Abstract
We report the case of a middle-aged man where a diagnosis of pulmonary embolism (PE) was delayed due to initial underestimation of risk and over-reliance on D-dimer testing. The patient presented with pleuritic chest pain after a 5 h domestic flight. The treating clinicians presumed that this duration of immobilisation was insufficient to cause a PE, D-dimer was not measured and the patient was discharged home. One week later, the patient re-presented due to persistence of chest pain. On this occasion, D-dimer was measured and it was normal, which was interpreted as excluding a PE. Subsequently, a CT pulmonary angiogram was performed, which demonstrated a subsegmental PE. This case highlights the importance of accurate assessment of PE-risk factors and following clinical guidelines, since a delayed diagnosis of PE is associated with increased mortality.
Background
Pulmonary embolism (PE) is a common cause of death in Western countries.1 However, presenting symptoms and signs can be non-specific and the diagnosis of PE is sometimes delayed or missed entirely.2 This is despite the establishment of several major validated clinical models endorsed by governing bodies such as the American College of Emergency Physicians and British Thoracic Society.3 4 A delay in the diagnosis or missed diagnosis of PE in the emergency department (ED) is associated with increased mortality.2
Our report illustrates that clinicians must be vigilant when performing history taking, physical examination and laboratory testing so that important clues are not missed, resulting in delays or missed diagnosis of PE.
Case presentation
A 57-year-old man presented to the ED of our institution with a 12 h history of persistent right-sided non-radiating chest pain. The pain was sudden in onset and pleuritic in nature (worse on inspiration). The patient denied any history of haemoptysis, night sweats, dyspnoea, cough or wheezing. There was no history of exposure to sick contacts, recent surgery or chest wall trauma. The patient was alert and oriented, with a temperature of 36.5°C, blood pressure 114/69 mm Hg, pulse rate 73 bpm, respiration rate 18 bpm and room air oxygen saturation 95%. His physical examination was unremarkable and there was no clinical evidence suggestive of deep vein thrombosis.
One day prior to presentation, the patient had returned home after visiting his relatives who lived approximately 4300 km away. He had travelled with a domestic airline and the total flight time was 5 h. The patient claimed that during the holiday he had spent a lot of time outdoors in the heat with minimal fluid replenishment and felt dehydrated at the time of the journey. His significant medical history was that he was diagnosed with epilepsy 30 years ago and since then had been prescribed phenytoin 100 mg daily and carbamazepine 400 mg mane and 200 mg nocte. He had not suffered a seizure for over 10 years. He had previously been reviewed by a cardiologist for an incidental finding of right bundle branch block. Following a comprehensive evaluation, it was deemed that the right bundle branch block was not of any clinical significance. There was no history of previous venous thromboembolism and the patient denied taking any other regular medications and was not aware of any allergies to medications. He identified himself as an ex-smoker who had ceased smoking 20 years ago and had a 15 pack-year smoking history. There was no significant family history of any medical illness.
Investigations
An ECG was performed which demonstrated first-degree heart block and right bundle branch block. Chest X-ray was within normal limits with no obvious pulmonary or cardiac anomalies identified. Blood tests demonstrated normal full blood count, biochemistry and C reactive protein 3.5 mg/L (<5). For further evaluation, a cardiac biomarker (troponin I) was measured. The troponin I was 0.017 μg/L (<0.04) and was determined to be within normal limits.
Differential diagnosis
Musculoskeletal chest pain
Acute coronary syndrome
PE
Treatment
The patient was evaluated by two emergency physicians. It was felt that since the patient had only travelled in a domestic flight and not an international flight, his risk for venous thromboembolism was low. In this clinical context of presumed low risk for venous thromboembolism, a D-dimer test was not performed. Furthermore, the normal cardiac biomarker and lack of new ECG findings was considered to exclude cardiac disease as a cause of patient’s chest pain. His chest pain was labelled as ‘musculoskeletal’, and he was prescribed oral analgesia (non-steroidal anti-inflammatory agent) and discharged from the ED.
Outcome and follow-up
Six days after the initial presentation, the patient re-presented to the ED reporting persistent chest pain. To determine the cause of the chest pain, troponin I and STA Liatest D-dimer (microlatex immunoassay) were measured. Troponin I was 0.011 μg/L (<0.04) and the D-dimer was 0.29 mg/L (<0.40). PE was considered unlikely to be the cause of the patient’s chest pain due to the negative D-dimer result. Since a cause for the patient's chest pain was not discernable, the patient was then referred to our department for further evaluation.
When we reviewed the history of the presenting symptom in conjunction with the results of the previously normal cardiac enzymes, it was felt that a pulmonary embolus was a plausible explanation for the symptoms. We also felt that since the D-dimer test was performed a week after the initial presentation, it did not definitively exclude thromboembolism. Hence a decision was made to perform a CT pulmonary angiogram (CTPA). The CTPA demonstrated a right-sided subsegmental pulmonary embolus (figure 1). The patient was then started on subcutaneous enoxaparin 1 mg/kg twice daily and warfarin. A screen for prothrombotic conditions was performed but was normal: lupus anticoagulant, factor V Leiden and prothrombin 20210G>A were negative; activated protein C resistance ratio was 5.76 (>1.80), antithrombin III 0.98 U/mL (0.70–1.30), protein C 1.19 U/mL (0.70–1.30) and protein S 1.09 U/mL (0.60–1.40).
Figure 1.
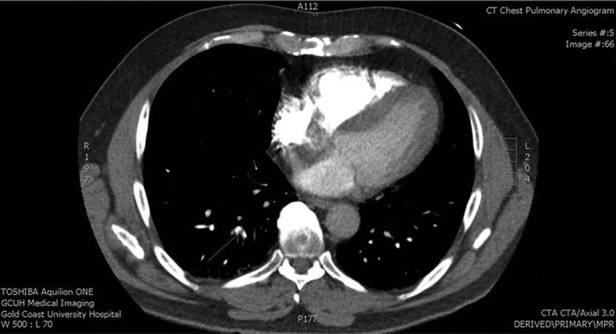
Diagnostic chest imaging performed in the patient. Representative slice of CT of the thorax showing a right subsegmental pulmonary embolus (white arrow).
The patient was educated about self-administering subcutaneous enoxaparin and arrangements were made for community monitoring and supervision of warfarin dosing. The patient was advised that the duration of warfarin treatment will be for 6 months and was discharged from hospital 3 days later with ongoing follow-up arranged.
Discussion
Thromboembolism during air travel on flights is due to the prothrombotic situation created by venous stasis in the lower limbs, which has been attributed to prolonged immobilisation, obstruction of venous return due to compression of lower limb veins and reduction in blood flow due to inflight dehydration. Nevertheless, thromboembolism is an uncommon event in healthy travellers, even on long-haul flights.5 The risk increases with the duration of the flight and certain physical attributes, such as female gender, oral contraceptive use, inherited or acquired states that predispose to venous thromboembolism and a history of thromboembolism.6 The risk of thromboembolism, albeit low, increases progressively after 4 h or 4000 km.5
D-dimer if formed when cross-linked fibrin is broken down by plasmin. Elevated levels of D-dimer are found in conditions that lead to activation of coagulation and fibrin formation and hence almost always increased in venous thromboembolism. Currently, there are several different types of D-dimer assays that are commercially available.7 However, the D-dimer assays use different methodologies and exhibit variable performance. Generally, the D-dimer assays based on an ELISA have the highest sensitivity (94–100%) and are considered to be the gold standard D-dimer test.8 Immunoturbidimetric assays, such as the STA Liatest assay have a lower sensitivity (80–95%) compared with an ELISA-based D-dimer assay, but their main advantage is their rapid turn-around time, making these tests highly attractive for use in an ED.7 One limitation of moderately sensitive assays (ie, immunoturbidimetric assay) is that they have a lower negative predictive value compared with a highly sensitive assay (ELISA-based assay) and therefore rules out PE only in patients with a low clinical pretest probability.9 Conversely, highly sensitive assays can rule out PE in patients with low or moderate clinical pretest probability. Our patient based on Well’s criteria had a moderate clinical probability of PE and a negative D-dimer result in this situation does not necessarily have a high-negative predictive value and does not definitively exclude a PE.
It is now recognised that there are instances when D-dimer may be normal even in the presence of venous thromboembolism and may be due to late presentation (patients present many days after the onset of symptoms), treatment with anticoagulation prior to D-dimer testing (can occur within 24 h after receiving heparin therapy), interindividual variation (clot breakdown capacity varies between individuals) and small size of clot burden.10 The phenomenon of a normal D-dimer in patients with prolonged symptoms (prior to testing) is well recognised with D’Angelo et al11 demonstrating a negative relationship between D-dimer levels and time elapsed since onset of symptoms. The relationship between D-dimer and size of PE clot burden was highlighted by De Monye et al,12 who reported that the diagnostic utility of D-dimer assay is considerably lower in patients with subsegmental PE (50%) compared with segmental or larger emboli (93%). Another possible explanation for a normal D-dimer result in patients with delayed presentation may be the complete or almost complete resolution of the pulmonary embolus. Stein et al13 and Aghayev et al14 performed retrospective reviews of patients diagnosed with PE and had a follow-up CTPA. Stein et al13 found that there was complete PE resolution within 2–7 days of initial imaging in 40% of patients and Aghayev et al14 report complete PE resolution within 14 days in 57% of patients. In all the time intervals studied, both studies found that decrease in clot burden and resolution was higher in the peripheral arterial emboli compared with central pulmonary artery emboli.
There are now several reports of patients with PE having a negative D-dimer result.15–17 A recent study by Zhiguo et al17 evaluated the clinical characteristics of 29 patients with PE and normal D-dimer values. They found that this cohort of patients were more likely to have had previous episodes of PE, less likely to have fever, tachypnoea and tachycardia and more likely to have had a time delay between symptom onset and D-dimer testing. Interestingly, in this study, almost 90% of patients were assessed to have low or moderate clinical probability of PE.
Another approach that may have alerted the ED clinicians to the possibility of a PE may have been to measure the brain natriuretic peptide (BNP) during the initial presentation. Plasma BNP is elevated in acute PE and is probably caused by right ventricular myocardial stress.18 Furthermore, in patients with acute PE, plasma BNP levels also have prognostic utility, with higher levels being associated with adverse clinical outcomes.18 However, the relative high cost of performing a BNP test precludes it from being used as a screening test in patients with suspected PE.
Common symptoms of PE are dyspnoea, pleuritic chest pain, cough and orthopnoea and signs of PE include tachypnoea, tachycardia, rales and distended jugular venous pulse. Our patient did not experience any of these symptoms or signs except pleuritic chest pain, which may have led the emergency clinician not to consider PE as a diagnosis. In the first presentation PE was considered unlikely based on an erroneous underestimation of risk of venous thromboembolism. The significance of total flight time of 5 h on a history of dehydration was not taken into consideration during PE-risk assessment and consequently a D-dimer was not requested. During the second presentation a D-dimer test was performed but the result which was within normal limits was erroneously interpreted as excluding a PE. We hypothesise that the reason for a normal D-dimer result in our patient is the time delay of 6 days between symptom onset and D-dimer testing in the presence of a subsegmental PE.
Learning points.
In patients with clinical risk factors of pulmonary embolism (PE), accurate assessment of the risk must be performed and established models of clinical assessment must be employed accordingly.
The identification of a normal D-dimer does not necessarily exclude a diagnosis of PE, particularly in patients where symptoms have been present for a week or more.
In clinical situations of persistent symptoms at risk of PE, a definitive test such as a CT pulmonary angiogram may be preferred to D-dimer testing as a means of ruling our PE.
Footnotes
Contributors: SB and KBS contributed in the patient management, literature review, manuscript preparation proofreading and editing.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple-cause mortality data. Arch Intern Med 2003;163:1711–17 [DOI] [PubMed] [Google Scholar]
- 2.Jelinek GA, Ingarfield SL, Mountain D, et al. Emergency department diagnosis of pulmonary embolism is associated with significantly reduced mortality: a linked data population study. Emerg Med Australas 2009;21:269–76 [DOI] [PubMed] [Google Scholar]
- 3.Writing Group for the Christopher Study I. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA 2006;295:172–9 [DOI] [PubMed] [Google Scholar]
- 4.Group BTSSoCCPEGD. British Thoracic Society guidelines for the management of suspected acute pulmonary embolism. Thorax 2003;58:470–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuipers S, Cannegieter SC, Middeldorp S, et al. The absolute risk of venous thrombosis after air travel: a cohort study of 8,755 employees of international organisations. PLoS Med 2007;4:e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Firkin F, Nandurkar H. Flying and thromboembolism. Aust Prescr 2009;32:148–50 [Google Scholar]
- 7.Ghanima W, Abdelnoor M, Mowinckel MC, et al. The performance of STA-Liatest D-dimer assay in out-patients with suspected pulmonary embolism. Br J Haematol 2006;132:210–15 [DOI] [PubMed] [Google Scholar]
- 8.Perrier A. D-dimer for suspected pulmonary embolism: whom should we test? Chest 2004;125:807–9 [DOI] [PubMed] [Google Scholar]
- 9.Lapner ST, Kearon C. Diagnosis and management of pulmonary embolism. BMJ 2013;346:f757. [DOI] [PubMed] [Google Scholar]
- 10.Thachil J. Over-reliance of D-dimer in isolation to exclude venous thrombosis should be avoided. Br J Gen Pract 2012;62:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Angelo A, D'Alessandro G, Tomassini L, et al. Evaluation of a new rapid quantitative D-dimer assay in patients with clinically suspected deep vein thrombosis. Thromb Haemost 1996;75:412–16 [PubMed] [Google Scholar]
- 12.De Monye W, Sanson B-J, Mac Gillavry MR, et al. Embolus location affects the sensitivity of a rapid quantitative D-dimer assay in the diagnosis of pulmonary embolism. Am J Respir Crit Care Med 2002;165:345–8 [DOI] [PubMed] [Google Scholar]
- 13.Stein PD, Yaekoub AY, Matta F, et al. resolution of pulmonary embolism on CT pulmonary angiography. Am J Roentgenol 2010;194:1263–8 [DOI] [PubMed] [Google Scholar]
- 14.Aghayev A, Furlan A, Patil A, et al. The rate of resolution of clot burden measured by pulmonary CT angiography in patients with acute pulmonary embolism. Am J Roentgenol 2013;200:791–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breen ME, Dorfman M, Chan SB. Pulmonary embolism despite negative ELISA D-dimer: a case report. J Emerg Med 2009;37:290–2 [DOI] [PubMed] [Google Scholar]
- 16.Dunn KL, Wolf JP, Dorfman DM, et al. Normal D-dimer levels in emergency department patients suspected of acute pulmonary embolism. J Am Coll Cardiol 2002;40:1475–8 [DOI] [PubMed] [Google Scholar]
- 17.Zhiguo G, Qingbian M, Yaan Z, et al. Normal blood D-dimer concentrations: do they exclude pulmonary embolism? Chin Med J 2014;127:18–22 [PubMed] [Google Scholar]
- 18.Kucher N, Printzen G, Goldhaber SZ. Prognostic role of brain natriuretic peptide in acute pulmonary embolism. Circulation 2003;107:2545–7 [DOI] [PubMed] [Google Scholar]


