Partitiviridae by M. Nibert et al. in 2013; assigned code, 2013.001a-kkF [http://talk.ictvonline.org/files/proposals/taxonomy_proposals_fungal1/m/fung02/4734.aspx]). Briefly, trees were generated at the website http://www.hiv.lanl.gov/content/sequence/PHYML/interface.html using the LG substitution model, empirical equilibrium frequencies, a program-estimated invariant-proportion value of 0.013, a gamma-shape value of 1.509, and 4 rate categories. The starting trees were obtained by BioNJ, optimized by both branch length and tree topology, and improved according to the best of nearest-neighbor interchange (NNI) and subtree pruning and regrafting (SPR). Branch support values (in percent) were estimated by the approximate likelihood ratio test (aLRT) with SH-like criteria. The secondary structures of the terminal sequences of the dsRNAs were determined online at the website http://mfold.rna.albany.edu/?q=DINAMelt/Quickfold) (26). The open reading frame (ORF) was deduced using the DNAMAN DNA analysis software package (DNAMAN, version 6.0; Lynnon Biosoft, Montreal, Quebec, Canada).
Protoplast transfection.
Protoplast preparation and transfection were performed according to a previous method (22). Virus-free strain HL-1 was transfected with the purified virus particles of BdPV1 extracted from LW-1-9a or mixed particles of BdPV1 and BdCV1 extracted from strain LW-1. Mycelium colonies generated from the protoplasts were individually transferred to new PDA plates. The dishes were incubated at 28°C in the dark for 7 days, and the resulting cultures were screened for the presence of dsRNAs.
Data analysis.
The data were statistically analyzed using the SPSS Statistics (version 17.0) program (WinWrap Basic) with descriptive statistics, chi-square test, one-way analysis of variance, and the Tukey post hoc test, and P values of 0.05 were considered significant.
Nucleotide sequence accession numbers.
The sequences of the full-length cDNAs of dsRNAs 1 to 7 have been deposited in GenBank with accession numbers KF688736 to KF688742, respectively.
RESULTS
Strain LW-1 displays hypovirulence traits in vitro and in vivo.
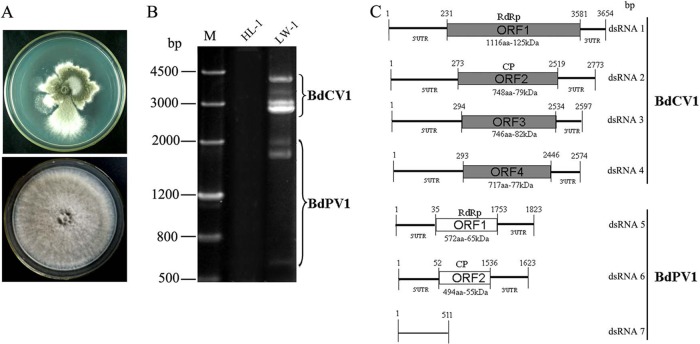
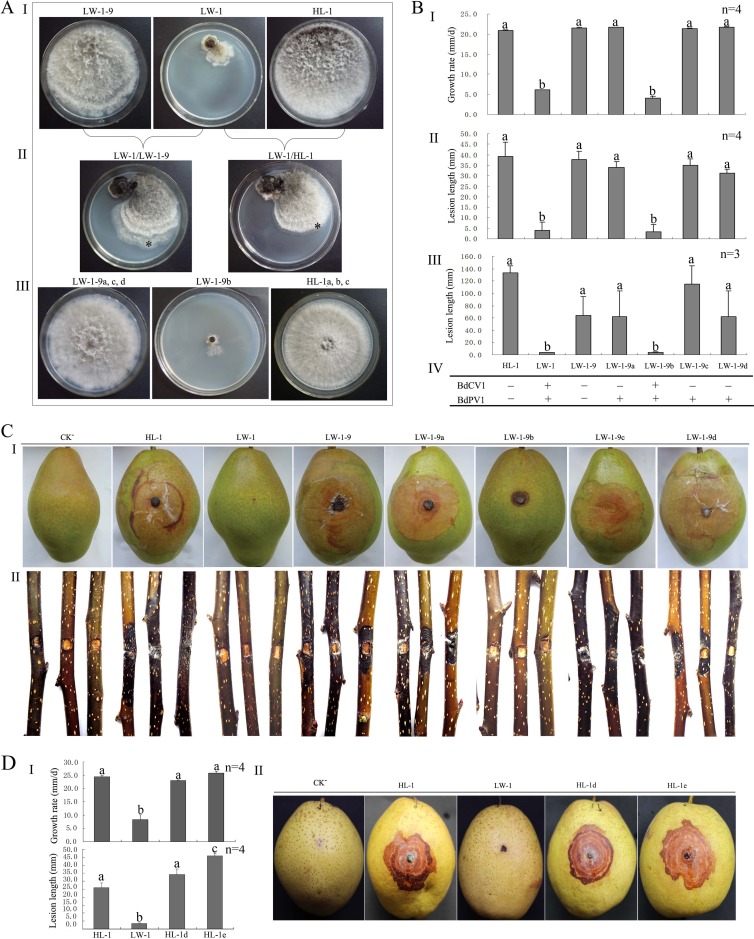
Compared to standard B. dothidea strains HL-1 and JS-1, LW-1 shows an abnormal phenotype with irregular colony margins with sectored regions (Fig. 1A, top). At 28°C in darkness, the in vitro growth rate of strain LW-1 was 1.5 mm per day, whereas the growth rates for the standard strains ranged from 20.8 to 21.9 mm per day. Importantly, strain LW-1 exhibited no or very weak virulence on sandy pear, with a lesion size of less than 5.0 mm on the fruits and branches, whereas for the standard strains, the lesion sizes were more than 30.0 mm on fruits and 60.0 mm on branches.
FIG 1.
Properties of the seven dsRNAs extracted from the mycelium of strain LW-1 of B. dothidea. (A) Colony morphologies of LW-1 (top) and HL-1 (bottom). (B) Electrophonic profiles on a 1.2% agarose gel of dsRNA preparations extracted from LW-1 and HL-1 after digestion with DNase I and S1 nuclease. Lane M, DNA size marker. (C) Genomic organization of dsRNAs 1 to 4 of BdCV1 and of dsRNAs 5 to 7 of BdPV1.
Strain LW-1 is associated with a complex pattern of dsRNAs.
To investigate whether one or more mycoviruses were responsible for the abnormal phenotype of strain LW-1, mycelia of this strain and strain HL-1 were subjected to dsRNA extraction, digestion with DNase I and S1 nuclease, and agarose gel electrophoresis. While seven dsRNAs (termed dsRNAs 1 to 7 according to their decreasing sizes) were detected in preparations of strain LW-1, no dsRNA was observed in preparations from strain HL-1 (Fig. 1B).
The sequences of the full-length cDNAs of dsRNAs 1 to 7 were determined by assembling partial-length cDNAs amplified from the purified dsRNAs using RT-PCR with tagged random primers and rapid amplification of cDNA ends (RACE) protocols.
Analysis of dsRNAs 1 to 4 reveals that they are the genomic components of a novel chrysovirus.
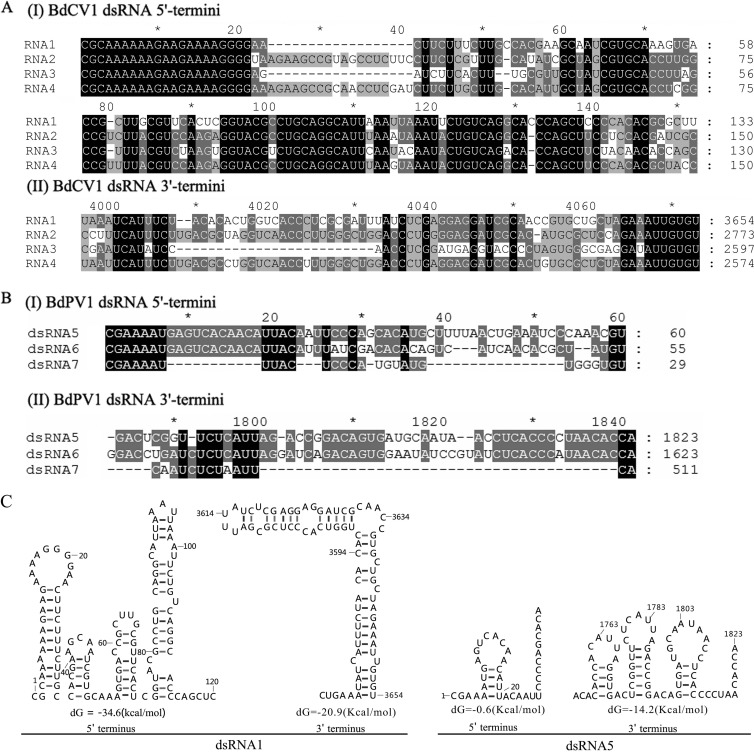
Sequence analysis of the full-length cDNAs of dsRNAs 1 to 4 showed that they were 3,654, 2,773, 2,597, and 2,574 bp, respectively, and that each contained a single ORF in one of the strands (Fig. 1C). The 5′ untranslated regions (UTRs) of the coding strands of dsRNAs 1 to 4 were 231, 273, 294, and 293 nucleotides (nt) long, respectively (Fig. 1C), and shared 53.3 to 72.5% identity, while the corresponding 3′ UTRs were 73, 254, 63, and 128 nt long, respectively (Fig. 1C), and shared 41.7 to 75% identity. Both termini of the coding strands of the four dsRNAs contained conserved sequences, 21 nt (CGCAAAAAAGAAGAAAAGGGG) at the 5′ termini and 7 nt (AUUGUGU) at the 3′ termini (Fig. 2A), and were predicted to fold into stable stem-loop structures, as illustrated Fig. 2C (left) for dsRNA1.
FIG 2.
Multiple-sequence alignments and predicted secondary structures for the terminal regions of the coding strand of dsRNAs of BdCV1 and BdPV1. (A and B) Conserved sequences of the 5′ termini (I) and 3′ termini (II) of the dsRNAs of BdCV1 and BdPV1, respectively. Black, gray, and light gray backgrounds, nucleotide identities of no less than 100%, 80%, and 60%, respectively. (C) Secondary structures proposed for dsRNAs 1 and 5 with the lowest energies (http://mfold.rna.albany.edu/?q=DINAMelt/Quickfold).
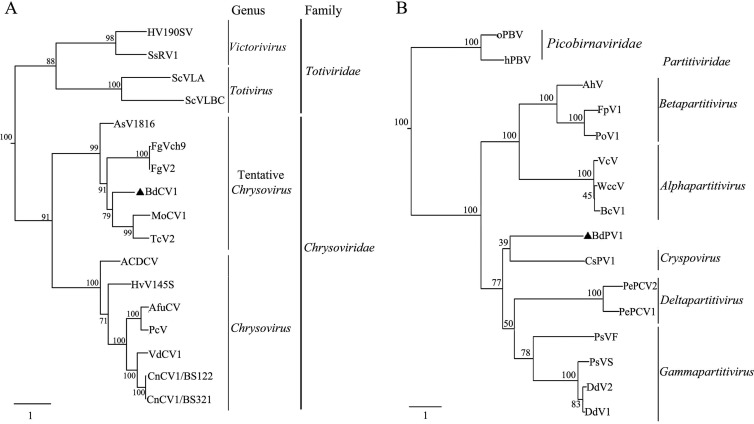
BLASTp searches of the deduced amino acid sequences of ORF1 unveiled the highest identity (45 to 35%) with the RdRps of some tentative members of the family Chrysoviridae. Specifically, alignment of the amino acid sequence of the putative RdRp encoded by ORF1 revealed the eight motifs conserved in members of the family Chrysoviridae (see Fig. S1A in the supplemental material) (27–30). Moreover, phylogenetic reconstruction of the complete sequence of the RdRp encoded by ORF1 with the RdRps of selected members of the families Totiviridae and Chrysoviridae indicated that the former clustered together with the tentative members of the family Chrysoviridae (Fig. 3A). In agreement with this view, BLASTp searches of the deduced amino acid sequences of ORFs 2 to 4 showed the highest identity (27%, 31%, and 33%, respectively) with the homologous ORFs of a tentative member (Magnaporthe oryzae chrysovirus 1 [MoCV1]) of the family Chrysoviridae. Based on this evidence, we propose that dsRNAs 1 to 4 are the genomic components of a novel chrysovirus designated Botryosphaeria dothidea chrysovirus 1 (BdCV1).
FIG 3.
Phylogenetic analysis of the RdRp sequences of BdCV1, BdPV1, and selected members of the families Totiviridae, Chrysoviridae, and Partitiviridae listed in Table 1. The phylogenetic trees for RdRp sequences for BdCV1 (A) and BdPV1 (B) were constructed completely according to M. Nibert et al. (2013). Two picobirnavirus sequences were used as the outgroups for phylogenetic analysis of BdPV1.
The genetic organization of dsRNAs 5 to 7 reveals their partitivirus-related origin.
Analysis of the full-length cDNA sequences of dsRNA5 and dsRNA6 showed that they were 1,823 and 1,623 bp, respectively, and that each contained a single ORF in the protein-coding strand (Fig. 1C). The 5′ UTRs of dsRNA5 and dsRNA6 were 35 and 52 nt long (Fig. 1C), respectively, and they shared 85.3% identity, while the corresponding 3′ UTRs were 70 and 87 nt long (Fig. 1C), respectively, and they shared 62.9% identity. More specifically, they contained the conserved sequences CGAAAAUGAGUCACAACAUUACA and CUCACCCMUAACACCA at their 5′ and 3′ termini, respectively (Fig. 2B). The 5′ UTRs of both dsRNAs are predicted to fold into unstable stem-loop structures, in contrast to the corresponding 3′ UTRs, which adopt stable stem-loop structures, as illustrated for dsRNA5 (Fig. 2C, right).
BLASTp searches of the deduced amino acid sequence of the dsRNA5 ORF1 revealed low identity (22 to 29%) with partial RdRps of members of the families Partitiviridae and Totiviridae and with those of a few unassigned mycovirus taxa; it also shared a similar low identity (23 to 28%) with polyproteins or NIb proteins of plant or animal viruses in the families Potyviridae, Caliciviridae, and Astroviridae (see Table S1 in the supplemental material). However, alignment of the putative RdRp encoded by dsRNA5 ORF1 revealed six motifs conserved in members of the family Partitiviridae (see Fig. S1B in the supplemental material) (31–33).
Moreover, a phylogenetic tree of the putative RdRp encoded by dsRNA5 ORF1 with the RdRps of representative members of the family Partitiviridae (Table 1) indicated that the former is clustered as a separate clade together with members of the five genera of the family Partitiviridae (Fig. 3B). BLASTp searches of the deduced amino acid sequence encoded by dsRNA6 ORF2 revealed no detectable sequence similarity with any mycovirus protein, while, remarkably, it displayed 46% identity and 59% similarity with a hypothetical protein of Exophiala dermatitidis (EHY58581.1; score, 343; E value, 2e−109; coverage, 91%).
TABLE 1.
Information on the virus isolates used for sequence alignment and phylogenetic analysis of their RdRpsa
| Virus name | Abbreviation | GenBank accession no. | Family | Genus |
|---|---|---|---|---|
| Magnaporthe oryzae chrysovirus 1 | MoCV1 | AB560761 | Chrysoviridae | Tentative Chrysovirus |
| Aspergillus mycovirus 1816 | AsV1816 | ABX79996 | Chrysoviridae | Tentative Chrysovirus |
| Helminthosporium victoriae 145S virus | HvV145S | YP_052858 | Chrysoviridae | Tentative Chrysovirus |
| Tolypocladium cylindrosporum virus 2 | TcV2 | CBY84993 | Chrysoviridae | Tentative Chrysovirus |
| Fusarium graminearum dsRNA mycovirus 2 | FgV2 | ADW08802 | Chrysoviridae | Tentative Chrysovirus |
| Fusarium graminearum dsRNA mycovirus China 9 | FgVch9 | ADU54123 | Chrysoviridae | Tentative Chrysovirus |
| Verticillium dahliae chrysovirus 1 | VdCV1 | ADG21213.1 | Chrysoviridae | Chrysovirus |
| Amasya cherry disease-associated chrysovirus | ACDCV | YP_001531163 | Chrysoviridae | Chrysovirus |
| Penicillium chrysogenum virus | PcV | YP_392482 | Chrysoviridae | Chrysovirus |
| Cryphonectria nitschkei chrysovirus 1 | CnCV1/BS321 | ACT79258 | Chrysoviridae | Chrysovirus |
| Cryphonectria nitschkei chrysovirus 1 | CnCV1/BS122 | ACT79255 | Chrysoviridae | Chrysovirus |
| Aspergillus fumigatus chrysovirus | AfuCV | CAX48749 | Chrysoviridae | Chrysovirus |
| Helminthosporium victoriae virus 190S | Hv190SV | NP_619670 | Totiviridae | Victorivirus |
| Sphaeropsis sapinea RNA virus 1 | SsRV1 | NP_047558 | Totiviridae | Victorivirus |
| Saccharomyces cerevisiae virus L-BC | ScVLBC | NP_042581 | Totiviridae | Totivirus |
| Saccharomyces cerevisiae virus L-A | ScVLA | NP_620495 | Totiviridae | Totivirus |
| White clover cryptic virus 1 | WccV | AAU14888 | Partitiviridae | Alphapartitivirus |
| Beet cryptic virus 1 | BcV1 | ACA81389 | Partitiviridae | Alphapartitivirus |
| Vicia cryptic virus | VcV | AAX39023 | Partitiviridae | Alphapartitivirus |
| Atkinsonella hypoxylon virus | AhV | AAA61829 | Partitiviridae | Betapartitivirus |
| Pleurotus ostreatus virus 1 | PoV1 | AAT07072 | Partitiviridae | Betapartitivirus |
| Fusarium poae virus 1 | FpV1 | AAC98734 | Partitiviridae | Betapartitivirus |
| Pepper cryptic virus 1 | PePCV1 | AEJ07890 | Partitiviridae | Deltapartitivirus |
| Pepper cryptic virus 2 | PePCV2 | AEJ07892 | Partitiviridae | Deltapartitivirus |
| Penicillium stoloniferum virus S | PsVS | YP_052856 | Partitiviridae | Gammapartitivirus |
| Discula destructiva virus 1 | DdV1 | NP_116716 | Partitiviridae | Gammapartitivirus |
| Discula destructiva virus 2 | DdV2 | NP_620301 | Partitiviridae | Gammapartitivirus |
| Penicillium stoloniferum virus F | PsVF | YP_271922 | Partitiviridae | Gammapartitivirus |
| Cryptosporidium parvum partitivirus | CsPV | O15925 | Partitiviridae | Cryspovirus |
| Human picobirnavirus | hPBV | AB517737 | Picobirnaviridae | Picobirnavirus |
| Otarine picobirnavirus | oPBV | AFJ79071 | Picobirnaviridae | Picobirnavirus |
The phylogenetic analysis of the RdRps is shown in Fig. 3, and the sequence alignment is presented in Fig. S1 in the supplemental material.
dsRNA7, of 511 bp, did not encode an ORF and had 5′ (CGAAAAU) and 3′ (CA) termini whose sequences were identical to those of dsRNA5 and dsRNA6. Its sequence shared no similarity with the sequences deposited in the NCBI database and those of the coinfecting dsRNAs, and it is predicted to fold into a highly branched secondary structure (data not shown).
Based on these analyses, we propose that dsRNA5 and dsRNA6 are the genomic components and dsRNA7 is a related dsRNA of a novel partitivirus, Botryosphaeria dothidea partitivirus 1 (BdPV1).
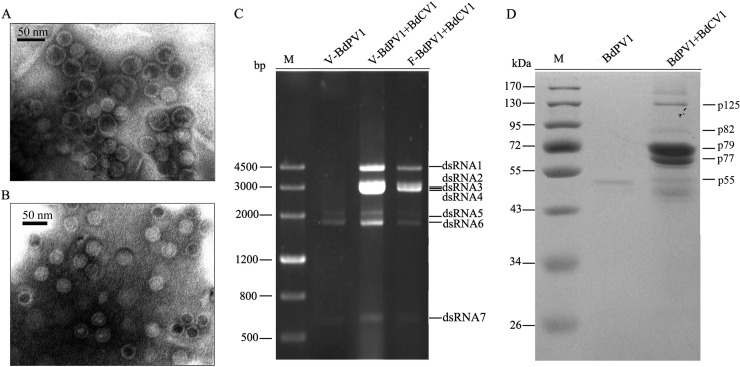
Sucrose gradient centrifugation reveals that dsRNAs 1 to 7 are encapsidated in two different kinds of virus-like particles.
To determine whether the dsRNAs associated with strain LW-1 were encapsidated, the presumed virus particles were analyzed by sucrose gradient centrifugation. Examination of the gradient fractions by TEM revealed two kinds of isometric virus-like particles with diameters of ∼35 and ∼40 nm in the fractions corresponding to 400 and 500 mg/ml sucrose, respectively (Fig. 4A). Agarose gel electrophoresis of the dsRNAs extracted from each fraction revealed that dsRNAs 1 to 7 were concentrated in the fractions containing the virus-like particles (Fig. 4C, lane V-BdPV1+BdCV1). SDS-PAGE analysis of the proteins of the fraction corresponding to 400 mg/ml of sucrose (containing both kinds of virus-like particles) revealed the presence of seven bands, five of which had estimated molecular masses of 125, 82, 72, 70, and 54 kDa, in very good agreement with the deduced molecular masses of 125, 82, 79, 77, and 55 kDa, respectively, for the proteins encoded by the ORFs of dsRNA1, dsRNA3, dsRNA2, dsRNA4, and dsRNA6, respectively (Fig. 4D, lane BdPV1+BdCV1).
FIG 4.
Virus-like particles, dsRNAs, and proteins extracted from the fraction corresponding to 400 mg/ml sucrose following sucrose gradient centrifugation. (A and B) Electron micrographs of virus-like particles purified from strain LW-1 coinfected by BdPV1 and BdCV1 (A) and from overcultured LW-1 or LW-1-9a containing only BdPV1 (B). (C) Agarose gel electrophoresis analysis of the dsRNAs extracted from purified virus-like particles of BdPV1 from overcultured LW-1 (lane V-BdPV1), mixed virus-like particles of BdPV1 and BdCV1 from LW-1 (lane V-BdPV1+BdCV1), and mycelia of strain LW-1 (lane F-BdPV1+BdCV1). V and F, dsRNAs extracted from virus-like particles and fungal mycelia, respectively. Lane M, DNA size marker. (D) SDS-PAGE analysis of proteins extracted from purified particles of BdPV1 from overcultured LW-1 (lane BdPV1 and mixtures of particles of BdPV1 and BdCV1 from LW-1 (lane BdPV1+BdCV1). Lane M, protein molecular mass marker.
When the analysis was extended to overcultured (about 1.5 months) mycelium of LW-1 in liquid medium or to the derived isolates with only BdPV1 obtained from the horizontal transmission assay (see below), only the ∼35-nm virus-like particles were observed by TEM (Fig. 4B), and only dsRNAs 5 to 7 were detected by agarose gel electrophoresis (Fig. 4C, lane V-BdPV1). Moreover, SDS-PAGE analysis of proteins from the purified particles revealed a major band of 54 kDa, similar to the size deduced for the capsid protein (CP; 55 kDa) of BdPV1 (Fig. 4D, lane BdPV1). When the extracted proteins were stored over a long time, e.g., more than 2 days at 4°C, an additional band corresponding to a protein with a molecular mass of about 50 kDa was observed (data not shown); this protein is most likely a degradation product of p55 and corresponds to the lowest band observed in the SDS-PAGE protein analysis of the sample containing BdCV1 and BdPV1 (Fig. 4D, lane BdPV1+BdCV1).
To further verify the presence of the deduced proteins on SDS-PAGE analysis, those two (p72 and p70) with sizes different from the deduced ones were purified and subjected to PMF analysis. The results showed that p72 and p70 generated a total of 18 and 17 peptide fragments, respectively (see Tables S2 and S3 in the supplemental material). Of these peptide fragments, the sequences of 12 fragments from p72 matched the partial sequence encoded by ORF2 of dsRNA2 delimited between amino acids 2 and 591, accounting for 25% of the entire coverage. The sequences of 14 peptide fragments from p70 matched the partial sequence encoded by ORF4 of dsRNA4 between amino acids 6 and 591, accounting for 21% of the entire coverage. All peptide fragments matched the deduced sequences with ions scores higher than 37 (see Tables S2 and S3 in the supplemental material); therefore, p72 and p70 were confirmed to correspond to the deduced 79- and 77-kDa proteins encoded by the ORFs of dsRNA2 and dsRNA4, respectively (Fig. 4D, lane BdPV1+BdCV1).
The hypovirulence of B. dothidea is associated with the horizontal transmission of BdCV1.
In contact cultures between strains LW-1 and LW-1-9 (a virulent subisolate obtained from LW-1 by hyphal tipping) and between LW-1 and HL-1 (also virulent), strains LW-1-9 and HL-1 grew rapidly and covered the entire plates after 7 days, like in single cultures, while strain LW-1 grew slowly and formed small colonies in dual or single cultures (Fig. 5AI and II).
FIG 5.
Horizontal transmission of BdCV1 and BdPV1, growth rate on PDA, and fruit lesion length and tests of virulence of different strains of B. dothidea on pear. (A) Colony morphology of LW-1, LW-1-9, and HL-1 in single culture (I) and contact culture (II) and of subisolates derived from the colony margins of the recipient strains (III). *, location from which a mycelial agar plug was removed for the generation of a derivative isolate for HL-1 or LW-1-9. (B) Histograms of growth rates of subisolates derived from the contact cultures and the parent strains (I), of the length of lesions induced on fruits (II) and branches (III) of pear (P. pyrifolia nakai cv. ‘Hongxiangsu'), and of the presence of BdPV1 and BdCV1 (IV). + and −, the presence and absence of BdPV1 or BdCV1, respectively, on the basis of the results of dsRNA detection by 1.2% agarose gel electrophoresis. (C) Virulence of HL-1, LW-1, and subisolates on fruits (I) and branches (II) of pear (P. pyrifolia nakai cv. ‘Hongxiangsu'). (D) Histograms of the growth rates of subisolates derived from protoplast transfection and the parent strains (I) and of the length of lesions (II) shown by virulence tests (III) on fruits of pear (P. bretschneideri Rehd. cv. ‘Mili'). CK−, treatments inoculated with noncolonized PDA plugs. Bars in each histogram labeled with the same letters are not significantly different (P > 0.05) according to the least-significant difference test.
Additionally, four mycelial derivative subisolates (LW-1-9a, LW-1-9b, LW-1-9c, and LW-1-9d) were obtained from four subcolonies of LW-1-9 in four contact cultures of LW-1/LW-1-9 (Fig. 5AIII). Isolate LW-1-9b was similar to donor strain LW-1 in mycelial growth on PDA (2.9 to 4.2 mm/day), morphological features (small colonies), and pathogenicity on pear (fruits and branches had lesions of 0 to 10 mm and 2.3 to 5.5 mm, respectively) (Fig. 5BI to III and C). However, the other three subisolates showed no significant difference in growth (21.25 to 21.81 mm/day) or virulence on pear (fruits and branches had lesions of 28.0 to 41.5 mm and 61.7 to 115.0 mm, respectively) from their parental strain, LW-1-9 (Fig. 5BI to III and C). Examination by agarose gel electrophoresis disclosed the presence of dsRNAs 1 to 4 and dsRNAs 5 to 7 (associated with BdCV1 and BdPV1, respectively) in LW-1-9b but only the dsRNAs associated with BdPV1 in subisolates LW-1-9a, LW-1-9c, and LW-1-9d (Fig. 5BIV). Therefore, BdCV1 and BdPV1 from strain LW-1 can be horizontally cotransmitted to strain LW-1-9 through hyphal contact, and the hypovirulence and impaired growth rate appear to be qualitatively correlated with this transmission. On the other hand, when only BdPV1 was horizontally transmitted to strain LW-1-9, no obvious change in virulence or phenotype was observed in the derivatives, thus indicating the major role of BdCV1 in the change of biological properties of B. dothidea.
In another experiment, three mycelial subisolates of strain HL-1 (HL-1a, HL-1b, and HL-1c) were obtained from the three colonies of strain HL-1 in contact cultures with LW-1 (Fig. 5A). Three subisolates were similar to parental strain HL-1 both in mycelial growth rate on PDA and in virulence on pear fruits and branches (Fig. 5BI to III and C). However, dsRNAs 1 to 7 were never detected in these subisolates or in their parental strain, HL-1 (Fig. 5BIV), thus confirming the association of at least some of these dsRNAs with the hypovirulence of B. dothidea.
Protoplast transfection.
As hypovirulence-associated dsRNAs could not be transmitted from strain LW-1 to strain HL-1, we tried to transfect protoplasts of HL-1 with the mixed particles of BdPV1 and BdCV1. Twenty-two smaller mycelium colonies generated from the protoplasts were individually cultured and screened for the presence of dsRNAs. The results indicated that except for the 3,654-bp dsRNA of BdCV1 that was transmitted to a derivative colony HL-1d together with BdPV1, BdCV1 was not transmitted to these derivative isolates, while BdPV1 was transmitted to seven derivative colonies. In the transfection with only the BdPV1 particles, 3 of 12 derivative colonies contained the expected dsRNAs, and 1 of them, HL-1e, was chosen as a representative isolate for further assays. A test for the mycelial growth rates of HL-1d (22.97 mm/day) and HL-1e (25.80 mm/day) revealed that they had growth rates similar to the growth rate of HL-1 (24.40 mm/day), which was considerably higher than that of LW-1 (8.35 mm/day). A pathogenicity test on pear fruits (Pyrus bretschneideri Rehd. cv. ‘Mili') for 5 days revealed that the lesions induced by HL-1e (45.91 mm) were significantly longer than those induced by LW-1 (6.00 mm), HL-1 (25.83 mm), and HL-1d (34.25 mm).
DISCUSSION
In this study, we also tried to isolate DNA of viral origin or single-stranded RNA of viral origin from B. dothidea strain LW-1, but neither of them was found in this strain (data not shown), suggesting the involvement of the mycoviral dsRNAs in the hypovirulence and abnormal phenotypes. Two virus-like particles (diameters, ∼35 and ∼40 nm) were purified (and visualized via TEM) from the mycelia of strain LW-1 coinfected by BdCV1 and BdPV1, while only the ∼35-nm particles were observed from the strain containing just BdPV1 (Fig. 4A and B). Additionally, dsRNAs 1 to 7 were recovered from preparations containing both particles, whereas only dsRNAs 5 to 7 were recovered from the ∼35-nm particles (Fig. 4C). Furthermore, proteins with sizes corresponding to those of the ORFs of BdCV1 and BdPV1 were revealed by SDS-PAGE or PMF analysis (see Tables S2 and S3 in the supplemental material), with the predicted CPs matching the major protein bands in the gel (Fig. 4D). Altogether these results support the suggestion that dsRNAs 1 to 4 are encapsidated in the ∼40-nm viral particles of BdCV1 and dsRNAs 5 to 7 are encapsidated in the ∼35-nm viral particles of BdPV1; their putative RdRps and CPs are encoded by the ORFs of dsRNAs 1 and 2 (BdCV1), respectively, and the ORFs of dsRNAs 5 and 6 (BdPV1), respectively. Two major structural proteins encoded by BdCV1 dsRNAs 2 and 4 suggest an icosahedral T=1 capsid consisting of 60 coat protein heterodimers. This situation is similar to that for some recently discovered dsRNA mycoviruses, such as Botrytis porri RNA virus 1 (BpRV1) (22) and quadriviruses (34) but distinct from that for typical chrysoviruses, including Penicillium chrysogenum virus (PcV) (35) and Cryphonectria nitschkei virus 1 (CnV1) (36), reported to have a T=1 capsid made up of 60 copies of a single polypeptide. Multiple protein components of chrysovirus-like viruses were also reported for MoCV1 (28, 37).
Therefore, on the basis of the dsRNA number, RdRp global amino acid sequence similarity, the presence of specific motifs, genomic organization, and virion size, BdCV1 and BdPV1 were identified as belonging to the families Chrysoviridae and Partitiviridae, respectively, according to the demarcation criteria for these mycoviruses (1). Phylogenetic analysis of the deduced RdRp of BdCV1 suggested that it is a new tentative member of the family Chrysoviridae that differs from the members of the genus Chrysovirus, a view further supported by the absence of the (CAA)n repeats at the 5′ UTRs of the genomic RNAs characteristic of typical members of this genus (27, 29). In the phylogenetic tree, BdCV1 is most closely related to MoCV1. Furthermore, we observed that while only BdPV1 remained in mycelia after prolonged culturing, coinfecting BdCV1 was not detected. This suggests that the behavior of BdCV1 is similar to that of MoCV1 that is present in culture medium after long culturing (28). It revealed that BdCV1 bears molecular and biological features similar to those of MoCV1, even though they were isolated from unrelated fungi.
Evaluation of the taxonomic status of BdPV1 revealed that it cannot be assigned to any known genera of the family Partitiviridae according to the new proposed criteria (see the taxonomic proposal for the family Partitiviridae by M. Nibert et al. in 2013; assigned code, 2013.001a-kkF [http://talk.ictvonline.org/files/proposals/taxonomy_proposals_fungal1/m/fung02/4734.aspx]). First, in the phylogenetic tree inferred from the RdRp amino acid sequences, BdPV1 forms a clade apart from representative members of the known genera (Fig. 3B; Table 1). Second, the RdRp amino acid sequence of BdPV1 shows low identity (10.7 to 21.7%) with the RdRp amino acid sequences of the other members of the family, and this level of identity is less than that (>24.7%) shared by the members within each genus (see Table S4 in the supplemental material). Third, the sizes of the dsRNAs and proteins from BdPV1 do not match the range of sizes characteristic of each known genus (see Table S5 in the supplemental material). Moreover, the RdRp of BdPV1 has a characteristic substitution (L instead of F) in one of the six conserved motifs (motif IV) (see Fig. S1B in the supplemental material), and a parallel analysis with the putative CP of BdPV1 produced essentially the same results (see Tables S4 and Fig. S2 in the supplemental material).
Together with Penicillium stoloniferum virus F (PsVF), BdPV1 is the only partitivirus whose putative CP shares no significant identity with any mycovirus protein (38). Consequently, BdPV1 appears to be separate from the other members of the family Partitiviridae in the phylogenetic tree of putative CP sequences (see Fig. S2 in the supplemental material), suggesting that the CP of BdPV1 might have a unique origin. Moreover, the CP of BdPV1 shares 46% amino acid sequence identity with a hypothetical protein of E. dermatitidis, suggesting that horizontal gene transmission may have occurred between the mycovirus and its host (39–41). Regarding dsRNA7, it is encapsidated in BdPV1 but lacks detectable identity with the coinfecting dsRNAs and with sequences deposited in the NCBI database, indicating that dsRNA7 is not a defective interfering RNA. However, unlike the small dsRNA F3 (670 bp, GenBank accession no. AY738338) of PsVF (38) and dsRNA4 (308 bp, GenBank accession no. AF316995) of Discula destructiva virus 1 (DdV1) (42) encoding a small putative protein, dsRNA7 encodes no protein, suggesting that it could be a noncoding satellite RNA. Although a noncoding satellite RNA (1,970 bp, no. L39127) had been detected in Atkinsonella hypoxylon virus (AhV) (43), it was deduced to encode five putative proteins ranging from 2.9 to 4.5 kDa using DNAMAN software.
It is worth noting that dsRNAs 1 to 7 are specifically encapsidated in their own viral particles, most likely because, like many other multipartite RNA viruses (6, 22, 27, 28, 38), they contain unique conserved terminal sequences playing an important role in packaging of viral RNA, in addition to transcription and replication (44, 45). BdCV1 dsRNAs contain the sequence CGCAAAAAAGAAGAAAAG at the 5′ termini, and this sequence is similar to that (GCAAAAAAGAGAAUAAAG) of MoCV1, a close tentative member of the genus Chrysovirus (28), and to that (GAUAAAAAAA) of some other members of this genus, e.g., Aspergillus fumigatus chrysovirus (AfuCV) (46), PcV (27), and Helminothosporium victoriae 145S virus (HvV145S) (47). BdCV1 dsRNAs also contain a defined sequence (GUGU) at their 3′ termini that is the same as that of the dsRNAs of PcV, AfuCV, and HvV145S. We therefore conclude that these 5′ and 3′ termini most likely mediate packaging of the genomic components of the proposed chrysovirus BdCV1. On the other hand, because dsRNAs 5, 6, and 7 contain identical sequences at their 5′ (CGAAAAU) and 3′ (CA) termini (Fig. 2B), we conclude that these conserved sequences act as a signal for guiding their copackaging in the virions of BdPV1 instead of BdCV1.
In the horizontal transmission experiments with contact culture, neither BdPV1 nor BdCV1 from strain LW-1 infected HL-1 of B. dothidea. This negative result might be due to the vegetative incompatibility between the different stains, as proposed before for BpRV1 (22). In contrast, the two viruses were successfully transmitted from strain LW-1 to LW-1-9. Coinfection by BdPV1 and BdCV1 resulted in significant alterations in growth rate, virulence, and phenotype, while infection by only BdPV1 induced no obvious changes to these biological features, suggesting that BdCV1 is responsible for the phenotypic alterations observed. In the transfection assays with purified virions, BdPV1 particles were transmitted to HL-1, while BdCV1 particles were not. The unsuccessful transfection was not due to the failure of transfection manipulations, as BdPV1 particles could be successfully transmitted to HL-1 when it was mixed with those of BdCV1. The reason for the unsuccessful transfection of BdCV1 needs further study, as there are no reports of successful chrysovirus transfection. The transfection assays using purified virions also indicated that BdPV1 has no attenuating effect on the hypovirulence of its host fungus. It further supports the suggestion that BdCV1 is closely associated with the hypovirulence of the phytopathogenic fungus, although we cannot eliminate the possibility of synergistic effects on the attenuation of virulence by the two viruses at this stage.
Many partitiviruses and chrysoviruses have been identified to infect phytopathogenic fungi, but few of them have been involved in the hypovirulence of their host fungi (1, 48–50). Some partitiviruses infect fruit-pathogenic fungi, e.g., Helicobasidium mompa virus (HmV) (6, 17, 51), Rosellinia necatrix partitivirus 1 (RnPV1) (17), RnPV2 (32), and tentative species RnPV3, RnPV4, and RnPV5 (52), while no chrysovirus is known to infect fruit-pathogenic fungi (12–17, 45). To our knowledge, this is the first report of a chrysovirus and a partitivirus infecting B. dothidea and the first report of a chrysovirus associated with the hypovirulence of a phytopathogenic fungus (50). This chrysovirus therefore appears to be a good candidate for the biological control of a serious disease induced by B. dothidea.
Supplementary Material
ACKNOWLEDGMENTS
This research was financially supported by the Chinese Ministry of Agriculture, Industry Technology Research Project (no. 201203034-02 and 200903004-05), and the National Natural Science Foundation of China (no. 31201488).
We declare that we have no competing interests.
We thank Ricardo Flores, Instituto de Biología Moleculary Celular de Plantas (UPV-CSIC), Valencia, Spain, for kind suggestions on the manuscript.
Footnotes
Published ahead of print 23 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.00538-14.
REFERENCES
- 1.Ghabrial SA, Suzuki N. 2009. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 47:353–384. 10.1146/annurev-phyto-080508-081932 [DOI] [PubMed] [Google Scholar]
- 2.Anagnostakis SL. 1982. Biological control of chestnut blight. Science 215:466–471. 10.1126/science.215.4532.466 [DOI] [PubMed] [Google Scholar]
- 3.MacDonald W, Fulbright D. 1991. Biological control of chestnut blight: use and limitations of transmissible hypovirulence. Plant Dis. 75:656–661 [Google Scholar]
- 4.Nuss DL. 1992. Biological control of chestnut blight: an example of virus-mediated attenuation of fungal pathogenesis. Microbiol. Mol. Biol. Rev. 56:561–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nuss DL. 2011. Mycoviruses, RNA silencing, and viral RNA recombination. Adv. Virus Res. 80:25–48. 10.1016/B978-0-12-385987-7.00002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba S, Salaipeth L, Lin YH, Sasaki A, Kanematsu S, Suzuki N. 2009. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 83:12801–12812. 10.1128/JVI.01830-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu X, Li B, Fu Y, Xie J, Cheng J, Ghabrial SA, Li G, Yi X, Jiang D. 2013. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. U. S. A. 110:1452–1457. 10.1073/pnas.1213755110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J, Wang Z, Shi Z, Zhang S, Ming R, Zhu S, Khan MA, Tao S, Korban SS, Wang H, Chen NJ, Nishio T, Xu X, Cong L, Qi K, Huang X, Wang Y, Zhao X, Deng C, Gou C, Zhou W, Yin H, Qin G, Sha Y, Tao Y, Chen H, Yang Y, Song Y, Zhan D, Wang J, Li L, Dai M, Gu C, Shi D, Wang X, Zhang H, Zeng L, Zheng D, Wang C, Chen M, Wang G, Xie L, Sovero V, Sha S, Huang W, Zhang M, Sun J, Xu L, Li Y, Liu X, et al. 2013. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 23:396–408. 10.1101/gr.144311.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slippers B, Wingfield MJ. 2007. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol. Rev. 21:90–106. 10.1016/j.fbr.2007.06.002 [DOI] [Google Scholar]
- 10.Sinclair WA, Lyon HH. 2005. Disease of trees and shrubs. Cornell University Press, Ithaca, NY [Google Scholar]
- 11.Matsumoto N. 1998. Biological control of root diseases with dsRNA based on population structure of pathogens. Jpn. Agric. Res. Q. 32:31–35 [Google Scholar]
- 12.Ikeda K, Nakamura H, Arakawa M, Matsumoto N. 2004. Diversity and vertical transmission of double-stranded RNA elements in root rot pathogens of trees, Helicobasidium mompa and Rosellinia necatrix. Mycol. Res. 108:626–634. 10.1017/S0953756204000061 [DOI] [PubMed] [Google Scholar]
- 13.Nomura K, Osaki H, Iwanami T, Matsumoto N, Ohtsu Y. 2003. Cloning and characterization of a totivirus double-stranded RNA from the plant pathogenic fungus, Helicobasidium mompa Tanaka. Virus Genes 26:219–226. 10.1023/A:1024453111809 [DOI] [PubMed] [Google Scholar]
- 14.Osaki H, Nakamura H, Sasaki A, Matsumoto N, Yoshida K. 2006. An endornavirus from a hypovirulent strain of the violet root rot fungus, Helicobasidium mompa. Virus Res. 118:143–149. 10.1016/j.virusres.2005.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Osaki H, Nomura K, Matsumoto N, Ohtsu Y. 2004. Characterization of double-stranded RNA elements in the violet root rot fungus Helicobasidium mompa. Mycol. Res. 108:635–640. 10.1017/S095375620400005X [DOI] [PubMed] [Google Scholar]
- 16.Sasaki A, Kanematsu S, Onoue M, Oyama Y, Yoshida K. 2006. Infection of Rosellinia necatrix with purified viral particles of a member of Partitiviridae (RnPV1-W8). Arch. Virol. 151:697–707. 10.1007/s00705-005-0662-2 [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A, Miyanishi M, Ozaki K, Onoue M, Yoshida K. 2005. Molecular characterization of a partitivirus from the plant pathogenic ascomycete Rosellinia necatrix. Arch. Virol. 150:1069–1083. 10.1007/s00705-005-0494-0 [DOI] [PubMed] [Google Scholar]
- 18.Kanematsu S, Arakawa M, Oikawa Y, Onoue M, Osaki H, Nakamura H, Ikeda K, Kuga-Uetake Y, Nitta H, Sasaki A, Suzaki K, Yoshida K, Matsumoto N. 2004. A reovirus causes hypovirulence of Rosellinia necatrix. Phytopathology 94:561–568. 10.1094/PHYTO.2004.94.6.561 [DOI] [PubMed] [Google Scholar]
- 19.Yaegashi H, Kanematsu S, Ito T. 2012. Molecular characterization of a new hypovirus infecting a phytopathogenic fungus, Valsa ceratosperma. Virus Res. 165:143–150. 10.1016/j.virusres.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Xie J, Xiao X, Fu Y, Liu H, Cheng J, Ghabrial SA, Li G, Jiang D. 2011. A novel mycovirus closely related to hypoviruses that infects the plant pathogenic fungus Sclerotinia sclerotiorum. Virology 418:49–56. 10.1016/j.virol.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Fu Y, Jiang D, Li G, Xie J, Peng Y, Yi X, Ghabrial SA. 2009. A novel mycovirus that is related to the human pathogen hepatitis E virus and rubi-like viruses. J. Virol. 83:1981–1991. 10.1128/JVI.01897-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu M, Jin F, Zhang J, Yang L, Jiang D, Li G. 2012. Characterization of a novel bipartite double-stranded RNA mycovirus conferring hypovirulence in the phytopathogenic fungus Botrytis porri. J. Virol. 86:6605–6619. 10.1128/JVI.00292-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Fu Y, Xie J, Jiang D, Li G, Yi X. 2009. A novel virus that infecting hypovirulent strain XG36-1 of plant fungal pathogen Sclerotinia sclerotiorum. Virol. J. 6:96. 10.1186/1743-422X-6-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholas KB, Nicholas HBJ, Deerfield DWI. 1997. GeneDoc: analysis and visualization of genetic variation. Embnew. News 4:14 [Google Scholar]
- 25.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599. 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- 26.Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415. 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang D, Ghabrial SA. 2004. Molecular characterization of Penicillium chrysogenum virus: reconsideration of the taxonomy of the genus Chrysovirus. J. Gen. Virol. 85:2111–2121. 10.1099/vir.0.79842-0 [DOI] [PubMed] [Google Scholar]
- 28.Urayama S, Kato S, Suzuki Y, Aoki N, Le MT, Arie T, Teraoka T, Fukuhara T, Moriyama H. 2010. Mycoviruses related to chrysovirus affect vegetative growth in the rice blast fungus Magnaporthe oryzae. J. Gen. Virol. 91:3085–3094. 10.1099/vir.0.025411-0 [DOI] [PubMed] [Google Scholar]
- 29.Covelli L, Coutts RHA, Di Serio F, Citir A, Acikgoz S, Hernández C, Ragozzino A, Flores R. 2004. Cherry chlorotic rusty spot and Amasya cherry diseases are associated with a complex pattern of mycoviral-like double-stranded RNAs. I. Characterization of a new species in the genus Chrysovirus. J. Gen. Virol. 85:3389–3397. 10.1099/vir.0.80181-0 [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Zhua X, Xiang Y, Li D, Yang J, Mao Q, Chen J. 2011. Genomic characterization of a novel dsRNA virus detected in the phytopathogenic fungus Verticillium dahliae Kleb. Virus Res. 159:73–78. 10.1016/j.virusres.2011.04.029 [DOI] [PubMed] [Google Scholar]
- 31.Bruenn JA. 1993. A closely related group of RNA-dependent RNA polymerases from double-stranded RNA viruses. Nucleic Acids Res. 21:5667–5669. 10.1093/nar/21.24.5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiba S, Lin YH, Kondo H, Kanematsu S, Suzuki N. 2013. Effects of defective interfering RNA on symptom induction by, and replication of, a novel partitivirus from a phytopathogenic fungus, Rosellinia necatrix. J. Virol. 87:2330–2341. 10.1128/JVI.02835-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghabrial SA. 1998. Origin, adaptation and evolutionary pathways of fungal viruses. Virus Genes 16:119–131. 10.1023/A:1007966229595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Y-H, Hisano S, Yaegashi H, Kanematsu S, Suzuki N. 2013. A second quadrivirus strain from the phytopathogenic filamentous fungus Rosellinia necatrix. Arch. Virol. 158:1093–1098. 10.1007/s00705-012-1580-8 [DOI] [PubMed] [Google Scholar]
- 35.Luque D, González JM, Garriga D, Ghabrial SA, Havens WM, Trus B, Verdaguer N, Carrascosa JL, Castón JR. 2010. The T=1 capsid protein of Penicillium chrysogenum virus is formed by a repeated helix-rich core indicative of gene duplication. J. Virol. 84:7256–7266. 10.1128/JVI.00432-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gómez-Blanco J, Luque D, González JM, Carrascosa JL, Alfonso C, Trus B, Havens WM, Ghabrial SA, Castón JR. 2012. Cryphonectria nitschkei virus 1 structure shows that the capsid protein of chrysoviruses is a duplicated helix-rich fold conserved in fungal double-stranded RNA viruses. J. Virol. 86:8314–8318. 10.1128/JVI.00802-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urayama S, Ohta T, Onozuka N, Sakoda H, Fukuhara T, Arie T, Teraoka T, Moriyama H. 2012. Characterization of Magnaporthe oryzae chrysovirus 1 structural proteins and their expression in Saccharomyces cerevisiae. J. Virol. 86:8287–8295. 10.1128/JVI.00871-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JW, Choi EY, Lee JI. 2005. Genome organization and expression of the Penicillium stoloniferum virus F. Virus Genes 31:175–183. 10.1007/s11262-005-1793-y [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Fu Y, Jiang D, Li G, Xie J, Cheng J, Peng Y, Ghabrial SA, Yi X. 2010. Widespread horizontal gene transfer from double-stranded RNA viruses to eukaryotic nuclear genomes. J. Virol. 84:11876–11887. 10.1128/JVI.00955-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreira D, López-García P. 2009. Ten reasons to exclude viruses from the tree of life. Nat. Rev. Microbiol. 7:306–311. 10.1038/nrmicro2108 [DOI] [PubMed] [Google Scholar]
- 41.Chiba S, Kondo H, Tani A, Saisho D, Sakamoto W, Kanematsu S, Suzuki N. 2011. Widespread endogenization of genome sequences of non-retroviral RNA viruses into plant genomes. PLoS Pathog. 7:e1002146. 10.1371/journal.ppat.1002146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rong R, Rao S, Scott SW, Carner GR, Tainter FH. 2002. Complete sequence of the genome of two dsRNA viruses from Discula destructiva. Virus Res. 90:217–224. 10.1016/S0168-1702(02)00178-8 [DOI] [PubMed] [Google Scholar]
- 43.Oh C-S, Hillman BI. 1995. Genome organization of a partitivirus from the filamentous ascomycete Atkinsonella hypoxylon. J. Gen. Virol. 76:1461–1470. 10.1099/0022-1317-76-6-1461 [DOI] [PubMed] [Google Scholar]
- 44.Attoui H, De Micco P, de Lamballerie X. 1997. Complete nucleotide sequence of Colorado tick fever virus segments M6, S1 and S2. J. Gen. Virol. 78:2895–2899 [DOI] [PubMed] [Google Scholar]
- 45.Wei CZ, Osaki H, Iwanami T, Matsumoto N, Ohtsu Y. 2003. Molecular characterization of dsRNA segments 2 and 5 and electron microscopy of a novel reovirus from a hypovirulent isolate, W370, of the plant pathogen Rosellinia necatrix. J. Gen. Virol. 84:2431–2437. 10.1099/vir.0.19098-0 [DOI] [PubMed] [Google Scholar]
- 46.Jamal A, Bignell EM, Coutts RH. 2010. Complete nucleotide sequences of four dsRNAs associated with a new chrysovirus infecting Aspergillus fumigatus. Virus Res. 153:64–70. 10.1016/j.virusres.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 47.Garibaldi D, Bertetti AP, Gullino ML. 2012. First report of fruit rot in pear caused by Botryosphaeria dothidea in Italy. Plant Dis. 96:910.1. 10.1094/PDIS-02-12-0130-PDN [DOI] [PubMed] [Google Scholar]
- 48.Nuss DL. 2005. Hypovirulence: mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 3:632–642. 10.1038/nrmicro1206 [DOI] [PubMed] [Google Scholar]
- 49.Pearson MN, Beever RE, Boine B, Arthur K. 2009. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 10:115–128. 10.1111/j.1364-3703.2008.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghabrial SA, Dunn SE, Li H, Xie J, Baker TS. 2013. Viruses of Helminthosporium (Cochlioblus) victoriae. Adv. Virus Res. 86:289–325. 10.1016/B978-0-12-394315-6.00011-8 [DOI] [PubMed] [Google Scholar]
- 51.Osaki H, Nomura K, Iwanami T, Kanematsu S, Okabe I, Matsumoto N, Sasaki A, Ohtsu Y. 2002. Detection of a double-stranded RNA virus from a strain of the violet root rot fungus Helicobasidium mompa Tanaka. Virus Genes 25:139–145. 10.1023/A:1020105701017 [DOI] [PubMed] [Google Scholar]
- 52.Yaegashi H, Nakamura H, Sawahata T, Sasaki A, Iwanami Y, Ito T, Kanematsu S. 2013. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. FEMS Microbiol. Ecol. 83:49–62. 10.1111/j.1574-6941.2012.01454.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.