Abstract
Most panuveitis in children are caused by infectious agents. A detailed clinical history and clinical examination are helpful in the diagnosis, but specific techniques are sometimes required to identify the causing specimen. We report the first published case of panuveitis in a child caused by simultaneous ocular infection by Toxocara canis and a fly larva and the innovative use of immunodiffusion technique in the vitreous for the diagnosis.
Background
Although important non-infectious aetiologies such as sarcoidosis, lymphoma or retinoblastoma must always be considered in paediatric uveitis, most unilateral posterior uveitis or panuveitis in children are caused by a limited number of infectious agents. Toxoplasma gondii (T. gondii) remains one of the most common, but Toxocara species—particularly Toxocara canis (T. canis)—are also very prevalent, accounting for 37% of childhood retinal diseases, making both, therefore, obligatory differential diagnosis in this setting.1 2
Toxocariasis is the clinical term used to describe human infection with either the dog ascarid T. canis or, less frequently, the feline ascarid Toxocara cati (T. cati). Humans can become infected by ingesting embryionated eggs from contaminated soils, hands or fomites. Children with geophagia and who live in rural areas or tend to play in public parks are a particular risk group. It is the immature nematode larvae migration that causes the local inflammation responsible for tissue damage and toxocariasis can be usually divided into two ‘larva migrans’ syndromes, visceral larva migrans (VLM) and ocular larva migrans (OLM). VLM syndrome tends to present more commonly in toddlers with a history of pica and is classically characterised by eosinophilia, fever and hepatomegaly. OLM, on the other hand, has been reported in older children usually without evidence of other organ involvement or an elevated eosinophil count in the peripheral blood, presenting only with ocular disease.3 4
Much less common that ocular toxoplasmosis and toxocariasis, however, is ocular myiasis, especially in developed countries such as Portugal. Infestation by fly larvae primarily affects cattle and livestock. In humans, it is much rarer and usually results from neglect and poor hygiene care, being more common in rural areas.5
To the best of our knowledge, the following case is the first to report a simultaneous intraocular infection by T. canis and a fly larva in a child who presented with panuveitis. We also report the use of an innovative counterimmunoelectrophoresis (CIEP) technique in vitreous samples as a diagnostic aid.
Case presentation
A 9-year-old boy presented with a supposedly ‘sudden’ vision loss in his right eye. There was neither history of known ametropia, squint nor any prior use of glasses. He denied any other ocular symptoms such as pain, hyperaemia, photophobia, floaters or photopsias. Apart from a previous adenoid surgery, he was otherwise healthy. He also lacked other concurrent systemic signs or symptoms such as fever, general malaise, sinusitis or cutaneous lesions. He had never travelled out of Portugal before and lived in a rural area near Lisbon with his parents. Apart from three puppies which had not been vaccinated or de-wormed, he denied having any other animals.
On examination, his visual acuity (VA) was severely reduced to light perception in his right eye and was normal (20/20) in his left eye. On slit-lamp examination, he presented with a quiet white eye with only subtle cellular reaction in the anterior chamber. An important vitritis made it impossible to visualise the fundus, but no ‘headlight in the fog’ appearance or other obvious lesions were noted. His left eye was completely normal.
Investigations
Owing to the dense vitritis, an ocular A+B mode ultrasound was performed which revealed numerous highly echogenic particles in the vitreous cavity with an apparently attached retina and no other obvious lesions. A complete blood work was requested, including antibodies and PCR testing to T. gondii and antibodies for T. canis and T. cati. The initial results were remarkable only for moderate eosinophilia (16.8%).
The child was submitted to therapeutic and diagnostic vitrectomy via pars plana, during which aqueous and vitreous samples were retrieved for analysis. Intraoperatively, a tractional retinal detachment was evident with marked proliferative vitreoretinopathy (PVR), rigid full-thickness retinal folds and heavy vitreous condensation.
Vitreous cytology analysis and immunophenotyping revealed an inflammatory process characterised mainly by T CD4 lymphocytes, neutrophils and macrophages. No neoplastic cells were detected. Vitreous PCR for T. gondii was requested, which proved to be negative, as in the peripheral blood. Serum and vitreous antibodies for T. canis were positive in ELISA and in immunoprecipitation assays, ID and CIEP, using an excretory–secretory antigen derived from second-stage larvae of T. canis (TES) prepared in-house according to the De Savigny protocol (1975).6 All immunoassays procedures were performed according to standardised protocols used in our laboratory (Rombert et al).7 Specific IgG antibodies in the two samples (at 1:200 dilution) were determined by ELISA and IgG titres above the established cut-off (0.4) were considered positive. To assess specific reactivity of antigen–antibody binding, undiluted serum and vitreous fluid samples were tested by ID and CIEP against TES and Ascaris suum crude adult worms antigen. In all assays, specific and total antibodies against Toxocara antigens were detected in both biological samples, but levels of IgG antibodies in ELISA were higher in vitreous fluid than in serum (0.523 and 0. 0.469, respectively). In accordance, a positive reaction in ID was found in the vitreous fluid and, although immune precipitate bands in CIEP were detected with both samples, they were more intense in the vitreous (figures 1 and 2), particularly against TES, which may reflect the intraocular immunostimulation induced by the Toxocara larvae.
Figure 1.
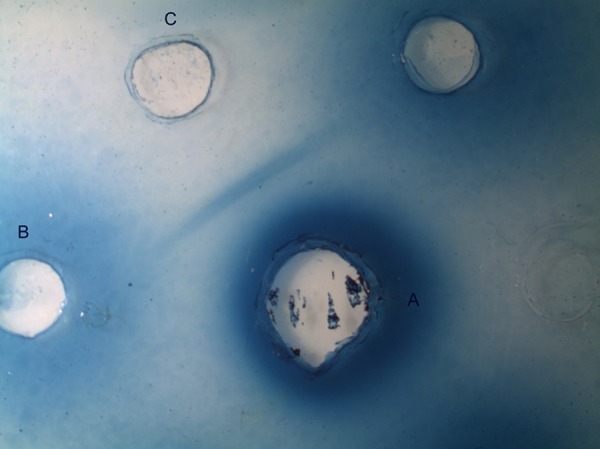
Immunodifusion in agarose gel. (A) Second-stage larvae of Toxocara canis antigen, (B) sera and (C) vitrous fluid.
Figure 2.
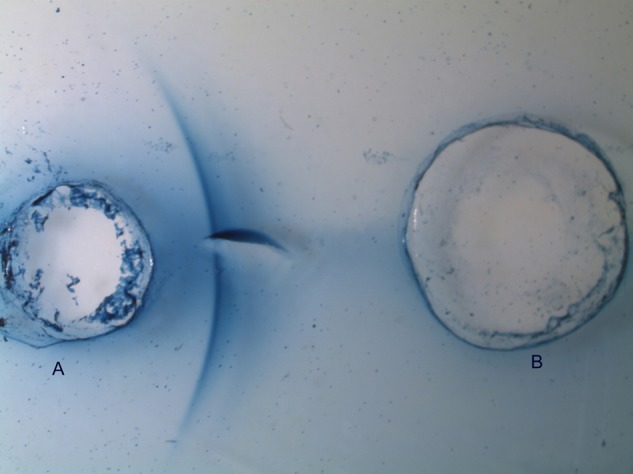
Counterimmunoelectrophoresis in agarose gel. (A) Second-stage larvae of Toxocara canis antigen and (B) vitreous fluid.
The most unexpected finding, however, was the presence of a single fly larva in the vitreous cavity (figure 3), even more so since no conjunctival, corneal or skin lesions were found on initial examination. The exact species was impossible to determine due to specimen damage during sample retrieval and conservation.
Figure 3.
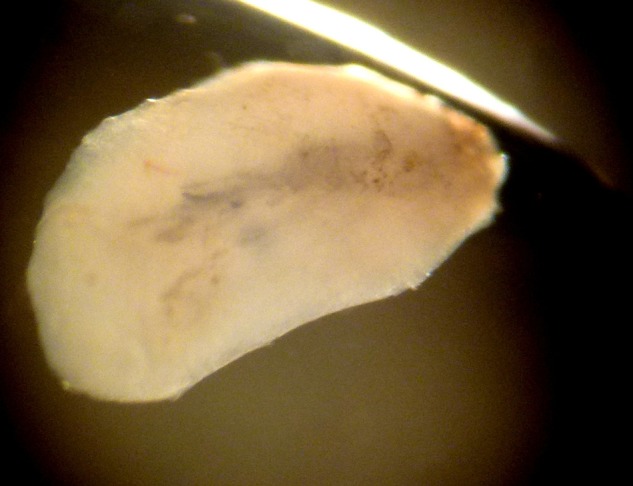
Fly larva obtained from the vitreous cavity.
Differential diagnosis
As mentioned, most unilateral posterior uveitis or panuveitis in children are caused by infectious agents. T. gondii must always be considered, as well as other less common causes, hence the requested cytology analysis for neoplastic cells. Although Toxoplasma uveitis had atypical presentations, PCR analysis of vitreous humour proved to be negative, excluding this hypothesis.8 9
OLM caused by Toxocara is another obligatory differential in the setting of a panuveitis in a young boy. It is usually reported in male children, without evidence of other organ involvement nor an elevated eosinophil count in the peripheral blood. Typically, it presents with retinal masses (which can be sometimes mistaken for retinoblastomas) caused by the local eosinophil-mediated granulomatous responses targeting the trapped Toxocara larvae which successfully reached the eye after inoculation. Less commonly, however, it can also manifest as a low-grade anterior uveitis or even endophthalmitis and the inflammatory response may result in secondary epiretinal membrane formation and tractional retinal detachment. The diagnosis is based on patient history and detailed examination, but additional tests may be required to confirm or support it in difficult cases. Serological testing is helpful and detection of serum antibodies directed against Toxocara antigens by ELISA has a reported sensitivity of approximately 73–78%, with a specificity of approximately 92% at a cut-off dilution value of 1:32.3 However, positive serum antibodies do not always correlate with patent disease, as they can be detectable for months or years after a previous infection, making them also unreliable to monitor treatment success. Furthermore, patients with OLM usually have low or even negative titres. So, detection of anti-Toxocara antibodies in aqueous or vitreous humour can be more sensitive in establishing the diagnosis due to local antibody production induced by metabolic products of Toxocara larvae.10
Although, in our case, Toxocara was a probable cause we were not expecting the additional finding of a fly larva in the vitreous cavity. Larvae deposited by flies preferentially infect necrotic tissue in various sites such as diabetic ulcers or bedsores but can also invade unbroken skin and living tissue, including the eyes, causing ocular myiasis.11 Cases of ocular infestation have been reported before with a clinical picture that can vary from an allergic conjunctivitis to a corneal ulcer, but rarely as endophthalmitis without apparent external eye disease.12–14 Had we not found the larva during vitrectomy, it would have probably gone undiagnosed.
Treatment
Although with unproven efficacy, after the initial vitrectomy the child was treated with oral albendazole (400 mg twice daily for 5 days), methylprednisolone (30 mg once a day)—associated with omeprazol for gastric protection—and routine postoperative topical antibiotics and corticosteroids.
Outcome and follow-up
Unfortunately, 1 month later routine ecographic examination revealed a retinal redetachment. He was again submitted to vitrectomy with membrane peeling, peripheral 360° retinectomy, endolaser and silicone oil tamponade, along with crystalline lens extraction. He subsequently developed ocular hypertension with associated iris bombe, for which he was submitted to laser iridotomy and later to silicone oil extraction. His retina remained attached on follow-up visits, but the boy developed band keratopathy and had no light perception in the affected eye in his last visit.
Discussion
This drastic case serves to emphasise the need to aggressively encourage animal control and public health measures in the general population in order to prevent associated causes of permanent vision loss (and other health problems) which, even nowadays, seem to continue to happen. Both Toxocara and fly larva infections could very possibly have been avoided if the boy’s family had better living conditions and hygiene care and if their puppies had been brought to the veterinary in due time.
Even with an established infection, however, the prognosis could have been better if the vision loss had been detected earlier as intraoperative findings were suggestive of long-lasting intraocular inflammation.
Nevertheless, it also serves to remind us of an important differential diagnosis in a child presenting with unilateral panuveitis or endophthalmitis. In such cases, it is important to exclude infectious aetiologies alongside other serious conditions, namely tumours. Serological testing, as noted before, is helpful but in some instances serum antibodies may not be present and not always correlate with actual disease. The physiological barrier between blood and ocular fluids may account for low or undetectable serum antibody levels in ocular toxocariasis.15 Aqueous or vitreous testing should, therefore, be requested in selected cases. Immunoprecitation techniques, particularly the CIEP, may be a valuable adjunct in the identification of visible reactivity against specific parasite antigens.
Learning points.
Most unilateral posterior uveitis and panuveitis in children are caused by infectious agents. Toxocara gondii and Toxocara canis should always be considered in the differential. Many of these cases are prevented with adequate personal and public health measures.
A detailed social and medical history with special regards to housing conditions, pets owned by the patient and recent trips abroad is an invaluable help to the correct diagnosis.
Although some posterior uveitis cases can be safely diagnosed solely on clinical findings, specific and sensible tests are sometimes needed when dealing with more serious or unclear cases. A multidisciplinary approach, with an infectious-diseases or microbiology expert, is also extremely helpful when dealing with such patients.
Acknowledgments
The authors are greatful to Mrs Isabel Clemente for the technical assistance in the helmintholgy laboratory, IHMT.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Huang JL, Gaudio PA. Ocular inflammatory disease and uveitis manual. USA: Lippincott Williams & Wilkins, 2010 [Google Scholar]
- 2.Olitsky SE, Nelson L. Pediatric clinical ophthalmology. London: Manson Publushing Ltd, 2012 [Google Scholar]
- 3.Guerrant RL, Walker DH, Weller PF. Tropical infectious diseases: principles, pathogens and practice. 3rd edn USA: Saunders Elsevier, 2011 [Google Scholar]
- 4.Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology. 5th edn USA: Elsevier Mosby, 2005 [Google Scholar]
- 5.Latif I, Qamar RR, Attaullah I, et al. Ocular myiasis. Pak J Ophthalmol 2008;24:151–2 [Google Scholar]
- 6.De Savigny DH. In vitro maintenance of Toxocara canis larvae and a simple method for production of Toxocara ES antigen for use in serodiagnostic tests for visceral larva migrans. J Parasitol 1975;61:781–2 [PubMed] [Google Scholar]
- 7.Rombert PC, Daniel F, Trinca A. Application of ELISA in the diagnosis of parasitic diseases. An Inst Hig Med Trop 1982;8:39–41 [Google Scholar]
- 8.Montoya JG, Parmley S, Liesenfeld O, et al. Use of the polymerase chain reaction for diagnosis of ocular toxoplasmosis. Ophthalmology 1999;106:1554–63 [DOI] [PubMed] [Google Scholar]
- 9.Rothova A, de Boer JH, Ten Dam-van Loon NH, et al. Usefulness of aqueous humor analysis for the diagnosis of posterior uveitis. Ophthalmology 2008;115:306–11 [DOI] [PubMed] [Google Scholar]
- 10.Rubinsky-Elefant G, Hoshino-Shimizu S, Jacob CMA, et al. Potential immunological markers for diagnosis and therapeutic assessment of toxocariasis. Rev Inst Med Trop São Paulo 2011;53:61–5 [DOI] [PubMed] [Google Scholar]
- 11.Dogra SS, Mahajan VK. Oral myiasis caused by Musca domestica larvae in a child. Int J Pediatr Otorhinolaryngol 2010;5:105–7 [Google Scholar]
- 12.Narayanan S, Jayaprakash K. Incidence of ocular myiasis due to infection with the larva of oestrus ovis (Oestridae Diptera). Indian J Ophthalmol 1991;39:176–8 [PubMed] [Google Scholar]
- 13.Corrin R, Scholten T, Earle J. Ocular myiasis: mobile conjunctival foreign body. Can Med Assoc J 1985;132:1291–2 [PMC free article] [PubMed] [Google Scholar]
- 14.Khurana S, Biswal M, Bhatti HS, et al. Ophthalmomyiasis: three cases from North India. Indian J Med Microbiol 2010;28:257–61 [DOI] [PubMed] [Google Scholar]
- 15.Benitez del Castillo JM, Herreros G, Guillen JL, et al. Bilateral ocular toxocariasis demonstrated by aqueous humor enzyme-linked immunosorbent assay. Am J Ophthalmol 1995;119:514–16 [DOI] [PubMed] [Google Scholar]


