Abstract
The purpose of this article is to review normal developmental bladder physiology in infants and bladder dysfunction in conditions such as neurogenic bladder, posterior urethral valves and high grade vesicoureteric reflux. We contrast the classical concept that bladder function in nontoilet-trained children is thought to be ‘reflexive’ or ‘uninhibited’, with the results of more recent research showing that infants most commonly have a stable detrusor. The infant bladder is physiologically distinct from the state seen in older children or adults. The voiding pattern of the infant is characterized by an interrupted voiding stream due to lack of proper urinary sphincter relaxation during voiding. This is called physiologic detrusor sphincter dyscoordination and is different from the pathologic ‘detrusor sphincter dyssynergy’ seen in patients with neurogenic bladder. Urodynamic abnormalities in neonates born with spina bifida are common and depend on the level and severity of the spinal cord malformation. Upper neuron lesions most commonly lead to an overactive bladder with or without detrusor sphincter dyssynergy while a lower neuron lesion is associated with an acontractile detrusor with possible denervation of the external urinary sphincter. In infants with neurogenic bladder, the role of ‘early prophylactic treatment (clean intermittent catheterization and anticholinergics)’ versus initial ‘watchful waiting and treatment as needed’ is still controversial and needs more research. Many urodynamic-based interventions have been suggested in patients with posterior urethral valves and are currently under scrutiny, but their impact on the long-term outcome of the upper and lower urinary tract is still unknown. Cumulative data suggest that there is no benefit to early intervention regarding bladder function in infants with high-grade vesicoureteric reflux.
Keywords: bladder function, children, infant, neurogenic bladder, pediatric, urodynamics
Introduction
The infant bladder is physiologically distinct from the state seen in older children or adults. Given that infants are not yet toilet trained, the assessment of bladder function can be somewhat challenging because adjuncts such as history and questionnaires are not easily obtained. Assessment of infant bladder function has become important given the realization that high bladder storage pressures place the upper tracts at risk [McGuire et al. 1981]. In addition, the association of bladder dysfunction with urinary tract pathology such as vesicoureteric reflux (VUR) and posterior urethral valves (PUVs) has significantly affected patient management [Abraham et al. 2009; Chandra et al. 1996]. It is essential that one keeps in mind the normal parameters of bladder function in infancy in order to contextualize findings from investigations such as urodynamics. In more direct terms, one would not want to call a normal finding ‘abnormal’ and then trigger inappropriate medical treatment or surgical intervention.
The purpose of this article is to review normal developmental bladder physiology in infants and the techniques at hand to allow for assessment of bladder function in this age group. Then we review pathological bladder physiology in infancy in the light of specific conditions such as neurogenic bladder, PUVs and VUR. We then focus on the implications of bladder function assessment on the treatment of these specific conditions. In the conclusion, future areas of investigation are discussed. It is anticipated that this review will provide the busy clinician with current concepts in the assessment and management of pathological bladder function in infancy in order to better serve their tiny charges.
Anatomy and neurophysiology of the lower urinary tract
The lower urinary tract includes the bladder and the urethra and has two major functions: the storage of urine at low pressure (filling phase) and intermittent expulsion of urine at an appropriate time and place (voiding phase). Lower urinary tract function requires coordinated activity of two anatomical compartments, the bladder and the sphincteric mechanism of the bladder outlet. The bladder is a hollow organ formed of three layers, including the outer serosa, the inner mucosa and the intermediate detrusor muscle. The latter is a syncytium of interlacing smooth muscle bundles. The fibers of the detrusor extend into the bladder neck and end in the posterior urethra, where they form the inner layer of the sphincteric mechanism. The sphincteric mechanism is much more developed and powerful in male than female individuals and its mass can further increase under certain conditions, inducing hypertrophy of the detrusor muscle such as in infants born with PUVs. The outer layer of the sphincteric mechanism is formed of striated musculature and is also called the rhabdosphincter. Next to this mechanism, other factors contribute to maintain continence, including the elastic properties of the urethra and the pelvic floor with the levator ani and coccygeus muscle that support and maintain the position of the pelvic organs.
The lower urinary tract has sensitive (afferent) and motor (efferent) innervation. The afferent activity mediates the desire to void. It starts from stretch receptors sited in the bladder wall and reaches the brain via the posterior root of the sacral cord and the spinothalamic tract.
The efferent innervation includes parasympathetic cholinergic nerves and sympathetic adrenergic nerves. The former originate from spinal segments S2–S4 and provide the main innervation to the detrusor muscle. They release acetylcholine which binds muscarinic receptors (M2 or M3) to stimulate detrusor contractions. Efferent sympathetic adrenergic nerve fibers originate from the spinal cord segment T10–L2. These nerves provide the main motor control for the smooth musculature of the bladder neck and posterior urethra. In particular, they release noradrenaline that binds α1A adrenoreceptors at this level, stimulating smooth muscle fiber contractions. Noradrenaline also influences detrusor activity, binding β adrenoreceptors sited in this muscle, which contributes to its relaxation [Franco, 2007]. Parasympathetic and sympathetic nerves are in close juxtaposition, as they merge in a plexus at the base of the bladder, and constantly interact. Finally, the rhabdosphincter is innervated by somatic pudendal nerve fibers that predominantly arise from a specific area, known as Onuf’s nucleus, located in the anterior horn of two segments of the sacral spinal cord. Rhabdosphincter activity is also coordinated with detrusor activity. Its tone increases during the storage phase and decreases during voiding [Thor, 2003; Thor and Donatucci, 2004].
In adults, filling is mainly under the control of the sympathetic nervous system that induces relaxation of the detrusor and contraction of the bladder outlet sphincter mechanisms. During filling the parasympathetic system is inhibited. Voiding is under the control of the pontine micturition centre, which coordinates the micturition reflex as follows. Afferent signals originating from stretch receptors in the bladder wall ascend via the posterior route and reach the micturition centre via the periaqueductal grey matter. The centre in turn activates the parasympathetic nerves, inhibits the sympathetic ones and sends inputs to Onuf’s nucleus [Thor, 2003]. Lower urinary tract function is eventually under the control of the brain. The cerebral cortex is able to determine voluntary contractions of the rhabdosphincter and levator ani to resist micturition and control the trigger inputs from the pontine micturition centre, thereby suppressing detrusor contractions.
In infants, this network is not fully developed. The parasympathetic nerves are not completely inhibited during bladder filling, so that bladder contractions can occasionally occur in this phase, and detrusor contraction and sphincteric relaxation are dyscoordinated during micturition, often resulting in incomplete bladder emptying [Neveus and Sillen, 2013].
Normal bladder function in infancy
The infantile bladder reflects the nature of the child who is growing and under constant body change and adaptation. The bladder capacity in the neonatal period is around 10 ml and it increases significantly in the first year of life. Bladder function in infants behaves very differently compared with older children and adults.
Classically, bladder function in nontoilet-trained children was thought to be ‘reflexive’ or ‘uninhibited’, resulting from a triggered spinal cord reflex arc without central nervous system (CNS) inhibition. This theory considers that the child voids when the bladder is full to the point when a spinal reflex is triggered leading to a reflex detrusor contraction [Hjalmas, 1976]. In contrast to classic theories, more recent studies have shown that bladder function in neonates and infants is stable [Yeung et al. 1995] or rarely demonstrates instability [Bachelard et al. 1999]. Urodynamic studies in infants showed that their bladders are frequently stable and do not show or show minimal uninhibited contractions during the filling phase. A premature contraction at the beginning of the cystometric phase may be the result of the catheter or the contact of the filling fluid [Bachelard et al. 1999; Neveus and Sillen, 2013]. It is now believed that under ideal conditions, most infant bladders should be stable and significant uninhibited contractions should be considered abnormal.
Another peculiar characteristic of bladder function in a healthy infant is the physiologic dyscoordination of the detrusor-sphincteric unit during micturition. It has been observed that the voiding pattern of the infant is characterized by an interrupted voiding stream due to lack of proper urinary sphincter relaxation during voiding. This is consequently accompanied by a higher detrusor pressure during micturition, especially in boys, and may be associated with incomplete bladder emptying. These changes improve as the child grows and are not commonly seen in older children. ‘Dyscoordinated voiding in infancy’ is a physiologic and transient event that subsides as the child grows, which is different from the ‘detrusor sphincter dyssynergy’ seen in patients with neurogenic bladder secondary to a neurological insult to the nervous system. The higher incidence of urinary tract infection (UTI) and reflux in male infants may be related to the elevated voiding pressures seen in this group [Chandra et al. 1996; Podesta et al. 2004]. It has been reported that the bladder volume at which the infant voids is not constant and may vary from 30% to 100% of the expected bladder capacity [Jansson et al. 2000; Holmdahl et al. 1996]. Essentially the bladder capacity in children grows from 10 ml in the neonate to around 48–60 ml in a 9 month old and will have another period of significant growth in the third year of life, which will reach 123–150 ml [Sillen 2004; Jansson et al. 2000].
Another piece of evidence against a totally uninhibited bladder in nontoilet-trained children came from natural filling urodynamic studies that used concomitant polysomnography to assess sleep pattern during voiding [Yeung et al. 1995]. It showed that the healthy infant has a stable bladder, voids with detrusor sphincter dyscoordination and most interestingly that voiding never happens during quiet sleep. The full bladder and micturition disturb the infant and neonate and they show signs of cortical arousal; however after voiding they go back to sleep.
The infantile bladder slowly progresses to the adult type of bladder which is characterized by socially conscious and voluntary voiding. This is achieved through an active learning behavioral process in which the child develops bladder continence and micturition control. This process is the result of the action of the parasympathetic and sympathetic central nervous systems on the detrusor and internal urethral sphincter, associated with voluntary control of the striated muscles of the pelvic floor and external urethral sphincter [Yeung et al. 2010].
Assessment of bladder function in infants and nontoilet-trained children
Challenges assessing bladder function in infants
For obvious reasons, many tools usually employed for the assessment of bladder function in older patients cannot be used in infants, such as questionnaires to assess symptoms or uroflowmetry. Many congenital anomalies of the urinary tract are diagnosed in the first year of life, triggered by an abnormal antenatal ultrasound (US) or UTI. Some of these congenital conditions may affect bladder and renal function; that is, massive VUR associated with high post-void residuals, PUVs and Prune–Belly syndrome. As upper tract dilatation and renal functional impairment secondary to prenatal maldevelopment are not uncommon in infants, it can be difficult to determine to what extent baseline abnormal renal function and renal functional deterioration in the first year of life are due to the bladder dysfunction itself or to the natural history of the associated condition. Video urodynamics has been used for investigation of selected older toilet-trained children with UTIs and bladder dysfunction [Glazier et al. 1997; Hoebeke et al. 2001]. Despite reported small series of video urodynamics in infants (median age 9 months) with VUR [Podesta et al. 2004], its role in nontoilet-trained infants has not been determined. Popoff mechanisms to relieve bladder pressure may be responsible for inaccuracy in detrusor pressure measurement. In such suspected cases, video urodynamics may help to account for this occurrence.
Technical aspects when performing urodynamics in infants
The general principles applied to perform urodynamics in older children and adults are also applied to infants, but some small adjustments must be made. In order to obtain an accurate urodynamic assessment of bladder function in children, one must provide a playful and relaxing environment for the patient during the test. In contrast to adults, children may react to an unfamiliar situation and not cooperate with the test. It is important that the professional performing urodynamics acts in a calm and gentle way with the child and is able to transmit trust to the parents. Anxious parents may make the child insecure and noncooperative. There is no need for sedation to perform urodynamics and most pediatric urologists agree that this may alter the results.
Cystometry
Getting prepared
Before bringing the child into the exam room, a quick briefing with the parents will provide the necessary explanation about what will happen within the urodynamic suite. It is important to remember that calm parents during the exam will result in a more cooperative child and more accurate results. Relaxing music, a comfortable temperature and warm room lights will make the child more content and cooperative.
Positioning
Urodynamics in older children may be performed with the patient in either the supine or sitting position; however, for obvious reasons infants are normally studied lying supine on the urodynamics exam table.
Catheters
The choice of urethral catheter is usually guided by the age of the patient. Infants should have a 6 or 7 Fr double lumen urodynamics catheter gently inserted using plenty of lubricant to minimize the discomfort. Catheters with balloons should not be used. For natural filling urodynamics a 5 Fr suprapubic catheter is utilized. A rectal catheter or sponge transducer is usually inserted to measure intra-abdominal pressure.
Filling the bladder
In general room temperature sterile water at a low filling rate should be used [Zerin, 1993]. It is interesting to note that a prospective, randomized study comparing 91 children with mean age 8.6 years undergoing urodynamics with room temperature versus prewarmed saline infusion showed that the systematic use of a warm infusion is not necessary on a routine basis. However, they found that prewarmed saline infusion is more physiologic in infants and they recommend its use for children younger than 2 years of age [Chin-Peuckert et al. 2004]. A filling rate of 10 ml/min is adequate for most children, but as a rule of thumb, calculated bladder capacity divided by 10 can be used [Neveus et al. 2006]. Most urodynamic machines have an injection pump with an adjustable filling rate, but the fluid bag can be placed at 30–40 cm height from the bladder level and gravity drip can be used. Gas (CO2) has been described, but it is an irritant to the bladder and is not commonly used.
Electromyography
There are some reports suggesting the possibility of dysfunctional voiding present in healthy nontoilet-trained infants [Jayanthi et al. 1997]. However, the use of EMG to rule out detrusor sphincter dyssynergism (DSD) in healthy infants with lower back stigmata of occult dysraphism is not well established. The interpretation of DSD in healthy infants with minimal neurologic conditions such as occult dysraphism is challenging due to transient detrusor sphincter dyscoordination, which is nonpathological and frequently observed in this age group. This dyscoordination pattern disappears in older children. Despite controversies, in a research and academic environment, EMG can be used during urodynamics in infants with established neuropathic lesions, such as open spinal dysraphism, in order to rule out DSD. Some infants with myelomeningocele may present rapid bladder function deterioration in the first few months of life and excessive EMG activity during voiding may help to identify children with high-risk bladders.
Uroflowmetry
Due to the technical challenge in collecting voided urine from infants in an electronic scale, uroflowmetry and flow/pressure studies are not usually obtained in infants. Custom-made US flow probes connected to a flowmeter mounted on the penis have previously been used to assess flow in infants [Olsen et al. 2010]. Assessing neonates using this method found that 34% had dyscoordinated flow and that voiding tended to occur with the child awake [Olsen et al. 2009].
Video urodynamics
The combination of fluoroscopy may aid in assessing the presence of reflux or bladder/urethral anomalies during filling and voiding. Although this technique may be indicated in older children, in our practice, it has limited use in infants.
Four-hour observational urodynamics
The four-hour voiding observational test is a noninvasive method described to assess bladder function in infants and nontoilet-trained children [Holmdahl et al. 1996]. The test consists of a 4 h observation period of the voiding pattern in an infant who is free to play in the observation area. The test tries to reproduce normal life as much as possible; milk feeding and oral fluid intake are provided as usual. The diapers are initially weighed. The infant is observed by the parent under the surveillance of a trained urodynamics nurse or urotherapist and as soon as the infant voids, the diaper is weighed and an US suprapubic bladder scan is done for assessment of postvoid residual. A voiding chart is recorded with the number of voids, voided volume, bladder capacity and postvoid residual volumes over 4 h. This test is easy to perform and can be used to monitor bladder function in this age group. In addition, it may help to identify infants who need treatment or bladder catheterization.
Natural filling cystometry
This test can be done using a suprapubic or urethral catheter. It was previously reported in a group of 21 children (mean age 5.4 months; 16 boys, five girls) who underwent open pyeloplasty or nephrectomy [Yeung et al. 1995]. The investigators inserted a 5 Fr suprapubic catheter during surgery and it was left in place postoperatively for bladder drainage. With Research Ethics Board approval, urodynamics were done 3 days after surgery to ensure bladder activity was restored. The suprapubic catheter and rectal catheters were used to measure bladder and rectal pressures during the ‘natural filling’ urodynamics using a portable ambulatory set connected to a notebook. The tests were typically started while the children were sleeping and continued after they were awake. The authors concluded that the normal infant bladder was stable and emptied almost completely, incomplete coordination between the bladder and sphincter can be normal in infants, and that micturition never occurs during quiet sleep in this age group. In another study, the authors used natural filling urodynamics in 16 boys (mean age 3.4 years) with a history of PUVs and showed that their bladders had pronounced instability during the day and were stable at night [Holmdahl et al. 1997].
Pathological bladder function in infants and specific conditions
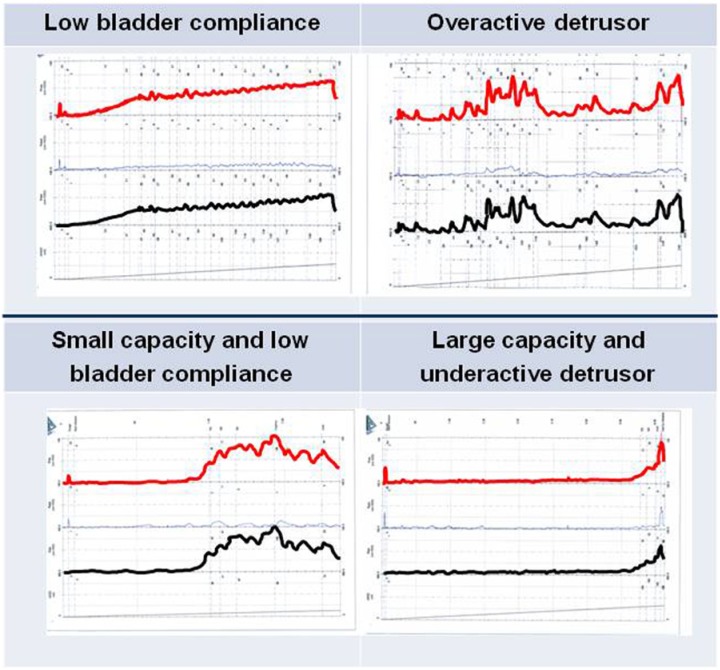
Given that there are reliable techniques for assessing bladder function in infants with pathological conditions such as neurogenic bladder and PUVs, we next examine the role of urodynamic assessment in such clinical situations. This will ultimately impact the clinical decision regarding whether or not intervention would be beneficial and what form such intervention would take. Figure 1 shows the most common pathological patterns found on urodynamics during infancy.
Figure 1.
Different urodynamic patterns in infancy.
Urodynamic abnormalities due to spine malformations
Myelomeningocele
Spinal dysraphism associated with myelomeningocele is the most common etiology of neurogenic bladder in children and it accounts for 80–90% of cases. The more severe cases are usually associated with lower extremity motor deficits and a complete neurological assessment should be performed. Urodynamic abnormalities in these neonates are common and will depend on the level and severity of the spinal cord malformation. Detrusor behavior may vary depending on the presence of either an upper or a lower motor neuron lesion, which can also be associated with dyssynergy or denervation of the urinary sphincter. Neurogenic bladder may be associated with long-term decrease in renal function and therefore it is important to obtain a baseline serum creatinine level as well as to monitor it over time. Torre and colleagues reported an incidence of up to 20% of some degree of renal impairment in 502 patients born with spinal dysraphism and neurogenic bladder [Torre et al. 2011]. US of the urinary tract should be obtained shortly after the child is born and repeated after the myelomeningocele closure. Up to 90% of neonates with spinal dysraphism have normal upper tracts on US in the first month of life [Bauer 2008]. However, 15% will have deterioration of their kidney function later in life and among them there is a significantly higher incidence of detrusor overactivity on childhood urodynamics (63%) compared with those with normal function of both kidneys (24%) [Thorup et al. 2011]. These findings indicate that early assessment of bladder function is recommended in order to identify the patients at risk of renal deterioration and to manage their condition accordingly. Upper tract abnormalities in a neonate with myelomeningocele are present in 15–20% of cases and can be secondary to functional bladder obstruction. A major limitation in obtaining a urodynamic assessment once a child with open spinal dysraphism is born is the urgent indication for surgical repair and the contraindication to lay the child on their back for the urodynamics. Positioning neonates on their back to perform this test may increase the chance of complications at the surgical site and skin dehiscence. For this reason, an US and postvoid residual measurement should be done initially and urodynamics and voiding cystourethrogram (VCUG) can be performed at around 2 months of life, once the child can be safely positioned for the test. The goal of the assessment of the infant with neurogenic bladder is to determine risk factors for upper tract deterioration, such as DSD, VUR, incomplete emptying with postvoid residual, low bladder compliance and functional bladder outlet obstruction. If high postvoid residual is confirmed, the child should be started on clean intermittent catheterization (CIC). VCUG rules out VUR, PUVs and potential ‘popoff mechanisms’ for bladder pressure relief in high-risk patients. In a study of 36 infants with myelodysplasia, urodynamic evaluation showed 50% with DSD, 25% with synergy and 25% with no activity of the sphincter; 13 (72%) with dyssynergy developed hydroureteronephrosis by 2 years of age compared with only 22% among the patients with synergy and 11% with absent activity [Bauer et al. 1984]. Infants with DSD are at high risk of deterioration of the urinary tract and should be followed closely. Early intervention is recommended in these patients. Radiological surveillance to detect upper tract deterioration in children with neurogenic bladder secondary to spina bifida is controversial. Kauffman and colleagues reported on 181 patients who were under radiological surveillance with urodynamics being performed only when there was upper urinary tract deterioration, recurrent UTI or urinary incontinence; 79 patients had upper tract deterioration and follow-up studies after CIC and pharmacologic treatment showed improvement in 69% of the upper tracts. However, bladder compliance improved in only 42% [Kaufman et al. 1996]. Furthermore, children with high-risk neurogenic bladder treated expectantly have greater chance of requiring enterocystoplasty compared with those who have early treatment with CIC and anticholinergics: 41% versus 17% respectively [Kaefer et al. 1999]. Children with a history of surgery for myelomeningocele closure should have yearly follow up with urodynamics and US until 5–6 years of age and then a yearly US. After this age, urodynamics should be repeated only if tethering of the spinal cord or a hostile bladder environment is suspected, such as magnetic resonance imaging (MRI) with low level of the spinal cord conus, hydronephrosis on the US, severe constipation, increased urinary accidents or recurrent UTIs.
Occult spinal dysraphism
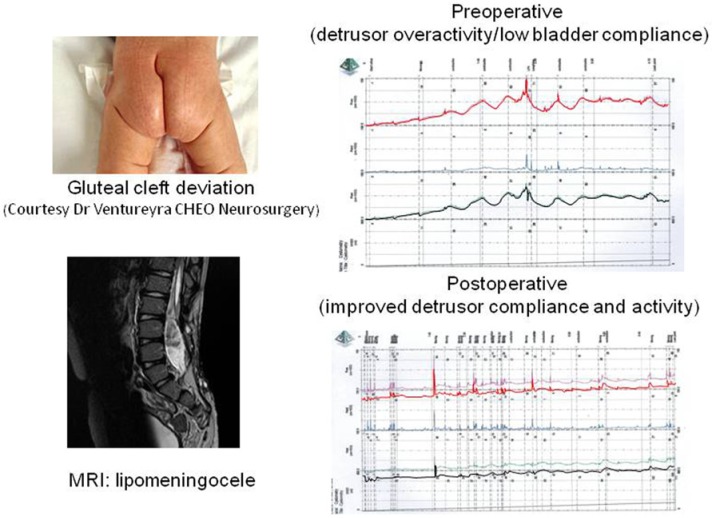
Some patients with milder degrees of spinal cord malformation may present with an occult or covered spinal dysraphism, such as lipomeningocele, split cord malformation, cauda equina lipoma, dorsal dermal sinus, sacral agenesis, neural enteric cyst or filum terminale syndrome. These children may have nerve root compression causing cauda equina or associated tethering of the spinal cord with clinical symptoms caused by abnormal spinal cord fixation. When the spinal cord is tethered, there is a limitation of its motion, which may lead to ischemic damage of the neural tissue secondary to neural root stretching and anaerobic metabolism. Healthy infants with occult spinal dysraphism are being diagnosed more frequently due to the wide use of US for assessment of lower back stigmata, such as skin dimple/tag, hairy tuft, vascular malformation, sacral dermal sinus or gluteal cleft deviation. Older children may be diagnosed due to lower back pain, leg weakness, foot asymmetry, chronic constipation or urinary incontinence. We are seeing an increasing number of normal infants born with significant lower back stigmata of occult spinal dysraphism who are referred for urological assessment to rule out bladder dysfunction that could be associated with a tethered cord. In the initial assessment of these patients one must obtain a history of previous urological problems such as UTI, abnormal voiding, severe constipation or abnormalities on the prenatal US. Physical exam will confirm the lower back stigmata and neurological exam is usually normal. In a study that assessed 123 infants with occult spinal dysraphism, urodynamics were abnormal in 35% versus 11% of the patients who underwent surgery for tethered cord release compared with those who did not have surgery (p < 0.002); the most common urodynamic abnormalities were low bladder capacity and involuntary detrusor contractions [Lavallee et al. 2013]. Children with neurogenic detrusor overactivity who undergo tethered cord release have 59% improvement in urodynamics postoperatively; however the level of the spinal conus on the MRI does not predict the urodynamic outcome (Figure 2) [Guerra et al. 2006].
Figure 2.
Lipomeningocele urodynamics pre and post tethered cord release. MRI, magnetic resonance imaging.
Anorectal malformation
Imperforate anus is a condition in which the child has a flattened perineum with no anal orifice and a possible cutaneous fistula. It may appear isolated or associated with other anomalies such as VATER or VACTERL syndromes. A fistulous communication with the urinary tract is commonly associated with a high rectal lesion. Imperforate anus has a 48% incidence of associated genitourinary anomalies and the severity of these anomalies is related to the level of the fistula between the blind-ending rectum and the genitourinary tract. A fistula between the rectum and the genitourinary tract was present in 89% of a series of 244 patients [Rich et al. 1988], especially in male patients with high anorectal malformation and female patients with cloaca. Thirty percent of the neonates with imperfortate anus have sacral dysplasia [Carson et al. 1984], which in contrast to sacral agenesis, is usually not associated with bladder dysfunction. A report of 90 children with anorectal malformations, who had 163 urodynamic studies at mean age 17 months, showed normal lower urinary tract function in 98% (46/52) of the children with normal sacrum or sacral dysplasia, and severe lower urinary tract dysfunction in 55% (21/38) of the patients with marked/partial sacral agenesis. There was no correlation between the type of sacral agenesis and a specific urodynamic pattern [Boemers et al. 1996]. US of the urinary tract is indicated for the initial assessment of infants with anorectal malformation in order to rule out congenital anomalies. US of the spinal cord before 6 months of life, while the spine is not completely calcified, can screen for spinal malformation, and if confirmed, MRI will show details of the abnormal anatomy. Early urodynamic studies in anorectal malformation are indicated when sacral agenesis is confirmed on the spinal MRI due to the higher association with neurogenic bladder in these cases. The most common pattern on urodynamics of patients with imperforate anus and anorectal malformation is an overactive detrusor due to an upper motor neuron lesion along with occasional DSD [Boemers et al. 1996]. However, an acontractile detrusor (lower motor neuron lesion) with denervated sphincter on the EMG may be present [MacLellan and Bauer, 2011]. Posterior sagittal anorectoplasty may lead to iatrogenic injury of the pelvic plexus and result in bladder denervation and acontractile detrusor; however the incidence of this complication is low [Boemers et al. 1996]. A specific type of myelodysplasia associated with anorectal malformation is seen in the Currarino syndrome, which includes sacrococcygeal anomaly, presacral mass (anterior meningocele, teratoma or cyst) and anorectal malformation. Urodynamics may show detrusor overactivity and DSD [Lee et al. 2012]. Table 1 summarizes common types of urodynamic patterns and treatments for different conditions in children.
Table 1.
Common types of urodynamic patterns and treatments for different conditions in children.
| Category | Possible conditions | Expected urodynamic pattern | Possible treatment in infancy |
|---|---|---|---|
| Urodynamic abnormalities due to malformations/damage of the lumbar-sacral spine | Open spina bifida (Myelomeningocele) | It depends on the level of the neurologic lesion:
|
Early prophylactic treatment (clean intermittent catheterization and anticholinergic) versus initial watchful waiting and treatment as needed |
| (In general, 50% have DSD, 25% synergy and 25% have no activity of the sphincter) [Bauer et al. 1984] | |||
| Tethered spinal cord | Low bladder capacity and overactive detrusor [Lavallee et al. 2013] | Tethered cord release | |
| Anorectal malformation (imperforate anus, Currarino syndrome, VACTERL, sacral agenesis, etc.) | Overactive detrusor due to an upper motor neuron lesion with occasional DSD. However, an acontractile detrusor (lower motor neuron lesion) with denervated sphincter on the EMG may be present | ||
| Urodynamic abnormalities due to damage of the CNS | Perinatal ischemia (cerebral palsy) | Safe storage pressure, good compliance and bladder sensation of fullness in older children. Overactive detrusor in 52% and DSD in 62% [Richardson and Palmer, 2009] | No urodynamic-based treatment generally recommended in infancy |
| Urodynamic abnormalities due to intrinsic bladder (detrusor, trigone, or bladder-neck) abnormalities | Primary VUR | In infant males with dilated reflux:
|
No urodynamic-based treatment generally recommended in infancy |
| (Female infants with dilating VUR were found to have a pattern consistent with the second group of infant males) | |||
| Posterior urethral valve | Infants and young boys: small, poorly compliant and overactive bladders | Alpha-blockers and anticholinergics, bladder-neck incision, intermittent catheterization, vesicostomy | |
| Post-pubertal age group: myogenic failure [Lal et al. 1999] | |||
CNS, central nervous system; DSD, detrusor sphincter dyssynergism; EMG, electromyography; VUR, vesicoureteric reflux.
Urodynamic abnormalities due to damage of the CNS (perinatal ischemia, trauma, meningitis, tumors)
A number of conditions can affect the CNS in infants, including perinatal asphyxia, incidents involving hypoxia to the brain (such as near drowning), encephalitis or meningitis, trauma including physical brain injury and the shaken baby syndrome, tumors, toxins, inborn metabolic errors and severe jaundice. Most patients with these conditions present with infantile cerebral palsy. Infantile autism is another condition that can have similar effects on the lower urinary tract. It is noteworthy that patients with CNS damage and autism can present with associated feeding problems, which may limit their fluid intake during the day and may cause low urine output that can at times be confused with urinary retention. Since the child is not voluntarily controlling their voids, CNS lesions have, in general, no major impact on lower urinary tract function in the first year of life. These patients can, instead, experience difficulties with toilet training [Wu, 2010; Silva et al. 2009], and show signs of severe lower urinary tract dysfunction with upper urinary tract deterioration later on in life [Richardson and Palmer, 2009; Murphy et al. 2012; Gundogdu et al. 2013]. The risk of lower urinary tract dysfunction increases along with the degree of motor functional impairment [Bross et al. 2007]. Clinical monitoring of UTIs and periodic US of the upper urinary tract and bladder should be recommended. Of note, US assessment of bladder wall thickness does not seem to be a good predictor of associated dysfunction [Silva et al. 2010].
Cerebral palsy
Children with cerebral palsy present with a nonprogressive motor disorder caused by a CNS insult, usually occurring during gestation or the perinatal period [Jones et al. 2009], which most often includes perinatal hypoxia. Due to the lack of continence in a nontoilet-trained infant, cerebral palsy usually does not merit investigation in this age group. Older patients will present with dysfunctional voiding symptoms, such as urinary frequency, incontinence and UTI in 30% of cases [McNeal et al. 1983; Decter et al. 1987]. Despite voluntary bladder control, previous series estimated that these children have a 75% incidence of neurogenic bladder, with upper motor neuron pattern the most common type of dysfunction (neurogenic overactive bladder) [Bauer, 1991]. In a recent study [Richardson and Palmer, 2009], voiding and urodynamic patterns in 31 children (median age 9.8 years) were reviewed and showed that 98% (28/31) voided spontaneously and only 10% (3/31) required CIC. However, 24 patients (77.4%) were using a diaper. Seven (22%) children were continent day and night, 12 (38.7%) had daytime incontinence and nocturnal enuresis, 11 (35.5%) had daytime incontinence and only one (3.2%) had nocturnal enuresis. During urodynamics, there was a safe storage pressure (mean 9.8 cm H2O), good compliance (mean 34.3 ml/cm H2O), sensation of bladder fullness (74%), uninhibited contractions (52%) and DSD (62%). Continent children tended to have a larger bladder, lower bladder pressure at capacity, higher compliance and fewer uninhibited contractions. However, sphincter activity was similar in both continent and incontinent groups. The only statistically significant difference was in bladder sensation, which was decreased in the incontinent group. Urodynamic studies have few indications in children with cerebral palsy as they are not predictive of continence and their use is reserved for cases in which high bladder storage pressure is suspected, including abnormal urinary tract findings on US.
Urodynamics in intrinsic bladder and urethral abnormalities (VUR and PUVs)
Vesicoureteric reflux
It has long been recognized that bladder dysfunction is prevalent in children with VUR [Seruca, 1989]. In infants, primary VUR was thought to be due to a congenital deficiency of the subureteric tunnel. However, seminal work by Sillen and others has shown that infants with VUR also have quantifiable bladder dysfunction. Sillen and colleagues studied five female and 11 male infants with dilating VUR by video cystometry and found three distinctive urodynamic patterns [Sillen et al. 1996]. In infant males with dilated reflux 50% had very high bladder pressures during voiding contractions, low bladder capacity and overactive detrusor activity during filling. This pattern was termed a ‘hypercontractile bladder’. The authors noted that 25% of infant males had overdistended, large-capacity bladders with normal pressure levels at contraction, high residual urine and detrusor overactivity. In both of these groups it was noted that increased pelvic floor activity was seen during voiding, indicating detrusor sphincter dyssynergy. There was a third group of infant males with reasonably normal urodynamics, who had voiding pressures minimally elevated over that seen in normal children. Female infants with dilating VUR were found to have a pattern consistent with the second group of infant males. It should be noted that an age-matched control group was not included in this study. In addition to invasive urodynamic testing, Sillen observed voiding patterns in 33 male and eight female infants with dilating reflux and compared them with their urodynamic findings [Sillen et al. 1999]. The noninvasive assessment included observation of the voiding pattern for 4 h and registration of postvoid residual by US after each void. Number of voids in 4 hours, voided volume, residual urine and functional bladder capacity (highest sum of voided volume and residual urine at one void) were recorded. The aforementioned hypercontractile urodynamic pattern could clearly be identified at free voiding by small, frequent voids with an increased rate of interrupted voiding. This same pattern may be seen in 15% of healthy infants, but the frequency and intensity of the pattern were more significant in refluxing male infants. Likewise the urodynamic pattern of high-capacity bladder could be identified on free voiding as increased capacity with increased voided volume and postvoid residual. Residual urine was increased in all groups of refluxing infants compared with healthy infants, who were historical controls [Holmdahl et al. 1996]. Thus, noninvasive observation could preclude the need for invasive studies in this cohort.
Chandra and colleagues studied 39 male and 22 female infants, including 40 with VUR and 21 with no reflux or obstruction [Chandra et al. 1996]. The findings differed strikingly between genders, although 97% of male and 77% of female infants had some form of bladder dysfunction. High voiding detrusor pressures (>40 cm H2O) were seen in 92% of male and 66% of female infants, whilst high postvoid residual urine was seen in 13% of male and 23% of female infants, and detrusor overactivity was seen in 30% of male infants. Voiding detrusor pressure was significantly greater in male than female infants and in male infants with dilating reflux compared with male infants with low-grade or no VUR. They followed up on 15 infants with high voiding detrusor pressures and documented normalization of the urodynamic findings and resolution of reflux in the majority. Of interest is the fact that Chandra and colleagues included age-matched controls to put the findings in refluxing infants in perspective.
Yeung and colleagues studied 42 infants with primary VUR and found six distinct urodynamic patterns [Yeung et al. 1998]. They described normal, normal immature, unstable, inadequate voiding, dyssynergic and obstructive patterns. Of those studied, 41% had the normal immature or dyssynergic patterns, which showed infrequent detrusor activity during filling, dyssynergic voiding with high elevations of detrusor pressure and pronounced interruptions to the urinary stream. Podesta and colleagues had findings similar to Yeung and colleagues when they evaluated 12 infants with VUR and 10 age-matched controls [Podesta et al. 2004]. He found low bladder capacities for age in 10 patients and that very few patients had detrusor overactivity during filling. In the majority, voiding alternated with peaks of high detrusor pressure associated with intermittent external sphincter contractions with normal coordinated micturition. Residual urine was only seen in three cases. In patients with VUR, reflux occurred in five during an increase in bladder pressure and in seven during stable filling. Of importance, Podesta and colleagues did not find any significant difference in the cystometric studies between patients with VUR and controls. They hypothesized that the pattern of decreased cystometric capacity, normal detrusor activity during filling and high voiding pressure with sphincteric overactivity coexistent with normal micturition represents a developmental stage of normal bladder control.
As can be seen from the above data, the results of these investigators are heterogeneous. This may be explained by patient selection and urodynamic technique, such as the use of urethral versus suprapubic catheter, or in the case of Chandra and colleagues, the use of CO2 cystometry. It is clear that the infant bladder undergoes normal developmental changes as the child matures. Some of these normal physiological changes may enhance the occurrence of VUR in susceptible individuals, as similar findings may be seen in infants without VUR. Of interest, careful observation of infant voiding patterns over 4 h may preclude the need for invasive studies.
Posterior urethral valves
PUVs cause bladder outlet obstruction, which may have upstream effects on the bladder and kidneys. After successful valve resection, the bladder may still demonstrate significant dysfunction. The urodynamic consequences of PUV are varied and may include normal bladders, overactive bladders, small capacity poorly compliant bladders and bladders with myogenic failure [Peters and Bauer, 1990]. In general, the small poorly compliant bladders and overactive bladders are seen in infants and young boys, whilst those with myogenic failure are seen in the postpubertal age group [Lal et al. 1999]. The group at Bambino Gesù hospital in Rome [De Gennaro et al. 2000] examined whether the urodynamic pattern from infancy to adolescence changed in a predictable manner. They studied 30 patients with PUV and no voiding symptoms with a mean of 2.8 urodynamic tests over the course of several years. Bladder dysfunction was found in 70% of boys at their first evaluation and in 60% at the last. They noted that the urodynamic pattern changed in 25 patients, with hypercontractility being the pattern most prevalent soon after valve ablation, and changing to a hypocontractile bladder after puberty. Thus they concluded that detrusor overactivity in infants and young boys with PUV leads eventually to detrusor failure in adolescence. The implications of bladder dysfunction in patients with valves are also well known. Poor bladder compliance and detrusor overactivity were shown to significantly correlate with renal functional impairment; however, normal urodynamic findings did not preclude upper tract deterioration [Ghanem et al. 2004].
It has long been recognized that pressure popoff mechanisms such as a large bladder diverticulum, patent urachus, unilateral VUR or urinoma may have beneficial effects on renal function [Hoover and Duckett, 1982]. This is thought to be the result of high bladder pressures being buffered and thus not affecting renal development. There is also evidence that such popoff mechanisms may have a beneficial effect on detrusor function in boys with PUV [Kaefer et al. 1995]. Amongst a cohort of 55 boys, popoff mechanisms were present in 39; of these 34 (87%) had favorable bladder outcomes. In contrast, of the 16 without such popoff mechanisms, only nine (55%) had favorable bladder outcomes. In fact, a direct correlation between the absolute number of popoff mechanisms and favorable bladder outcome was noted, further solidifying the relationship. In this study, bladder outcome was evaluated with clinical and urodynamic assessment.
Aside from the use of invasive urodynamics to assess the valve cohort, the Goteborg group advocated 4 h voiding observation, similar to that described above by Sillen and colleagues. In their study, 24 boys younger than 4 years with PUV were compared with healthy age-matched controls. The number of voids was higher, voided volume was lower and residual urine volume was higher in the PUV group. Interestingly, there was no difference in the pattern of voiding before and after removal of anatomical obstruction in the PUV group [Holmdahl et al. 1998].
Therefore, it can be seen that the bladder in patients with PUVs may be permanently affected by the obstruction, even if valve resection occurs early in life. Furthermore, bladder dysfunction evolves from a small-capacity, hypercontractile bladder in infancy to that of myogenic failure in adolescence. Although the focus of this paper is the infant, it is important to put the bladder dysfunction seen in PUV in full context.
Implications of urodynamic assessment for treatment
The detection of signature bladder dysfunction associated with underlying diagnoses is only of clinical relevance if management of such dysfunction benefits the course of the patient. In situations such as neurogenic bladder and PUVs that is arguably the case; whilst for VUR the situation is less convincing. In this section, we examine the impact of treatment of bladder dysfunction on the outcome of these varied clinical conditions.
Neurogenic bladder
The importance of urodynamic assessment of the neurogenic bladder in cases of spina bifida was brought to the fore by McGuire and colleagues when they described the concept of detrusor leak point pressure. They found that if the intravesical pressure at the time of urethral leakage was 40 cm H2O or less, VUR was not seen and only 2/20 patients showed ureteral dilatation on imaging studies. In contrast, in those with leak point pressures above 40 cm H2O, 69% showed VUR and 81% showed ureteral dilatation on imaging [McGuire et al. 1981]. Since the publication of this pioneering article, many other studies have confirmed the risk of high bladder storage pressure to the upper tracts. The important question that must be posed is whether early intervention to manage the bladder can improve outcomes [Morrisroe et al. 2005]. The answer is a resounding ‘yes’, as documented by the group at Boston Children’s Hospital. They studied 21 newborns with myelodysplasia with high bladder pressure and DSD treated prophylactically over 5 years. They compared them to a group of similar newborns with the same findings who were treated expectantly during the previous 7 years. The prophylactic intervention comprised CIC and oxybutynin. When comparing the intervention group with the control group, only 8% of the former had new onset hydroureteronephrosis versus 48% of controls. There were no significant patient harms seen with prophylactic therapy. It was the conclusion of these authors that expectant therapy could no longer be advocated when ‘at risk’ infants are identified, as the prophylactic treatment is so effective [Kasabian et al. 1992]. Subsequently, this strategy has been confirmed to also preserve renal function [Dik et al. 2006]. In addition, the Boston group assessed urological outcomes in patients with myelodysplasia who were documented to have high-risk bladders in infancy due to the presence of DSD or high filling or voiding pressure. Intervention in this group comprised intermittent catheterization and anticholinergic therapy. They were compared with controls with the same high-risk urodynamic parameters who did not receive early therapy. The authors found that the requirement for augmentation cystoplasty on long-term follow up was 17% in the prophylactic treatment group versus 41% in the observation group. Thus, not only can the upper tracts be protected by prophylactic therapy, but it also decreases the future need for bladder augmentation in this group of high-risk infants [Kaefer et al. 1999].
There are other methods one can use to remedy the hostile bladder in infants with spina bifida. The most common alternative would be the performance of a cutaneous vesicostomy. This procedure is often performed when management with intermittent catheterization and anticholinergics has failed. It has been shown that cutaneous vesicostomy can improve or completely resolve hydronephrosis, decrease the incidence of UTI, allow for resolution of VUR and lead to improvement or stabilization of renal function [Morrisroe et al. 2005]. Complications of cutaneous vesicostomy include stomal stenosis, prolapse and bladder calculi, and vesicostomies must ultimately be closed. At the time of closure, intermittent catheterization and anticholinergic therapy may be indicated, and in some cases bladder augmentation will be necessary. Although urethral dilatation has also been advocated to improve bladder storage parameters in patients with neurogenic bladder, there is not much experience in the neonate [Park et al. 2001]. Neither bladder augmentation nor supravesical diversion via bowel conduit should be utilized in the neonate with spina bifida.
In cases of tethered cord, it is generally accepted that the infant undergo a tethered cord release to treat their condition. This intervention has been shown to be beneficial, particularly if performed prior to 1 year of age [Hsieh et al. 2006]. However, some infants continue to have bladder dysfunction and should be managed as described above for neurogenic bladder.
Posterior urethral valves
The infant with PUVs usually has a small, hypercontractile bladder. The intervention of early transurethral PUV incision goes a long way towards rehabilitating the bladder by allowing it to cycle normally. Such normal cycling has been shown to increase bladder compliance and volume in infants treated for PUV in the first year of life [Close et al. 1997]. In situations when the infant is too small for transurethral incision with the instruments at hand, cutaneous vesicostomy is a viable alternative. It has been demonstrated that short-term outcomes are comparable in neonates treated with either transurethral incision of PUVs or vesicostomy [Narasimhan et al. 2004]. In fact, bladder function after vesicostomy closure was found to be normal in 75% of patients. These patients underwent vesicostomy at a mean of 15 weeks of age, and although their underlying diagnoses varied, 31/75 had PUVs [Jayanthi et al. 1995]. Another approach is the use of CIC to manage the hostile bladder in infants with PUVs. Holmdahl and colleagues started CIC on 19 infants with pronounced bladder dysfunction in the setting of PUVs [Holmdahl et al. 2003]. The mean age of the patients at the time CIC commenced was 8 months. Their bladder dysfunction was characterized by poor emptying, unsafe pressure levels, high-grade reflux and renal impairment. Only two patients did not continue with CIC due to parental noncompliance. On follow up to a median age of 8 years, detrusor instability decreased, bladder compliance improved and renal function was minimally improved compared with an age-matched control group with less severe bladder dysfunction. There is no significant experience with overnight indwelling catheterization to improve bladder function in infants, although it has proved beneficial in older children [Koff et al. 2002].
There has been some interest in the use of anticholinergic drugs to improve bladder function and clinical outcomes in infants after PUV resection. In one study, oxybutynin was started in 18 infants at a mean age of 3.4 months and continued for a mean of 2.2 years. Initial high voiding pressures improved from a mean of 148.5 to 49.9 cm H2O in 15/17 patients. All eight patients with initial poor bladder compliance demonstrated improvement on oxybutynin. In addition, all seven patients with low bladder capacity had significant improvement in this parameter [Casey et al. 2012]. Longer-term follow up was lacking in this series. There is some concern that treatment with anticholinergics may hasten the onset of myogenic failure, which is the ultimate fate of many valve bladders [Kim et al. 1997]. In this series, the authors noted that 2/21 boys treated with anticholinergic therapy developed new onset myogenic failure and required CIC. Glassberg’s group noted that the development of myogenic failure in their cohort of 51 patients with PUVs was uncommon. They noted its occurrence in 3/51 (5.9%) patients who all initially had poorly compliant bladders and new onset myogenic failure occurred only after anticholinergic therapy was initiated. The myogenic failure resolved after the anticholinergic therapy was discontinued [Misseri et al. 2002]. Therefore, regarding the use of anticholinergics in patients with PUVs, there are some realizable benefits, but the patients must be carefully watched for the onset of myogenic failure. If such an outcome occurs a decision to start CIC and continue the anticholinergics versus stopping the anticholinergics must be made.
There is emerging experience with the use of α1 adrenergic blockers such as terazosin in the management of poor bladder emptying in patients with PUVs [Abraham et al. 2009]. However, experience in infants is lacking. The use of simultaneous bladder neck incision at 06:00 at the time of PUV incision has also been demonstrated to improve bladder functional outcome versus PUV incision alone [Kajbafzadeh et al. 2007]. However, this was in a cohort of patients of average age 1.6 years at presentation. There are no significant data at hand for an infant population.
Vesicoureteric reflux
The finding of bladder dysfunction in infants with VUR has not as yet produced any significant change to clinical management. It is not commonplace to intervene with anticholinergic drugs, intermittent catheterization or vesicostomy. Sillen and colleagues documented that treatment of infants with high bladder capacity, high postvoid residual and dilating reflux by intermittent catheterization did not influence the spontaneous resolution rate of VUR [Sillen et al. 2007]. In addition, it has been shown that the abnormal urodynamic findings have a propensity to spontaneous resolution, as does the concurrent VUR in the first 18 months of life [Chandra et al. 1996]. Thus the impetus to aggressively investigate and manage the transient bladder dysfunction seen in infants with VUR has been tempered. Table 1 summarizes different bladder dysfunctions, expected urodynamic pattern and possible treatment in infants.
Conclusion and area for future investigations
Although only a modest number of publications on infant bladder function have appeared in the literature over the past two decades, significant new information has been reported. This innovative knowledge has increased our understanding of normal and abnormal bladder function in infants and we are currently in a better position to manage these children. Some issues are still controversial and need more research, such as ‘early prophylactic treatment (CIC and anticholinergic)’ versus initial ‘watchful waiting and treatment as needed’ in neonates with neurogenic bladder. However, more recent evidence seems to point towards early prophylactic management as a better way to maintain adequate renal function and prevent the need for bladder augmentation. Many urodynamic-based interventions have been attempted in infants with PUV and are currently under scrutiny. However, long-term data are still lacking to ascertain whether such interventions influence the natural evolution of the valve bladder and the stability of the upper tracts. Conversely, cumulative data suggest that there is no benefit to early intervention as regards bladder function in infants with high-grade VUR. There is a paucity of studies on long-term outcomes such as renal function and quality of life specifically related to infantile bladder dysfunction. Finally, molecular and genetic research in infant bladder dysfunction is a frontier still largely unexplored.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
Contributor Information
Luis Guerra, Division of Urology, Children’s Hospital of Eastern Ontario (CHEO), 401 Smyth Rd, Ottawa, ON, Canada K1H 8L1.
Michael Leonard, Department of Surgery, Division of Pediatric Urology, Children’s Hospital of Eastern Ontario, University of Ottawa, Ottawa, ON, Canada.
Marco Castagnetti, Section of Paediatric Urology, Urology Unit, University Hospital of Padova, Padua, Italy.
References
- Abraham M., Nasir A., Sudarsanan B., Puzhankara R., Kedari P., Unnithan G., et al. (2009) Role of alpha adrenergic blocker in the management of posterior urethral valves. Pediatr Surg Int 25: 1113–1115 [DOI] [PubMed] [Google Scholar]
- Bachelard M., Sillen U., Hansson S., Hermansson G., Jodal U., Jacobsson B. (1999) Urodynamic pattern in asymptomatic infants: siblings of children with vesicoureteral reflux. J Urol 162: 1733–1737 [DOI] [PubMed] [Google Scholar]
- Bauer S. (1991) Pediatric neuro-urology. In: Krane RJ., Siroky MB. (eds), Clinical Neuro-Urology, 2nd edn. Boston: Little, Brown and Company, pp. 375–409 [Google Scholar]
- Bauer S. (2008) Neurogenic bladder: etiology and assessment. Pediatr Nephrol 23: 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S., Hallett M., Khoshbin S., Lebowitz R., Winston K., Gibson S., et al. (1984) Predictive value of urodynamic evaluation in newborns with myelodysplasia. JAMA 252: 650–652 [PubMed] [Google Scholar]
- Boemers T., Beek F., van Gool J., de Jong T., Bax K. (1996) Urologic problems in anorectal malformations. Part 1: Urodynamic findings and significance of sacral anomalies. J Pediatr Surg 31: 407–410 [DOI] [PubMed] [Google Scholar]
- Bross S., Honeck P., Kwon S., Badawi J., Trojan L., Alken P. (2007) Correlation between motor function and lower urinary tract dysfunction in patients with infantile cerebral palsy. Neurourol Urodyn 26: 222-227 [DOI] [PubMed] [Google Scholar]
- Carson J., Barnes P., Tunell W., Smith E., Jolley S. (1984) Imperforate anus: the neurologic implication of sacral abnormalities. J Pediatr Surg 19: 838–842 [DOI] [PubMed] [Google Scholar]
- Casey J., Hagerty J., Maizels M., Chaviano A., Yerkes E., Lindgren B., et al. (2012) Early administration of oxybutynin improves bladder function and clinical outcomes in newborns with posterior urethral valves. J Urol 188(Suppl.): 1516–1520 [DOI] [PubMed] [Google Scholar]
- Chandra M., Maddix H., McVicar M. (1996) Transient urodynamic dysfunction of infancy: relationship to urinary tract infections and vesicoureteral reflux. J Urol 155: 673–677 [DOI] [PubMed] [Google Scholar]
- Chin-Peuckert L., Rennick J., Jednak R., Capolicchio J., Salle J. (2004) Should warm infusion solution be used for urodynamic studies in children? A prospective randomized study. J Urol 172: 1657–1661 [DOI] [PubMed] [Google Scholar]
- Close C., Carr M., Burns M., Mitchell M. (1997) Lower urinary tract changes after early valve ablation in neonates and infants: is early diversion warranted? J Urol 157: 984–988 [PubMed] [Google Scholar]
- Decter R., Bauer S., Khoshbin S., Dyro F., Krarup C., Colodny A., et al. (1987) Urodynamic assessment of children with cerebral palsy. J Urol 138: 1110–1112 [DOI] [PubMed] [Google Scholar]
- De Gennaro M., Capitanucci M., Mosiello G., Caione P., Silveri M. (2000) The changing urodynamic pattern from infancy to adolescence in boys with posterior urethral valves. BJU Int 85: 1104–1108 [DOI] [PubMed] [Google Scholar]
- Dik P., Klijn A., van Gool J., de Jong-de Vos van Steenwijk C., de Jong T. (2006) Early start to therapy preserves kidney function in spina bifida patients. Eur Urol 49: 908–913 [DOI] [PubMed] [Google Scholar]
- Franco I. (2007) Overactive bladder in children. Part 1: pathophysiology. J Urol 178: 761–768 [DOI] [PubMed] [Google Scholar]
- Ghanem M., Wolffenbuttel K., De Vylder A., Nijman R. (2004) Long-term bladder dysfunction and renal function in boys with posterior urethral valves based on urodynamic findings. J Urol 171: 2409–2412 [DOI] [PubMed] [Google Scholar]
- Glazier D., Murphy D., Fleisher M., Cummings K., Barone J. (1997) Evaluation of the utility of video-urodynamics in children with urinary tract infection and voiding dysfunction. Br J Urol 80: 806–808 [DOI] [PubMed] [Google Scholar]
- Guerra L., Pike J., Milks J., Barrowman N., Leonard M. (2006) Outcome in patients who underwent tethered cord release for occult spinal dysraphism. J Urol 176: 1729–1732 [DOI] [PubMed] [Google Scholar]
- Gundogdu G., Komur M., Avlan D., Sari F., Delibas A., Tasdelen B., et al. (2013) Relationship of bladder dysfunction with upper urinary tract deterioration in cerebral palsy. J Pediatr Urol 9: 659–664 [DOI] [PubMed] [Google Scholar]
- Hjalmas K. (1976) Micturition in infants and children with normal lower urinary tract. A urodynamic study. Scand J Urol Nephrol (Suppl. 37): 1–106 [PubMed] [Google Scholar]
- Hoebeke P., Van Laecke E., Van Camp C., Raes A., Van De, Walle J. (2001) One thousand video-urodynamic studies in children with non-neurogenic bladder sphincter dysfunction. BJU Int 87: 575–580 [DOI] [PubMed] [Google Scholar]
- Holmdahl G., Hanson E., Hanson M., Hellstrom A., Hjalmas K., Sillen U. (1996) Four-hour voiding observation in healthy infants. J Urol 156: 1809–1812 [PubMed] [Google Scholar]
- Holmdahl G., Hanson E., Hanson M., Hellstrom A., Sillen U., Solsnes E. (1998) Four-hour voiding observation in young boys with posterior urethral valves. J Urol 160: 1477–1481 [PubMed] [Google Scholar]
- Holmdahl G., Sillen U., Bertilsson M., Hermansson G., Hjalmas K. (1997) Natural filling cystometry in small boys with posterior urethral valves: unstable valve bladders become stable during sleep. J Urol 158: 1017–1021 [DOI] [PubMed] [Google Scholar]
- Holmdahl G., Sillen U., Hellstrom A., Sixt R., Solsnes E. (2003) Does treatment with clean intermittent catheterization in boys with posterior urethral valves affect bladder and renal function? J Urol 170: 1681–1685 [DOI] [PubMed] [Google Scholar]
- Hoover D., Duckett J., Jr (1982) Posterior urethral valves, unilateral reflux and renal dysplasia: a syndrome. J Urol 128: 994–997 [DOI] [PubMed] [Google Scholar]
- Hsieh M., Perry V., Gupta N., Pearson C., Nguyen H. (2006) The effects of detethering on the urodynamics profile in children with a tethered cord. J Neurosurg 105(Suppl.): 391–395 [DOI] [PubMed] [Google Scholar]
- Jansson U., Hanson M., Hanson E., Hellstrom A., Sillen U. (2000) Voiding pattern in healthy children 0 to 3 years old: a longitudinal study. J Urol 164: 2050–2054 [PubMed] [Google Scholar]
- Jayanthi V., Khoury A., McLorie G., Agarwal S. (1997) The nonneurogenic neurogenic bladder of early infancy. J Urol 158: 1281–1285 [DOI] [PubMed] [Google Scholar]
- Jayanthi V., McLorie G., Khoury A., Churchill B. (1995) The effect of temporary cutaneous diversion on ultimate bladder function. J Urol 154: 889–892 [DOI] [PubMed] [Google Scholar]
- Jones M., Morgan E., Shelton J., Thorogood C. (2009) Cerebral palsy: introduction and diagnosis (part I): in: Clinical and Urodynamic Spectrum of Bladder Function in Cerebral Palsy. Richardson I., Palmer L. J Urol 182: 1945–1948 [DOI] [PubMed] [Google Scholar]
- Kaefer M., Keating M., Adams M., Rink R. (1995) Posterior urethral valves, pressure pop-offs and bladder function. J Urol 154: 708–711 [DOI] [PubMed] [Google Scholar]
- Kaefer M., Pabby A., Kelly M., Darbey M., Bauer S. (1999) Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomentingocele. J Urol 162: 1068–1071 [DOI] [PubMed] [Google Scholar]
- Kajbafzadeh A., Payabvash S., Karimian G. (2007) The effects of bladder neck incision on urodynamic abnormalities of children with posterior urethral valves. J Urol 178: 2142–2147 [DOI] [PubMed] [Google Scholar]
- Kasabian N., Bauer S., Dyro F., Colodny A., Mandell J., Retik A. (1992) The prophylactic value of clean intermittent catheterization and anticholinergic medication in newborns and infants with myelodysplasia at risk of developing urinary tract deterioration. Am J Dis Child 146: 840–843 [DOI] [PubMed] [Google Scholar]
- Kaufman A., Ritchey M., Roberts A., Rudy D., McGuire E. (1996) Decreased bladder compliance in patients with myelomeningocele treated with radiological observation. J Urol 156: 2031–2033 [PubMed] [Google Scholar]
- Kim Y., Horowitz M., Combs A., Nitti V., Borer J., Glassberg K. (1997) Management of posterior urethral valves on the basis of urodynamic findings. J Urol 158: 1011–1016 [DOI] [PubMed] [Google Scholar]
- Koff S., Mutabagani K., Jayanthi V. (2002) The valve bladder syndrome: pathophysiology and treatment with nocturnal bladder emptying. J Urol 167: 291–297 [DOI] [PubMed] [Google Scholar]
- Lal R., Bhatnagar V., Agarwala S., Grover V., Mitra D. (1999) Urodynamic evaluation in boys treated for posterior urethral valves. Pediatr Surg Int 15: 358–362 [DOI] [PubMed] [Google Scholar]
- Lavallee L., Leonard M., Dubois C., Guerra L. (2013) Urodynamic testing – is it a useful tool in the management of children with cutaneous stigmata of occult spinal dysraphism? J Urol 189: 678–683 [DOI] [PubMed] [Google Scholar]
- Lee N., Gana R., Borer J., Estrada C., Khoshbin S., Bauer S. (2012) Urodynamic findings in patients with Currarino syndrome. J Urol 187: 2195–2200 [DOI] [PubMed] [Google Scholar]
- MacLellan DL., Bauer SB. (2011) Campbell Walsh Urology. In: Wein AJ., Kavoussi LR., Novick AC., Partin WA, Peters CA. (eds) Neuropathic dysfunction of the lower urinary tract. 10th ed. USA: Elsevier Saunders, pp. 3431–3456 [Google Scholar]
- McGuire E., Woodside J., Borden T., Weiss R. (1981) Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 126: 205–209 [DOI] [PubMed] [Google Scholar]
- McNeal D., Hawtrey C., Wolraich M., Mapel J. (1983) Symptomatic neurogenic bladder in a cerebral-palsied population. Dev Med Child Neurol 25: 612–616 [DOI] [PubMed] [Google Scholar]
- Misseri R., Combs A., Horowitz M., Donohoe J., Glassberg K. (2002) Myogenic failure in posterior urethral valve disease: real or imagined? J Urol 168: 1844–1848 [DOI] [PubMed] [Google Scholar]
- Morrisroe S., O’Connor R., Nanigian D., Kurzrock E., Stone A. (2005) Vesicostomy revisited: the best treatment for the hostile bladder in myelodysplastic children? BJU Int 96: 397–400 [DOI] [PubMed] [Google Scholar]
- Murphy K., Boutin S., Ide K. (2012) Cerebral palsy, neurogenic bladder, and outcomes of lifetime care. Dev Med Child Neurol 54: 945–950 [DOI] [PubMed] [Google Scholar]
- Narasimhan K., Kaur B., Chowdhary S., Bhalla A. (2004) Does mode of treatment affect the outcome of neonatal posterior urethral valves? J Urol 171: 2423–2426 [DOI] [PubMed] [Google Scholar]
- Neveus T., Sillen U. (2013) Lower urinary tract function in childhood; normal development and common functional disturbances. Acta Physiol 207: 85–92 [DOI] [PubMed] [Google Scholar]
- Neveus T., von Gontard A., Hoebeke P., Hjalmas K., Bauer S., Bower W., et al. (2006) The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol 176: 314–324 [DOI] [PubMed] [Google Scholar]
- Olsen L., Grothe I., Rawashdeh Y., Jorgensen T. (2009) Urinary flow patterns of healthy newborn males. J Urol 181: 1857–1861 [DOI] [PubMed] [Google Scholar]
- Olsen L., Grothe I., Rawashdeh Y., Jorgensen T. (2010) Urinary flow patterns in first year of life. J Urol 183: 694–698 [DOI] [PubMed] [Google Scholar]
- Park J., McGuire E., Koo H., Schwartz A., Garwood C., Bloom D. (2001) External urethral sphincter dilation for the management of high risk myelomeningocele: 15-year experience. J Urol 165: 2383–2388 [DOI] [PubMed] [Google Scholar]
- Peters C., Bauer S. (1990) Evaluation and management of urinary incontinence after surgery for posterior urethral valves. Urol Clin North Am 17: 379–387 [PubMed] [Google Scholar]
- Podesta M., Castera R., Ruarte A. (2004) Videourodynamic findings in young infants with severe primary reflux. J Urol 171: 829–833 [DOI] [PubMed] [Google Scholar]
- Rich M., Brock W., Peña A. (1988) Spectrum of genitourinary malformations in patients with imperforate anus. J Pediatr Surg Int 3: 110–113 [Google Scholar]
- Richardson I., Palmer L. (2009) Clinical and urodynamic spectrum of bladder function in cerebral palsy. J Urol 182(Suppl.): 1945–1948 [DOI] [PubMed] [Google Scholar]
- Seruca H. (1989) Vesicoureteral reflux and voiding dysfunction: a prospective study. J Urol 142: 494–498 [DOI] [PubMed] [Google Scholar]
- Sillen U. (2004) Bladder function in infants: urodynamics reveal high pressures and immature coordination. Scand J Urol Nephrol Suppl 38(Suppl. 215): 69–74 [DOI] [PubMed] [Google Scholar]
- Sillen U., Bachelard M., Hansson S., Hermansson G., Jacobson B., Hjalmas K. (1996) Video cystometric recording of dilating reflux in infancy. J Urol 155: 1711–1715 [PubMed] [Google Scholar]
- Sillen U., Hellstrom A., Hermanson G., Abrahamson K. (1999) Comparison of urodynamic and free voiding pattern in infants with dilating reflux. J Urol 161: 1928–1933 [PubMed] [Google Scholar]
- Sillen U., Holmdahl G., Hellstrom A., Sjostrom S., Solsnes E. (2007) Treatment of bladder dysfunction and high grade vesicoureteral reflux does not influence the spontaneous resolution rate. J Urol 177: 325–329 [DOI] [PubMed] [Google Scholar]
- Silva J., Alvares R., Barboza A., Monteiro R. (2009) Lower urinary tract dysfunction in children with cerebral palsy. Neurourol Urodyn 28: 959–963 [DOI] [PubMed] [Google Scholar]
- Silva J., Gonsalves M., Saverio A., Oliveira I., Carrerette F., Damiao R. (2010) Lower urinary tract dysfunction and ultrasound assessment of bladder wall thickness in children with cerebral palsy. Urology 76: 942–945 [DOI] [PubMed] [Google Scholar]
- Thor K. (2003) Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: implications for treating stress urinary incontinence. Urology 62(Suppl. 1): 3–9 [DOI] [PubMed] [Google Scholar]
- Thor K., Donatucci C. (2004) Central nervous system control of the lower urinary tract: new pharmacological approaches to stress urinary incontinence in women. J Urol 172: 27–33 [DOI] [PubMed] [Google Scholar]
- Thorup J., Biering-Sorensen F., Cortes D. (2011) Urological outcome after myelomeningocele: 20 years of follow-up. BJU Int 107: 994–999 [DOI] [PubMed] [Google Scholar]
- Torre M., Guida E., Bisio G., Scarsi P., Piatelli G., Cama A., et al. (2011) Risk factors for renal function impairment in a series of 502 patients born with spinal dysraphisms. J Pediatr Urol 7: 39–43 [DOI] [PubMed] [Google Scholar]
- Wu H. (2010) Achieving urinary continence in children. Nat Rev Urol 7: 371–377 [DOI] [PubMed] [Google Scholar]
- Yeung C., Barker G., Lackgren G. (2010) Pathophysiology of bladder dysfunction. In:Gearhart J., Rink R., Mouriquand P. (eds), Pediatric Urology, 2 edn. Philadelphia: Elsevier Saunders, pp. 353–365 [Google Scholar]
- Yeung C., Godley M., Dhillon H., Duffy P., Ransley P. (1998) Urodynamic patterns in infants with normal lower urinary tracts or primary vesico-ureteric reflux. Br J Urol 81: 461–467 [DOI] [PubMed] [Google Scholar]
- Yeung C., Godley M., Ho C., Ransley P., Duffy P., Chen C., et al. (1995) Some new insights into bladder function in infancy. Br J Urol 76: 235–240 [DOI] [PubMed] [Google Scholar]
- Zerin J. (1993) Impact of contrast medium temperature on bladder capacity and cystographic diagnosis of vesicoureteral reflux in children. Radiology 187: 161–164 [DOI] [PubMed] [Google Scholar]