Abstract
We report the first case of native aortic and mitral valve endocarditis due to Gemella bergeriae from the Middle East in a young patient with rheumatic heart disease. Our case illustrates a fulminant course of infection with G. bergeriae endocarditis that was complicated by embolic stroke, as well as intracerebral and subarachnoid haemorrhage secondary to rupture of a mycotic aneurysm in the right middle cerebral artery. This case highlights the dire, unreported neurological complications of infective endocarditis due to a rare causative organism—G. bergeriae.
Background
We describe a case that highlights the dire, unreported neurological complications of infective endocarditis (IE) due to a rare causative organism—Gemella bergeriae.
Case presentation
A 24-year-old Philipino man presented to the emergency department with left hemiparesis. The patient had been well until the night before, but woke up in the morning with headache, and left-sided weakness. He denied having fever, weight loss, dyspnoea, cough or chest pain. There was no history of any recent dental or periodontal infections. He denied any history of intravenous drug use, smoking, alcohol abuse, recent travel or hospitalisation. His medical history was significant only for anaemia, for which he was prescribed iron replacement.
He was afebrile on arrival with a blood pressure of 136/86 mm Hg, respiratory rate of 16 breath/min, heart rate was regular with 85 bpm and oxygen saturation of 98% in room air. His neurological examination showed a Glasgow Coma Scale (GCS) of 14/15. There was no evidence of neck stiffness, Kernig's sign or Brudzinski's sign. His motor examination was significant for grade 3/5 motor power in his left leg proximal and distal muscles and grade 4/5 in his left forearm. The sensory examination showed preservation of sensation to light touch. Cardiac examination revealed a grade IV pansystolic murmur in the mitral area and a late diastolic murmur in the aortic area. Other systemic examination showed no evidence of peripheral stigmata of IE.
Investigations
Laboratory data at the time of admission included a peripheral white cell count of 16.3×109/L with 87% neutrophils, haemoglobin level of 11.1 g/dL (normocytic normochromic), a platelet count of 437×109/L, serum procalcitonin level 0.32 ng/mL and C reactive protein level >120 mg/L. His blood glucose level, liver enzymes, coagulation profile, creatinine, electrolytes and cardiac enzymes were all within normal limits. Urine analysis showed microscopic haematuria, with crenated red blood cell and granular casts. HIV status was negative. ECG and chest X-ray were unremarkable.
A CT scan of his brain at arrival to the emergency revealed the presence of a faint hypodensity in the right parietal cortex in the territory of the right middle cerebral artery (MCA), which was suggestive of a very recent evolving infarct. There was also another hypodensity noted in the left posterior frontal cortex, which indicated another recent ischaemic infarct (figure 1). However, this infarct appeared to have been a clinically silent event.
Figure 1.
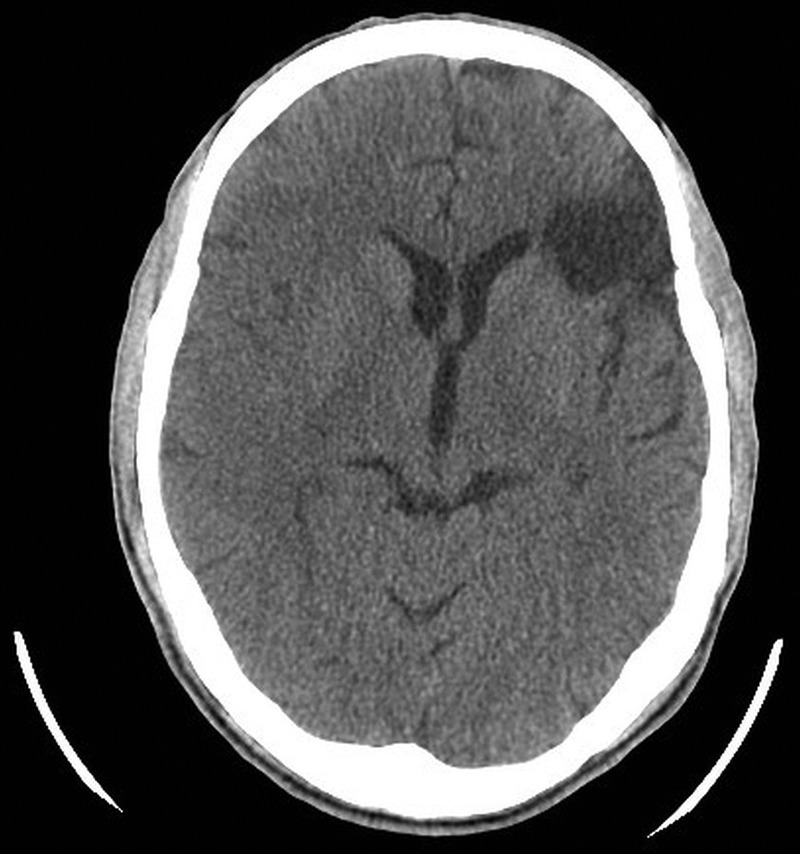
CT scan of the brain at arrival showing a faint hypodensity in the right parietal cortex in the territory of the right middle cerebral artery and another hypodensity noted in the left posterior frontal cortex.
Neurology consultation was requested and based on the clinical findings the patient was suspected to have cardioembolic stroke. An urgent cardiology consultation and cardiac echocardiogram was requested, which showed thickened and restricted aortic cusps with vegetations on them causing severe aortic regurgitation (figure 2). The anterior leaflet of the mitral valve was thickened with multiple vegetations and mild mitral regurgitation (figure 3). The left atrium and the left ventricle were dilated. Right ventricular systolic pressure was 45 mm Hg and left ventricular ejection fraction was 50%. There was no left ventricular clot. The findings of echo were consistent with rheumatic heart disease with IE.
Figure 2.
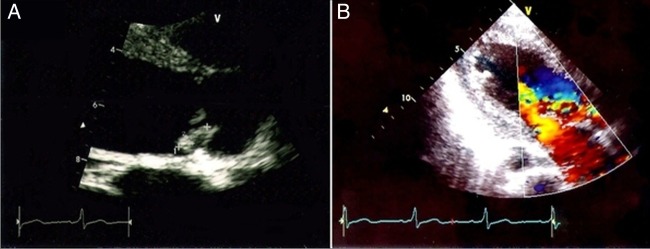
(A) Echocardiogram images showing vegetation on the aortic valve measuring 3mm×12 mm. (B) Doppler images showing moderately severe aortic regurgitation.
Figure 3.
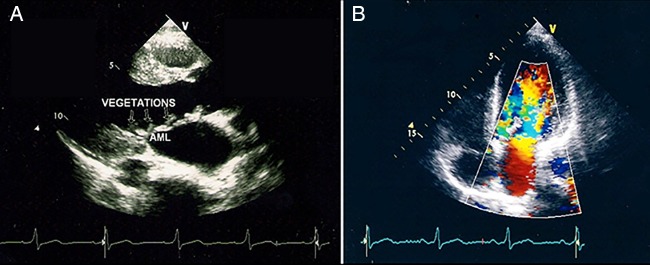
(A) Echocardiogram image showing multiple small vegetation on the anterior leaflet of the mitral valve. (B) Doppler images showing mild mitral regurgitation.
Treatment
As per guidelines for the diagnosis and management of IE, three sets of aerobic and anaerobic blood cultures were sent and the patient was started empirically on intravenous ceftriaxone 2 g once daily and gentamicin 60 mg every 8 h for IE. He was not given any antithrombotic therapy. The patient was admitted in high dependency ward with continuous haemodynamic monitoring and 2 hourly neurological observation. In view of the current diagnosis of IE complicated by embolic episodes, the patient was referred to the cardiothoracic surgeons for surgical intervention. Unfortunately, it was not possible to proceed with cardiac surgery as the patient was noted to develop progressive drowsiness over the next few hours. The GCS dropped to 8/15 and he was immediately intubated and taken for an urgent CT scan of the head, which showed acute right frontotemporoparietal intra-axial haematoma with surrounding low-density changes that caused effacement of the cortical sulci, the adjacent right sylvian fissure as well as the right lateral ventricle with a mild contralateral midline shift. Acute subarachnoid haemorrhage was also noted (figure 4). The neurosurgical team was informed and the patient was immediately taken to the operating theatre for right-sided decompression craniotomy and insertion of intracerebral pressure monitoring catheter. He was transferred to the intensive care unit (ICU) postoperatively for further management. On arrival to the ICU, the patient was sedated and ventilated with intracranial pressure (ICP) >30 cm H2O. He received all brain protective measures as per our ICU protocol for high ICP, which include head elevation, mild hypothermia, avoid hypercapnia, maintain PaO2 >80 mm Hg, mannitol, empiric phenytoin and mechanical device for prevention of deep venous thrombosis. He was started on enteral nutrition and received stress ulcer prophylaxis with proton pump inhibitor.
Figure 4.
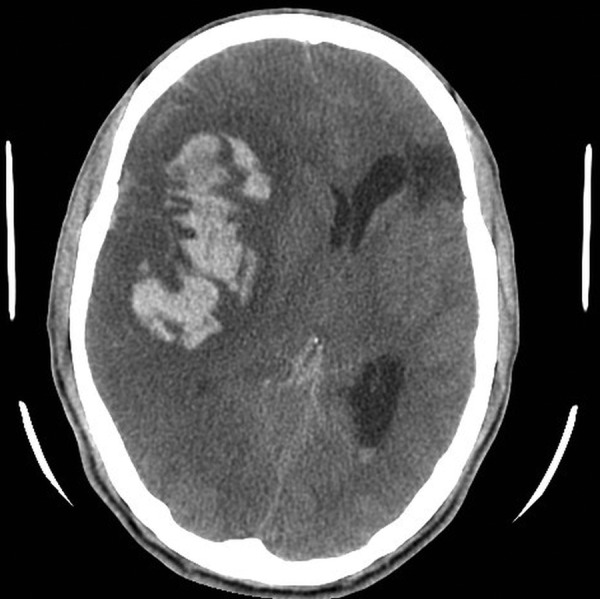
Repeat CT scan of the brain showing acute right frontotemporoparietal intra-axial haematoma and subarachnoid haemorrhage.
As per neurosurgical suggestion, CT cerebral angiography was performed which showed a large saccular aneurysm arising from the right MCA bifurcation with a narrow neck with complete occlusion of the right MCA branches beyond the bifurcation (figure 5).
Figure 5.
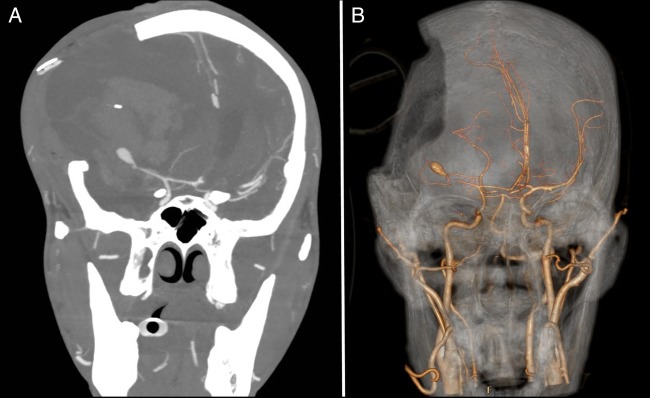
CT cerebral angiography showing a large saccular aneurysm arising from the right middle cerebral artery (MCA) bifurcation with a narrow neck and complete occlusion of the right MCA branches beyond the bifurcation.
On the fourth day of admission, aerobic and anaerobic vials of the three sets of blood culture showed growth of Gram-positive cocci after 70–78 h incubation in BacT/ALERT 3D automated microbial detection system (BioMerieux, USA). The Gram stain results were instantly informed to the treating physician and all the sets were subcultured on blood agar, chocolate agar and MacConkey’ agar plates for further identification and susceptibility testing. The blood agar was incubated at 37°C in aerobic and anaerobic atmosphere, the chocolate agar was incubated at 37°C in 5% CO2 atmosphere and the MacConkey's agar at 37°C aerobically. The aerobic, anaerobic blood agar plates and chocolate revealed pin head non-haemolytic colonies of Gram-positive cocci after around 40 h of incubation. MacConkey’s agar remained negative. The grown colonies were catalase negative, had negative agglutination with Streptococcus pneumonia antisera, the isolate was optochin disc resistant and had negative agglutination with Streptococci Lancefield grouping. The organism was later identified to be G. bergeriae using the VITEK II system (BioMerieux, USA). The Gram-positive identification card (VITEK II) reaction showed the isolate to be positive only for L-proline arylamidase and L-pyrrolidonyl-arylamidase and was able to utilise N-acetyl-D-glucosamine, D-mannitol and D-mannose as a carbon source with acid production. D-sorbitol, D-galactose, D-ribose, lactose, D-maltose, D-raffinose, sucrose and D-trehalose were not utilised. Susceptibility testing was performed on Mueller-Hinton agar supplemented with 5% defribrinated horse blood incubated at 37°C by E test for penicillin and Kirby-Bauer disc diffusion for ampicillin, amoxicillin/clavulanic acid, cefuroxime, ceftriaxone, clindamycin, erythromycin and vancomycin. The Clinical Laboratory Standards Institute interpretive was used for interpretation of results after 48 h incubation. The isolate was sensitive to penicillin, ampicillin, amoxicillin/clavulanic acid, erythromycin, clindamycin, ceftriaxone, cefuroxime and vancomycin.
Based on the susceptibility test of the organism, the same antibiotics were continued. Repeat blood cultures were negative after 2 days of antibiotic therapy.
Outcome and follow-up
The ICP continued to show refractory intracranial hypertension despite all the brain protective measures. Repeat brain CT scan showed a large haemorrhagic infarct in the right MCA territory with significant brain swelling and mass effect. The patient continued to remain hypotensive, requiring full inotropic support. He later lost all brain stem reflexes and subsequently succumbed to his illness after 20 days.
Discussion
Gemella species are relatively uncommon Gram-positive organisms that are increasingly being recognised as a causative agent in causing a wide range of infections in humans. Such infections include skeletal infections, pneumonia, pleural empyema, brain abscess, meningoencephalitis, endophthalmitis, septic shock and IE.1–9
Members of the genus Gemella consist of facultative anaerobic, slow growing fastidious, catalase negative, Gram-positive cocci.10 They are found as the normal resident flora in the oropharyngeal cavity, gastrointestinal and genitourinary tracts.11 Gemella species have a variable Gram staining property. This morphological polymorphism is responsible for misidentification of the organism as viridance group Streptococci and other related organisms, which may contribute to under-reporting of cases of Gemella infection.12 Before 1988, there were only two known Gemella species, G. haemolysans and G. morbillorum. The former was earlier classified as belonging to the genus Neisseria while the latter went through several classification: Diplococcus, Peptostreptococcus and Streptococcus before being finally classified to belong to Gemella.13 The species that are implicated in causing infections in humans include G. haemolysans, G. morbillorum, G. sanguinis, G. bergeriae and G. asaccharolytica. G. bergeriae is a relatively new member of this group that was added in 1998.14 In our case G. bergeriae was isolated from all the bottles of the three sets of blood cultures sent for the patients which confirms the aetiology of the condition.
One of the most recognised infections due to Gemella species is IE.9 There are several cases reported in the literature that have highlighted the pathogenicity of G. morbillorum and G. haemolysans in IE, especially in patients with pre-existing damaged heart valves or poor dental hygiene.15–23 Antimicrobial susceptibility testing of Gemella species has shown that they are highly sensitive to penicillin G and ampicillin and have low-level resistance to aminoglycosides. With appropriate antimicrobial treatment, the outcome is generally good.9 24
To the best of our knowledge there are only five previously reported cases of IE due to G. bergeriae. Collins et al14 reported a series of three cases of IE due to G. bergeriae in 1998, which led to the identification of this bacterium. Elsayed et al25 described another case of G. bergeriae endocarditis in an adult patient with a bicuspid aortic valve that was complicated by aortic valve ring abscess. He underwent aortic valve replacement with good outcome. Later, Logan et al26 reported the first case of paediatric IE by G. bergeriae in a 15-year-old boy with tetralogy of Fallot and pulmonary atresia who had a recent history of cardiac surgery. He was diagnosed to have subclinical IE involving the conduit inserted between his right ventricle and pulmonary artery. All previous cases showed good response to antimicrobial therapy with survival of all five patients.
In general, neurological complications are reported in 20–40% of cases of IE and they have been shown to be predictors of poor outcome.27 28 These complications may be the presenting manifestation in patients with IE. They include ischaemic events, intracerebral haemorrhage, mycotic aneurysm, meningitis, brain abscess or encephalopathy.29 The best way to reduce the risk of embolic complications in IE is by prompt initiation of appropriate antibiotics. It is noteworthy that the addition of aspirin has not been shown to reduce the risk of embolic events.28
Intracerebral haemorrhage is one of the most serious neurological complications and it occurs in about 5% of cases of IE.30 The aetiology is related to many factors including haemorrhagic transformation of the infarcted vessel secondary to septic embolisation, rupture of vessel due to pyogenic arteritis and rarely due to rupture of intracranial mycotic aneurysms. Although mycotic aneurysms can involve any cerebral vessel, the septic emboli are more likely to lodge in the distal part of MCA, especially at the bifurcation points. Hence, they are more likely to be found at these sites.27 30 31 This feature helps to distinguish it from congenital berry aneurysms which commonly tend to occur around the Circle of Willis.32 Intracranial mycotic aneurysms can either be clinically silent, or manifest as cerebral embolisation and intracranial haemorrhage.33 Unruptured aneurysms often heal with appropriate courses of antimicrobial therapy and can be followed up by serial imaging alone.31 However, ruptured aneurysms and IE are a devastating combination and can lead to significant morbidity or even death. They require management by a multidisciplinary team that provides a high level of supportive care, appropriate antibiotic coverage and prompt neurosurgical or even endovascular interventions.34 In cases of IE with intracerebral haemorrhage it is generally recommended to wait for a month prior to proceeding with cardiac surgery due to high risk of perioperative complications.28
All other reported cases of G. bergeriae endocarditis that are reported in the literature had good clinical outcome with appropriate treatment. Nevertheless, it is important to keep in mind that Gemella species can cause serious infections, which can be fatal. Our case illustrates a very fulminant case of G. bergeriae endocarditis that presented with severe, progressive neurological complications, which unfortunately led to a fatal outcome. Our aim is to highlight that not all cases of G. bergeriae endocarditis have a benign course and we recommend physicians to remain vigilant during follow-up of such cases in order to facilitate close monitoring for development of complications.
Learning points.
Gemella bergeriae endocarditis can lead to severe complications.
Consider the possibility of cardioembolic stroke in young patients with ischaemic stroke.
Infective endocarditis (IE) may present with neurological complications.
Patients with stroke secondary to IE should be closely followed for the occurrence of further neurological complications.
Footnotes
Contributors.: KH has written the manuscript. JA & RA were involved in the patient care and have reviewed the manuscript. ZOAD has reviewed the microbiology details of the case.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gatibelza ME, Laroye B, Lombard J, et al. Management of a ruptured infected abdominal aortic aneurysm and a spondylodiscitis due to Gemella haemolysans. Ann Vasc Surg 2009;23:536.e13–7 [DOI] [PubMed] [Google Scholar]
- 2.Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock. Clin Infect Dis 1996;22:1084–6 [DOI] [PubMed] [Google Scholar]
- 3.Roche M, Smyth E. A case of septic arthritis due to infection with Gemella morbillorum. J Infect 2005;51:e187–9 [DOI] [PubMed] [Google Scholar]
- 4.Eisenhut M, Jones C, Hughes D, et al. Acute renal failure associated with Gemella haemolysans pneumonia. Pediatr Nephrol 2004;19:448–50 [DOI] [PubMed] [Google Scholar]
- 5.Valipour A, Koller H, Setinek U, et al. Pleural empyema associated with Gemella morbillorum: report of a case and review of the literature. Scand J Infect Dis 2005;37:378–81 [DOI] [PubMed] [Google Scholar]
- 6.Benedetti P, Rassu M, Branscombe M, et al. Gemella morbillorum: an underestimated aetiology of central nervous system infection? J Med Microbiol 2009;58(Pt 12):1652–6 [DOI] [PubMed] [Google Scholar]
- 7.Galen BT, Banach DB, Gitman MR, et al. Meningoencephalitis due to Gemella haemolysans. J Med Microbiol 2014;63 1):138–9 [DOI] [PubMed] [Google Scholar]
- 8.Ascaso FJ, Cardeñosa E, Cascante JM, et al. Acute postoperative endophthalmitis caused by Gemella morbillorum. Eur J Ophthalmol 2010;20:608–11 [DOI] [PubMed] [Google Scholar]
- 9.Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev 2001;14:177–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger U. The genus Gemella. In: Balows A, Trüper HG, Dworkin M, et al. eds. The prokaryotes. 2nd edn. New York, NY: Springer-Verlag, 1992:1643–53 [Google Scholar]
- 11.Ruoff K. Aerococcus Abiotrophia, and other infrequently isolated aerobic catalase-negative, gram-positive cocci. In: Murray PR, Baron EJ, Jorgensen JH, et al. eds. Manual of clinical microbiology. 8th edn Washington, DC: American Society for Microbiology, 2003:434–44 [Google Scholar]
- 12.La Scola B, Raoult D. Molecular identification of Gemella species from three patients with endocarditis. J Clin Microbiol 1998;36:866–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilpper-Balz R, Schleifer KH. Transfer of Streptococcus morbillorum to the genus Gemella morbillorum comb. nov. Int J Syst Bacteriol 1988;38:442–3 [Google Scholar]
- 14.Collins MD, Hutson RA, Falsen E, et al. Gemella bergeriae sp. nov., isolated from human clinical specimens. J Clin Microbiol 1998;36:1290–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull JE. Multisystem organ failure due to Gemella morbillorum native valve endocarditis. Mil Med 2010;175:923–5 [DOI] [PubMed] [Google Scholar]
- 16.Al Chekakie MO, Heroux A, Montpetit M, et al. Gemella morbillorum prosthetic valve endocarditis. Congest Heart Fail 2009;15:291–2 [DOI] [PubMed] [Google Scholar]
- 17.Avgoustidis N, Bourantas CV, Anastasiadis GP, et al. Endocarditis due to Gemella haemolysans in a patient with systemic lupus erythematosus. J Heart Valve Dis 2011;20:107–9 [PubMed] [Google Scholar]
- 18.Taimur S, Madiha R, Samar F, et al. Gemella morbillorum endocarditis in a patient with a bicuspid aortic valve. Hellenic J Cardiol 2010;51:183–6 [PubMed] [Google Scholar]
- 19.Zheng M, Ng OT, Teo BW. Aortic and mitral valve endocarditis caused by Gemella morbillorum in a haemodialysis patient. Singapore Med J 2008;49:e385–7 [PubMed] [Google Scholar]
- 20.FitzGerald SF, Moloney AC, Maurer BJ, et al. Gemella endocarditis: consider the colon. J Heart Valve Dis 2006;15:833–5 [PubMed] [Google Scholar]
- 21.Murai M, Fukumoto H, Negoro N, et al. Evidence of active endocarditis, caused by Gemella morbillorum, related to acute embolic stroke. Int J Cardiol 2006;112:e17–18 [DOI] [PubMed] [Google Scholar]
- 22.Khan R, Urban C, Rubin D, et al. Subacute endocarditis caused by Gemella haemolysans and a review of the literature. Scand J Infect Dis 2004;36:885–8 [DOI] [PubMed] [Google Scholar]
- 23.Al Soub H, El-Shafie SS, Al-Khal AL, et al. Gemella morbillorum endocarditis. Saudi Med J 2003;24:1135–7 [PubMed] [Google Scholar]
- 24.Buu-Hoi A, Sapoetra A, Branger C, et al. Antimicrobial susceptibility of Gemella haemolysans isolated from patients with subacute endocarditis. Eur J Clin Microbiol 1982;1:102–6 [DOI] [PubMed] [Google Scholar]
- 25.Elsayed S, Zhang K. Gemella bergeriae endocarditis diagnosed by sequencing of rRNA genes in heart valve tissue. J Clin Microbiol 2004;42:4897–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan LK, Zheng X, Shulman ST. Gemella bergeriae endocarditis in a boy. Pediatr Infect Dis J 2008;27:184–6 [DOI] [PubMed] [Google Scholar]
- 27.Sonneville R, Mourvillier B, Bouadma L, et al. Management of neurological complications of infective endocarditis in ICU patients. Ann Intensive Care 2011;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habib G, Hoen B, Tornos P, et al. ; ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for infection and cancer. Eur Heart J 2009;30:2369–413 [DOI] [PubMed] [Google Scholar]
- 29.Chaudhary G, Lee JD. Neurologic complications of infective endocarditis. Curr Neurol Neurosci Rep 2013;13:380. [DOI] [PubMed] [Google Scholar]
- 30.Masuda J, Yutani C, Waki R, et al. Histopathological analysis of the mechanisms of intracranial hemorrhage complicating infective endocarditis. Stroke 1992;23:843–50 [DOI] [PubMed] [Google Scholar]
- 31.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394–434 [DOI] [PubMed] [Google Scholar]
- 32.Jones HR, Jr, Siekert RG. Neurological manifestations of infective endocarditis. Review of clinical and therapeutic challenges. Brain 1989;112(Pt 5):1295–315 [DOI] [PubMed] [Google Scholar]
- 33.Moskowitz MA, Rosenbaum AE, Tyler HR. Angiographically monitored resolution of cerebral mycotic aneurysms. Neurology 1974;24:1103–8 [DOI] [PubMed] [Google Scholar]
- 34.Ducruet AF, Hickman ZL, Zacharia BE, et al. Intracranial infectious aneurysms: a comprehensive review. Neurosurg Rev 2010;33:37. [DOI] [PubMed] [Google Scholar]


