Abstract
Actinomyces meyeri is a Gram-positive anaerobic forming bacterium of the genus Actinomyces, part of the oral cavity's flora, and its classification remains an unresolved issue. It is an extremely rare cause of disease, occurring in middle-aged immunocompetent patients and frequently misdiagnosed as malignancy or lung abscess. A 56-year-old man diagnosed with oesophageal squamous cell carcinoma had an endoscopically placed stent to palliate his dysphagia. Two weeks later he presented with thoracalgia and fever, interpreted as a common lung infection. Owing to lack of improvement, additional examinations were undertaken revealing mediastinum involvement. Unlike the good prognosis usually associated with this infection, the patient eventually died, reflecting the aggressive nature of his underlying condition. To our knowledge, this is the first report of mediastinitis by A. meyeri, supporting the described propensity of this agent to disseminate, particularly to the thoracic cavity, although probably in this case with an iatrogenic contribution.
Background
Since its description in 1911, Actinomyces meyeri went through several name and classification changes; its current denomination dates from 1977 as described by Holdeman et al.1 Originally confused with a fungus (the term Actinomycete derives from the Greek ‘ray fungus’), it was later reclassified as Actinobacterium meyeri in 1938 and is nowadays integrated in the order Actinomycetales. There are still considerable difficulties in properly distinguishing this species from others belonging to the same genus, even with specific 16S ribosomal RNA sequencing.2
From a clinical point of view, A. meyeri is a very uncommon agent of human disease. It manifests as a chronic infection and, contrary to other Actinomyces spp, has a special tendency to disseminate to distant organs. According to a recent review,3 pulmonary infections are the most common clinical conditions caused by this agent, different from other species part of the same genus, which are more frequently associated with cervicofacial disease (‘lumpy jaw’ syndrome).
We report a case of mediastinitis following the placement of an oesophageal stent in a patient with stage IV oesophageal squamous cell carcinoma and an oesophagomediastinal fistula.
Case presentation
A 56-year-old patient with a recent diagnosis of stage IV oesophageal squamous cell carcinoma was admitted to our gastroenterology department due to worsening dysphagia and intense coughing. Medical history included hypertension, anaemia, chronic alcohol abuse and a 30-pack-year smoking habit.
At the time of diagnosis the thoracic CT and positron emission tomography scan identified bone metastases but there was no evidence of pulmonary involvement. The clinical suspicion of an oesophagotracheal fistula was confirmed by upper digestive endoscopy and flexible bronchoscopy. In order to close the fistula and improve the dysphagia, a covered oesophageal stent was placed under direct endoscopic view. This resulted in clinical improvement, with the patient being discharged 5 days later.
In the 3 weeks following discharge the patient was admitted twice in the emergency room with a history of intermittent fever, dyspnoea, cough and bilateral pleuritic chest pain. On the first occasion the clinical manifestations were interpreted as a common nosocomial infection, resulting in a 2-week empirical course of broad spectrum antibiotics (ceftriaxone+vancomycin) with incomplete clinical response. In the second admission to our hospital, he was clearly septic and with severe upper limbs and facial oedema. Chest examination revealed diffuse rhonchi and diminished heart sounds. The patient had mild leukocytosis (11.3/mm3; normal range: 4–10/mm3) and elevated C reactive protein (11.7 mg/dL; cut-off: <0.5 mg/dL), as well as hypoxaemia and hypocapnia in the arterial blood gas analysis. The chest radiograph suggested a widened mediastinum (figure 1), which was confirmed by a thoracic CT scan that identified the presence of a diffuse collection with a hydroaerial level, causing mass effect, shifting thoracic vascular structures (figure 2). The patient had a prior history of an oesophageal fistula, a water-soluble contrast oesophagram was carried out, confirming the presence of a new fistula proximal to the upper side of the prosthesis and extending into the mediastinum (figure 3). About 2 weeks later blood cultures collected at admission became positive for A. meyeri.
Figure 1.
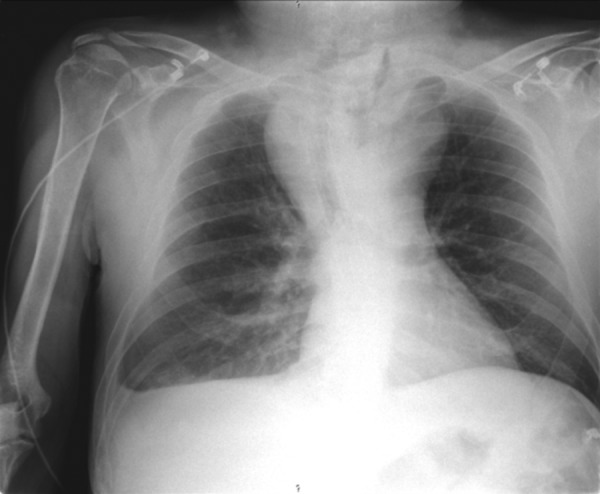
Chest radiograph revealing an enlarged upper mediastinum.
Figure 2.
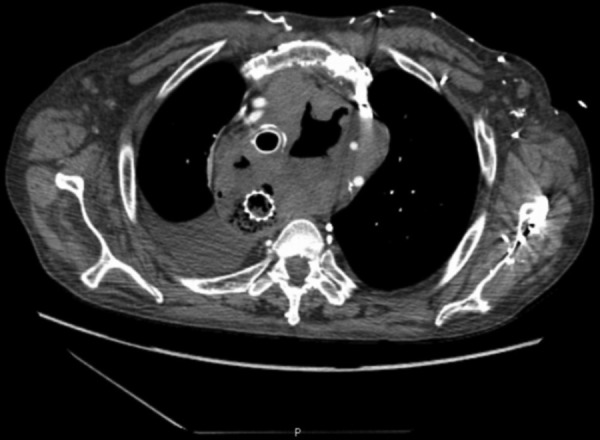
CT scan documented a mediastinal 8 cm diameter collection with a hydroaerial level shifting vascular structures.
Figure 3.
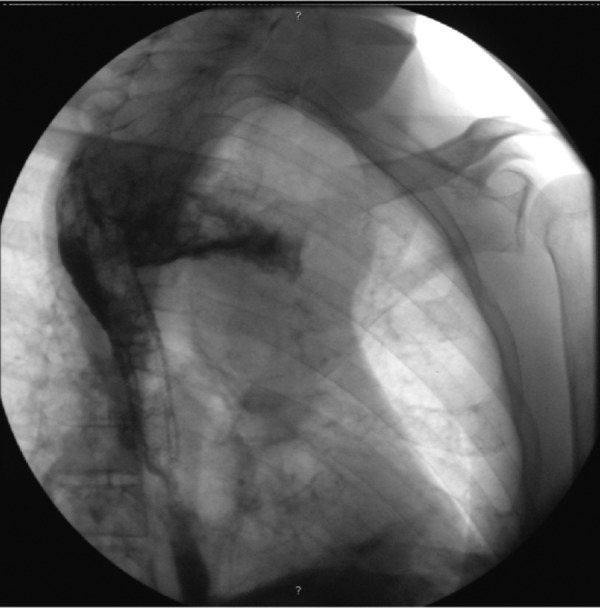
A large contrast extravasation to the mediastinum at the upper top of the metallic stent.
Outcome and follow-up
Exclusive parenteral nutrition and an intravenous antibiotic regimen consisting of imipenem/cilastatin and metronidazole were started, achieving modest clinical response. A gradual worsening of the patient's condition was noted despite a decrease in inflammation markers. Another endoscopic intervention to close the fistula was not undertaken because it probably could not have resolved the septic focus. As an anaerobic agent, A. meyeri infection usually requires surgical debridement. Despite potentially lifesaving, and after multidisciplinary evaluation by the anaesthetic and surgical team, such an intervention was not an option and it was decided to maintain only medical treatment due to his fragile condition.
Unfortunately, about 2 weeks after admission the patient died as a result of serious mediastinal infection, as his prognosis was already dim due to the aggressive nature of his underlying condition.
Discussion
Actinomyces are a group of filamentous bacteria belonging to the class Actinobacteria. In the past, microorganisms under this classification were wrongly classified as fungi because of their tendency to produce branching filaments.4 Sequencing of the 16S rRNA gene led to increased recognition of novel bacterial species, and some of them may become part of Actinomyces, as it has the second most repeatedly encountered novel taxa,5 renewing the interest for this genus' pathological and clinical relevance.
In contrast to other Actinomyces species, A. meyeri is a strict anaerobe, and is part of the normal flora of the oral, gastrointestinal and genital tract. Its pathogenic action is enhanced by other organisms (eg, Fusobacterium spp and Bacteroides spp), by creating an anaerobic environment in which phagocytic activity and mediators are inhibited.6 The Actinomyces species requires enriched culture and its growth is enhanced by a carbon dioxide enriched atmosphere. Colonies appear as Gram-positive bacilli.
The most frequent manifestation of A. meyeri is pulmonary infection, frequently complicated by an empyema. Liver, brain and long bone abscesses are also described,7 reflecting the capacity of this agent to disseminate and to induce necrosis and cavitation. Although the exact mechanism is not well known, it may be related to the high bacteraemia rate associated with A. meyeri—as was the case of our patient.
From a clinical point of view, infection by this agent is especially hard to diagnose as it leads frequently to confusion with other chronic suppurative lung infections8—tuberculosis, histoplasmosis, blastomycosis or with non-infectious entities such as primary or metastatic malignancy, arteriovenous malformation or benign hamartomas. In this clinical setting, differential diagnosis with metastatic spread from the oesophageal cancer was particularly difficult.
The cornerstone of treatment includes a long course antibiotic therapy as well as surgical debridement to remove any necrotic or devitalised tissue. Reflecting this agent's tendency to cause chronic infection, additional 6–12 months of oral penicillin is recommended. Considering its anaerobic nature, metronidazole is frequently added.9
Unlike the good prognosis described in A. meyeri infections,10 this patient undertook a downward clinical course despite empiric antibiotic therapy (actually covering the implicated agent as it included a β-lactam and metronidazole).
In conclusion it seems safe to affirm that several known risk factors were present—his history of alcoholism and the endoscopic procedure undertaken about 3 weeks before the beginning of clinical manifestation. The latter may have led to aspiration of oral and gastric contents, which are A. meyeri natural reservoirs. The presence of an oesophagomediastinal fistula adds to the probable multifactorial nature of this disease in our patient, enabling A. meyeri a direct pathway from his digestive tract to the mediastinum.
Endoscopic procedures in the setting of advanced oesophageal cancer, even if only with a palliative intent, carry high risk of infection and perforation due to the aggressive growth of the tumour into sensible structures like the upper airways and the mediastinum. Such procedures should be undertaken by an experienced team, with the ability of quickly identifying and managing treatable complications, always taking into account the patient's medical condition and informed choices.
Learning points.
Mediastinitis by Actinomyces meyeri is a never before described clinical entity.
New methods of gene sequencing allow relevant bacteria species to be accurately identified and classified.
Infection by A. meyeri should be included in the differential diagnosis of thoracic chronic suppurative infections and pulmonary nodules.
It takes an experienced team to correctly diagnose and treat even unusual complications of endoscopic procedures.
The decision to engage into invasive procedures in patients with advanced oesophageal cancer is frequently difficult, as our objective is to palliate dysphagia or dyspnoea, trying to avoid medical futility and dysthanasia.
Footnotes
Contributors: DFB gathered the data and drafted the manuscript. DRA made significant contributions to the content of the manuscript. NA was involved in critically reviewing the data. CS gave final approval of the version to be published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Holdeman LV, Cato EP, Moore WE. Anaerobe laboratory manual. 4th edn Blacksburg, VA: Virginia Polytechnic Institute and State University, 1977: 61–2 [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing: approved guideline. CLSI Document 18-A Wayne, PA, 2008 [Google Scholar]
- 3.Fazili T, Blair D, Riddell S, et al. Actinomyces meyeri infection: case report and review of the literature. J Infect 2012;65:357–61 [DOI] [PubMed] [Google Scholar]
- 4.Gomes J, Pereira T, Carvalho A, et al. Primary cutaneous actinomycosis caused by Actinomyces meyeri as first manifestation of HIV infection. Dermatol Online J 2011;17:5. [PubMed] [Google Scholar]
- 5.Schlaberg R, Simmon K, Fisher M. A systematic approach for discovering novel, clinically relevant bacteria. Emerg Infect Dis 2012;18:422–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J 2003;21:545–51 [DOI] [PubMed] [Google Scholar]
- 7.Apotheloz C, Regamey C. Disseminated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis 1996;22:621–5 [DOI] [PubMed] [Google Scholar]
- 8.Costiniuk CT, Nha Voduc F, et al. Pulmonary actinomycosis in a male patient with a tracheal bronchus. Can Respir J. 2011;18:84–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeil MM, Schaal KP. Actinomyces species (Actinomycoses) Antimicrobial therapy and vaccines. Volume I: Microbes. 2nd edn Antimicrobe. 2012. [Google Scholar]
- 10.Fahim A, Teoh R, Kastelik J, et al. Case series of thoracic actinomycosis presenting as a diagnostic challenge. Respir Med CME 2009;2:47–50 [Google Scholar]


