Abstract
We report that primary human vaginal dendritic cells (DCs) display a myeloid phenotype and express CD4, CCR5, and CXCR4. Vaginal CD13+ CD11c+ DCs rapidly and efficiently bound transmitted/founder (T/F) CCR5-tropic (R5) viruses, transported them through explanted vaginal mucosa, and transmitted them in trans to vaginal and blood lymphocytes. Vaginal myeloid DCs may play a key role in capturing and disseminating T/F R5 HIV-1 in vivo and are candidate “gatekeeper” cells in HIV-1 transmission.
TEXT
Dendritic cells (DCs) play a critical role in HIV-1 transmission (1–12). We recently reported that DCs in human intestinal mucosa rapidly captured HIV-1, transported the virus through the intestinal lamina propria, and transmitted it in trans to peripheral blood and intestinal lymphocytes, implicating intestinal DCs in HIV-1 entry through gut mucosa (13). In contrast, surprisingly little is known about the role of human vaginal DCs in heterosexual HIV-1 transmission. Using primary human vaginal cells and explanted vaginal mucosa, we have shown that vaginal myeloid DCs (mDCs) efficiently capture, transport, and transmit transmitter/founder (T/F) viruses in trans to vaginal lamina propria and systemic lymphocytes, suggesting that vaginal DCs are candidate “gatekeeper” cells in heterosexual HIV-1 transmission.
Human vaginal lamina propria contains myeloid DCs that express HIV-1 receptors.
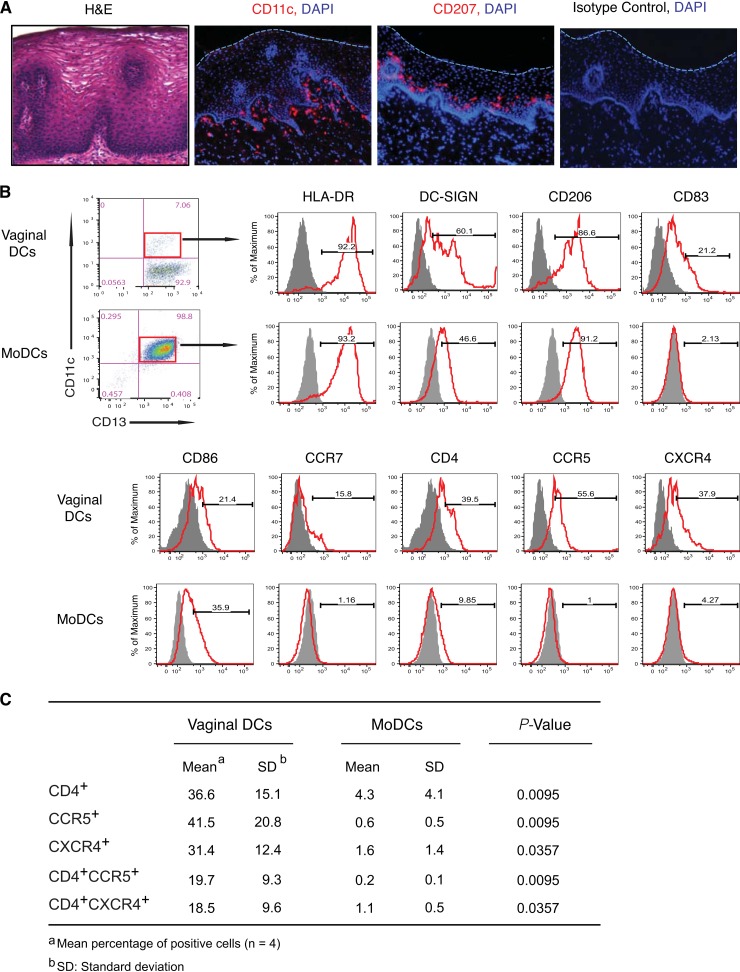
In normal vaginal mucosa from healthy women not receiving hormone therapy, CD11c+ DCs were located predominantly in the basilar lamina propria and infrequently in the epithelium, whereas CD207 (langerin)+ Langerhans cells were present predominantly in the lower region of the epithelium and not in the lamina propria (Fig. 1A). Among isolated vaginal mononuclear cells (MNLs) (12, 14) that expressed myeloid marker CD13, 9.1% ± 3.0% (n = 11) expressed DC marker CD11c, indicating a myeloid DC population. Most vaginal CD13+ CD11c+ DCs expressed HLA-DR, and a substantial proportion expressed DC-SIGN, mannose receptor CD206, maturation marker CD83, costimulatory molecule CD86, and lymph node homing receptor CCR7 (Fig. 1B). Importantly, vaginal DCs also expressed CD4 (36.6%), CCR5 (41.5%), and CXCR4 (31.4%), and a substantial proportion coexpressed CD4 plus CCR5 (19.7%) and CD4 plus CXCR4 (18.5%) (Fig. 1B and C). Thus, vaginal CD13+ CD11c+ DCs express receptors that bind HIV-1 (12, 15–18). Except for DC-SIGN, the percentage of vaginal DCs that express these receptors was significantly different from that of control monocyte-derived DCs (MoDCs) (n = 4; data not shown), underscoring the importance of using primary vaginal DCs in vaginal transmission studies.
FIG 1.
Tissue localization and phenotype of human vaginal DCs. (A) Vaginal DCs (CD11c+) and Langerhans cells (CD207+) display distinct localization in intact vaginal mucosa. H&E, hematoxylin and eosin; DAPI, 4′,6-diamidino-2-phenylindole. (B) CD13+ CD11c+ vaginal DCs display myeloid features and differ from control CD13+ CD11c+ monocyte-derived DCs (MoDCs) in the levels of receptor expression, maturation, and activation. Profiles are from a representative donor (n = 4). (C) Expression of HIV-1 receptor and coreceptor on vaginal DCs and MoDCs. MNLs were isolated from hysterectomized vaginal tissue from otherwise healthy women not receiving hormone therapy, stained with fluorescence-conjugated antibodies to CD11c and the indicated markers, and analyzed by flow cytometry by gating on the CD13+ CD11c+ population.
Vaginal myeloid DCs efficiently capture transmitted/founder (T/F) R5 virus.
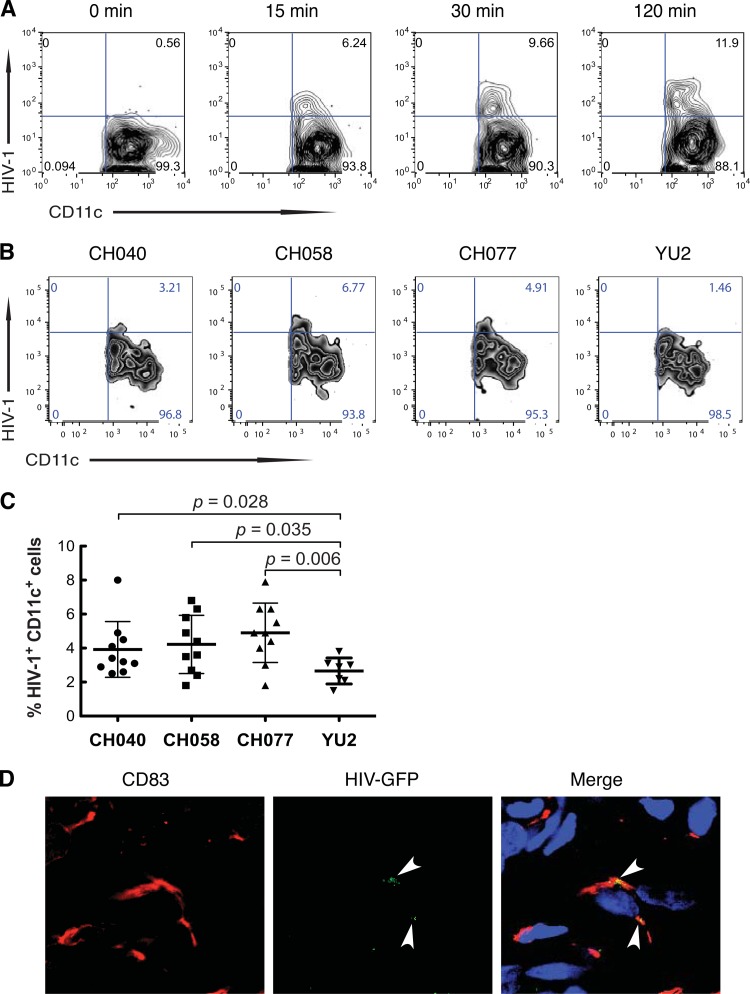
In cultures of freshly isolated human vaginal MNLs inoculated with YU2 Env-pseudotyped green fluorescent protein (GFP)-Gag virus-like particles (VLPs) (19) at a multiplicity of infection (MOI) of 10, VLPs were detected in 6.2% of CD13+ CD11c+ DCs at 15 min, increasing to 11.9% of the DCs at 2 h postinoculation by flow cytometry gating on the CD13+ cells for CD11c and GFP (Fig. 2A). Next, we determined the ability of isolated vaginal DCs to capture T/F viruses. To more closely mimic natural transmission, we used a predetermined optimal MOI of 0.1 (from MOI 0.01 to 1.0) for infectious T/F viruses. Among vaginal MNLs isolated from a representative donor, 3.21% to 6.77% of the CD11c+ DCs bound T/F viruses and 1.46% bound control YU2 at an optimal MOI of 0.1 at 2 h (Fig. 2B). The T/F viruses CH040, CH058, and CH077, which were used in these experiments, are subtype B viruses and were cloned from Fiebig stage II/III donors (20, 21). Vaginal CD11c+ DCs from 10 different donors captured T/F viruses CH040, CH058, and CH077 1.5-, 1.6-, and 1.8-fold more efficiently, respectively, than the capture of YU2 (P = 0.028, 0.035, and 0.006, respectively) (Fig. 2C), indicating that vaginal DCs efficiently captured T/F viruses. Interestingly, the magnitude of vaginal DC capture of T/F viruses is similar to the magnitude recently reported by Parrish and colleagues (22) for MoDC capture of T/F viruses. Finally, we investigated whether DCs in vaginal mucosa using tissue explants (13, 14, 23) could take up virus in situ. HIV-1–GFP VLPs inoculated onto explants were detected in cells expressing DC marker CD83 in vaginal mucosa 30 min postinoculation (Fig. 2D). Thus, both isolated and tissue vaginal DCs capture HIV-1.
FIG 2.
Vaginal myeloid DCs efficiently take up T/F R5 virus. (A) DCs among isolated vaginal MNLs capture HIV-1. Cultures of isolated vaginal MNLs were inoculated with YU2 envelope-pseudotyped GFP-Gag virus-like particles (VLPs) and incubated at 37°C. At 0, 15, 30, and 120 min postinoculation, cells were harvested and analyzed by flow cytometry gating on CD13+ cells for CD11c and GFP. (B and C) Vaginal myeloid DCs efficiently capture T/F R5 viruses. Vaginal MNLs from a representative donor (B) or 7 to 10 donors (C) were incubated for 2 h with T/F viruses CH040, CH058, and CH077 or control virus YU2 at an MOI of 0.1 and then analyzed by flow cytometry for CD13+ CD11c+ cells containing HIV-1, gating on CD13+ myeloid cells positive for KC57-FITC intracellular staining. Values in panel C are percent HIV-1+ CD13+ CD11c+ cells among vaginal MNLs from individual donors; bars correspond to means ± SD; P values were calculated by the Mann-Whitney test. (D) Vaginal myeloid DCs capture HIV-1 in explanted mucosa. YU2-GFP VLPs were inoculated onto the apical surface of explanted vaginal mucosa, and 30 min postinoculation, explants were harvested, sectioned, stained, and analyzed by confocal microscopy for CD83+ DCs containing GFP-tagged VLPs.
Vaginal myeloid DCs capture and transport HIV-1 through vaginal mucosa.
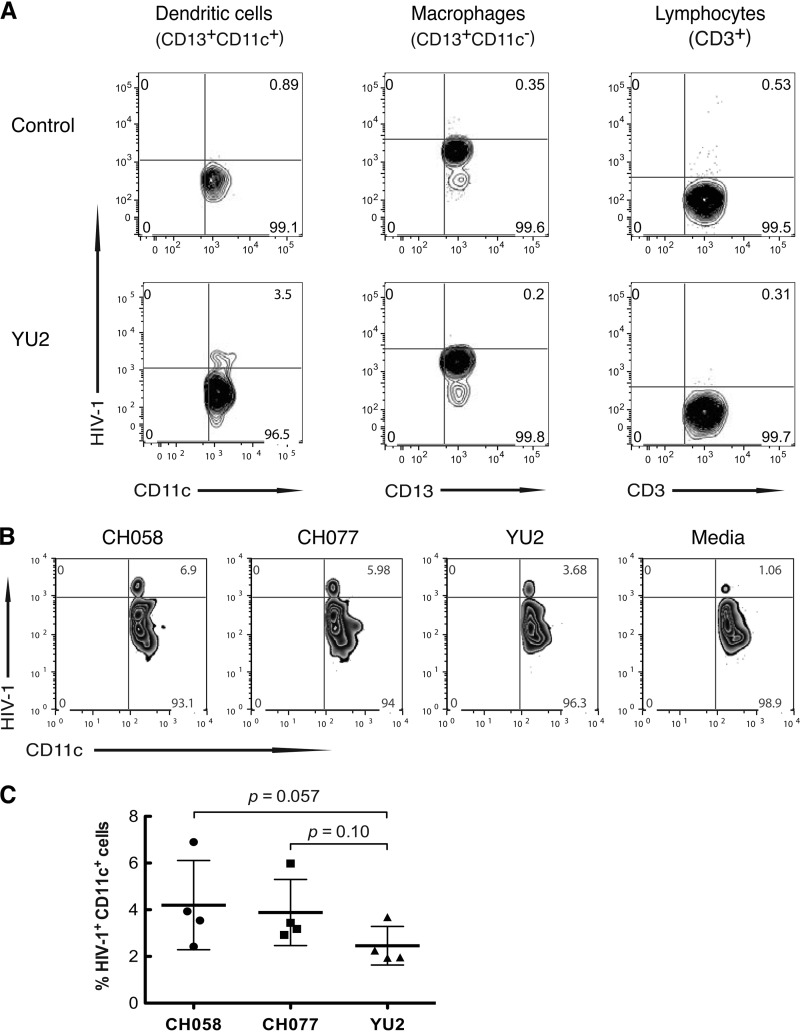
In contrast to nonmigrating Langerhans cells (24), mucosal DCs migrate to draining lymph nodes to present antigen to naive T cells (25). Therefore, we determined whether vaginal DCs could capture HIV-1 inoculated onto freshly constructed, leak-proof explants of vaginal mucosa, as we have previously described (14, 23), and transport the virus through the mucosa. YU2 or medium was inoculated onto vaginal explants, and 2 h later, the cells that had migrated through the tissue into the lower chamber were collected and analyzed for HIV-1 by flow cytometry gating on CD13+ CD11c+, CD13+ CD11c−, and CD3+ populations for DCs, macrophages, and lymphocytes, respectively, and using KC57-fluorescein isothiocyanate (FITC) to detect intracellular virus. Among the cells in the lower chamber, only CD13+ CD11c+ cells, and neither CD13+ CD11c− macrophages nor CD3+ lymphocytes, contained HIV-1 (Fig. 3A). The migrating CD13+ CD11c+ cells also expressed HLA-DR, DC-SIGN, CD206, CD83, CD86, and CCR7 (data not shown). Thus, the cells that capture and transport HIV-1 through vaginal mucosa within the first 2 h after inoculation are myeloid DCs. We next inoculated the apical surface of explants with T/F viruses CH058 and CH077 and 2 h later detected both viruses in CD13+ CD11c+ DCs in the lower chamber (Fig. 3B). In four separate vaginal tissues, DCs captured and transported T/F virus CH058 1.7-fold and CH077 1.6-fold more efficiently than chronic virus YU2 (Fig. 3C).
FIG 3.
Vaginal myeloid DCs capture and transport T/F HIV-1 through vaginal mucosa. (A) Vaginal myeloid DCs, not macrophages or lymphocytes, capture and transport HIV-1 through vaginal mucosa. YU2 was inoculated onto the apical surface of explanted vaginal mucosa, and after 2 h of incubation, the cells that had migrated into the lower chamber were harvested and analyzed by flow cytometry using antibodies CD11c-allophycocyanin (APC), CD13-APC, and CD3-phycoerythrin (PE) to identify CD13+ CD11c+ DCs, CD13+ CD11c− macrophages, and CD3+ lymphocytes, respectively, and KC57-FITC to detect intracellular virus. (B and C) Vaginal myeloid DCs capture and transport T/F R5 virus CH058 1.7-fold and CH077 1.6-fold more efficiently than control virus YU2 (n = 4; P = 0.057 and 0.10, Mann-Whitney test). Profiles in panel B were from a representative donor; values in panel C are from 4 donors, with means ± SD.
Vaginal myeloid DCs efficiently transmit T/F HIV-1 in trans to lymphocytes.
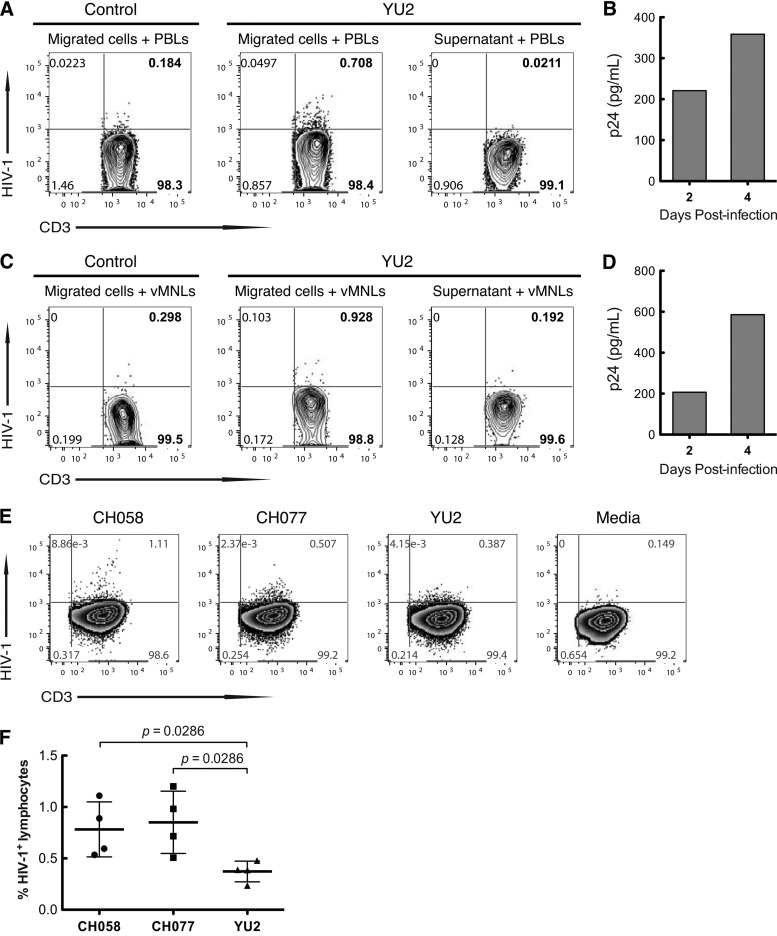
Having shown that mDCs are the only lamina propria cells that capture and transport T/F HIV-1 through explanted vaginal mucosa (Fig. 3), we next explored whether the DCs that migrated through the mucosa could transmit infectious virus to target mononuclear cells. YU2 HIV-1 was applied to the apical surface of vaginal explants, and 2 h later, cells that had migrated into the lower chamber were collected and cocultured with phytohemagglutinin (PHA)-stimulated heterologous peripheral blood lymphocytes (PBLs) or autologous vaginal lamina propria MNLs for up to 4 days, after which the cells were analyzed for HIV-1 replication by flow cytometry, using KC57-FITC intracellular staining of CD3+ T cells and p24 enzyme-linked immunosorbent assay (ELISA). Cells that migrated into the lower chamber of explants inoculated with YU2, but not supernatant or cells from medium-inoculated explants, infected both blood (Fig. 4A) and vaginal T cells (Fig. 4C) in trans, resulting in progressive p24 production in the cultures of migrated DCs plus either PBLs (Fig. 4B) or mucosal MNLs (Fig. 4D). Virus replication occurred exclusively in the PBLs and MNLs, since ≥95% of mucosal DCs die within 24 of culture (data not shown). Thus, infectious R5 virus inoculated onto explanted vaginal tissue was taken up by vaginal DCs and transported through the mucosa and trans infected systemic and mucosal mononuclear target cells. We performed the same trans infection assay with T/F virus or chronic R5 virus. Due to the small size of each vaginal specimen, only a limited number of explants were established, allowing evaluation of only two T/F viruses and one control virus per donor tissue. CD11c+ DCs in vaginal mucosa from four separate donors transmitted T/F viruses CH058 and CH077 2.0-fold and 1.9-fold, respectively, compared with transmission of the chronic virus YU2 (n = 4; P = 0.029), to blood lymphocytes (Fig. 4E and F). Thus, vaginal DCs efficiently captured and transported T/F virus through the mucosa and trans infected lymphocytes.
FIG 4.
Vaginal myeloid DCs that capture and transport T/F HIV-1 through vaginal mucosa efficiently transmit T/F viruses to lymphocytes. (A to D) Vaginal myeloid DCs transmit HIV-1 to peripheral blood lymphocytes (PBLs) and vaginal lymphocytes. YU2 was inoculated onto the apical surface of explanted vaginal mucosa, and 2 h later, the cells in the lower chamber were harvested and cocultured with PHA-stimulated PBLs or vaginal lamina propria MNLs. After 4 days of incubation, cells were analyzed by flow cytometry for HIV-1 replication using KC57-FITC intracellular staining and gating on CD3+ T cells (A and C). YU2 replication also was determined by p24 ELISA on days 2 and 4 (B and D). (E and F) Vaginal DCs transmitted T/F viruses CH058 and CH077 2.0-fold and 1.96-fold more efficiently, respectively, than control virus YU2 (n = 4; P = 0.029 and 0.029, Mann-Whitney test). The assay was performed with explanted vaginal mucosa as for panels A and B with the indicated viruses. Profiles in panel E are from a representative donor. Values in panel F are from 4 donors, with bars corresponding to means ± SD.
Here we used primary human vaginal cells and tissues to elucidate the role of vaginal DCs in HIV-1 transmission biology. Vaginal DCs captured HIV-1 severalfold more efficiently than MoDCs, possibly due to the higher expression level of receptors that bind HIV-1, and trans infected lymphocytes (2, 15–18). Vaginal myeloid DCs also captured T/F viruses more efficiently and transported T/F viruses through vaginal mucosa more efficiently than control YU2 virus. Further, vaginal myeloid DCs transmitted T/F viruses to lymphocytes with significantly higher efficiency than that for YU2. Together, these findings suggest that vaginal DCs play a key role in HIV-1 genital transmission and could contribute to the selection of founder virus in heterosexual HIV-1 transmission.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI093151 and AI106395 (to R.S.); grants AI083127, RR-20136, and DK064400 (Mucosal HIV and Immunobiology Center) (to P.D.S.); UAB Center for AIDS Research (CFAR) and Comprehensive Cancer Center Pilot Grant Program (to R.S.); Immunology, Autoimmunity and Transplantation Strategic Planning (IAT) and amfAR, the Foundation for AIDS Research 108015-49-RGRL (to P.D.S.); and the Research Service of the Veterans Administration (to P.D.S. and J.C.K.).
Footnotes
Published ahead of print 16 April 2014
REFERENCES
- 1.Piguet V, Steinman RM. 2007. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28:503–510. 10.1016/j.it.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L, KewalRamani VN. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859–868. 10.1038/nri1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teleshova N, Frank I, Pope M. 2003. Immunodeficiency virus exploitation of dendritic cells in the early steps of infection. J. Leukoc. Biol. 74:683–690. 10.1189/jlb.0403178 [DOI] [PubMed] [Google Scholar]
- 4.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215–225. 10.1084/jem.183.1.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J, Gardner MB, Miller CJ. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087–6095. 10.1128/JVI.74.13.6087-6095.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257–270. 10.1016/j.immuni.2007.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawamura T, Gulden FO, Sugaya M, McNamara DT, Borris DL, Lederman MM, Orenstein JM, Zimmerman PA, Blauvelt A. 2003. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc. Natl. Acad. Sci. U. S. A. 100:8401–8406. 10.1073/pnas.1432450100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reece JC, Handley AJ, Anstee EJ, Morrison WA, Crowe SM, Cameron PU. 1998. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J. Exp. Med. 187:1623–1631. 10.1084/jem.187.10.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen R, Richter HE, Smith PD. 1 April 2014. Interactions between HIV-1 and mucosal cells in the female reproductive tract. Am. J. Reprod. Immunol. 10.1111/aji.12244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. 2005. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J. Virol. 79:5762–5773. 10.1128/JVI.79.9.5762-5773.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. 2002. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J. Virol. 76:1866–1875. 10.1128/JVI.76.4.1866-1875.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen R, Richter HE, Smith PD. 2011. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am. J. Reprod. Immunol. 65:261–267. 10.1111/j.1600-0897.2010.00939.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen R, Smythies LE, Clements RH, Novak L, Smith PD. 2010. Dendritic cells transmit HIV-1 through human small intestinal mucosa. J. Leukoc. Biol. 87:663–670. 10.1189/jlb.0909605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. 2009. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J. Virol. 83:3258–3267. 10.1128/JVI.01796-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, Cornelissen ILMHA, Nottet HSLM, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587–597. 10.1016/S0092-8674(00)80694-7 [DOI] [PubMed] [Google Scholar]
- 16.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135–144. 10.1016/S1074-7613(02)00259-5 [DOI] [PubMed] [Google Scholar]
- 17.Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. 2002. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3:975–983. 10.1038/ni841 [DOI] [PubMed] [Google Scholar]
- 18.Freer G, Matteucci D. 2009. Influence of dendritic cells on viral pathogenicity. PLoS Pathog. 5:e1000384. 10.1371/journal.ppat.1000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forthal DN, Landucci G, Ding H, Kappes JC, Wang A, Thung I, Phan T. 2011. IgG2 inhibits HIV-1 internalization by monocytes, and IgG subclass binding is affected by gp120 glycosylation. AIDS 25:2099–2104. 10.1097/QAD.0b013e32834b64bd [DOI] [PubMed] [Google Scholar]
- 20.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. 2010. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology 408:1–13. 10.1016/j.virol.2010.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557. 10.1073/pnas.0802203105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, Hora B, Berg A, Cai F, Hopper J, Denny TN, Ding H, Ochsenbauer C, Kappes JC, Galimidi RP, West AP, Jr, Bjorkman PJ, Wilen CB, Doms RW, O'Brien M, Bhardwaj N, Borrow P, Haynes BF, Muldoon M, Theiler JP, Korber B, Shaw GM, Hahn BH. 2013. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. U. S. A. 110:6626–6633. 10.1073/pnas.1304288110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. 2010. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J. Immunol. 184:3648–3655. 10.4049/jimmunol.0903346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merad M, Ginhoux F, Collin M. 2008. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 8:935–947. 10.1038/nri2455 [DOI] [PubMed] [Google Scholar]
- 25.Coombes JL, Powrie F. 2008. Dendritic cells in intestinal immune regulation. Nat. Rev. Immunol. 8:435–446. 10.1038/nri2335 [DOI] [PMC free article] [PubMed] [Google Scholar]