Abstract
In its 2012 global report on tuberculosis, the World Health Organization estimated that 3–7% (range 2.1–5.2%) of new cases and 20% (range 13–26%) of previously treated cases had multidrug-resistant tuberculosis (defined as tuberculosis caused by Mycobacterium tuberculosis isolates that are resistant to rifampicin and isoniazid). In many countries in Eastern Europe and central Asia, 9–32% of new patients and more than 50% of previously treated patients have multidrug-resistant tuberculosis.
Ninety-three patients with suspected prostate tuberculosis were enrolled in this study and all underwent prostate biopsy. This method allowed confirmation of diagnosis in 32 patients (34.4%): 23 by histology, six by culture and five by polymerase chain reaction (PCR) (among them, two also had positive culture).
The efficiency of an optimized scheme for the therapy of prostate tuberculosis (the second part of the study) was estimated in 53 patients. The first group (25 patients) was treated with a standard scheme of chemotherapy; the second group (28 prostate tuberculosis patients) received ofloxacin in addition for 2 months during the intensive phase. The phase continuation in both groups was identical, with rifampicin and isoniazid administered for 6 months. Optimization of the standard therapy by additional administration of ofloxacin improved results of the treatment in 33.8% of patients.
Keywords: diagnosis, infection, prostate, prostatitis, therapy, tuberculosis, urogenital
Introduction
In its 2012 global report on tuberculosis (TB), the World Health Organization (WHO) estimated that 3–7% (range 2.1–5.2%) of new cases and 20% (range 13–26%) of previously treated cases had multidrug-resistant (MDR) TB [defined as TB caused by Mycobacterium tuberculosis (Mtb) isolates that are resistant at least to rifampicin and isoniazid]. In many countries in Eastern Europe and central Asia, 9–32% of new patients and more than 50% of previously treated patients had MDR-TB [WHO, 2012]. Extrapulmonary TB (EPTB), except in a small number of patients, plays a significant role in both phthisiatry and urology, mostly because of a greater frequency of fatal complications, more severe reduction in quality of life, and more common association with HIV-infection, than pulmonary TB.
Prostate TB (PTB) is difficult to diagnosis early because both clinical features and laboratory findings (excluding Mtb) are non-specific. PTB seems to be a rare disease. Nevertheless, 70% of men who died from TB of all localizations had PTB, which was mostly overlooked during their lifetimes [Kamyshan, 2003]. For example, in Russia it is diagnosed in more than 10,000 men annually – PTB is a sexually transmitted disease and one of the main reasons for infertility in both men and women [Kulchavenya and Krasnov, 2010].
Traditionally, the term ‘prostatitis’ has included both acute and chronic bacterial prostatitis, in which an infective origin is accepted, and the term ‘prostatitis syndrome’ or, more recently, ‘chronic pelvic pain syndrome’, is where no infective agent can be found and whose origin is multifactorial and in most cases obscure [Nickel and Weidner, 2000]. Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a complex condition for which the etiological determinants are still poorly defined. To better characterize the diagnostic and therapeutic profile of patients, an algorithm known as UPOINT was created, addressing six major phenotypic domains of CP/CPPS, specifically the urinary (U), psychosocial (P), organ-specific (O), infection (I), neurological/systemic (N) and muscular tenderness (T) domains. An additional sexual dysfunction domain may be included in the UPOINT(S) system [Magri et al. 2013].
Prostatitis and CPPS are diagnosed by symptoms and evidence of inflammation and infection localized to the prostate. A causative pathogen, however, is detected by routine methods in only 5–10% of cases, and for whom antimicrobial therapy therefore has a rational basis. The remainder of patients are treated empirically with numerous medical and physical modalities. Cure of CPPS can be attempted by long-term therapy with antibacterial agents, but relapses are frequent. Few antibacterial agents are able to distribute to the prostatic tissue and achieve sufficient concentrations at the site of infection. These agents include fluoroquinolones, macrolides, tetracyclines and trimethoprim [Perletti et al. 2013].
Although abacterial prostatitis implies an absence of any microbes, guidelines recommend a prescription of a fluoroquinolone or another antibiotic for a significant time. Very often we receive a good result and symptoms resolve. Why? What is the point of fluoroquinolones if it is real abacterial prostatitis? Escherichia coli seems to be the most common infection agent for CP [Wagenlehner et al. 2014], but prostate inflammation may also be caused by intracellular parasites, like Chlamydia trachomatis or Mtb. Usual standard bacteriological tests don’t reveal these infection agents and actual PTB hides under the guise of abacterial prostatitis [Kulchavenya and Krasnov, 2010]. Therefore, abacterial prostatitis means a latent or unusual infection, for example, caused by Mtb. Late diagnosed PTB means a presence of cavities in the prostate, and at this stage the disease cannot ever be fully cured; caverns will be preserved for the patient’s lifetime.
The results of therapy closely depend on in-time diagnosis – if a patient is revealed to be in the cavern stage, he will never be fully cured. Standard chemotherapy for PTB is insufficiently effective: only 22.8% of patients are cured with isoniazid, rifampicin, pyrazinamid and streptomycin, and in 77.2% the disease becomes chronic [Kulchavenya and Khomyakov, 2006]. Thus, we aimed to improve both diagnosis and therapy of patients with PTB.
Material and methods
Our study consisted of two parts. The first part was to estimate the value of prostate biopsy, and the second part to estimate the efficiency of optimized therapy. For the first part, 93 patients with suspected PTB were enrolled. They were aged between 27 and 64 years (average 39.7 ± 6.4) and all were inpatients of Novosibirsk Research TB Institute between 2006 and 2011. All underwent standard ultrasound guided core prostate biopsy (middle size needle 18 g, 19 mm length) with local anaesthesia at six points. Straws were investigated by polymerase chain reaction (PCR), pathomorphology and by culture.
The efficiency of an optimized scheme for the therapy of PTB (the second part of the study) was estimated in 53 patients aged between 24 and 69 years (average 32.1 ± 7.2). Infiltrative PTB was diagnosed in 24 patients and cavernous PTB was revealed in 29 patients. All patients were new-revealed, and all had a long history (up to 14 years) in which they were managed as ‘chronic prostatitis’. Mtb was found in 21 patients (39.6%): in six patients by culture and in 15 patients by PCR; pathomorphological verification was in eight patients (15.1%), in 29 patients (54.7%) diagnosis was established by X-ray (prostate caverns were found on the urethrogram); in three patients diagnosis was confirmed both by histology and bacteriology.
All patients were randomized to two identical groups. The first group (25 patients) was treated with a standard scheme of chemotherapy, approved by the Russian Ministry of Public Health. This scheme has two phases of therapy: intensive, in which the patient is treated by four anti-TB drugs daily (isoniazid 10mg/kg + pyrazinamid 25 mg/kg + streptomycin 1000 mg i.m. + rifampicin 10 mg/kg), and phase continuation, where the therapy includes two main anti-TB drugs only (isoniazid and rifampicin) in a daily or intermittent regimen (3 times a week). The second group (28 PTB patients), alongside standard chemotherapy (isoniazid 10mg/kg + pyrazinamid 25 mg/kg + streptomycin 1000 mg i.m. + rifampicin 10 mg/kg), additionally received ofloxacin 10 mg/kg during the intensive phase. The phase continuation was identical in both groups, with rifampicin and isoniazid administered for 6 months. One patient in the second group had severe side effects on pyrazinamid and rifampicin and was excluded from the study.
The efficiency was estimated at two points: after the intensive phase and after ending phase continuation (accordingly, at 2 and 8 months of therapy). The criteria of efficacy were: resolution of pain, dysuria, pyospermia and negative results of bacteriological investigations. A ‘good result’ meant resolution of pain and dysuria, a normal amount of leucocytes in the ejaculate and the absence of Mtb by any method. A ‘moderate result’ meant a normal or insignificantly above normal amount of leucocytes in the ejaculate, absence of Mtb by any method, but chronic pelvic pain or moderate dysuria presented. A ‘poor result’ noted pain, dysuria, pyospermia or Mtb in the ejaculate.
In phthisiopulmonology a very important criterion is the disappearance of the lung’s caverns. Unfortunately, in urology this criterion doesn’t work. Once established, caverns of the kidneys or prostate remain forever. Uroflowmetry, and decreasing prostate size, reflected inflammatory edema in PTB patients, and were estimated as surrogate secondary criteria.
Statistical analysis was made by estimation of z-criteria of significance of proportions. Differences were considered significant at p < 0.05.
Results
Among 93 patients with suspected PTB, who were admitted to the Urogenital Clinic of Novosibirsk Research TB Institute between 2006 and 2011, 36 (38.7%) had TB history and 31 (33.3%) had active TB of another localization, mostly pulmonary. Common complaints were pain (90 patients; 96.8%), and dysuria (74 patients; 79.6%). In laboratory findings, leucospermia was found in 68 patients (73.1%) and haemospermia in 48 patients (51.6%). Prostate specific antigen (PSA) was normal (less than 4.0 ng/ml) in 76 patients (81.7%) and in 17 patients (18.3%) was, on average, 7.2 ± 1.25 ng/ml (range 4.0–9.6 ng/ml)
For confirmation of the diagnosis, all patients underwent prostate biopsy. Pathomorphologically, in 88 patients (94.6%) inflammation was found, fibrosis in 61 patients (65.6%), intraprostatic neoplasia in nine patients (9.7%) and cancer in five patients (5.4%). Tuberculous inflammation with Pirogov-Langhans cells presented in 23 patients (24.7%). Figures 1–2 demonstrate typical histology.
Figure 1.

TB granulomas in prostate tissue. X100. Hematoxylin and eosin.
Figure 2.
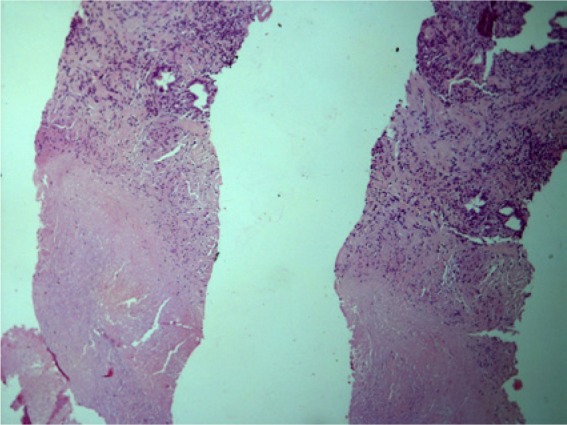
Caseous zones in prostate tissue. X100. Hematoxylin and eosin.
PCR of the biopsies revealed human papilloma virus in 10 patients (10.7%), ureaplasma urealythicum in two patients (2.2%) and Mtb in five patients (5.4%). Mtb culture was positive in six patients (6.4%).
Thus, prostate biopsy allowed confirmation of a diagnosis of ‘PTB’ in 32 patients (34.4%): 23 by histology, six by culture and five by PCR (among them, two also had positive culture). Comparison of results of the standard therapy and optimized therapy is shown in Tables 1 and 2.
Table 1.
Dynamic of clinical features in PTB patients in groups with standard therapy (n=25) and optimized therapy (n=27).
| Symptoms | Group | Base-line |
In 2 months |
In 8 months |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Dysuria | optimized | 25 | 92.6 | 11 | 40.7*! | 4 | 14.8*! |
| standard | 23 | 92.0 | 12 | 48.0 | 7 | 28.0 | |
| Perineal pain | optimized | 27 | 100 | 14 | 51.8*! | 6 | 22.2*! |
| standard | 25 | 100 | 14 | 56.0 | 8 | 32.0 | |
| Pyospermia | optimized | 24 | 88.9 | 7 | 25.9*! | 2 | 7.4*! |
| standard | 22 | 88.0 | 18 | 72.0 | 9 | 36.0 | |
| Mtb+ | optimized | 11 | 40.7 | 0 | 0*! | 0 | 0*! |
| standard | 8 | 32.0 | 2 | 8.0 | 1 | 4.0 | |
Notes:
Differences are statistically significant in comparison with base-line.
Differences are statistically significant in comparison between groups.
Table 2.
Dynamic of prostate volume and uroflowmetry in groups with standard therapy (n=25) and optimized therapy (n=27).
| Sign | Group | Base-line | In 2 months | In 8 months |
|---|---|---|---|---|
| Prostate volume (ml) | optimized | 36.9±0.3 | 25.2±0.1*! | 23.8±0.07*! |
| standard | 32.7±0.4 | 29.4±0.3 | 28.9±0.6 | |
| Q max (ml/s) | optimized | 14.8±0.2 | 22.7±0.09*! | 25.2±0.1*! |
| standard | 14.4±0.4 | 17.1±0.7 | 19.2±0.6 | |
| Q ave (ml/s) | optimized | 7.2±0.1 | 10.9±0.07*! | 12.6±0.07*! |
| standard | 8.0±0.2 | 9.1±0.3 | 9.9±0.2 |
Notes:
Differences are statistically significant in comparison with base-line.
Differences are statistically significant in comparison between groups.
Optimized therapy improved dysuria, perineal pain, pyospermia and presence of Mtb significantly faster than the standard scheme. Patients who took the optimized scheme had faster positive dynamics concerning pain, dysuria, pyospermia and negative growths on Mtb as well as PCR in ejaculate; in 8 months the best results of optimized therapy were shown after 8 months.
Patients in the optimized therapy group demonstrated decreasing prostate size, improving micturition and increasing Qmax and Qave. We can explain these findings mostly in terms of a reduction of inflammatory edema of the prostate gland. We didn’t estimate these parameters as criteria of the efficiency of the therapy of PTB, but they reflected a condition of the gland as whole.
In the group receiving optimized therapy, 21 patients (77.8%) had good results based on resolution of pain and dysuria, a normal amount of leucocytes in their ejaculate, absence of Mtb by any method; six patients (22.2%) had moderate results. In the control group, who received standard therapy, good results were found in 11 patients (44.0%) only, and moderate results in 13 patients (52.0%); one patient (4.0%) had a poor result after completion of a full course of standard therapy.
We noted severe side effects in one patient on pyrazinamid and rifampicin and he was excluded from the study. The tolerability of the therapy, including ofloxacin, in the remaining patients was good.
Thus, optimization of the standard therapy by additional administration of ofloxacin improved results of the treatment in 33.8% of patients. In the optimized group we achieved good results in 77.8% but in the standard group only in 44.0% of patients.
Discussion
Diagnosis of PTB is important as (a) it is a sexually transmitted disease; (b) it leads to infertility; (c) it results, like any prostatitis, in chronic pelvic pain which significantly reduces quality of life and (d) it decreases sexual function, and this reduces quality of life again.
The problem of early diagnosis is acute, since 54.7% of patients were revealed with caverns, in which case full, complete convalescence is impossible and relapse and a chronic course are probable. For in-time diagnosis we have to follow some main principles: careful study of the history of the patient, 3-glass test [Kulchavenya et al. 2012] performed before digital rectal examination, and investigation of expressed prostatic fluid and ejaculate as well as prostate biopsies on Mtb by PCR.
Lee and colleagues [Lee et al. 2001] evaluated 18 patients (mean age 66.7 ± 10.2 years) with PTB to characterize the clinical features and to evaluate the short- and long-term results of antituberculous chemotherapy. The median pretreatment PSA level was 2.7 ng/mL (range 0.3–31). Eight patients (44.4%) received a triple-drug regimen of rifampin, ethambutol, and isoniazid for more than 6 months. The mean duration of chemotherapy was 7.6 months (range 6–12). Of the eight patients, three underwent chemotherapy for longer because of concurrent TB of other organs. Follow-up studies included digital rectal examination, total PSA determination, and transrectal prostate biopsy. Ten patients were eligible for regular follow-up. The average number of follow-up transrectal prostate biopsies was 2.4 (range 2–3). The follow-up histologic findings showed nodular hyperplasia in seven patients and chronic inflammatory cell infiltration in three patients. No acid-fast bacillus was found in any follow-up specimen [Lee et al. 2001].
Kim and colleagues [Kim et al. 2011] described miliary TB as a complication of transrectal ultrasonography(TRUS)-guided prostate biopsy. A 75-year-old patient, who presented with high fever and cough following TRUS-guided prostate biopsy for his high serum PSA level (13.104 ng/ml), was diagnosed with miliary TB after clinical, laboratory, and radiological assessments. Histopathological examination of the prostate revealed TB with acid-fast bacilli. We would like to emphasize that, at a glance, this patient had contraindications for invasive procedure due to fever. He actually had miliary TB before prostate biopsy, not as a result of the operation.
Gupta and colleagues [Gupta et al. 2008] found that PTB is uncommon and is usually found incidentally following transurethral resection; nevertheless, there are no reports on transurethral resection for the surgery for PTB.
The therapy of prostatitis of any etiology – both non-specific and tuberculous – is very difficult as few antibacterial agents are able to distribute to the prostatic tissue and achieve sufficient concentrations at the site of infection. These agents include fluoroquinolones, macrolides, tetracyclines and trimethoprim [Perletti et al. 2013]. Standard anti-TB drugs, excluding rifampicin, have suboptimal concentrations in prostate tissue [Kulchavenya and Krasnov, 2010] as opposed to ofloxacin. Ofloxacin has wide antibacterial activity, including a bactericidal effect on Mtb, so it is an optimal drug for patients with PTB. Our results confirmed the superiority of optimized polychemotherapy for patients with PTB in comparison with standard treatment.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Ekaterina Kulchavenya, Novosibirsk Medical University, Okhotskaya 81-a, Novosibirsk, Russian Federation.
Elena Brizhatyuk, Novosibirsk Research TB Institute, Novosibirsk, Russian Federation.
Victor Khomyakov, Novosibirsk Research TB Institute, Novosibirsk, Russian Federation.
References
- Gupta N., Mandal A., Singh S. (2008) Tuberculosis of the prostate and urethra: A review. Indian J Urol 24: 388–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamyshan I. (2003) Guideline on tuberculosis of urogenital organs. Kiev: Medicine, pp. 47–52 [Google Scholar]
- Kim C., Sano T., Takimoto K. (2011) Miliary tuberculosis following transrectal ultrasonography (TRUS)-guided prostate biopsy. Korean J Urol 52: 425–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulchavenya E., Azizoff A., Brizhatyuk E., Khomyakov V., Kholtobin D., Breusoff A., et al. (2012) Improved diagnostics of chronic inflammatory prostatitis. Minerva Urol Nefrol 64: 273–278 [PubMed] [Google Scholar]
- Kulchavenya E., Khomyakov V. (2006) Male genital tuberculosis in Siberians. World J Urol 24: 74–78 [DOI] [PubMed] [Google Scholar]
- Kulchavenya E., Krasnov V. (2010) Selected Topics of Phthysiourology. Novosibirsk: Nauka [Google Scholar]
- Lee Y., Huang W., Huang J., Wang J., Yu C., Jiaan B., et al. (2001) Efficacy of chemotherapy for prostatic tuberculosis – a clinical and histologic follow-up study. Urology 57: 872–877 [DOI] [PubMed] [Google Scholar]
- Magri V., Wagenlehner F.M., Marras E., van Till J.W., Houbiers J., Panagopoulos P., et al. (2013). Influence of infection on the distribution patterns of NIH-Chronic Prostatitis Symptom Index scores in patients with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Exp Ther Med 6: 503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J., Weidner W. (2000) Chronic prostatitis: Current concepts and antimicrobial therapy. Infect Urol 13/5A: s22–s28 [Google Scholar]
- Perletti G., Marras E., Wagenlehner F., Magri V. (2013) Antimicrobial therapy for chronic bacterial prostatitis. Cochrane Database Syst Rev 8: CD009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenlehner F., Weidner W., Pilatz A., Naber K. (2014) Urinary tract infections and bacterial prostatitis in men. Curr Opin Infect Dis 27: 97–101 [DOI] [PubMed] [Google Scholar]
- WHO (2012) Global tuberculosis report 2012. Geneva: World Health Organization; http://www.who.int/tb/publications/global_report/en/index.html (accessed 27 December 2012). [Google Scholar]


