ABSTRACT
The production of neutralizing antibodies (NAbs) is a correlate of protection for many human vaccines, including currently licensed vaccines against flaviviruses. NAbs are typically measured using a plaque reduction neutralization test (PRNT). Despite its extensive use, parameters that impact the performance of the PRNT have not been investigated from a mechanistic perspective. The results of a recent phase IIb clinical trial of a tetravalent dengue virus (DENV) vaccine suggest that NAbs, as measured using a PRNT performed with Vero cells, do not correlate with protection. This surprising finding highlights the importance of understanding how well the PRNT captures the complexity of the NAb response to DENV. In this study, we demonstrated that the structural heterogeneity of flaviviruses arising from inefficient virion maturation impacts the results of neutralization assays in a cell type-dependent manner. Neutralization titers of several monoclonal antibodies were significantly reduced when assayed on Vero cells compared to Raji cells expressing DC-SIGNR. This pattern can be explained by differences in the efficiency with which partially mature flaviviruses attach to each cell type, rather than a differential capacity of antibody to block infection. Vero cells are poorly permissive to the fraction of virions that are most sensitive to neutralization. Analysis of sera from recipients of live-attenuated monovalent DENV vaccine candidates revealed a strong correlation between the sensitivity of serum antibodies to the maturation state of DENV and cell type-dependent patterns of neutralization. Cross-reactive patterns of neutralization may be underrepresented by the “gold-standard” PRNT that employs Vero cells.
IMPORTANCE Cell type-dependent patterns of neutralization describe a differential capacity of antibodies to inhibit virus infection when assayed on multiple cellular substrates. In this study, we established a link between antibodies that neutralize infection in a cell type-dependent fashion and those sensitive to the maturation state of the flavivirus virion. We demonstrated that cell type-dependent neutralization reflects a differential capacity to measure neutralization of viruses that are incompletely mature. Partially mature virions that most efficiently bind maturation state-sensitive antibodies are poorly represented by assays typically used in support of flavivirus vaccine development. The selection of cellular substrate for neutralization assays may significantly impact evaluation of the neutralization potency of the polyclonal response. These data suggest that current assays do not adequately capture the full complexity of the neutralizing antibody response and may hinder the identification of correlates of protection following flavivirus vaccination.
INTRODUCTION
Flaviviruses are a group of single-stranded RNA viruses responsible for considerable annual mortality and morbidity. These viruses are responsible for a variety of severe diseases in humans that include encephalitis, hemorrhage, and shock. Members of this genus that significantly impact global health include yellow fever virus (YFV), dengue virus (DENV), Japanese encephalitis virus (JEV), and West Nile virus (WNV) (1). Many flaviviruses are emerging pathogens. WNV was first observed in the Western Hemisphere in the summer of 1999 and has since spread throughout the United States and into Canada and Central America. While WNV is now endemic in the United States, under favorable conditions, the potential exists for intense localized outbreaks associated with significant mortality (2, 3). More globally, DENV has spread extensively across tropical and subtropical regions during the last ∼80 years. Roughly one-third of the global population now lives in a region with endemic DENV; up to 390 million DENV infections occur each year (4). Fortunately, vaccines have proven to be effective at controlling flaviviruses. Vaccines against YFV, JEV, and tick-borne encephalitis viruses (TBEV) have been used extensively (5–7). In each instance, neutralizing antibodies have been established as a correlate of protection (8–10).
Flaviviruses are spherical particles composed of three viral proteins (capsid, premembrane [prM], and envelope [E]), a host-derived lipid membrane, and an ∼11-kb RNA genome of positive-sense polarity (11). On newly synthesized virions, viral E proteins are incorporated into the virus particle as 60 heterotrimeric complexes with the prM protein (12). The function of prM on immature virions is to prevent adventitious fusion of the virus particle during egress from infected cells (13). Cleavage of prM is the defining event of the virion maturation process (reviewed in reference 14). Genetic studies demonstrate that cleavage of at least some prM during transit through acidic compartments of the cellular secretory pathway is required for the production of infectious virions (15). In contrast to the “spiky” appearance of immature virions, mature virus particles released from cells are relatively smooth. The 180 copies of E protein on mature virions exist as antiparallel dimers that lay flat against the surface of the virus particle (16). Several lines of evidence indicate that the virion maturation process may be quite inefficient, resulting in the generation of infectious partially mature virus particles with structural features of both mature and immature virions (reviewed in reference 14).
Antibodies play an important role in the immune control of flavivirus infections via several mechanisms that include the direct neutralization of virus infectivity. The E protein is the principal target of neutralizing antibodies (17). The E protein ectodomain is composed of three structurally distinct domains connected by flexible hinges: a central domain I (DI) that is connected on one side to an elongated domain II (DII) containing a hydrophobic fusion loop (fl) and on the other side to domain III (DIII). DIII is an immunoglobulin-like fold structure hypothesized to participate in interactions with cellular factors (18). Neutralizing antibodies have been identified that bind all three domains of the E protein and may interfere with infection at several distinct steps of the virus entry process, including blocking virus attachment or viral membrane fusion (19–21). Antibodies that recognize prM protein have also been identified but are typically characterized by low neutralizing activity (22–24).
Assays that measure antibody-mediated neutralization are designed to capture the outcome of antibody occupancy of the virion. Virus neutralization is thought to occur once an individual virion is bound by a critical number of antibodies. The extent of virion maturation is one factor shown to govern how many antibodies bind the virion at a given concentration of antibody (25). In several instances, the results of neutralization assays have been shown to vary when the assays are performed on multiple cellular substrates (26–32). Interest in cell type-dependent patterns of neutralization is heightened by results of the first large-scale phase IIb clinical study of a tetravalent DENV vaccine (33). This live-attenuated vaccine was shown to elicit only modest protection when administered in dengue-experienced populations (34). Curiously, and in contrast to experience with other flavivirus vaccines, protection did not appear to correlate with neutralizing antibody titers as measured by a plaque reduction neutralization test (PRNT) using Vero cells. In this study, we detail the mechanism of cell type-dependent neutralization of flaviviruses and its significance for measuring the antibody response to vaccination.
MATERIALS AND METHODS
Immune sera from recipients of candidate flavivirus vaccines.
Sera from volunteers of phase I studies of candidate monovalent DENV serotype 1 (DENV1) (35) or DENV2 (36) vaccines were obtained for study. Clinical studies were conducted at the Center for Immunization, Johns Hopkins Bloomberg School of Public Health, under an investigational new-drug application reviewed by the U.S. Food and Drug Administration. The clinical protocol and consent forms were reviewed and approved by the NIAID Regulatory Compliance and Human Subjects Protection Branch, the NIAID Data Safety Monitoring Board, the Western Institutional Review Board, and the Johns Hopkins University Institutional Biosafety Committee (ClinicalTrials.gov identifiers NCT00473135 and NCT00920517).
Sera from recipients of a candidate WNV DNA vaccine were obtained for use in neutralization studies. A phase I single-site, open-label clinical study (NCT00106769) to evaluate the safety and tolerability of a recombinant nucleic acid vaccine has been described previously (37). The protocol and conduct of the clinical research adhered to the experimental guidelines of the U.S. Department of Health and Human Services and was approved by the NIAID Intramural Institutional Review Board. Sera from 12 subjects collected at 12 weeks after the final vaccination were selected for study because they were shown previously to exhibit patterns of neutralization sensitive to the maturation state of the virion (25).
For all three clinical studies, written informed consent was obtained from each participant in accordance with the Code of Federal Regulations (21 CFR 50) and International Conference on Harmonisation Guidelines for Good Clinical Practice (ICH E6).
Cell lines.
HEK-293T, BHK-21, and Vero cells were maintained in Dulbecco's modified Eagle medium (DMEM) containing Glutamax (Invitrogen, Carlsbad, CA) supplemented with 7% fetal bovine serum (FBS) (HyClone, Logan, UT) and 100 U/ml penicillin-streptomycin (PS) (Invitrogen, Carlsbad, CA). Raji cells expressing the flavivirus attachment factor DC-SIGNR (dendritic cell-specific ICAM-3-grabbing non-integrin-related protein) (Raji-DC-SIGNR) were maintained in RPMI medium containing Glutamax (Invitrogen, Carlsbad, CA) supplemented with 7% FBS and 100 U/ml PS. Adherent cell lines overexpressing human furin or DC-SIGNR (HEK-293T-FIRB and Vero-DC-SIGNR, respectively) were maintained in DMEM as described above but with the addition of 5 μg/ml blasticidin S (Invitrogen, Carlsbad, CA). All cells were maintained at 37°C in the presence of 7% CO2.
Generation of the HEK-293T-FIRB line.
A HEK-293T cell line that stably expresses human furin was generated by transfection with a bicistronic expression vector encoding furin and a drug resistance gene (pFIRB). pFIRB was generated by cloning a 966-bp fragment encoding the blasticidin S resistance gene under the translational control of foot-and-mouth disease virus internal ribosome entry site (IRES) into a human furin-expressing pcDNA3.1 vector. The resulting construct was transfected into HEK-293T cells. Furin-expressing clones were isolated by limiting dilution, screened, and characterized by immunoblotting using the furin-reactive antibody MON-152 (Enzo Life Sciences, Farmingdale, NY).
Generation of the Vero-DC-SIGNR line.
Vero cells were transfected with a previously described DC-SIGNR expression construct (38). Vero cells expressing DC-SIGNR were isolated by cell sorting. DC-SIGNR expression on Vero-DC-SIGNR cells was measured by flow cytometry using a phycoerythrin (PE)-conjugated DC-SIGNR-reactive monoclonal antibody (MAb), 120604 (R&D Systems, Minneapolis, MN).
WNV production.
WNV was produced using an infectious clone of WNV lineage I (NY99) engineered to express green fluorescent protein (GFP) as described previously (39). Virus stocks were produced by transfection of HEK-293T cells using Lipofectamine LTX (Invitrogen, Carlsbad, CA), followed by a single passage in HEK-293T cells to create working stocks of suitable scale. Virions were harvested, clarified through a 0.22-μm filter (Millipore, Billerica, MA), and frozen at −80°C until further use.
DENV reporter virus particle production.
Reporter virus particles (RVPs) were generated by genetic complementation of a WNV subgenomic replicon carrying a GFP reporter gene with the viral structural genes expressed from a DNA expression plasmid as described previously (40, 41). DENV serotype 2 strain 16681 (DENV2) RVPs were produced by transfecting HEK-293T cells with plasmids carrying the structural genes and WNV subgenomic replicon at a 3:1 ratio by mass. RVPs were harvested at 96 and 120 h posttransfection. Particles were clarified through a 0.22-μm filter (Millipore, Billerica, MA) and frozen at −80°C until further use.
Manipulation of the level of prM on virions by increasing or decreasing the efficiency of furin cleavage.
Standard preparations of DENV RVPs or infectious WNV retain detectable levels of uncleaved prM (25, 39). Homogenous populations of mature virions that retain reduced levels of uncleaved prM (WNV-Furin and DENV-Furin) were generated in HEK-293T-FIRB cells using the transfection methods described above (39, 40). To generate virus populations with markedly increased levels of uncleaved prM (WNV-prM and DENV-prM), the medium from infected or transfected cells was exchanged with medium containing 20 mM NH4Cl and 50 μM furin inhibitor Dec-RVKR-CMK (for WNV-prM) or 50 μM furin inhibitor Dec-RVKR-CMK (for DENV-prM RVPs) (Enzo Life Sciences, Farmingdale, NY).
The efficiency of prM cleavage in preparations of RVPs was determined by Western blotting as previously described (25, 39, 42). Briefly, RVPs were partially purified by ultracentrifugation through 20% sucrose. Pelleted viruses were lysed with buffer containing 1% Triton, 100 mM Tris, 2 M NaCl, and 100 mM EDTA and analyzed using E and prM protein-reactive MAbs. E protein was detected using the DII-fl-reactive MAb 4G2 at 1 μg/ml, whereas WNV prM was detected using a MAb recognizing residues 8 to 27 of the WNV M protein at 1 μg/ml (Abcam, Cambridge, MA). DENV prM was detected on Western blots using MAb GTX128093 (Genetex) at 1 μg/ml.
Calculation of specific infectivity of WNV on different cell types.
The infectious titer of WNV preparations was determined by infecting Raji-DC-SIGNR, Vero, and Vero-DC-SIGNR cells with serial dilutions of virus at 37°C for 16 h. Virus-infected cells were fixed and infection scored by flow cytometry as a function of GFP expression. The RNA content of each WNV stock was determined by quantitative real-time reverse transcription-PCR (qRT-PCR) as described previously (43). Briefly, WNV supernatant was incubated with 100 U of recombinant DNase I (Roche, Indianapolis, IN), and viral RNA was isolated using the QIAamp viral RNA kit (Qiagen, Valencia, CA). Amplification of viral genomic RNA was accomplished using the Superscript III one-step RT-PCR system (Invitrogen, Carlsbad, CA) and primers specific for the 3′ untranslated region of WNV (43).
To calculate the specific infectivity of a virus preparation, infectivity data obtained during virus titration experiments were plotted against the number of RNA copies present in each dilution of virus studied. Specific infectivity was calculated as the number of RNA molecules required for each infection event. Only linear segments of the virus titration curve were used in calculations. Comparisons of specific infectivity were performed using a pairwise t test (GraphPad Prism; GraphPad Software Inc., San Diego, CA).
Virus binding assays.
Virus binding to cells was quantified by measuring the cell-associated viral RNA as described previously (41). Target cells were pretreated with 30 mM NH4Cl to prevent virus fusion and genomic RNA replication. Virus was incubated with cells at 37°C for 3 h to allow virus-cell binding to occur; this time interval has been shown previously to allow for maximal binding to cells at this assay volume/temperature (41). Thereafter, the cells were washed twice with phosphate-buffered saline (PBS) supplemented with 30 mM NH4Cl and lysed. Total RNA was isolated using the RNeasy minikit (Qiagen, Valencia, CA), and the amount of viral RNA was determined using qRT-PCR. Comparisons of the extent of virus binding on each cell type were performed using an unpaired t test (GraphPad Prism; GraphPad Software, San Diego, CA).
Neutralization studies.
Neutralization assays were performed using Raji-DC-SIGNR, BHK-21, Vero, and Vero-DC-SIGNR cells. Neutralization assays were performed as previously described (25, 41, 44). Briefly, target cells were plated in 96-well flat-bottom plates overnight. The next day, serial dilutions of MAbs or immune sera were incubated with DENV RVPs or infectious WNV for 1 h at room temperature. The resulting immune complexes were then added to the cells and incubated for 48 or 16 h for DENV RVPs or infectious WNV, respectively. Infection was monitored as a function of GFP expression as measured using flow cytometry. The neutralization-resistant fraction of virions and the 50% effective concentrations (EC50) were predicted by a nonlinear regression analysis. Comparisons of neutralization potency and the resistant fraction of virions between cell types were performed using a pairwise t test (GraphPad Prism; GraphPad Software Inc., San Diego, CA).
Neutralization titer values for assays with WNV and DENV vaccine sera were predicted by analyzing the dose-response profiles using a nonlinear regression analysis. Comparisons of the neutralization titers of WNV and DENV immune sera were performed using an unpaired t test (GraphPad Prism; GraphPad Software Inc., San Diego, CA).
RESULTS
Cell type-dependent neutralization of WNV.
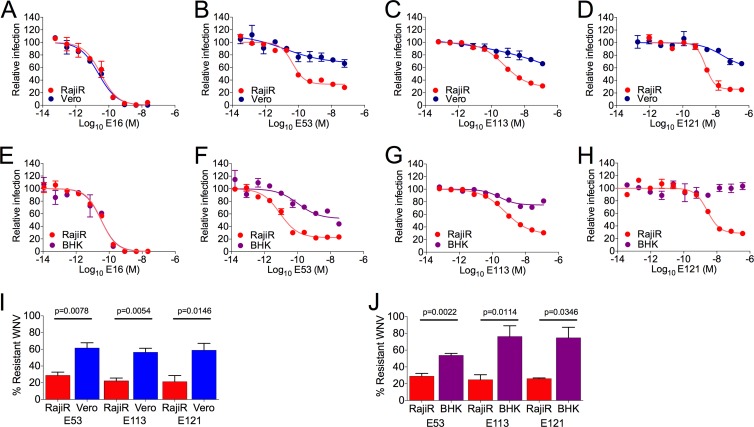
Antibody-mediated neutralization of viruses may be influenced by the cellular substrates used in in vitro assays, including those employed in the evaluation of vaccines (26–32). To investigate the underlying mechanism for cell type-dependent neutralization of flaviviruses, we studied the potency of previously characterized monoclonal antibodies (MAbs) on multiple cell types (25, 31). Serial dilutions of MAb were mixed with WNV for 1 h at room temperature and then added to either Vero or BHK-21 cells or a Raji B-cell line expressing DC-SIGNR (Raji-DC-SIGNR) (44). The virus used in these studies was an infectious GFP-expressing lineage I WNV produced in HEK-293T cells (39). MAb E16 is a potently neutralizing WNV-specific antibody that binds the lateral ridge (lr) of E-DIII (45, 46). The neutralization potency of E16 was not significantly different when assayed on these two cell types (Fig. 1A) (P = 0.225, n = 5). Similar results were obtained with BHK-21 cells (Fig. 1E) (P = 0.59, n = 4). In contrast, MAbs E53 (E-DII-fl), E113 (E-DII), and E121 (E-DI-lr) exhibited significantly reduced neutralization activity when assayed on Vero cells compared to Raji-DC-SIGNR cells (Fig. 1B, C, and D). In each instance, inspection of antibody dose-response curves revealed significant differences in the size of the population of virions resistant to neutralization; this was estimated using the size of the fraction of virions resistant to neutralization at saturating concentrations of antibody (25). For example, the fraction of virions resistant to neutralization by MAb E53 was roughly 2-fold greater when assayed using Vero cells then when using Raji-DC-SIGNR cells (61% and 29%, respectively; n = 5, P = 0.0078) (Fig. 1B and I). Similar results were observed in comparisons of BHK-21 and Raji-DC-SIGNR cells (53% and 28%, respectively; n = 4, P = 0.0022) (Fig. 1F and J) and also with MAbs E113 (Fig. 1C, G, I, and J) and E121 (Fig. 1D, H, I, and J).
FIG 1.
Cell type-dependent neutralization of WNV. A GFP-expressing variant of an infectious lineage I WNV (39) was incubated with serial 5-fold dilutions of the indicated MAbs for 1 h at room temperature and then added to Vero, BHK-21 (BHK), or Raji-DC-SIGNR (RajiR) cells. Infectivity was measured as a function of GFP expression at 16 h postinfection using flow cytometry. Data were analyzed using nonlinear regression (GraphPad Prism). (A to H) Antibody dose-response curves generated with the DIII-lr-specific MAb E16 (A and E), the DII-fl-specific MAb E53 (B and F), the DII-hinge interface-specific MAb E113 (C and G), and the DI-lr-specific MAb E121 (D and H) are shown. Results are representative of four or five independent pairwise experiments conducted on Vero and RajiR cells or BHK and RajiR cells, respectively. Error bars represent the range of data from duplicate wells within a single experiment. (I and J) Summary of the fraction of infectious WNV resistant to neutralization at saturating concentrations of the indicated antibody when assayed pairwise on RajiR and Vero cells (I) or RajiR and BHK cells (J). Error bars represent standard errors from four or five independent experiments. The fractions of neutralization-resistant WNV on the different cell types were statistically evaluated using a paired t test.
Maturation state-dependent neutralization of WNV.
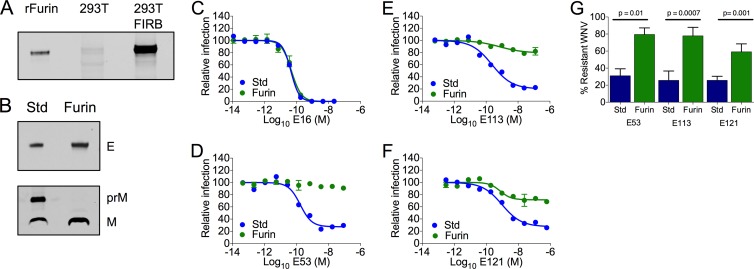
Flavivirus-infected cells release a heterogeneous population of virions that vary with respect to the efficiency of virion maturation (47). Using WNV RVPs, we have shown previously that the efficiency of virion maturation modulates the potency of many neutralizing antibodies through changes in epitope accessibility (25). To confirm that maturation state-dependent neutralization occurs with infectious WNV, we produced populations of WNV that vary with respect to the efficiency of prM cleavage. To increase the efficiency of maturation, virions were produced in an HEK-293T cell line engineered to stably express high levels of human furin, 293T-FIRB (Fig. 2A); virions produced using these methods are referred to here as WNV-Furin. The enhanced efficiency of prM cleavage in WNV-Furin populations was confirmed using Western blotting by comparison to WNV generated using standard virus production conditions (WNV-Std) (Fig. 2B). Neutralization studies were performed using MAbs E16, E53, E113, and E121. MAb E16 has been shown previously to be insensitive to the maturation state of the virion because of the relative accessibility of the E-DIII-lr epitope on the virion (44). As anticipated, neutralization studies with MAb E16 revealed no difference in the sensitivities of WNV-Std and WNV-Furin to neutralization (Fig. 2C) (P = 0.588, n = 4). In contrast, E53, E113, and E121 recognize epitopes not predicted to be accessible for antibody binding on the mature virion (31). In each instance, neutralization was significantly reduced by increases in the efficiency of virion maturation, as manifested by a significant increase in the fraction of virions resistant to neutralization (Fig. 2D to G) (P = 0.01, P = 0.0007, and P = 0.001 for MAbs E53, E113, and E121, respectively).
FIG 2.
Antibodies exhibiting cell type-dependent neutralization patterns are sensitive to changes in the maturation state of the virion. The efficiency of maturation of infectious WNV was increased by propagation in an HEK-293T cell line that overexpresses human furin (293T-FIRB). Viruses produced in these cells are referred to as WNV-Furin. (A) The levels of furin expression in 293T-FIRB and HEK-293T cells were evaluated along with recombinant furin (rFurin) (R&D Systems, Minneapolis, MN) by Western blotting using the furin-reactive antibody MON-152 (Enzo Life Sciences, Farmingdale, NY). (B) The efficiency of prM cleavage in preparations of WNV-Furin was compared to that in standard WNV produced using HEK-293T cells (WNV-Std) by Western blotting of pelleted virions using an anti-prM antibody (Abcam, Cambridge, MA) and the E protein-specific MAb 4G2. (C to F) Neutralization studies with the indicated antibodies were performed using WNV-Std and WNV-Furin on Raji-DC-SIGNR cells as described for Fig. 1. Dose-response curves of MAbs E16 (C), E53 (D), E113 (E), and E121 (F) are shown. In each case, experiments are representative of four or five independent experiments. Error bars represent the range of data from duplicate wells within a single experiment. (G) Summary of the fractions of neutralization-resistant WNV-Std or WNV-Furin at saturating concentrations of the indicated antibodies. Error bars represent standard errors from four or five independent experiments. A paired t test was used to compare the sizes of the neutralization-resistant fractions of WNV-Std and WNV-Furin.
The extent of prM cleavage modulates the ability of WNV to interact with target cells.
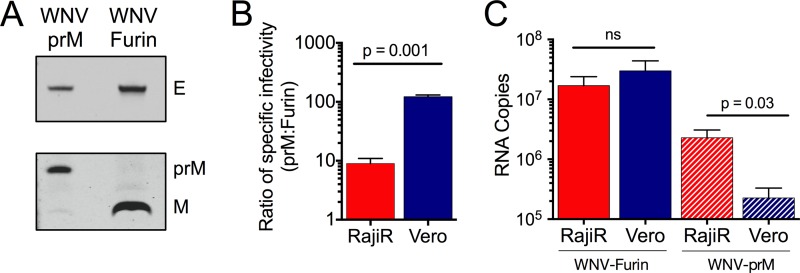
Because of similarities in the patterns of maturation state- and cell type-dependent neutralization, we next explored how the prM content of virions impacts their capacity to interact with Vero and Raji-DC-SIGNR cells. Virions that differed substantially in the efficiency of prM cleavage (WNV-Furin and WNV-prM) were produced and characterized by Western blotting (Fig. 3A). The efficiency of virion maturation of WNV was reduced by treating HEK-293T cells used for virus propagation with ammonium chloride (NH4Cl) and an inhibitor of furin-like proteases (42, 48, 49). The specific infectivities of WNV-Furin and WNV-prM populations were determined using Raji-DC-SIGNR and Vero cells in parallel. The ratio of the specific infectivities of WNV-Furin and WNV-prM on both cell substrates is presented in Fig. 3B. These results indicate that the infectivity of WNV-prM on Raji-DC-SIGNR cells was reduced by roughly 10-fold compared with that of WNV-Furin. Of interest, on Vero cells, the infectivity of prM-containing WNV (WNV-prM) was reduced much more dramatically (∼100-fold). This cell type-dependent difference in specific infectivity was found to be statistically significant using three independent preparations of virus (P = 0.001, n = 4). The reduced specific infectivity of WNV-prM when measured on Vero cells correlated with a diminished capacity of this population of WNV to bind to these cells (Fig. 3C) (P = 0.03, n = 5).
FIG 3.
Incomplete virion maturation differentially impacts specific infectivity and binding of WNV on different cellular substrates. Populations of relatively homogenous mature WNV (WNV-Furin) were produced in 293T-FIRB cells, while WNV that retains a significant amount of uncleaved prM was produced in HEK-293T cells treated with ammonium chloride and furin inhibitor (WNV-prM). (A) The efficiency of prM cleavage in preparations of WNV-Furin and WNV-prM used in subsequent experiments was confirmed by Western blotting using anti-prM antibody (Abcam, Cambridge, MA) and the E protein-specific MAb 4G2. (B) The specific infectivities of WNV-Furin and WNV-prM populations were measured on Vero and Raji-DC-SIGNR (RajiR) cells as defined by the number of viral RNA molecules associated with each infection event. The ratio of the specific infectivity of WNV-prM to WNV-Furin on RajiR and Vero cells is displayed. Error bars represent the standard errors from four independent experiments; statistical comparisons were performed using a paired t test. (C) Binding of WNV-Furin (solid red and blue bars) and WNV-prM (hatched red and blue bars) to RajiR and Vero cells. Viruses were allowed to bind to cells pretreated with 30 mM NH4Cl for 3 h at 37°C; virus binding was measured as a function of the copies of viral genome detected using quantitative real-time PCR. Error bars represent the standard errors from five independent experiments. Statistical comparisons were made using an unpaired t test.
Virion maturation governs cell type-dependent neutralization of WNV.
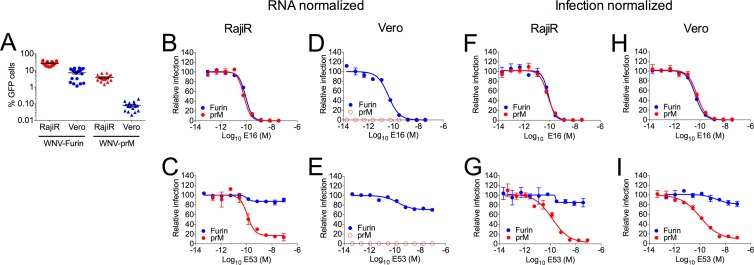
Because of the low specific infectivity of WNV-prM on Vero cells, we hypothesized that prM-containing virions are underrepresented in neutralization studies performed with the heterogeneous mixture of virions released from cells. To evaluate this possibility, we performed neutralization studies in which the amounts of WNV-Furin and WNV-prM used in the assay were normalized in two different ways. First, experiments were performed in which the virus inoculum was normalized by the RNA content of the virus stock (Fig. 4A to E). As expected, the level of infection achieved in the absence of antibody differed markedly as a function of the prM content of the virus preparation; this difference was more pronounced in studies with Vero cells (Fig. 4A). While the neutralization potency of MAbs E16 and E53 for WNV-prM could be determined with confidence using Raji-DC-SIGNR cells (Fig. 4B and C), evaluation of studies performed with WNV-prM on Vero cells (Fig. 4D and E) was not possible because of the low specific infectivity of the virus on this cell type.
FIG 4.
Incomplete virion maturation differentially impacts cell type-dependent neutralization of WNV. (A) Infectivities of WNV-Furin and WNV-prM on Raji-DC-SIGNR (RajiR) and Vero cells when virus input was normalized to equivalent RNA copies. Error bars represent the standard errors from 16 to 18 independent experiments. (B to E) Neutralization assays were performed using RNA-normalized stocks of WNV-Furin and WNV-prM preparations with the indicated antibodies on Raji-DC-SIGNR (RajiR) cells (B and C) or Vero cells (D and E) as described in Materials and Methods. Open symbols in panels D and E indicate that the level of infection was below the limit of detection. (F to I) Neutralization studies were repeated with the indicated MAbs on RajiR cells (F and G) and Vero cells (H and I) in which virus input was normalized to achieve similar levels of infectivity. Dose-response curves are representative of four or five independent experiments. Error bars represent the range of the data from duplicate measurements.
Next we performed studies in which virus input was normalized to achieve an equivalent level of infection by both WNV-Furin and WNV-prM virions (Fig. 4F to I). Due to the difference in specific infectivity detailed above, this translated into a requirement to add substantially more WNV-prM (∼90-fold) to assays using Vero cells. Under these conditions, cell-type dependent patterns of neutralization were no longer apparent. Neutralization of WNV-prM by MAb E53 was similar when assayed on both Raji-DC-SIGNR and Vero cells (Fig. 4G and I) (P = 0.812). Similar results were obtained with MAbs E113 and E121 (data not shown) (P = 0.908 and 0.216, respectively).
Neutralization of WNV on Vero cells expressing DC-SIGNR.
To confirm that the apparent reduced sensitivity of WNV to neutralization by maturation state-sensitive antibodies on Vero cells reflects differences in the mode of virus attachment to cells, we created a stable Vero cell line that expresses DC-SIGNR. The addition of the DC-SIGNR attachment factor to Vero cells significantly increased the specific infectivity of WNV-prM to levels that approach results obtained with Raji-DC-SIGNR cells (Fig. 5A) (P = 0.31, n = 3). Neutralization studies were then performed with standard preparations of WNV (WNV-Std) and MAbs E16 (Fig. 5B) and E53 (Fig. 5C) using Vero-DC-SIGNR cells and compared to experiments with parental Vero and Raji-DC-SIGNR cells performed in parallel. The sensitivity of WNV to neutralization by E53 was similar on both Vero-DC-SIGNR and Raji-DC-SIGNR cells (P = 0.516, n = 3); the expression of DC-SIGNR eliminated the cell type-dependent pattern of neutralization observed on Raji-DC-SIGNR and Vero cells. Thus, increasing the specific infectivity of prM-containing WNV on Vero cells correlates with a reduction in cell type-dependent neutralization.
FIG 5.
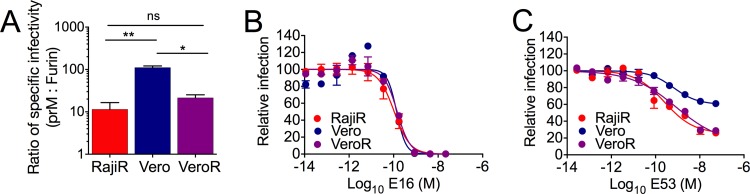
Cell type-dependent neutralization can be eradicated by expressing DC-SIGNR on Vero cells. The role of lectin-mediated attachment in the cell type-dependent neutralization of WNV was explored using a Vero cell line that was engineered to express DC-SIGNR (Vero-DC-SIGNR). (A) The specific infectivities of WNV-Furin and WNV-prM were measured on Vero, Vero-DC-SIGNR (VeroR), and Raji-DC-SIGNR (RajiR) cells. The ratio of the specific infectivity of WNV-prM to WNV-Furin virus was calculated for each cell type. Error bars display the standard errors from three experiments performed using independent stocks of viruses. Statistical comparisons were performed using a paired t test. (B and C) Neutralization of WNV-Std on the three cell types was evaluated using MAbs E16 (B) and E53 (C). Error bars display the range of the data from duplicate measurements. Data are representative of three independent experiments; statistical comparisons were performed using a paired t test.
Polyclonal antibody responses of flavivirus vaccine recipients may be sensitive to the cellular substrate used for neutralization assays.
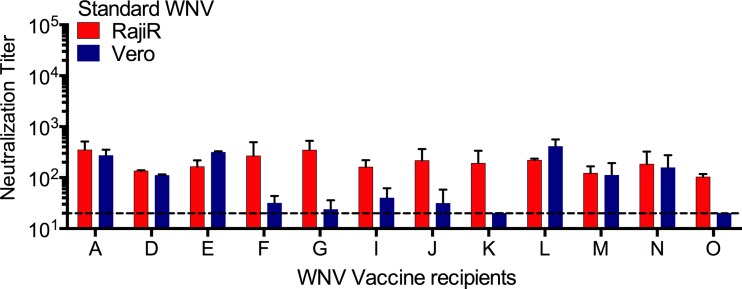
We have shown previously that the neutralizing antibody response elicited by WNV candidate vaccines may be sensitive to the maturation state of the virion (25, 37). Therefore, we next investigated whether maturation state-sensitive polyclonal antibody responses may also be sensitive to the cellular substrate used in neutralization assays. Sera from 12 volunteers participating in a WNV DNA vaccine trial were tested for sensitivity to the cell type used in neutralization assays; half of these samples (those from volunteers F, G, I, J, K, and O) have been shown previously to exhibit a significantly reduced capacity to neutralize mature WNV (25). Studies comparing neutralization sensitivity on Vero and Raji-DC-SIGNR cells revealed a perfect correlation between sera with neutralizing activity sensitive to the maturation state of the virion and cell type-dependent patterns of neutralization (Fig. 6). Neutralization titers were significantly reduced on Vero cells for all six maturation state-sensitive serum samples (P = 0.0003, n = 6) but not for those insensitive to the prM content of the virion (P = 0.5925, n = 6).
FIG 6.
Polyclonal responses from West Nile virus vaccine recipients are sensitive to the virion maturation state and the cellular substrate used in the assay. Neutralization dose-response profiles of WNV-Std particles were generated with previously characterized maturation state-sensitive sera from 12 recipients of WNV DNA vaccine (25). Cell-type dependent neutralization of WNV-Std by WNV vaccine sera was assayed on Raji-DC-SIGNR (Raji-R) cells (red bars) and Vero cells (blue bars). Neutralization assays were performed according to methods described in the legend to Fig. 1. Data were analyzed by nonlinear regression using GraphPad Prism 6.0, and the reciprocal of the average EC50 obtained is displayed. Error bars represent the range in the data. The dashed line indicates the limit of detection in the neutralization assays.
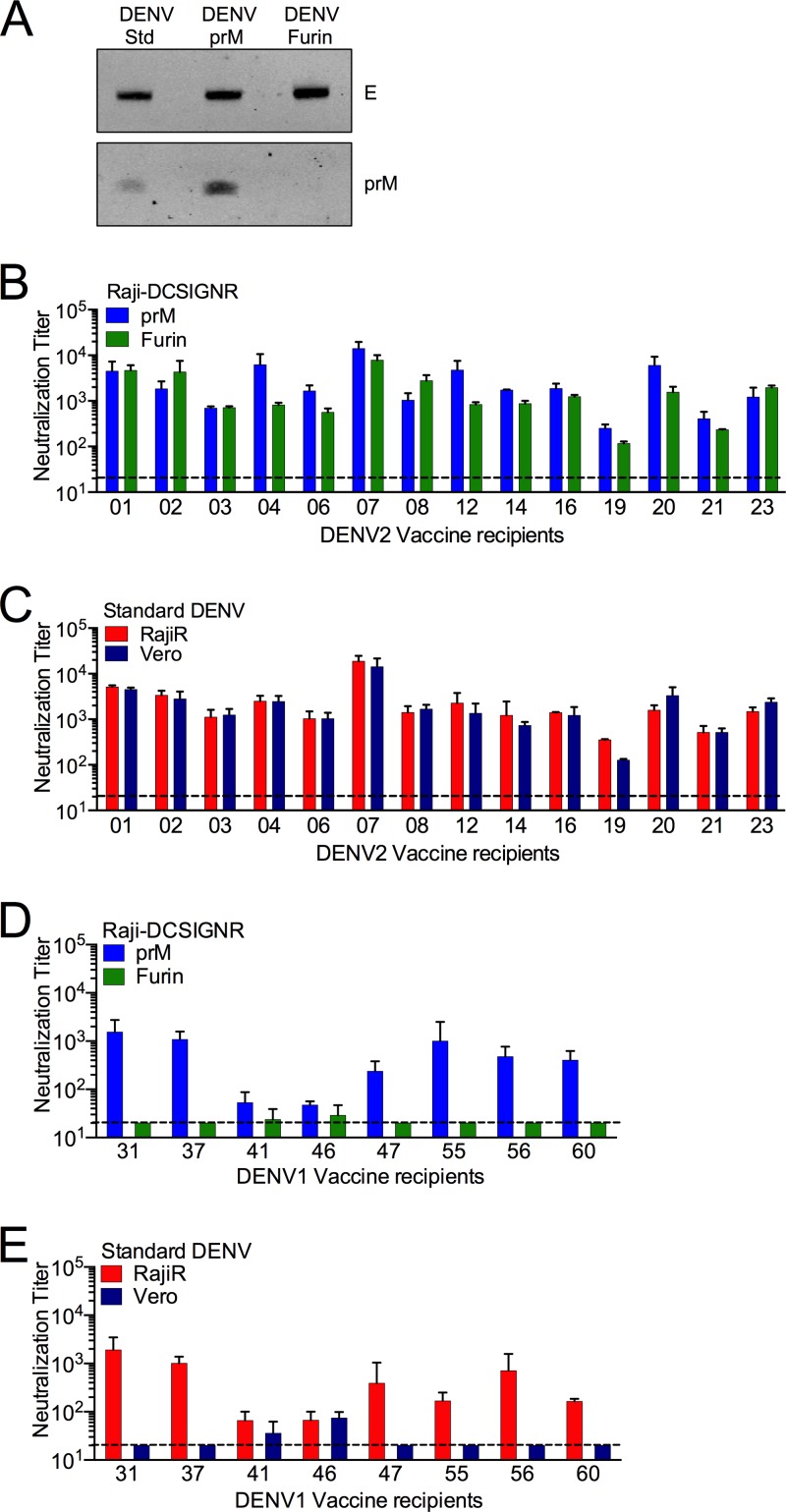
The neutralization activities of many antibodies that bind cross-reactive DENV epitopes, including the DII-fl and those on prM, are sensitive to the maturation state of the virion (25; S. Mukherjee and T. C. Pierson, unpublished data). Because these would be predicted to confer a cell type-sensitive pattern of neutralization, we sought to determine how the cell type typically used in the evaluation of DENV vaccines impacts the interpretation of neutralization tests. For this purpose, DENV serotype 2 (strain 16681) RVP populations where the efficiency of virion maturation was unmodified (DENV-Std), reduced (DENV-prM), or enhanced (DENV-Furin) were produced (Fig. 7A). Neutralization tests were performed using DENV-prM and DENV-Furin RVP populations and sera from 14 recipients of a monovalent DENV2 vaccine candidate on Raji-DC-SIGNR cells (Fig. 7B, blue versus green bars). Neutralization titers were similar among sera from all volunteers tested (Fig. 7B) (P = 0.277). Similar results were obtained from the analysis of type-specific neutralizing antibody present in DENV1-immune sera (50). The sensitivity of DENV-Std to neutralization was also similar for all volunteers when analyzed using Raji-DC-SIGNR cells and Vero cells (Fig. 7C, red versus blue bars) (P = 0.834), indicating that the type-specific DENV2 response is not markedly sensitive to the maturation state of the virion or the cellular substrate used in the assay.
FIG 7.
Cross-reactive polyclonal responses from dengue vaccine recipients are sensitive to the virion maturation state and cellular substrate used in the assay. DENV RVPs (DENV2 strain 16681) that differ with respect to the efficiency of virion maturation (DENV-Std, DENV-Furin, and DENV-prM) were generated using biochemical modifications as described in Materials and Methods. (A) The prM contents of the DENV-Std, DENV-Furin and DENV-prM RVPs were evaluated using Western blotting with an anti-E MAb and an anti-prM MAb. The efficiency of prM cleavage was evaluated on blots normalized by loading equivalent levels of E protein. (B to E) Neutralization profiles of DENV-prM (blue bars) and DENV-Furin (green bars) (B and D) and of DENV-Std on Raji-DC-SIGNR (RajiR) (red bars) and Vero (blue bars) particles (C and E) were generated with sera from 14 recipients of a live-attenuated monovalent DENV2 vaccine (B and C) or eight recipients of a live-attenuated monovalent DENV1 vaccine (D and E) using RajiR cells (red bars) or Vero cells (blue bars). Neutralization assays were performed as described in Materials and Methods. Data were analyzed by nonlinear regression using GraphPad Prism, and the reciprocal of the average neutralization titer values obtained is displayed. Error bars represent the range in the data from independent experiments. Dashed lines indicate the limit of detection of the assay.
We next performed similar studies to probe the maturation state and cell type dependence of cross-reactive responses to DENV using sera from recipients of a DENV1 monovalent vaccine. In contrast to type-specific DENV polyclonal responses, the cross-reactive response has the potential to be quite cell type dependent. In six of eight samples studied, neutralization titers of cross-reactive antibody were significantly greater when measured using DENV-prM virions than when using DENV-Furin virions (Fig. 7D, blue versus green bars) (P = 0.003). In each of these instances, the neutralization potency was also very sensitive to the cell type used in the assay (Fig. 7E, red versus blue bars) (P = 0.025). Thus, the selection of cellular substrate for neutralization assays may significantly impact our ability to evaluate the neutralization potency of the polyclonal response due to the differential capacity of cells to bind neutralization-sensitive populations of virus that retain significant prM.
DISCUSSION
Identifying a correlate of protection is a critical aspect of vaccine development. A vaccine-induced neutralizing antibody response predicts protection against several flaviviruses, including YFV, TBEV, and JEV (8–10). The selection of a cellular substrate is an important practical consideration in the design of assays to support vaccine development. Attention to these issues was increased significantly with the publication of the results of the first large clinical trial of a tetravalent DENV vaccine (34). This trial revealed inefficient protection against DENV that did not correlate with vaccine-induced antibody in the study population, particularly in the case of DENV2. While many parameters may have contributed to this outcome, one possibility is that the Vero cell-based neutralization test used to measure neutralizing antibodies does not capture the full breadth of the neutralization activity in each sample.
Cell type-dependent neutralization describes the differential capacity of antibodies to inhibit virus infection when assayed using different cellular substrates, and it has been observed in multiple viral systems (26–30, 32), including flaviviruses (31). Because of the potential for cell type-dependent patterns of neutralization to impact assays of vaccine efficacy, we investigated the underlying mechanism of this phenomenon, of which many have been proposed. First, antibodies may neutralize infection by blocking distinct steps in the virus entry pathway, resulting in distinct requirements for antibody occupancy on the virion (51). For example, antibodies that neutralize flaviviruses may do so by blocking attachment to target cells (19, 45, 52) or inhibiting membrane fusion in the endosome (20, 21, 53). The concentration of antibody associated with these distinct mechanisms may differ, as we and others have shown with WNV MAb E16 (41, 45). Alternatively, cell type-dependent neutralization could be apparent when different cell types are not equally susceptible to virus infection. A requirement to add substantially more virus on one cell type than another may change the apparent neutralizing activity of an antibody as an artifact of violating the “percentage law” (54, 55). The use of a very high concentration of virions in neutralization studies may markedly reduce the concentration of free antibody and prevent steady-state binding conditions; under these conditions, viruses escape from neutralization simply because free antibody is depleted (56). Neutralizing antibodies that recognize epitopes uniquely displayed on the virion during the process of viral entry may also contribute to patterns of antibody recognition as a result of virus-cellular factor interactions (28, 57).
Our data suggest that cell type-dependent neutralization of flaviviruses is explained by the interplay between how the extent of prM cleavage governs the number of antibody molecules docked on the virion (via changes in epitope accessibility) and the efficiency of infection of target cells. With few exceptions, partially mature virions have the potential to bind more antibody than mature virions and thus are more readily neutralized (25). Here, we demonstrate that partially mature virions infect Raji-DC-SIGNR cells more efficiently than Vero cells. Thus, the heterogeneous population of virions sampled by neutralization assays employing Raji-DC-SIGNR cells not only is larger than the populations of viruses measured using Vero cells but also is more sensitive to neutralization because of greater epitope accessibility. In agreement with this model, simply adding more prM-containing virions to Vero cells dramatically increased the observed neutralization potency of antibodies that otherwise display a cell type-dependent pattern of neutralization. That the neutralization activity of antibodies in this context is similar to that observed with Raji-DC-SIGNR suggests that the mechanism of inhibition does not actually differ between these two cell types or involve blocking interactions with DC-SIGNR. Instead, the prM on infectious virions reduces the efficiency of binding to cells that do not express the C-type lectin DC-SIGNR, as discussed below. The novel mechanism of cell-type dependent neutralization defined here highlights the functional consequences of differential interactions of a heterogeneous population of virions on various cellular substrates.
The molecular basis for the difference in specific infectivity observed with prM-containing viruses on Vero and Raji-DC-SIGNR cells requires additional study. DC-SIGNR has been shown to promote more efficient attachment of flaviviruses to cells via interactions with asparagine-linked (N-linked) sugars of the prM and E proteins (38, 58). Our studies suggest that the prM-E heterotrimeric spikes found on partially mature virions reduce the efficiency of virus interactions with Vero cells via a mechanism that can be rescued by DC-SIGNR expression. Recent studies suggest that WNV and DENV bind Vero cells via interactions between TIM or TAM receptors and phosphatidylserine lipids incorporated into the viral membrane (59). It is possible that a high density of prM-E spikes projecting off the surfaces of partially mature virions reduces the efficiency of these or other interactions critical for stable attachment to the cell surface. In contrast, recognition of N-linked sugars by DC-SIGNR may prove to be a more accommodating mode for binding pleomorphic virions. Whether all cells that express C-type lectins implicated in flavivirus attachment (such as DC-SIGN and the mannose receptor) bind prM-containing virions efficiently remains unexplored, as does the converse. Furthermore, it will be important to determine how other cellular factors modulate the cell type-dependent patterns conferred by DC-SIGNR expression described here. For example, the high-affinity Fc-γ receptor (CD64) expressed by the U937-DC-SIGN cellular substrate used extensively to study antibody-mediated neutralization of DENV may provide an alternative antibody-dependent mechanism for the attachment of virions that vary with respect to prM cleavage (60, 61). Because the cell biology of flavivirus attachment and entry is not well understood, it will be important to explore mechanisms underlying cell-type dependent neutralization patterns as they are identified; progress in this area to date has been limited because the number of cellular substrates studied in quantitative neutralization assays is very limited.
To explore the significance of cell type, we extended our mechanistic studies with MAbs to explore how cellular substrates impact neutralization assays with polyclonal immune sera. We first studied the neutralizing antibody response to a candidate WNV DNA vaccine, as half the recipients of either a live-attenuated or DNA vaccine displayed maturation state-sensitive patterns of neutralization (25). In agreement with our studies with MAbs, we observed perfect agreement between samples sensitive to the maturation state and those differentially neutralizing WNV on multiple cell types (25, 37). Similar studies were performed using sera obtained from recipients of two monovalent DENV candidate vaccines (35, 36). Our data established that serum responses that were sensitive to the maturation state of the virion were also sensitive to the substrate used in a neutralization assay. DENV type-specific neutralizing antibodies neither were sensitive to the maturation state of the virion nor displayed cell type-dependent patterns of neutralization. In contrast, estimates of cross-reactive antibodies in DENV2-immune sera were impacted significantly by the cellular substrate used in the neutralization experiment. This was perhaps not surprising, as many of the antibodies that bind cross-reactive epitopes (e.g., the DII-fl) are maturation state sensitive. In this context, the use of Vero cells to measure neutralizing activity may underestimate the response to vaccination. Our data suggest that simply adding DC-SIGNR to existing substrates for neutralization assays or developing methods to produce a more structurally homogenous virus population (such as the use of cells that express furin at high levels) may be beneficial for assay standardization purposes.
The implications of our work for identifying the most appropriate clinical assay to evaluate DENV antibody responses are limited. An assay to predict protective responses can be identified only via study of samples from individuals who were protected or nonprotected from infection, which are unavailable. Our results highlight two variables (the extent of virion maturation and the mode of viral attachment to cells) that when combined significantly impact the results of neutralization tests. While we have not performed experiments with the sera of volunteers of the phase II tetravalent DENV trial in Thailand, we hypothesize that the neutralization activity reported for that trial (measured by PRNT assays employing Vero cells) largely reflects type-specific antibodies previously thought to be highly protective (62, 63). DENV type-specific neutralizing antibodies are largely insensitive to both the maturation state of the virion and the cell substrate used in the neutralization assay (Fig. 7B and C) (50). In contrast, our results predict that the extent of cross-reactive antibody present in this DENV-immune population is underrepresented by the PRNT assay, although the significance of this is purely speculative.
This study highlights the complexity arising from the structural heterogeneity of flaviviruses released from cells. While individual virions that retain a significant number of uncleaved prM proteins bind more antibody due to increased epitope accessibility (25), they are significantly less infectious than more mature virions on Vero cells and thus may not contribute functionally to neutralization assays on this substrate. Critically, our data suggest that assays performed using Vero cells fail to measure the contribution of the population of virus particles most sensitive to neutralization. The role of cross-reactive antibody in protection in humans may thus be underappreciated and requires further study.
ACKNOWLEDGMENTS
This work was funded by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
We thank members of our laboratories for critically reading the manuscript and for helpful discussions.
Footnotes
Published ahead of print 16 April 2014
REFERENCES
- 1.Gould EA, Solomon T. 2008. Pathogenic flaviviruses. Lancet 371:500–509. 10.1016/S0140-6736(08)60238-X [DOI] [PubMed] [Google Scholar]
- 2.Beasley DW, Barrett AD, Tesh RB. 2013. Resurgence of West Nile neurologic disease in the United States in 2012: what happened? What needs to be done? Antiviral Res. 99:1–5. 10.1016/j.antiviral.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 3.Petersen LR, Roehrig JT, Hughes JM. 2002. West Nile virus encephalitis. N. Engl. J. Med. 347:1225–1226. 10.1056/NEJMo020128 [DOI] [PubMed] [Google Scholar]
- 4.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead SB, Thomas SJ. 2011. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev. Vaccines 10:355–364. 10.1586/erv.11.7 [DOI] [PubMed] [Google Scholar]
- 6.Heinz FX, Holzmann H, Essl A, Kundi M. 2007. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 25:7559–7567. 10.1016/j.vaccine.2007.08.024 [DOI] [PubMed] [Google Scholar]
- 7.Monath TP. 2012. Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev. Vaccines 11:427–448. 10.1586/erv.12.6 [DOI] [PubMed] [Google Scholar]
- 8.Heinz FX, Stiasny K. 2012. Flaviviruses and flavivirus vaccines. Vaccine 30:4301–4306. 10.1016/j.vaccine.2011.09.114 [DOI] [PubMed] [Google Scholar]
- 9.Julander JG, Trent DW, Monath TP. 2011. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine 29:6008–6016. 10.1016/j.vaccine.2011.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinz FX, Stiasny K. 2012. Flaviviruses and their antigenic structure. J. Clin. Virol. 55:289–295. 10.1016/j.jcv.2012.08.024 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. 2007. Structure of immature West Nile virus. J. Virol. 81:6141–6145. 10.1128/JVI.00037-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. 2008. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319:1834–1837. 10.1126/science.1153264 [DOI] [PubMed] [Google Scholar]
- 14.Pierson TC, Diamond MS. 2012. Degrees of maturity: the complex structure and biology of flaviviruses. Curr. Opin. Virol. 2:168–175. 10.1016/j.coviro.2012.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elshuber S, Allison SL, Heinz FX, Mandl CW. 2003. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J. Gen. Virol. 84:183–191. 10.1099/vir.0.18723-0 [DOI] [PubMed] [Google Scholar]
- 16.Perera R, Khaliq M, Kuhn RJ. 2008. Closing the door on flaviviruses: entry as a target for antiviral drug design. Antiviral Res. 80:11–22. 10.1016/j.antiviral.2008.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roehrig JT. 2003. Antigenic structure of flavivirus proteins. Adv. Virus Res. 59:141–175. 10.1016/S0065-3527(03)59005-4 [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay S, Kuhn RJ, Rossmann MG. 2005. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 3:13–22. 10.1038/nrmicro1067 [DOI] [PubMed] [Google Scholar]
- 19.Crill WD, Roehrig JT. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769–7773. 10.1128/JVI.75.16.7769-7773.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roehrig JT, Bolin RA, Kelly RG. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317–328. 10.1006/viro.1998.9200 [DOI] [PubMed] [Google Scholar]
- 21.Thompson BS, Moesker B, Smit JM, Wilschut J, Diamond MS, Fremont DH. 2009. A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes. PLoS Pathog. 5:e1000453. 10.1371/journal.ppat.1000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvert AE, Kalantarov GF, Chang GJ, Trakht I, Blair CD, Roehrig JT. 2011. Human monoclonal antibodies to West Nile virus identify epitopes on the prM protein. Virology 410:30–37. 10.1016/j.virol.2010.10.033 [DOI] [PubMed] [Google Scholar]
- 23.Chan AH, Tan HC, Chow AY, Lim AP, Lok SM, Moreland NJ, Vasudevan SG, MacAry PA, Ooi EE, Hanson BJ. 2012. A human PrM antibody that recognizes a novel cryptic epitope on dengue E glycoprotein. PLoS One 7:e33451. 10.1371/journal.pone.0033451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748. 10.1126/science.1185181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS, Pierson TC. 2008. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 4:e1000060. 10.1371/journal.ppat.1000060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldinotti F, Matteucci D, Mazzetti P, Giannelli C, Bandecchi P, Tozzini F, Bendinelli M. 1994. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J. Virol. 68:4572–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grady LJ, Kinch W. 1985. Two monoclonal antibodies against La Crosse virus show host-dependent neutralizing activity. J. Gen. Virol. 66:2773–2776. 10.1099/0022-1317-66-12-2773 [DOI] [PubMed] [Google Scholar]
- 28.Kjellen L. 1985. A hypothesis accounting for the effect of the host cell on neutralization-resistant virus. J. Gen. Virol. 66:2279–2283. 10.1099/0022-1317-66-10-2279 [DOI] [PubMed] [Google Scholar]
- 29.Mann AM, Rusert P, Berlinger L, Kuster H, Gunthard HF, Trkola A. 2009. HIV sensitivity to neutralization is determined by target and virus producer cell properties. AIDS 23:1659–1667. 10.1097/QAD.0b013e32832e9408 [DOI] [PubMed] [Google Scholar]
- 30.Mannini-Palenzona A, Moschella A, Costanzo F, Manservigi R, Monini P. 1995. Cell-type dependent sensitivity of herpes simplex virus 1 mutants to plaque development inhibition by an anti-gD monoclonal antibody. New Microbiol. 18:351–358 [PubMed] [Google Scholar]
- 31.Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, Pierson TC, Diamond MS. 2006. Antibody recognition and neutralization determinants on domains I and II of West Nile virus envelope protein. J. Virol. 80:12149–12159. 10.1128/JVI.01732-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhai W, Zhang DN, Mai C, Choy J, Jian G, Sra K, Galinski MS. 2012. Comparison of different cell substrates on the measurement of human influenza virus neutralizing antibodies. PLoS One 7:e52327. 10.1371/journal.pone.0052327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halstead SB. 2012. Dengue vaccine development: a 75% solution? Lancet 380:1535–1536. 10.1016/S0140-6736(12)61510-4 [DOI] [PubMed] [Google Scholar]
- 34.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567. 10.1016/S0140-6736(12)61428-7 [DOI] [PubMed] [Google Scholar]
- 35.Durbin AP, Whitehead SS, Shaffer D, Elwood D, Wanionek K, Thumar B, Blaney JE, Murphy BR, Schmidt AC. 2011. A single dose of the DENV-1 candidate vaccine rDEN1Delta30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS Negl. Trop. Dis. 5:e1267. 10.1371/journal.pntd.0001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durbin AP, McArthur JH, Marron JA, Blaney JE, Thumar B, Wanionek K, Murphy BR, Whitehead SS. 2006. rDEN2/4Delta30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naive adults. Hum. Vaccines 2:255–260. 10.4161/hv.2.6.3494 [DOI] [PubMed] [Google Scholar]
- 37.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M, Fay M, Koup RA, Roederer M, Bailer RT, Gomez PL, Mascola JR, Chang GJ, Nabel GJ, Graham BS. 2007. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J. Infect. Dis. 196:1732–1740. 10.1086/523650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, Pierson TC. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290–1301. 10.1128/JVI.80.3.1290-1301.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin TY, Dowd KA, Manhart CJ, Nelson S, Whitehead SS, Pierson TC. 2012. A novel approach for the rapid mutagenesis and directed evolution of the structural genes of West Nile virus. J. Virol. 86:3501–3512. 10.1128/JVI.06435-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ansarah-Sobrinho C, Nelson S, Jost CA, Whitehead SS, Pierson TC. 2008. Temperature-dependent production of pseudoinfectious dengue reporter virus particles by complementation. Virology 381:67-74. 10.1016/j.virol.2008.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dowd KA, Jost CA, Durbin AP, Whitehead SS, Pierson TC. 2011. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog. 7:e1002111. 10.1371/journal.ppat.1002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee S, Lin TY, Dowd KA, Manhart CJ, Pierson TC. 2011. The infectivity of prM-containing partially mature West Nile virus does not require the activity of cellular furin-like proteases. J. Virol. 85:12067–12072. 10.1128/JVI.05559-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geiss BJ, Pierson TC, Diamond MS. 2005. Actively replicating West Nile virus is resistant to cytoplasmic delivery of siRNA. Virol. J. 2:53. 10.1186/1743-422X-2-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierson TC, Xu Q, Nelson S, Oliphant T, Nybakken GE, Fremont DH, Diamond MS. 2007. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 1:135–145. 10.1016/j.chom.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. 2005. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 437:764–769. 10.1038/nature03956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522–530. 10.1038/nm1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, Kasinrerk W, Malasit P, Kuhn RJ, Sittisombut N. 2010. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J. Virol. 84:8353–8358. 10.1128/JVI.00696-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozden S, Lucas-Hourani M, Ceccaldi PE, Basak A, Valentine M, Benjannet S, Hamelin J, Jacob Y, Mamchaoui K, Mouly V, Despres P, Gessain A, Butler-Browne G, Chretien M, Tangy F, Vidalain PO, Seidah NG. 2008. Inhibition of Chikungunya virus infection in cultured human muscle cells by furin inhibitors: impairment of the maturation of the E2 surface glycoprotein. J. Biol. Chem. 283:21899–21908. 10.1074/jbc.M802444200 [DOI] [PubMed] [Google Scholar]
- 49.Randolph VB, Winkler G, Stollar V. 1990. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 174:450–458. 10.1016/0042-6822(90)90099-D [DOI] [PubMed] [Google Scholar]
- 50.VanBlargan LA, Mukherjee S, Dowd KA, Durbin AP, Whitehead SS, Pierson TC. 2013. The type-specific neutralizing antibody response elicited by a dengue vaccine candidate is focused on two amino acids of the envelope protein. PLoS Pathog. 9:e1003761. 10.1371/journal.ppat.1003761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Outlaw MC, Dimmock NJ. 1991. Insights into neutralization of animal viruses gained from study of influenza virus. Epidemiol. Infect. 106:205–220. 10.1017/S0950268800048354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He RT, Innis BL, Nisalak A, Usawattanakul W, Wang S, Kalayanarooj S, Anderson R. 1995. Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J. Med. Virol. 45:451–461. 10.1002/jmv.1890450417 [DOI] [PubMed] [Google Scholar]
- 53.Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, Nelson S, Pierson TC, Wilschut J, Throsby M, Diamond MS. 2009. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J. Virol. 83:6494–6507. 10.1128/JVI.00286-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrewes CH, Elford WJ. 1933. Observations on anti-phage sera. I. “The percentage law.” Br. J. Exp. Pathol. 14:367–376 [Google Scholar]
- 55.Klasse PJ, Sattentau QJ. 2001. Mechanisms of virus neutralization by antibody. Curr. Top. Microbiol. Immunol. 260:87–108 [DOI] [PubMed] [Google Scholar]
- 56.Pierson TC, Sanchez MD, Puffer BA, Ahmed AA, Geiss BJ, Valentine LE, Altamura LA, Diamond MS, Doms RW. 2006. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology 346:53–65. 10.1016/j.virol.2005.10.030 [DOI] [PubMed] [Google Scholar]
- 57.Flynn DC, Meyer WJ, Mackenzie JM, Jr, Johnston RE. 1990. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J. Virol. 64:3643–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis CW, Mattei LM, Nguyen HY, Ansarah-Sobrinho C, Doms RW, Pierson TC. 2006. The location of asparagine-linked glycans on West Nile virions controls their interactions with CD209 (dendritic cell-specific ICAM-3 grabbing nonintegrin). J. Biol. Chem. 281:37183–37194. 10.1074/jbc.M605429200 [DOI] [PubMed] [Google Scholar]
- 59.Meertens L, Carnec X, Lecoin MP, Ramdasi R, Guivel-Benhassine F, Lew E, Lemke G, Schwartz O, Amara A. 2012. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12:544–557. 10.1016/j.chom.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283. 10.1016/j.chom.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kraus AA, Messer W, Haymore LB, de Silva AM. 2007. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J. Clin. Microbiol. 45:3777–3780. 10.1128/JCM.00827-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabin AB. 1952. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 1:30–50 [DOI] [PubMed] [Google Scholar]
- 63.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. 2007. Prospects for a dengue virus vaccine. Nat. Rev. Microbiol. 5:518–528. 10.1038/nrmicro1690 [DOI] [PubMed] [Google Scholar]