ABSTRACT
Several arenaviruses are known to cause viral hemorrhagic fever (VHF) in sub-Saharan Africa and South America, where VHF is a major public health and medical concern. The biosafety level 4 categorization of these arenaviruses restricts their use and has impeded biological studies, including therapeutic drug and/or vaccine development. Due to difficulties associated with handling live viruses, pseudotype viruses, which transiently bear arenavirus envelope proteins based on vesicular stomatitis virus (VSV) or retrovirus, have been developed as surrogate virus systems. Here, we report the development of a pseudotype VSV bearing each envelope protein of various species of arenaviruses (AREpv), including the newly identified Lujo virus (LUJV) and Chapare virus. Pseudotype arenaviruses generated in 293T cells exhibited high infectivity in various mammalian cell lines. The infections by New World and Old World AREpv were dependent on their receptors (human transferrin receptor 1 [hTfR1] and α-dystroglycan [αDG], respectively). However, infection by pseudotype VSV bearing the LUJV envelope protein (LUJpv) occurred independently of hTfR1 and αDG, indicating that LUJpv utilizes an unidentified receptor. The pH-dependent endocytosis of AREpv was confirmed by the use of lysosomotropic agents. The fusion of cells expressing these envelope proteins, except for those expressing the LUJV envelope protein, was induced by transient treatment at low pH values. LUJpv infectivity was inhibited by U18666A, a cholesterol transport inhibitor. Furthermore, the infectivity of LUJpv was significantly decreased in the Niemann-Pick C1 (NPC1)-deficient cell line, suggesting the necessity for NPC1 activity for efficient LUJpv infection.
IMPORTANCE LUJV is a newly identified arenavirus associated with a VHF outbreak in southern Africa. Although cell entry for many arenaviruses has been studied, cell entry for LUJV has not been characterized. In this study, we found that LUJpv utilizes neither αDG nor hTfR1 as a receptor and found unique characteristics of LUJV glycoprotein in membrane fusion and cell entry. Proper exclusion of cholesterol or some kinds of lipids may play important roles in LUJpv cell entry.
INTRODUCTION
Arenaviruses belong to the family Arenaviridae and are classified into two complexes, New World and Old World arenaviruses, based on serological, genetic, and geographical relationships and the rodent hosts (1). New World arenaviruses are further divided into 3 major clades (A, B, and C). Clade B contains 5 hemorrhagic fever-causing arenaviruses that are known to cause South American hemorrhagic fever in humans: Junin virus (JUNV), Guanarito virus (GTOV), Sabia virus (SABV), Machupo virus (MACV), and Chapare virus (CHPV) (2). Of the Old World arenaviruses, Lassa virus (LASV) is endemic in western Africa and is known to cause viral hemorrhagic fever (VHF) in humans (3). Recently, another Old World arenavirus, Lujo virus (LUJV), was identified as a cause of VHF with a high case fatality rate of 80% (4).
Arenaviruses are enveloped negative-strand RNA viruses with a genome that is bisegmented into S and L segments. The S segment encodes a nucleocapsid protein (NP) and an envelope glycoprotein precursor (GPC); the L segment encodes a matrix protein (Z) and an RNA-dependent RNA polymerase (L). The GPC is synthesized as a single polypeptide and undergoes processing by the host cell signal peptidase (SPase) and subtilisin-like kexin isozyme-1/site-1-protease (SKI-1/S1P), yielding typical receptor-binding (G1), transmembrane fusion (G2), and stable signal peptide (SSP) subunits, respectively (5). Viral entry into target cells is initiated by the binding of G1 to appropriate cell surface receptors. The first cellular receptor for arenavirus to be identified was α-dystroglycan (αDG), a ubiquitous receptor for extracellular matrix proteins (6). αDG is a binding receptor for Old World arenaviruses, such as LASV and lymphocytic choriomeningitis virus (LCMV), and some of the clade C New World arenaviruses (7). Among the New World arenaviruses, many pathogenic viruses use human transferrin receptor 1 (hTfR1) as a receptor for cell entry (8, 9). A number of additional receptor candidates for JUNV, LASV, and LCMV have recently been reported, including the C-type lectin family, dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN), liver and lymph node sinusoidal endothelial calcium-dependent lectin (LSECtin), and two members of the TAM family (Axl and Tyro3) (10–13). In the case of LUJV, the receptor candidate (or candidates) is at this time largely unknown.
The categorization in many countries of VHF-causing arenaviruses as biosafety level 4 (BSL4) pathogens means that they may only be handled in specific institutions that are equipped with BSL4 facilities. This has impeded analysis of the life cycle and pathogenesis of these pathogenic arenaviruses and the development of therapeutic agents and vaccines for arenaviral hemorrhagic fevers. The analysis of the initial steps of viral infection, including identification of the entry receptors, is important for understanding the life cycle of these viruses and for further developing entry inhibitors. Several alternative research tools for the viruses have therefore been developed. A cell fusion assay was established to examine the membrane fusion activities of viral envelope proteins (14). The assay is sensitive and can easily determine cell fusion using reporter genes. Pseudotype virus systems based on vesicular stomatitis virus (VSV) or lentivirus and retrovirus have also been established to examine viral entry mechanisms and to identify putative entry receptors (15). These systems are beneficial in the study of highly pathogenic arenavirus infections.
In the present study, we generated pseudotype VSVs bearing envelope proteins of several arenaviruses, including LUJV. In particular, the characteristics of envelope proteins of LUJV were determined for the first time with respect to their glycosylation, fusion activities, and utilization of known arenaviral receptors and the involvement of cholesterol or sphingolipid in cell entry.
MATERIALS AND METHODS
Plasmids and cells.
The cDNAs of the SABV, MACV, CHPV, and LUJV GPCs were obtained by chemical synthesis (Codon Devices, Cambridge, MA). The GPC cDNAs of JUNV (strain MC2) and LASV (strain Josiah) were supplied by V. Romanowski (Universidad Nacional de La Plata) and C. J. Peters (University of Texas Medical Branch), respectively. The GenBank accession numbers of the nucleotide sequences of the SABV, MACV, CHPV, LUJV, JUNV, and LASV GPC genes are NC_006317, NC_005078, NC_010562, FJ952384, U70799, and J04324, respectively. The GPC cDNAs of SABV, MACV, CHPV, LUJV, JUNV, and LASV were cloned into the expression vector pKS336 (16). The resulting plasmids were designated pKS-SABV-GP, pKS-MACV-GP, pKS-CHPV-GP, pKS-LUJV-GP, pKS-JUNV-GP, and pKS-LASV-GP. Each GP sequence of the plasmids was confirmed to be identical to the original cDNA sequence using an ABI Prism 3100-Avant Genetic Analyzer (Applied Biosystems). The plasmid pKS-EBOV-GP (Reston) was prepared as described previously (17).
FLAG/One-STrEP (FOS)-tagged fusion protein expression vectors were also constructed. cDNAs of the CHPV-GP, LUJV-GP, JUNV-GP, and LASV-GP with the stop codon deleted were synthesized by PCR from each of the above-described cDNAs. The PCR products were cloned into pCAG-MCS2-FOS, which expresses carboxyl-terminally FOS-tagged fusion protein (provided by E. Morita, Osaka University). The resulting plasmids were designated pCAG-CHPV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-JUNV-GP-FOS, and pCAG-LASV-GP-FOS. The cDNA encoding G protein (G) of VSV was amplified from pCAG-VSV-G by PCR and cloned into pCAG-MCS2-FOS to construct pCAG-VSV-G-FOS in order to express carboxyl-terminally FOS-tagged VSV G protein.
A plasmid carrying hTfR1 cDNA was generated. Total RNAs were isolated from 293T cells using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The cDNA was synthesized by reverse transcription with Superscript III reverse transcriptase (Invitrogen) using random hexamer primers (Invitrogen). The cDNA encoding hTfR1 was amplified by PCR using an Expand High Fidelity PCR System (Roche Applied Science, Indianapolis, IN) with cDNA synthesized from the 293T RNAs. The amplified cDNA was cloned into pKS336. The resulting plasmid was designated pKS-hTfR1. The cloned sequence was confirmed to be identical to the hTfR1 cDNA sequence using an ABI Prism 3100-Avant Genetic Analyzer.
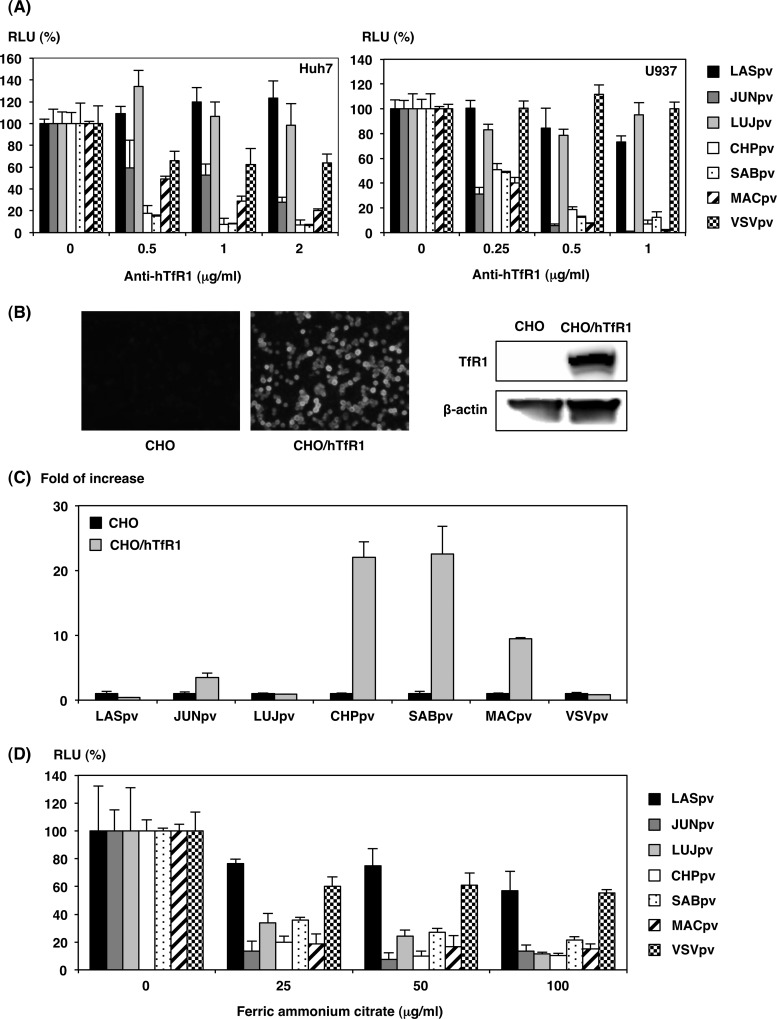
Hamster (BHK and CHO), mouse (NIH 3T3, NMuLi, and P388), rabbit (PK15), monkey (VeroE6, COS7, and MARC), and human (Huh7, HepG2, Hep3B, 293T, HeLa, Saos-2, Raji, U937, Molt-4, and Jurkat) cell lines were obtained from the American Type Culture Collection (Manassas, VA) or DS Pharma Biomedical Co. Ltd. (Osaka, Japan). All of the cell lines, with the exceptions of Raji, Molt-4, P388, and Jurkat cells, were grown in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, St. Louis, MO) containing 10% heat-inactivated fetal bovine serum (FBS). Raji, Molt-4, P388, and Jurkat cells were grown in RPMI 1640 (Sigma-Aldrich) containing 10% FBS. To establish a CHO cell line that stably expresses hTfR1 (CHO/hTfR1), CHO cells were transfected with pKS-hTfR1 using Fugene HD (Roche) reagent. The transfected CHO cells were selected with DMEM containing 10% FBS and 2 μg/ml of Blasticidin S-HCl (Invitrogen). When clusters of the cells appeared, some clusters of the cells were cloned and subcultured to establish CHO/hTfR1. The αDG knockout embryonic stem (ES) cell clone 354.B11 was provided by K. P. Campbell (Howard Hughes Medical Institute, University of Iowa) and was cultured using Esgro Complete Plus Clonal Grade Medium (Merck Millipore, Darmstadt, Germany). A CHO cell mutant defective in the NPC1 gene (CHO/A101) and CHO/A101 cells stably expressing FLAG-tagged NPC1 (CHO/A101/NPC1-KI) had been previously generated and were maintained in Ham's F-12 containing 10% FBS (18).
Generation of pseudotype VSVs.
Pseudotype VSVs bearing the GPC of arenaviruses (AREpv), GP of ebolavirus (EBOpv), G of VSV (VSVpv), and murine leukemia virus envelope protein (MLVpv) were generated as described previously (17, 19, 20). Briefly, 293T cells were grown to 70% confluence on collagen-coated tissue culture plates and then transfected with pKS-LASV-GP, pKS-JUNV-GP, pKS-LUJV-GP, pKS-CHPV-GP, pKS-SABV-GP, pKS-MACVGP, pKS-EBOV-GP, pCAG-CHPV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-JUNV-GP-FOS, pCAG-LASV-GP-FOS, pCAG-VSV-G, pCAG-VSV-G-FOS, or pFBASALF (which expresses murine leukemia virus envelope proteins; provided by T. Miyazawa, Kyoto University). After 24 h of incubation, the transfected cells were infected with G-complemented (*G) VSVΔG/Luc (*G-VSVΔG/Luc) (21) at a multiplicity of infection (MOI) of 0.1. The virus was adsorbed and then extensively washed four times with serum-free DMEM. After 24 h of incubation, the culture supernatants containing pseudotype VSVs were centrifuged to remove cell debris and stored at −80°C until use. The infectious titers of the pseudotype VSVs were also determined by a focus-forming assay, as described below, and were measured as focus-forming units (FFU). AREpv, EBOpv, VSVpv, and MLVpv infectivities were assessed by luciferase activity. The relative light unit (RLU) value of luciferase was determined using a Bright-Glo luciferase assay system (Promega Corporation, Madison, WI), according to the manufacturer's protocol.
Focus-forming assay.
Huh7 or Vero cells were transfected with pCAG-VSV-G. At 24 h posttransfection, the cells infected with the pseudotype viruses were cultured with 0.8% methylcellulose in 10% FBS DMEM for 48 h. FFU were determined by counting visible foci.
Immunofluorescence assays.
For immunofluorescence staining of the cells with antibodies, Huh7 cells transfected with pCAG-LASV-GP-FOS, pCAG-JUNV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-CHPV-GP-FOS, or pCAG-VSV-G-FOS or CHO/hTfR1 cells were fixed with acetone at room temperature for 5 min. To stain the FOS-tagged protein and hTfR1, the fixed transfected Huh7 and CHO/hTfR1 cells were incubated with mouse monoclonal anti-FLAG primary antibody (Sigma) and mouse monoclonal anti-hTfR1 antibody (BD Biosciences, San Jose, CA), respectively. The CHO cells were also prepared and stained as hTfR1 negative-control cells. All of the cells were rinsed with phosphate-buffered saline (PBS) and incubated with goat anti-mouse Alexa Fluor 488 (Invitrogen). After washing with PBS, the stained cells were observed under a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan).
Immunoblotting.
293T cells were transfected with each of the plasmids pCAG-CHPV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-JUNV-GP-FOS, pCAG-LASV-GP-FOS, and pCAG-VSV-G-FOS. At 24 h posttransfection, the cells were collected and lysed in PBS containing 1% NP-40. The lysates were centrifuged to separate insoluble pellets from supernatants. The supernatants were used as samples. Pseudotype VSVs bearing FOS-tagged GPCs generated as described above were pelleted through a 20% (wt/vol) sucrose cushion at 25,000 rpm for 2 h in an SW28 rotor (Beckman Coulter, Tokyo, Japan). The pellets were resuspended in PBS and used as additional samples. Each sample, boiled in loading buffer, was subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were electrophoretically transferred to a methanol-activated polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA) and reacted with mouse monoclonal anti-FLAG antibody (Sigma). Immune complexes were visualized with SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL) and detected by an LAS3000 analyzer (Fuji Film, Tokyo, Japan).
The lysates of CHO/hTfR1 and CHO cells were also treated as described above. To detect the bands of hTfR1 and β-actin, mouse monoclonal anti-hTfR1 antibody (BD Biosciences) and mouse monoclonal anti-β-actin antibody (Sigma), respectively, were used as described above.
Effects of treatment with antiserum on LUJpv infectivity.
To characterize the infection of LUJpv, the pseudotype was preincubated with serially diluted anti-LUJV-GP polyclonal antibody for 1 h at 37°C and then inoculated into Huh7 cells. Anti-LUJV-GP polyclonal antibody was prepared by immunization of rabbits with the expression plasmid pKS-LUJV-GP, as described previously (22). After 2 h of adsorption at 37°C, the cells were washed with DMEM containing 10% FBS, and infectivity was determined after 24 h of incubation at 37°C.
Involvement of hTfR1 in AREpv infections.
To determine the involvement of hTfR1 in viral entry, Huh7 or U937 cells were pretreated with various concentrations of anti-hTfR1 for 1 h at 37°C and inoculated with a series of AREpv, VSVpv, or MLVpv at an MOI of 1. After 1 h of adsorption at 37°C, the cells were washed and cultured for 24 h at 37°C. Pseudotype infectivities were determined by measuring luciferase activities after 24 h of incubation at 37°C.
The CHO and CHO/hTfR1 cells were infected with a series of AREpv or VSVpv, respectively, at an MOI of 1. Pseudotype infectivities were determined by measuring luciferase activities after 24 h of incubation at 37°C. AREpv infectivity for CHO/hTfR1 cells was normalized to the infectivity for CHO cells.
Huh7 cells were incubated at 37°C for 2 h in the presence of various concentrations of ferric ammonium citrate (FAC), which is rich in iron and is known to decrease TfR mRNA in the cells (23). The cells were then infected with the series of AREpv or VSVpv, as described above. Pseudotype infectivities were determined by measuring luciferase activities after 24 h of incubation at 37°C.
Involvement of αDG in AREpv infection.
To examine the involvement of αDG in viral entry, Raji, Jurkat, or αDG knockout ES cells expressing like-acetylglucosaminyltransferase (LARGE) or αDG were prepared by infection with lentiviral vectors encoding LARGE, DG, or control (fCD2) genes as constructed previously (11). The cells were infected with a series of AREpv or VSVpv at an MOI of 1. Pseudotype infectivities were determined by measuring luciferase activities after 24 h of incubation at 37°C.
Effects of enzymes, chemicals, and low-pH exposure on AREpv infection.
To examine the effects of oligosaccharide modification of arenavirus GPs or VSV-G, cell lysates and purified pseudotype virions were digested with endoglycosidase H (Endo H) or peptide-N-glycosidase F (PNGase F) (Roche) in accordance with the manufacturer's protocol and analyzed by immunoblotting.
To examine the effects of endosomal acidification of cells on viral entry, Huh7 cells were treated with various concentrations of inhibitors of endosomal acidification—bafilomycin A1 from Streptomyces griseus (Sigma), ammonium chloride (Sigma), and chloroquine (Sigma)—for 1 h at 37°C. The cells were then infected with the series of AREpv, VSVpv, or MLVpv at an MOI of 1. Pseudotype infectivity was determined by measuring luciferase activities after 24 h of incubation at 37°C.
To examine the pH dependence of AREpv cell entry, the series of AREpv or VSVpv was exposed to citrate-phosphate buffer (0.1 M citric acid, 0.2 M sodium dihydrogenorthophosphate) adjusted to the indicated pH level (pH 7, 6, 5, 4, or 3) for the indicated time (0, 15, 30, 60, 90, or 120 s). After pH neutralization with a 100× volume of DMEM containing 10% FBS, the viruses were inoculated into Huh7 cells. After 24 h of incubation at 37°C, residual infectivity was determined by measuring luciferase activities and comparison with the infectivities of the pseudotypes exposed to the buffer adjusted to pH 7.
To examine the involvement of cholesterol and sphingolipids in viral entry, Huh7 cells were treated with various concentrations of chlorpromazine, imipramine, desipramine, amitriptyline, or U18666A for 1 h at 37°C. The cells were then infected with the series of AREpv, EBOpv, VSVpv, or MLVpv at an MOI of 1. Pseudotype infectivity was determined by measuring luciferase activities after 24 h of incubation at 37°C.
To examine the involvement of NPC1 in viral entry, wild-type CHO (CHO/wt), CHO/A101, and CHO/A101/NPC1-KI cells were infected with the series of AREpv, EBOpv, or VSVpv at an MOI of 1. Pseudotype infectivity for these cells was determined by measuring luciferase activities after 24 h of incubation at 37°C. Pseudotype infectivity for CHO/A101 and CHO/A101/NPC1-KI cells was normalized to the infectivity for CHO/wt cells.
Syncytium formation and quantitative reporter assays for cell fusion.
To examine whether syncytium formation of the cells expressing arenavirus GPC is induced by low-pH exposure, Huh7 cells were transfected with pCAG-CHPV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-JUNV-GP-FOS, pCAG-LASV-GP-FOS, or pCAG-VSV-G-FOS. At 24 h posttransfection, the cells were rinsed once with PBS and then incubated with citrate-phosphate buffers adjusted to the indicated pH value (pH 7, 6, 5, 4, or 3) for 2 min. The citrate-phosphate buffers were then replaced with DMEM containing 10% FBS and incubated for 24 h for LASV-GP/JUNV-GP- and LUJV-GP/CHPV-GP-expressing cells or 8 h for VSV-G-expressing cells. The cell monolayers were then observed for the induction of cell fusion under a phase-contrast microscope.
To quantify cell fusion induced by arenavirus GPs, a reporter assay was carried out as follows. 293T cells were grown on 35-mm tissue culture plates and transfected with pCAG-CHPV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-JUNV-GP-FOS, pCAG-LASV-GP-FOS, or pCAG-VSV-G-FOS, together with pCAGT7pol (provided by Y. Matsuura, Osaka University), an expression plasmid carrying the T7 RNA polymerase gene under the control of the CAG promoter (14). The Huh7 target cells were grown on 35-mm tissue culture plates and transfected with pT7EMCVLuc (provided by Y. Matsuura), a reporter plasmid carrying a firefly luciferase gene under the control of the T7 promoter. At 24 h posttransfection, the target cells were collected by trypsinization and regrown in a 96-well plate. The 293T cells were treated with 0.05% EDTA in PBS and suspended in DMEM containing 10% FBS. The 293T cells were overlaid onto the target Huh7 cells and incubated for 4 h. The cocultured cells were bathed in citrate-phosphate buffers adjusted to the indicated pH values for 2 min and then incubated with DMEM containing 10% FBS for 12 h. Cell fusion activity was quantitatively determined by measuring luciferase gene expression in the lysates of the cocultured cells. The RLU values of luciferase were determined using a Bright-Glo luciferase assay system and normalized to the values of cells treated with pH 7 buffer.
RESULTS
Production and characterization of AREpv.
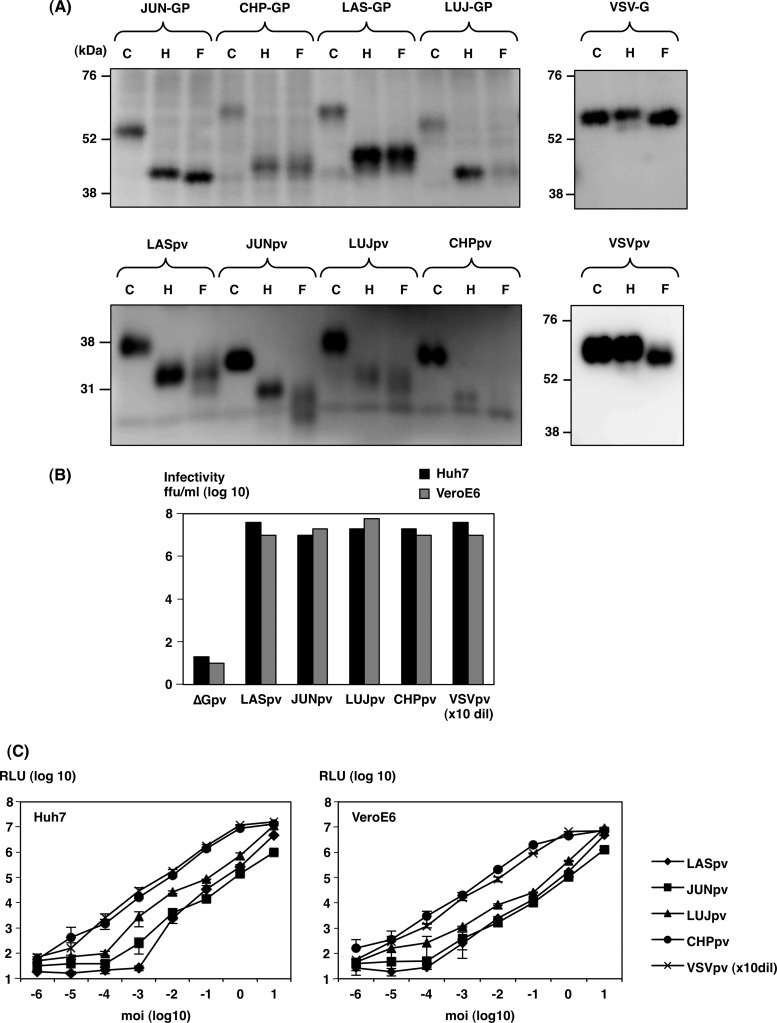
LASpv, JUNpv, LUJpv, CHPpv, and VSVpv were generated in 293T cells transiently expressing the carboxyl-terminally FOS-tagged envelope glycoproteins of LASV, JUNV, LUJV, CHAPV, and VSV, respectively, upon infection of *G pseudotype VSV, as previously reported (21, 24). To examine the properties of the arenaviral GPs incorporated into AREpv particles, the arenaviral GPs expressed in 293T cells and incorporated into the viral particles were digested with Endo H and PNGase F and then examined by immunoblotting using anti-FLAG monoclonal antibody (Fig. 1A). Since the arenaviral GPCs were FOS tagged at the carboxyl terminus, the GPC and processed G2 were detected in the immunoblotting. All of the GPCs in the lysates of the cells transfected with GPC-expressing plasmids and G2 incorporated into the viral particles that were examined in the present study were sensitive to both Endo H and PNGase F treatments, suggesting that both immature and mature GPs exhibited high-mannose-type glycosylation. In contrast, the sugar chains of VSV-G protein in the cells and on the virions were sensitive to PNGase F but resistant to Endo H, indicating that the sugar chains of VSV-G protein were modified to the complex- or hybrid-type glycans, which is consistent with previous reports (25, 26) (Fig. 1A).
FIG 1.
Glycosylation of glycoproteins in AREpv. (A) The GPC or G2 proteins of arenaviruses (LAS-GP, JUN-GP, LUJ-GP, and CHP-GP) or G protein of VSV expressed in 293T cells (top) and incorporated into the particles of AREpv (LASpv, JUNpv, LUJpv, and CHPpv) or VSVpv (bottom) were either untreated (lanes C) or treated with endoglycosidase H (lanes H) or peptide-N-glycosidase F (lanes F). Following fractionation on SDS-PAGE gels, the glycoproteins were detected by immunoblotting using anti-FLAG monoclonal antibody. (B) Infectivities of AREpv generated in 293T cells were determined in Huh7 and VeroE6 cells by focus-forming assay. (C) Dose-(MOI)-dependent relative luciferase activities (RLU) were determined in Huh7 and VeroE6 cells by luciferase assay. The MOI of each virus was determined on the basis of the titer in a focus-forming assay in Huh7 cells. The results shown are from three independent assays, with error bars representing standard deviations. VSV without envelope (ΔG) was used as a negative control. VSVpv (VSV) was used at 10-fold dilution (dil).
To evaluate the correlation of infectious titers and luciferase activities in AREpv infection, a focus-forming assay was performed (Fig. 1B). VSV lacking an envelope protein (ΔGpv) was used as a negative control. The infectious titers of all of the AREpv were shown to be high (∼1 × 107 to 5 × 107 IU/ml) on both Huh7 and VeroE6 cells. The luciferase activities of all of the AREpv-infected cells were correlated with the MOI (Fig. 1C).
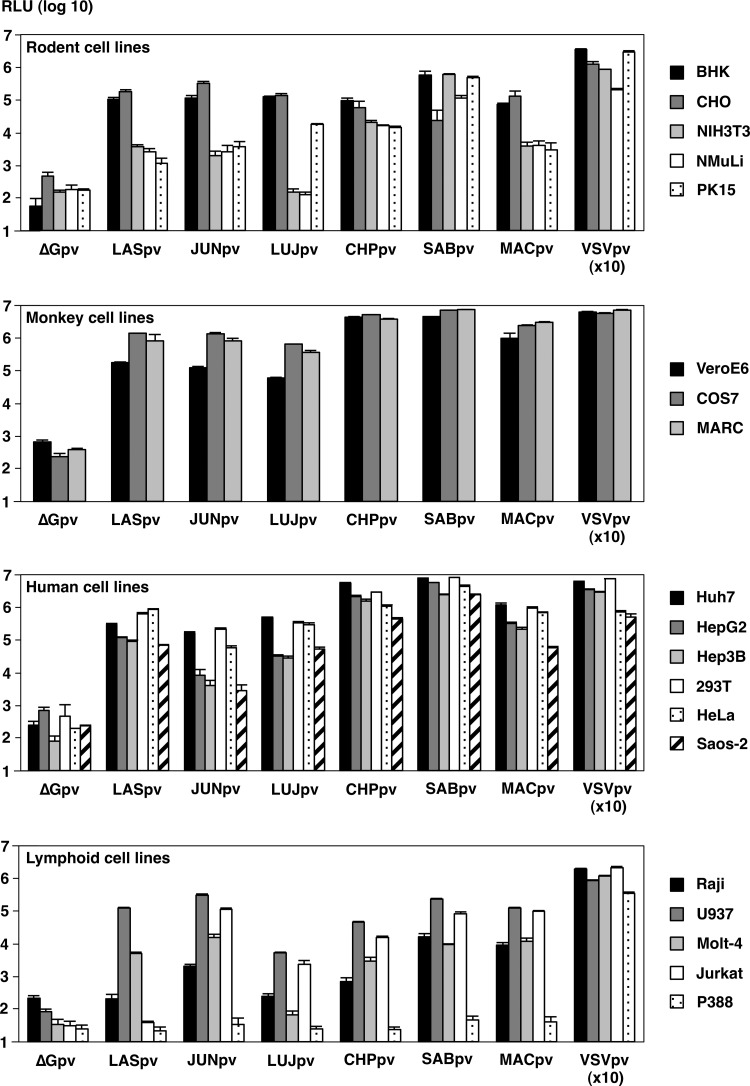
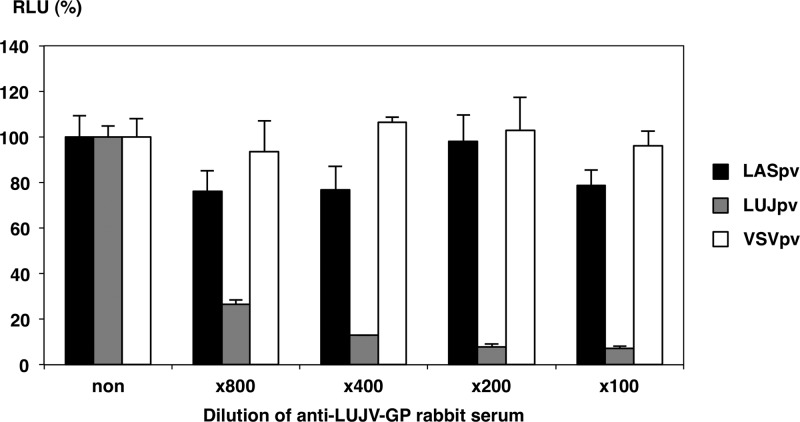
To further examine AREpv infectivity for various mammalian cell lines, LASpv, JUNpv, LUJpv, CHPpv, SABpv, and MACpv were inoculated into the indicated cell lines (Fig. 2). Almost all of the cell lines were susceptible to AREpv infection. Among them, COS7, MARC, Huh7, 293T, and HeLa cells were highly susceptible to AREpv infection, followed by BHK, CHO, VeroE6, HepG2, Hep3B, Saos-2, and U937. Other cell lines, NIH 3T3, NMuLi, PK15, Raji, Molt-4, and Jurkat cells, were less susceptible to infection by many of the AREpv, while P388 showed no susceptibility to AREpv infection. It is noteworthy that NIH 3T3, NMuLi, and Molt-4 cells or Jurkat cells were not susceptible to infection with LUJpv or LASpv, respectively. To determine the specificity of infection of LUJpv, neutralization assays of the pseudotypes were performed using anti-LUJV-GP rabbit serum. The infectivity of LUJpv, but not that of LASpv and VSVpv, for Huh7 cells was clearly inhibited by anti-LUJV-GP rabbit serum in a dose-dependent manner (Fig. 3). These data indicated that LUJpv infection exhibited GP-mediated entry.
FIG 2.
Efficiency of gene transduction into various mammalian cell lines by AREpv. The AREpv (LASpv, JUNpv, LUJpv, CHPpv, SABpv, and MACpv) generated by 293T cells were inoculated into the indicated cell lines at an MOI of 1. The MOI of each virus was determined on the basis of the titer in a focus-forming assay in Huh7 cells. At 24 h postinfection, the infectivities of the viruses were determined as RLU. The results shown are from three independent assays, with error bars representing standard deviations. ΔG was used as a negative control, and VSV was used at 10-fold dilution.
FIG 3.
Neutralization of AREpv infection by Lujo virus GP antibody. Shown is the effect of anti-LUJ-GP rabbit serum on the infectivity of LASpv, LUJpv, and VSVpv for Huh7 cells. The viruses were preincubated for 1 h at room temperature with the indicated dilution of antibody before infection of Huh7 cells. Luciferase activities were determined at 24 h postinfection. The results shown are from three independent assays, with error bars representing standard deviations.
Entry pathways of the AREpv.
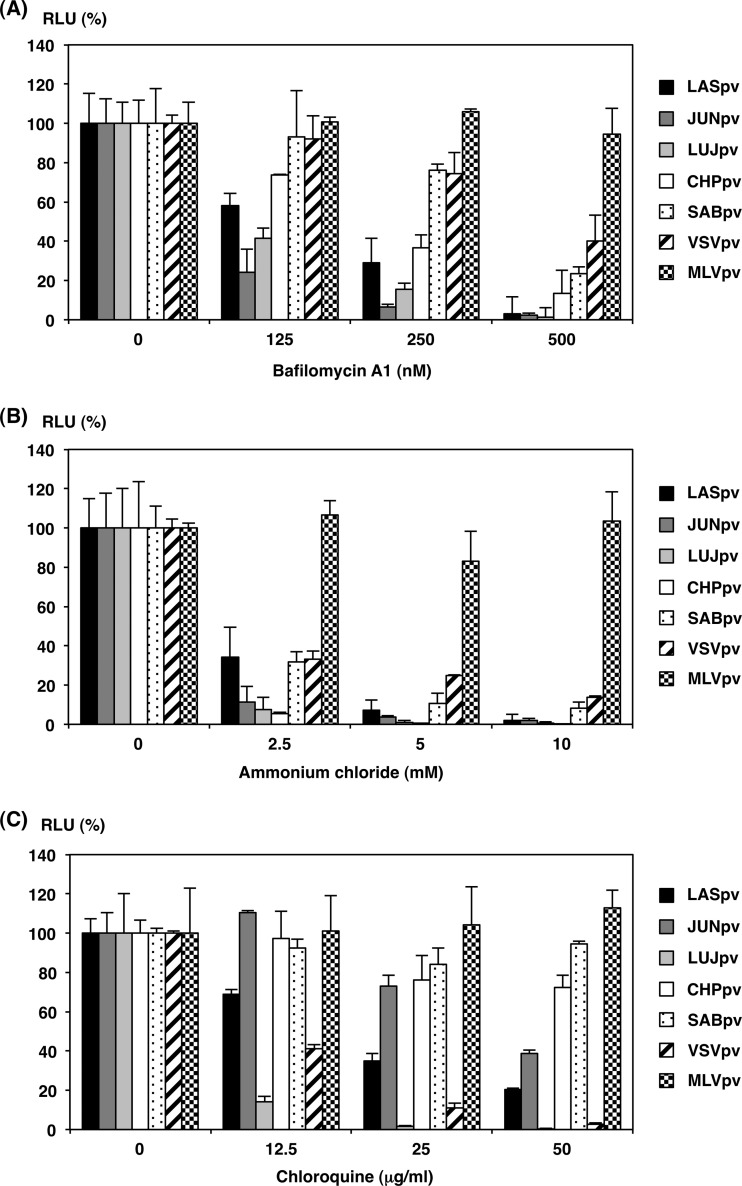
Previous studies showed that infections by LASV, JUNV, and some other arenaviruses were inhibited by treatment with lysosomotropic agents, such as ammonium chloride or bafilomycin A1, suggesting that arenaviruses enter target cells via pH-dependent endocytosis (27–29). To examine the pH-dependent entry pathway of the AREpv, Huh7 cells were pretreated with various concentrations of bafilomycin A1, ammonium chloride, or chloroquine, and then the cells were inoculated with a series of AREpv, VSVpv, and MLVpv (Fig. 4). As expected, the treatment of the cells with these reagents did not affect the infectivity of MLVpv, which enters cells through a pH-independent direct fusion of the viral membrane and plasma membrane. In contrast, infections of all of the AREpv and VSVpv, which enter cells through pH-dependent endocytosis, were inhibited by treatment of the cells with all of the aforementioned reagents in a dose-dependent manner. This result suggests that AREpv enter cells through pH-dependent endocytosis. It is noteworthy that treatment of cells with chloroquine dramatically reduced the infectivity of LUJpv, even at lower concentrations, while the infectivities of CHPpv and SABpv were slightly reduced with higher concentrations of chloroquine (Fig. 4C).
FIG 4.
Inhibition of AREpv infection by H+-ATPase inhibitors. AREpv (LASpv, JUNpv, LUJpv, CHPpv, and SABpv), VSVpv, and MLVpv were inoculated into Huh7 cells after treatment with various concentrations of bafilomycin A1 (A), ammonium chloride (B), or chloroquine (C). Luciferase activities were determined at 24 h postinfection. The results shown are from three independent assays, with error bars representing standard deviations.
Involvement of TfR1 in AREpv infection.
Among the candidates for cellular receptors for arenaviruses, hTfR1 was shown to be the principal receptor for the infection of New World clade B arenaviruses. It is not known whether LUJV exhibits hTfR1-dependent infection. To determine the involvement of hTfR1 in AREpv infection, Huh7 or U937 cells were pretreated with anti-hTfR1 monoclonal antibody and infected with the pseudotypes. JUNpv, CHPpv, SABpv, and MACpv infections in Huh7 and U937 cells were inhibited by anti-hTfR1 monoclonal antibody in a dose-dependent manner, whereas no inhibition of infection was observed with LASpv, LUJpv, or VSVpv (Fig. 5A). To further confirm the involvement of hTfR1, infectivities of AREpv for CHO cells stably expressing hTfR1 were examined. The expression of hTfR1 was confirmed by immunofluorescence assay and immunoblotting (Fig. 5B) (24). The expression of hTfR1 in CHO cells conferred increased susceptibility to JUNpv, CHPpv, SABpv, and MACpv infection, but not to LASpv, LUJpv, or VSVpv infection (Fig. 5C). FAC is known to downregulate TfR1 expression and inhibit infection by pseudotype viruses of the New World arenaviruses (9). In this study, infections by pseudotype viruses of the New World arenaviruses, namely, JUNpv, CHPpv, SABpv, and MACpv, were inhibited by FAC treatment (Fig. 5D). Infections by LASpv and VSVpv, which do not utilize hTfR1 as a receptor, were not affected by FAC treatment (Fig. 5D). It is noteworthy that infection with LUJpv was also inhibited by FAC treatment. These results suggest that LUJV does not utilize hTfR1 as a receptor but utilizes an unknown receptor, which is affected by FAC treatment. The results of the present study confirmed TfR1-dependent entry in New World arenavirus infections. In contrast, LUJpv and LASpv infections exhibited TfR1-independent entry.
FIG 5.
Involvement of human TfR1 in AREpv infection. (A) Inhibition of AREpv infection by anti-hTfR1 antibody. Huh7 or U937 cells were pretreated with various concentrations of anti-hTfR1 antibody for 1 h and inoculated with LASpv, JUNpv, LUJpv, CHPpv, SABpv, MACpv, or VSVpv, and infectivities were determined at 24 h postinfection by measuring luciferase activity. (B) Expression of hTfR1 on CHO cell lines constitutively expressing hTfR1 (CHO/hTfR1) was examined by immunofluorescence assay (left) or immunoblotting assay (right). (C) Infectivities of pseudotype immunoviruses for CHO cells expressing hTfR1. CHO or CHO/hTfR1 cells were infected with LASpv, JUNpv, LUJpv, CHPpv, SABpv, MACpv, or VSVpv, and infectivities were determined at 24 h postinfection by measuring luciferase activities. The infectivities of AREpv for CHO/hTfR1 cells were normalized to the infection of parental CHO cells. (D) Huh7 cells were pretreated with the indicated concentration of FAC and incubated at 37°C. After 1 h of incubation, the cells were inoculated with LASpv, JUNpv, LUJpv, CHPpv, SABpv, MACpv, or VSVpv, and infectivities were determined at 24 h postinfection by measuring luciferase activities. The results shown are from three independent assays, with error bars representing standard deviations.
Involvement of αDG in AREpv infection.
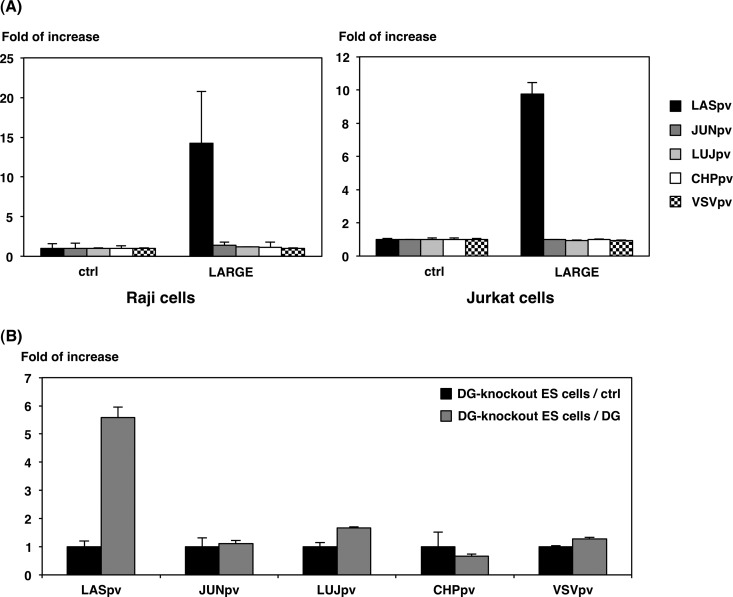
To further examine the involvement of another arenavirus receptor candidate, αDG, in arenavirus infection, a series of AREpv were inoculated into LARGE-expressing Raji and Jurkat cells. LARGE is a putative N-acetylglucosaminyltransferase whose expression compensates for any lack of O-mannosylation of DG (30). Expression of O-mannosylated DG in these cells was examined as previously described (reference 11 and data not shown). The expression of LARGE in both Raji and Jurkat cells conferred susceptibility to LASpv infection, but not to JUNpv, LUJpv, CHPpv, or VSVpv infection (Fig. 6A). To further confirm this result, the infectivities of AREpv were examined in DG knockout ES cells transfected with DG-expressing or control plasmids. The expression of DG in the DG knockout ES cells resulted in an increase in LASpv infection, whereas no significant increases were observed in the other pseudotype infections (Fig. 6B). These results confirmed that LASpv exhibited αDG-dependent cell entry, while LUJpv and New World AREpv infections exhibited αDG-independent cell entry.
FIG 6.
Involvement of αDG in AREpv infection. (A) Infectivities of AREpv in Jurkat or Raji cells expressing LARGE. Raji and Jurkat cells were transfected with the plasmid encoding LARGE or empty vector (ctrl). (B) Infectivities of AREpv in DG knockout ES cells expressing αDG. DG knockout ES cells were transfected with the plasmid encoding αDG or empty vector (ctrl). At 24 h posttransfection, the cells were infected with LASpv, JUNpv, LUJpv, CHPpv, or VSVpv, and infectivities were determined by measurement of luciferase activities. The results shown are from three independent assays, with error bars representing standard deviations.
Syncytium formation and cell fusion activity in cells expressing arenaviral GPs.
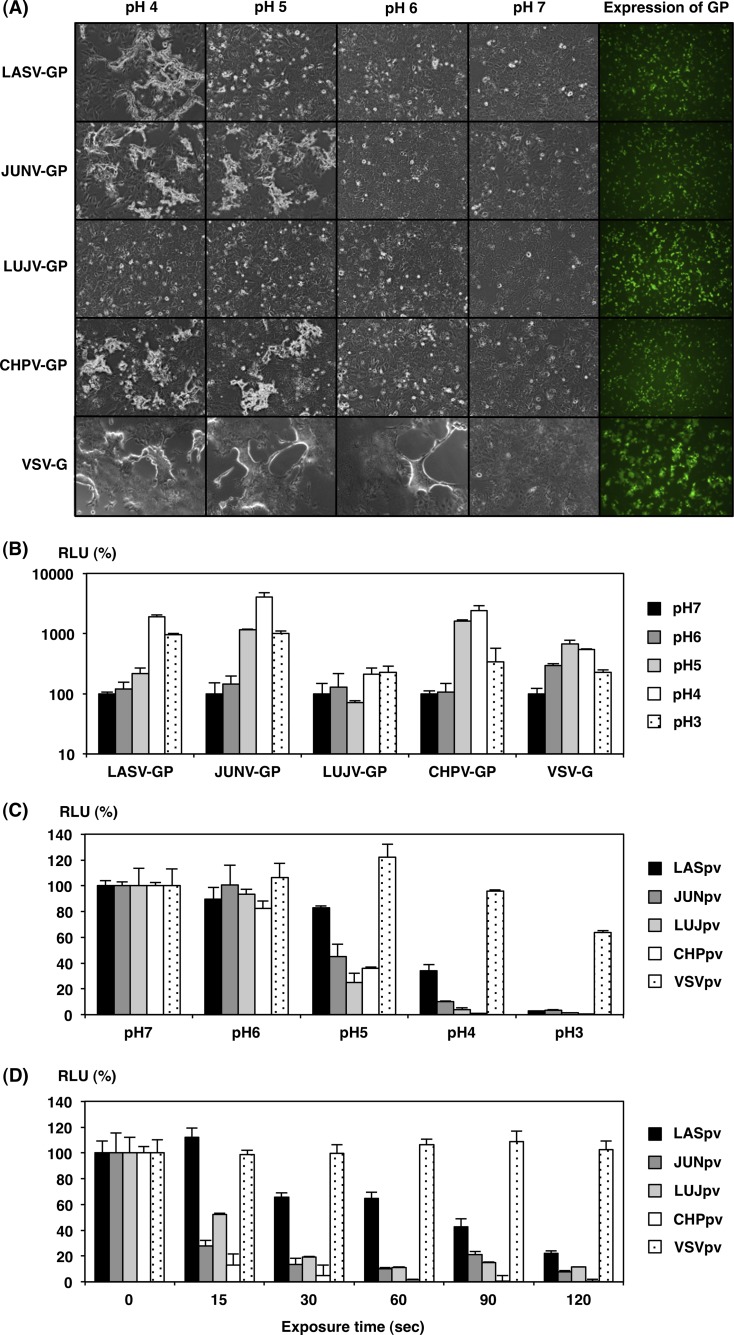
To examine syncytium formation in the arenaviral-GP-expressing cells, Huh7 cells were transfected with expression plasmids encoding various arenaviral GPs or VSV-G and cultured for 24 h. The cells were then treated with the indicated pH buffer for 2 min. The expression of each viral GP was confirmed by immunofluorescence assay with anti-FLAG antibody 24 h after the transfection of the expression plasmids. Synctia were observed after treatment with buffer at pH 4 in LASV-GP-expressing cells, below pH 5 in JUNV-GP- and CHPV-GP-expressing cells, and below pH 6 in VSV-G-expressing cells. In contrast, no syncytium formation was observed in LUJV-GP-expressing cells, even by treatment with buffer at pH 4 (Fig. 7A).
FIG 7.
pH dependence on arenavirus GP for cell fusion and entry. (A) Syncytium formation of Huh7 cells transiently expressing arenaviral GPs or VSV-G after treatment with low-pH buffer. Huh7 cells were transfected with pCAG-LASV-GP-FOS, pCAG-JUNV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-CHPV-GP-FOS, or pCAG-VSVG-FOS. At 24 h posttransfection, the cells were treated with citrate-phosphate buffer adjusted to the indicated pH value (pH 7, 6, 5, 4, or 3) for 2 min. Syncytium formation was determined by microscopic examination after 24 h (arenaviral GPs) or 8 h (VSV-G) incubation. Expression of arenaviral GPs or VSV-G at 24 h posttransfection was examined by immunofluorescence assay using anti-FLAG monoclonal antibody (right). (B) Quantitative cell fusion reporter assay. 293T cells transfected with pCAG-LASV-GP-FOS, pCAG-JUNV-GP-FOS, pCAG-LUJV-GP-FOS, pCAG-CHPV-GP-FOS, or pCAG-VSVG-FOS, together with a plasmid encoding T7 RNA polymerase, were cocultured with Huh7 cells, which were transfected with a plasmid carrying a luciferase gene under the control of the T7 promoter. Cell fusion activity after exposure at the indicated pH for 2 min was determined by measuring RLU. (C) The relative infectivities of AREpv after exposure at the indicated pHs. LASpv, JUNpv, LUJpv, CHPpv, or VSVpv was exposed at the indicated pHs for 2 min. After neutralization with DMEM containing 10% FCS, the remaining infectivities of the pseudotypes for Huh7 cells were measured. The luciferase activities were determined at 24 h postinfection. (D) Effects of exposure time in low-pH buffer on AREpv infectivity. LASpv, JUNpv, LUJpv, CHPpv, or VSVpv was pretreated with pH 4 citrate-phosphate buffer for the indicated time (0, 15, 30, 60, 90 or 120 s). After neutralization, the pseudotypes were inoculated into Huh7 cells. The luciferase activities were determined at 24 h postinfection. The results shown are from three independent assays, with error bars representing standard deviations.
To further examine cell fusion activities of the arenaviral GPs, we utilized a previously established, highly sensitive, and quantitative reporter gene activation method (14). Cell fusion was induced in LASV-GP-expressing cells below pH 4, in JUNV-GP- and CHPV-GP-expressing cells below pH 5, and in VSV-G-expressing cells below pH 6. Cell fusion was not induced in cells expressing LUJV-GP, even by treatment with the buffer at pH 4 (Fig. 7B). The luciferase activities in the VSV-G-expressing cells exhibited moderate enhancement, even though syncytium formation was clearly observed. This may be due to cytotoxicity of the VSV-G in the expressing cells. Similarly, pH 3 treatment of the cells induced cytotoxicity, and thus, the expression of luciferase may have been affected.
In general, low-pH exposure is known to change the conformation of some viral GP in the absence of cellular receptors and then to abolish viral infectivity. To examine the effect of low-pH exposure on AREpv infection, the viruses were treated with the indicated pH buffer or for the indicated exposure times before infection. Infectivities of all of the AREpv, including LUJpv, were abolished after low-pH treatment and within 2 min of exposure (Fig. 7C and D). Notably, LASpv was relatively resistant to low-pH exposure in comparison to other AREpv, suggesting that conformational change of LASV-GP may be induced under more acidic pH conditions or by longer-duration exposure.
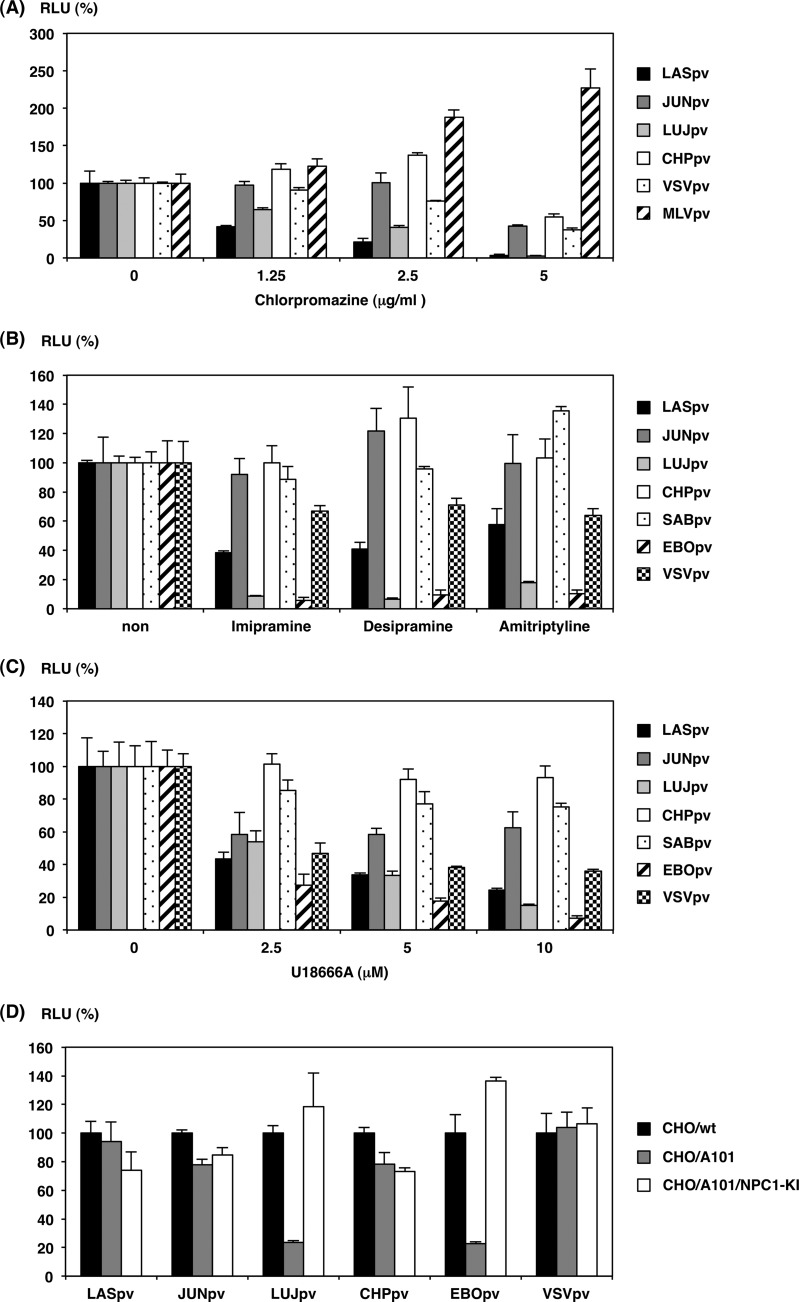
Involvement of cholesterol and sphingolipids in AREpv infection.
Recently, it has been reported that Ebola virus infection requires cholesterol transporter protein, Niemann-Pick C1 (NPC1), and acid sphingomyelinase, which is the hydrolase enzyme involved in sphingolipid metabolism (31–33). In the present study, LUJV-GP-expressing cells did not exhibit cell fusion upon treatment at low pH, indicating the necessity for additional cellular factors for LUJV infection. To further examine the entry pathway and roles of lipid metabolism in AREpv infection (especially LUJpv infection), we examined the effect of chlorpromazine treatment of the cells. This chemical is widely known, not only as an inhibitor of clathrin-mediated endocytosis, but also as an inducer of lipidosis (34, 35). Even though infections by all of the AREpv were inhibited by chlorpromazine treatment in a dose-dependent manner, LUJpv and LASpv infections were drastically reduced in comparison with the other AREpv (Fig. 8A). The effects of other lipidosis-inducing drugs, imipramine, desipramine, and amitriptyline, which are known as antidepressant drugs and which cause lipid accumulation in vitro (33), were also examined. LUJpv and EBOpv were drastically inhibited by all of these drugs (Fig. 8B). Furthermore, a cholesterol transport inhibitor, U18666A, which is known to inhibit the function of NPC1 (36, 37), was utilized. Treatment with the chemical resulted in a dose-dependent decrease in LUJpv and EBOpv infectivities (Fig. 8C). Interestingly, these inhibitors also partially decreased LASpv infectivity, suggesting that the entry mechanisms of LASpv are similar, in part, to those of LUJpv (Fig. 8B and C). To further examine the involvement of NPC1, NPC1-deficient CHO mutant cells, CHO/A101 cells, were utilized. The infectivities of LUJpv and EBOpv were decreased in CHO/A101 cells and were recovered by the replenishment of NPC1 in CHO/A101-expressing NPC1 cells (Fig. 8D).
FIG 8.
Involvement of cholesterol and sphingolipids in AREpv infection. (A to C) Infectivities of AREpv in Huh7 cells pretreated with the indicated concentrations of chlorpromazine (A); 10 μM imipramine, desipramine, or amitriptyline (B); or the indicated concentrations of U18666A (C). Huh7 cells were treated with each reagent and incubated at 37°C for 1 h. The cells were then inoculated with the pseudotype arenaviruses, EBOpv, MLVpv, or VSVpv, respectively. Their relative infectivities for the nontreated cells were determined at 24 h postinfection by measuring the luciferase activities. (D) Infectivities of pseudotype arenaviruses in wild-type CHO cells (CHO/wt), NPC1-deficient CHO cell mutants (CHO/A101), and CHO/A101 cells stably expressing FLAG-tagged NPC1 (CHO/A101/NPC1-KI). Their relative infectivities for the CHO/wt cells were obtained. The results shown are from three independent assays, with error bars representing standard deviations.
DISCUSSION
The development of safe alternative research tools for pathogenic arenaviruses that cause VHF in humans is very important for research on arenavirus diagnosis and for the development of prevention systems because classification of the viruses as BSL4 pathogens impedes their use in many countries. Toward this end, pseudotype virus systems based on VSV and retroviruses have been established. In the present study, we generated pseudotype VSVs bearing various arenaviral GPs, including the GP of a novel arenavirus (Lujo virus), and analyzed the involvement of arenaviral receptor candidates, fusion activities, and entry-mediated molecules.
Both VSV and retroviruses normally bud from the plasma membranes of the infected cells. The foreign viral envelope proteins expressed on the cell surface are therefore thought to be incorporated into the pseudotype particles during their budding. In the present study, although the presence of G1 could not be detected due to the absence of detectable antibodies, all of the carboxyl-terminally FOS-tagged arenaviral GPCs and G2s were detected in the cell lysates and purified virions, respectively (Fig. 1A). Arenaviral GPs may have been efficiently incorporated by VSV because of the presence of mature arenaviral GPs on the plasma membrane, which would make all of the pseudotype VSVs possessing arenaviral GPs highly infectious to the target cells. The fact that mature G2, but not GPC, was detected in the AREpv is consistent with a previous report that showed that only cleaved G1 and G2 were incorporated into the virions (38).
Previous studies have demonstrated the glycosylation of both LASV-GP and JUNV-GP by N-linked high-mannose-type oligosaccharide (39, 40). The glycosylation of VSV-G by complex-type oligosaccharide has also been demonstrated (26, 41). In the present study, all of the arenaviral GPs expressed in the cells and incorporated into the virions were sensitive to Endo H and PNGase F treatment (Fig. 1A), showing that they were glycosylated by high-mannose-type oligosaccharide. Though analysis of the glycosylation of live LUJV is needed, these data indicate that LUJV-GP was modified by the same glycosylation as the AREpv in the above-mentioned reports.
Previous studies have also demonstrated that several arenaviruses, including LASV, JUNV, and CHPV, and pseudotype viruses bearing their GPs were able to infect various types of cell lines. The AREpv were also able to infect almost all of the cell lines examined in the present study. However, LUJpv failed to infect NIH 3T3, NMuLi, or Molt-4 cells, while LASpv failed to infect Jurkat cells. It is known that Jurkat cells are not susceptible to LASV infection because they lack the function of O-mannosylation of αDG (11, 42). These results indicate that LASpv generated in this study represents the tropism of live LASV. Although comparison with live viruses is difficult, the entry mechanisms and characteristics of the envelope proteins of other pseudotype viruses are also believed to mimic those of live viruses. LUJV is known to be able to propagate in VeroE6 cells (43). The present study showed that, in addition to VeroE6 cells, both COS7 and Huh7 cells were highly susceptible to LUJpv infection. In contrast, no susceptibility was observed in mouse-derived cell lines (NIH 3T3 and NMuLi), but hamster-derived cell lines (BHK and CHO) were susceptible. The receptors and reservoirs of LUJV remain unclear. Although the characteristics of established cell lines may differ from those of the primary host cells, these results provide a hint for identifying the LUJV receptor and allow us to speculate with regard to its reservoir, since the expression of TfR1 from the reservoir rodent was shown to confer the highest susceptibility to many New World clade B pathogenic arenaviruses (44).
In the present study, we demonstrated that LUJpv entry was drastically decreased by the treatment of cells with chloroquine or chlorpromazine. These chemicals are widely known, not only as inhibitors of H+-ATPase activation and clathrin-mediated endocytosis, but also as inducers of lipidosis. These phenomena make sense, because our data suggest that lipid metabolism plays an important role in LUJpv cell entry. Furthermore, given the use of chloroquine in the prevention and treatment of malaria in some areas of the world, it might be also available as a medicine to patients infected with LUJV.
Several studies have revealed αDG and TfR1 to be major cellular receptors for pathogenic Old World and New World clade B arenaviruses, respectively. In the present study, we demonstrated that LUJpv cell entry occurred independently of both αDG and TfR1. Although further experiments utilizing live LUJV to examine the entry and/or propagation mechanisms are needed, our results suggest the possibility that LUJV utilizes one or more novel receptors, but not αDG or TfR1.
Observation of syncytium formation and the cell-cell fusion assay provide simple, quantitative, and versatile tools to study viral-glycoprotein-mediated cell fusion (14, 45, 46). Low-pH-induced membrane fusion by arenaviral GPs has been the subject of previous studies (47, 48). In the present study, with the exception of LUJV-GP, cell fusion induced by arenaviral GPs initiated by treatment with low-pH buffer was clearly observed, both in syncytium formation and in reporter gene activities. As for LUJV-GP, no syncytium formation or reporter gene activities were observed in the cells treated with low-pH buffer, despite the abundant expression of GP. Furthermore, immunofluorescence assays with and without permeabilized conditions revealed no difference between the cellular localization of GP in LUJV-GP and that of the other arenaviral GPs (data not shown). Moreover, LUJV-GP is considered to be present in the plasma membrane, because LUJV-GP was efficiently incorporated into virions and LUJpv, as well as other AREpv that exhibited high infectivity. In some of the enveloped viruses, such as Ebola virus or severe acute respiratory syndrome coronavirus (SARS-CoV), it is known that cleavage of the GP with the cysteine proteases cathepsins B and L is a prerequisite for membrane fusion (49–51). As far as we examined, the infectivity of LUJpv in Huh7 cells was not influenced by treatment with cathepsin B or L inhibitors (data not shown). Although we need to clarify whether extra cleavage or modification of LUJV-GP is necessary for membrane fusion, it is suggested that LUJV-GP shows unique characteristics among arenaviral GPs.
No visible syncytium formation was observed in the cells expressing Ebola virus GP under similar experimental conditions (data not shown). It was recently reported that in Ebola virus infection, the interaction of NPC1 with Ebola virus GP activated by the cathepsins is required for endosomal membrane fusion (31, 32). The results of the present study showed that LUJpv infection was inhibited, not only by lipidosis-inducing drugs, but also by the drug U18666A, which causes NPC1-like organelle defects. Furthermore, both LUJpv and EBOpv infections were abolished in NPC1-deficient cells, and these infectivities were recovered by the replenishment of NPC1 expression. Although it is currently not known whether LUJpv infection is directly involved in interaction with the NPC1 protein, the accumulation of cholesterol or some kinds of lipids may play important roles in the inhibition of LUJpv infection. Generally, NPC1 presents on cellular endosomes but not on the cell surface (52). If Lujo virus GP needs to interact with NPC1 in the same way as Ebola virus GP, this could be one of the reasons why cell-to-cell fusion did not occur in GP-expressing cells.
In conclusion, we generated replication-incompetent pseudotype arenaviruses possessing each arenaviral envelope glycoprotein, in particular, LUJpv, which is a novel surrogate model for the study of LUJV entry. In the present study, some of the analyses of LUJpv revealed that LUJV and its glycoprotein have unique characteristics for entry and fusion. Although LUJV is classified as a BSL4 pathogen, which makes it difficult to handle the authentic live virus, LUJpv and other pseudotype arenaviruses are quite useful for further detailed examination of arenaviral entry mechanisms.
ACKNOWLEDGMENTS
We gratefully acknowledge Momoko Ogata for her technical and secretarial assistance.
This work was supported in part by a grant-in-aid for Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a grant-in-aid from the Ministry of Health, Labor, and Welfare of Japan (grants H22-shinkou-ippan-006, H24-shinkou-wakate-016, H25-shinkou-ippan-008, and H25-shinkou-ippan-004).
Footnotes
Published ahead of print 16 April 2014
REFERENCES
- 1.Bowen MD, Peters CJ, Nichol ST. 1997. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol. Phylogenet. Evol. 8:301–316. 10.1006/mpev.1997.0436 [DOI] [PubMed] [Google Scholar]
- 2.Delgado S, Erickson BR, Agudo R, Blair PJ, Vallejo E, Albarino CG, Vargas J, Comer JA, Rollin PE, Ksiazek TG, Olson JG, Nichol ST. 2008. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 4:e1000047. 10.1371/journal.ppat.1000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormick JB, Fisher-Hoch SP. 2002. Lassa fever, p 75–109 In Oldstone MBA. (ed), Arenaviruses I. Current topics in microbiology and immunology, vol 262 Springer, New York, NY. 10.1007/978-3-642-56029-3_4 [DOI] [PubMed] [Google Scholar]
- 4.Briese T, Paweska JT, McMullan LK, Hutchison SK, Street C, Palacios G, Khristova ML, Weyer J, Swanepoel R, Egholm M, Nichol ST, Lipkin WI. 2009. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 5:e1000455. 10.1371/journal.ppat.1000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burri DJ, da Palma JR, Kunz S, Pasquato A. 2012. Envelope glycoprotein of arenaviruses. Viruses 4:2162–2181. 10.3390/v4102162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079–2081. 10.1126/science.282.5396.2079 [DOI] [PubMed] [Google Scholar]
- 7.Rojek JM, Spiropoulou CF, Campbell KP, Kunz S. 2007. Old World and clade C New World arenaviruses mimic the molecular mechanism of receptor recognition used by alpha-dystroglycan's host-derived ligands. J. Virol. 81:5685–5695. 10.1128/JVI.02574-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flanagan ML, Oldenburg J, Reignier T, Holt N, Hamilton GA, Martin VK, Cannon PM. 2008. New world clade B arenaviruses can use transferrin receptor 1 (TfR1)-dependent and -independent entry pathways, and glycoproteins from human pathogenic strains are associated with the use of TfR1. J. Virol. 82:938–948. 10.1128/JVI.01397-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radoshitzky SR, Abraham J, Spiropoulou CF, Kuhn JH, Nguyen D, Li W, Nagel J, Schmidt PJ, Nunberg JH, Andrews NC, Farzan M, Choe H. 2007. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 446:92–96. 10.1038/nature05539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimojima M, Kawaoka Y. 2012. Cell surface molecules involved in infection mediated by lymphocytic choriomeningitis virus glycoprotein. J. Vet. Med. Sci. 74:1363–1366. 10.1292/jvms.12-0176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimojima M, Stroher U, Ebihara H, Feldmann H, Kawaoka Y. 2012. Identification of cell surface molecules involved in dystroglycan-independent Lassa virus cell entry. J. Virol. 86:2067–2078. 10.1128/JVI.06451-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez MG, Bialecki MA, Belouzard S, Cordo SM, Candurra NA, Whittaker GR. 2013. Utilization of human DC-SIGN and L-SIGN for entry and infection of host cells by the New World arenavirus, Junin virus. Biochem. Biophys. Res. Commun. 441:612–617. 10.1016/j.bbrc.2013.10.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncalves AR, Moraz ML, Pasquato A, Helenius A, Lozach PY, Kunz S. 2013. Role of DC-SIGN in Lassa virus entry into human dendritic cells. J. Virol. 87:11504–11515. 10.1128/JVI.01893-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takikawa S, Ishii K, Aizaki H, Suzuki T, Asakura H, Matsuura Y, Miyamura T. 2000. Cell fusion activity of hepatitis C virus envelope proteins. J. Virol. 74:5066–5074. 10.1128/JVI.74.11.5066-5074.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tani H, Morikawa S, Matsuura Y. 2011. Development and applications of VSV vectors based on cell tropism. Front. Microbiol. 2:272. 10.3389/fmicb.2011.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saijo M, Qing T, Niikura M, Maeda A, Ikegami T, Sakai K, Prehaud C, Kurane I, Morikawa S. 2002. Immunofluorescence technique using HeLa cells expressing recombinant nucleoprotein for detection of immunoglobulin G antibodies to Crimean-Congo hemorrhagic fever virus. J. Clin. Microbiol. 40:372–375. 10.1128/JCM.40.2.372-375.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayama Y, Demetria C, Saito M, Azul RR, Taniguchi S, Fukushi S, Yoshikawa T, Iizuka I, Mizutani T, Kurane I, Malbas FF, Jr, Lupisan S, Catbagan DP, Animas SB, Morales RG, Lopez EL, Dazo KR, Cruz MS, Olveda R, Saijo M, Oshitani H, Morikawa S. 2012. A seroepidemiologic study of Reston ebolavirus in swine in the Philippines. BMC Vet. Res. 8:82. 10.1186/1746-6148-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higaki K, Ninomiya H, Sugimoto Y, Suzuki T, Taniguchi M, Niwa H, Pentchev PG, Vanier MT, Ohno K. 2001. Isolation of NPC1-deficient Chinese hamster ovary cell mutants by gene trap mutagenesis. J. Biochem. 129:875–880. 10.1093/oxfordjournals.jbchem.a002932 [DOI] [PubMed] [Google Scholar]
- 19.Tani H, Komoda Y, Matsuo E, Suzuki K, Hamamoto I, Yamashita T, Moriishi K, Fujiyama K, Kanto T, Hayashi N, Owsianka A, Patel AH, Whitt MA, Matsuura Y. 2007. Replication-competent recombinant vesicular stomatitis virus encoding hepatitis C virus envelope proteins. J. Virol. 81:8601–8612. 10.1128/JVI.00608-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi S, Sayama Y, Nagata N, Ikegami T, Miranda ME, Watanabe S, Iizuka I, Fukushi S, Mizutani T, Ishii Y, Saijo M, Akashi H, Yoshikawa Y, Kyuwa S, Morikawa S. 2012. Analysis of the humoral immune responses among cynomolgus macaque naturally infected with Reston virus during the 1996 outbreak in the Philippines. BMC Vet. Res. 8:189. 10.1186/1746-6148-8-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tani H, Shiokawa M, Kaname Y, Kambara H, Mori Y, Abe T, Moriishi K, Matsuura Y. 2010. Involvement of ceramide in the propagation of Japanese encephalitis virus. J. Virol. 84:2798–2807. 10.1128/JVI.02499-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukushi S, Tani H, Yoshikawa T, Saijo M, Morikawa S. 2012. Serological assays based on recombinant viral proteins for the diagnosis of arenavirus hemorrhagic fevers. Viruses 4:2097–2114. 10.3390/v4102097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward JH, Kushner JP, Kaplan J. 1982. Transferrin receptors of human fibroblasts. Analysis of receptor properties and regulation. Biochem. J. 208:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iha K. 2013. Pseudotyped vesicular stomatitis virus for analysis of entry of arenaviruses and its application to serodiagnosis of Argentine hemorrhagic fever. Ph.D. thesis University of Tokyo, Tokyo, Japan [Google Scholar]
- 25.Spiro MJ, Spiro RG. 2000. Use of recombinant endomannosidase for evaluation of the processing of N-linked oligosaccharides of glycoproteins and their oligosaccharide-lipid precursors. Glycobiology 10:521–529. 10.1093/glycob/10.5.521 [DOI] [PubMed] [Google Scholar]
- 26.Spiro MJ, Spiro RG. 2001. Release of polymannose oligosaccharides from vesicular stomatitis virus G protein during endoplasmic reticulum-associated degradation. Glycobiology 11:803–811. 10.1093/glycob/11.10.803 [DOI] [PubMed] [Google Scholar]
- 27.Glushakova SE, Lukashevich IS. 1989. Early events in arenavirus replication are sensitive to lysosomotropic compounds. Arch. Virol. 104:157–161. 10.1007/BF01313817 [DOI] [PubMed] [Google Scholar]
- 28.Castilla V, Palermo LM, Coto CE. 2001. Involvement of vacuolar proton ATPase in Junin virus multiplication. Arch. Virol. 146:251–263. 10.1007/s007050170173 [DOI] [PubMed] [Google Scholar]
- 29.Cosset FL, Marianneau P, Verney G, Gallais F, Tordo N, Pecheur EI, ter Meulen J, Deubel V, Bartosch B. 2009. Characterization of Lassa virus cell entry and neutralization with Lassa virus pseudoparticles. J. Virol. 83:3228–3237. 10.1128/JVI.01711-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunz S, Rojek JM, Kanagawa M, Spiropoulou CF, Barresi R, Campbell KP, Oldstone MB. 2005. Posttranslational modification of alpha-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J. Virol. 79:14282–14296. 10.1128/JVI.79.22.14282-14296.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, Dal Cin P, Dye JM, Whelan SP, Chandran K, Brummelkamp TR. 2011. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 477:340–343. 10.1038/nature10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller EH, Obernosterer G, Raaben M, Herbert AS, Deffieu MS, Krishnan A, Ndungo E, Sandesara RG, Carette JE, Kuehne AI, Ruthel G, Pfeffer SR, Dye JM, Whelan SP, Brummelkamp TR, Chandran K. 2012. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J. 31:1947–1960. 10.1038/emboj.2012.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller ME, Adhikary S, Kolokoltsov AA, Davey RA. 2012. Ebolavirus requires acid sphingomyelinase activity and plasma membrane sphingomyelin for infection. J. Virol. 86:7473–7483. 10.1128/JVI.00136-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange Y, Ye J, Steck TL. 2012. Activation mobilizes the cholesterol in the late endosomes-lysosomes of Niemann Pick type C cells. PLoS One 7:e30051. 10.1371/journal.pone.0030051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez MG, Cordo SM, Candurra NA. 2007. Characterization of Junin arenavirus cell entry. J. Gen. Virol. 88:1776–1784. 10.1099/vir.0.82808-0 [DOI] [PubMed] [Google Scholar]
- 36.Liscum L, Faust JR. 1989. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. J. Biol. Chem. 264:11796–11806 [PubMed] [Google Scholar]
- 37.Cenedella RJ. 2009. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids 44:477–487. 10.1007/s11745-009-3305-7 [DOI] [PubMed] [Google Scholar]
- 38.Lenz O, ter Meulen J, Klenk HD, Seidah NG, Garten W. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. U. S. A. 98:12701–12705. 10.1073/pnas.221447598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albarino CG, Bergeron E, Erickson BR, Khristova ML, Rollin PE, Nichol ST. 2009. Efficient reverse genetics generation of infectious Junin viruses differing in glycoprotein processing. J. Virol. 83:5606–5614. 10.1128/JVI.00276-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eichler R, Lenz O, Garten W, Strecker T. 2006. The role of single N-glycans in proteolytic processing and cell surface transport of the Lassa virus glycoprotein GP-C. Virol. J. 3:41. 10.1186/1743-422X-3-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marozin S, Altomonte J, Apfel S, Dinh PX, De Toni EN, Rizzani A, Nussler A, Kato N, Schmid RM, Pattnaik AK, Ebert O. 2012. Posttranslational modification of vesicular stomatitis virus glycoprotein, but not JNK inhibition, is the antiviral mechanism of SP600125. J. Virol. 86:4844–4855. 10.1128/JVI.06649-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rojek JM, Campbell KP, Oldstone MB, Kunz S. 2007. Old World arenavirus infection interferes with the expression of functional alpha-dystroglycan in the host cell. Mol. Biol. Cell 18:4493–4507. 10.1091/mbc.E07-04-0374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergeron E, Chakrabarti AK, Bird BH, Dodd KA, McMullan LK, Spiropoulou CF, Nichol ST, Albarino CG. 2012. Reverse genetics recovery of Lujo virus and role of virus RNA secondary structures in efficient virus growth. J. Virol. 86:10759–10765. 10.1128/JVI.01144-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham J, Kwong JA, Albarino CG, Lu JG, Radoshitzky SR, Salazar-Bravo J, Farzan M, Spiropoulou CF, Choe H. 2009. Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic new world clade B arenaviruses. PLoS Pathog. 5:e1000358. 10.1371/journal.ppat.1000358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, Arase H. 2010. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. U. S. A. 107:866–871. 10.1073/pnas.0913351107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka Y, Suenaga T, Matsumoto M, Seya T, Arase H. 2013. Herpesvirus 6 glycoproteins B (gB), gH, gL, and gQ are necessary and sufficient for cell-to-cell fusion. J. Virol. 87:10900–10903. 10.1128/JVI.01427-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castilla V, Mersich SE. 1996. Low-pH-induced fusion of Vero cells infected with Junin virus. Arch. Virol. 141:1307–1317. 10.1007/BF01718832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klewitz C, Klenk HD, ter Meulen J. 2007. Amino acids from both N-terminal hydrophobic regions of the Lassa virus envelope glycoprotein GP-2 are critical for pH-dependent membrane fusion and infectivity. J. Gen. Virol. 88:2320–2328. 10.1099/vir.0.82950-0 [DOI] [PubMed] [Google Scholar]
- 49.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. 2005. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 102:11876–11881. 10.1073/pnas.0505577102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. 2005. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 308:1643–1645. 10.1126/science.1110656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. 2006. Role of endosomal cathepsins in entry mediated by the Ebola virus glycoprotein. J. Virol. 80:4174–4178. 10.1128/JVI.80.8.4174-4178.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garver WS, Heidenreich RA, Erickson RP, Thomas MA, Wilson JM. 2000. Localization of the murine Niemann-Pick C1 protein to two distinct intracellular compartments. J. Lipid Res. 41:673–687 [PubMed] [Google Scholar]