Abstract
Background
There were 1.2 million registered heroin users in China by the end of 2011, but little research in the country has focused on the neuropsychological functioning of these individuals.
Aim
Assess error-related negativity (ERN) – an important indicator of high-level cognitive functioning – among males with heroin dependence undergoing rehabilitation.
Method
Twenty male patients in a rehabilitation center who met DSM-IV criteria for heroin dependence and 15 healthy male controls completed 800 trials of Erikson flanker tasks to provoke ERN waves on the 32-electrode electroencephalograph (EEG) when erroneous responses are induced by presenting incongruent flankers around the target stimulus. Mean error rates and reaction times for the task, and the amplitude and latency of crude ERN waves and standardized ERN waves (created by subtracting the wave for correct responses from that for incorrect responses) at three frontal midline EEG electrodes (Fz, FCz, and Cz) were compared between patients and controls.
Results
There was no significant difference in error rates between patients and controls but the reaction times for both erroneous and correct responses were shorter in patients than controls. The amplitude of both crude and standardized ERN waves was lower in patients than controls, but this difference became non-significant after adjustment for the lower educational level in the patient group. The latency of the peak value in both the crude and standardized ERN waves was significantly shorter in the patient group; this difference remained significant after adjustment for educational level. There was a significant correlation between the negative amplitude of the standardized ERN wave and the duration of heroin use.
Conclusion
These findings suggest impaired impulse control and abnormal error-monitoring functions in persons with a history of heroin dependence and add to the growing literature on the neurological mechanisms related to cognitive dysfunction in individuals with addictive disorders.
Abstract
背景
截止2011年底,全国登记吸毒人群中滥用海洛因人员共115.6万人,然而国内对海洛因依赖者神经心理功能的研究较少。
目的
探讨康复期男性海洛因依赖者高级认知功能的重要指标之一,即错误相关负电位(error-related negativity,ERN)的特征。
方法
符合DSM-IV诊断标准的男性海洛因依赖者20例和健康对照者15人完成视觉Eriksen Flanker刺激任务。该任务共800个刺激,在目标刺激两侧呈现与目标不完全一致的干扰刺激,以此诱发错误应答及ERN。记录被试头皮32导脑电图(EEG)信号。比较患者组与对照组的平均错误率和执行任务的反应时,并比较额叶中线3处EEG电极位置(Fz、FCz和Cz)原始ERN以及ERN差异波(在错误应答波基础上减去正确应答波后的负波)的波幅和潜伏期。
结果
两组的错误率无明显差异,但与对照组相比,患者组的正确反应时和错误反应时均缩短。无论是原始ERN还是ERN差异波,患者组的波幅均低于对照组。由于患者组受教育年限较低,经校正后,两组波幅差异无统计学意义。患者组的原始ERN和ERN差异波的潜伏期均比对照组短,校正受教育水平后差异仍有统计学意义。海洛因依赖组ERN差异波波幅与吸毒年限呈正相关。
结论
研究结果提示有海洛因依赖史的个体存在冲动控制能力受损以及错误监控功能异常。关于成瘾障碍个体认知功能障碍的神经机制的研究日益增加,本研究也为这一文献库添砖加瓦。
1. Introduction
By the end of 2011 there were 1.2 million registered heroin users in China.[1] Heroin dependence has, thus, become a serious public health problem for the country. But research on the causes, characteristics and course of heroin addiction in China is quite limited, so there are no culture-specific theoretical models that can help inform preventive and therapeutic interventions.
One area of interest is the cognitive functioning of persons who become addicted to heroin. Previous studies have found impairments of several dimensions of cognitive functioning among individuals with heroin dependence, including impulse control, attention, and the processing of emotional or cognitive stimuli.[2]–[4] But few studies have assessed high-level cognitive functions. One measure of high-level cognitive functioning is error-related negativity (ERN), an indicator of the self-monitoring mechanisms of the human brain after the occurrence of an error.[5] ERN is measured by assessing the sharp negative electroencephalographic (EEG) signal when an incorrect motor response is made during behavioral tests of event-related potentials (ERP). This ERN wave typically occurs concurrently with or 50-100 ms after the erroneous response, has an amplitude of about 10 µv, and is most prominent in the frontal and central EEG electrodes.[5],[6] Most studies of ERN have been conducted in persons without mental disorders,[7],[8] though some studies have been reported in persons with schizophrenia[9] and anxiety disorders.[10] We have found no reports of ERP-based studies about self-monitoring mechanisms among individuals with heroin dependence either in China or in other countries. With the goal of advancing understanding of the neurobiological mechanisms of heroin dependence, this study uses high-density ERP techniques to compare the characteristics of ERN among males undergoing rehabilitation for heroin dependence and non-dependent, male community members.
2. Methods
2.1. Sample
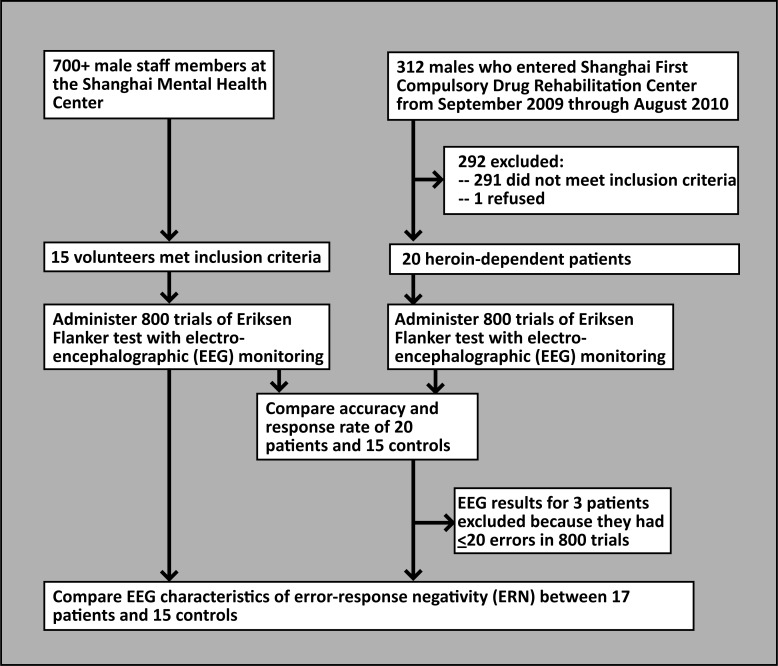
The enrolment of subjects is shown in Figure 1. The heroin-dependent group consisted of 20 men who entered the heroin dependence rehabilitation program at the First Compulsory Rehabilitation Center of Shanghai from September 2009 to August 2010. Inclusion criteria were: (a) 18-55 years of age, (b) meets diagnostic criteria of substance dependence listed in the fourth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-IV),[11] (c) no history of use of other drugs, (d) no history of mental retardation, serious physical illness, or serious mental illness, and (e) right-handedness. The mean (sd) age of the 20 individuals was 37.1 (9.5) years, the mean duration of formal education was 8.4 (3.0) years, the mean duration of prior heroin use was 12.4 (3.1) years, and the mean time of residential treatment in the current rehabilitation program was 8.2 (2.0) months (range, 4-10 months). Four were in their first episode of the mandated two-compulsory inpatient treatment (which lasts two years), 16 had had prior periods of compulsory treatment.
Figure 1. Flowchart of the study.
The control group consisted of 15 individuals recruited from male staff members in the Shanghai Mental Health Center. Inclusion criteria included (a) 18-55 years of age, (b) no current or prior mental illness or mental retardation identified during a clinical interview, (c) no history of drug abuse, (d) no serious physical illness, and (e) right-handedness. The mean age of the control group was 32.5 (9.9) years and they had a mean of 13.7 (4.6) years of education. There was no significant difference in the mean age between cases and controls but the duration of formal schooling in the controls was significantly longer than in the cases (Z=4.08, df=33, p=0.001).
The current study was approved by the Institutional Review Board of Shanghai Mental Health Center. All participants were informed about the procedures of the study and signed consent forms.
2.2. Assessment
All participants filled out a general information questionnaire, which collected demographic information including age, level of education and marital status. In the patient group it also collected information related to drug use including age at the time of the first use of illegal drugs, duration of usage, frequency of use, route(s) of administration, number of attempts at quitting, and time in current compulsory inpatient treatment program.
ERN induced by Eriksen flanker tests[12] were recorded using EEG. All tests were conducted using standardized instructions and parameters by a single trained technician using equipment provided by the Brain Products (Germany) Company. Tests were conducted in a private room that was quiet and had low lighting. During the test, participants wore an electroencephalography cap and sat in front of a monitor at a distance of 100 cm. The ERP were recorded using the international standard 32-channel Ag-Agcl EEG cap. The fronto-central electrode was used as the reference and the anterior midline frontal (AFz) electrode was used as the ground. All electrodes were connected to the scalp via conductive gel. (In order to reduce interference while wearing the EEG cap, all participants washed their hair prior to the test.) Resistance was lower than 5 kΩ, sampling rate was 1000 Hz, and the range of the recording band width was 0.01-200 Hz.
During each trial, a “+” symbol was initially shown on the screen for a random duration that varied in length between 400 and 700 ms. This was followed by a 125ms presentation of one of four stimuli (a string of five letters in the form of ‘HHHHH’, ‘AAAAA’, ‘HHAHH’, or ‘AAHAA’) that were presented as images 1.5 cm x 6 cm on the monitor. At the beginning of the test, participants were instructed to react to these stimuli as follows: “Use the left hand to press ‘1’ (on the keyboard) when the letter in the middle is ‘H’ and use the right hand to press ‘5’ when the letter in the middle is ‘A’. Please press the button as quickly as possible.” Each presentation of a stimulus was followed by a black screen for 475 ms, during which time the participant was expected to react to the stimulus as requested. This is followed by a black screen for a further 500 ms before beginning the next trial; if the participant did not react to the stimulus, the words ‘hurry up’ were shown on the screen for 500 ms before the next stimulus was presented. A brief pilot test was conducted first to familiarize the participant with the process and requirements of the test. The main test included 800 trails in eight blocks with 100 trails in each block and a 30-second rest between blocks. Each of the four stimuli was presented 200 times during the entire test, which took 25 minutes to complete.
2.3. Data analysis
The Analyzer 1.02 software provided by the Brain Products Company was used to conduct offline analyses of the ERN data. All data were referenced to the average of the reference electrodes and then band-pass filtered between 0.5 and 30 Hz. The time point when the participant pressed the button served as the baseline time of the response. EEG data were segmented for each stimulus beginning 150ms before the onset of the response and continuing until 550 ms after the response. Ocular corrections, and baseline corrections were made automatically, and artifacts (>100 µV) were automatically deleted. Data for responses that occurred after erroneous responses (minimum of 20 erroneous responses) were then pooled to quantify the ERN measures for each of the three frontal midline EEG electrodes (Fz,FCz and Cz). Tests during which the respondent made less than 21 errors were excluded from the analysis. The ERN was the EEG wave that occurred during the time window of 0-100 ms after the incorrect motor response. The amplitude of the crude ERN wave (i.e., the maximum value of the mean negative wave following incorrect responses) and latency of these waves (i.e., the time at which the mean raw ERN waves reached their maximum values) were measured. To increase the sensitivity of the analysis, we also computed the amplitude and latency of a ‘standardized’ ERN wave, a composite wave, which subtracts the mean wave after correct responses from the mean wave after incorrect responses. These four values were used in the current analysis.
SPSS 13.0 statistical software was used for data analysis. Non-parametric tests and t-tests were used to analyze demographic data and behavioral data; repeated measures analysis of variance was used to analyze the EEG data. Because EEG data did not follow normal distributions, Spearman correlation coefficients were used to estimate the correlation between EEG measures and other variables. Level of education was categorized into six groups (i.e., 0 years, 1-6 years, 7-9 years, 10-12 years, 13-15 years, and 16+ years) and was entered in the analyses as a covariate. The level of statistical significance was set at p<0.05.
3. Results
3.1. Comparison of behavioral data for the Eriksen flanker tests
Comparison of test results of patients and controls is shown in Table 1. There were no statistically significant differences between the two groups in non-response rates, error rates, or accuracy. There was, however, a wider variation in the number of errors in the heroin group (ranging from 18 to 373 errors in the 800 trials) than in the control group (38 to 107 errors). Compared to the control group, heroin-dependent patients had shorter response times both for correct and erroneous responses. After including levels of education as a covariate, these differences in response times remained statistically significant (F=51.55, p<0.001 for correct responses; and F=40.97, p<0.001 for incorrect responses). The reaction time for erroneous responses was shorter than that for correct responses for both patients (paired t=5.75, p<0.001) and for controls (paired t=8.85, p<0.001).
Table 1. Comparison of behavioral results for the Erickson flanker tests between heroin-dependent patients and controls.
| heroin- dependent patients (n=20) | controls (n=15) | statistic | p | |
| % non-response, median (IQR) | 4.0 (1.3-11.4) | 4.6 (2.0-5.8) | Za=0.13 | 0.894 |
| % errors, median (IQR) | 9.8 (4.9-28.1) | 8.5 (6.8-11.8) | Za=0.75 | 0.453 |
| % correct, median (IQR) | 84.9 (55.5-91.0) | 87.1(82.6-90.4) | Za=1.03 | 0.301 |
| Mean (sd) reaction time for incorrect answers (ms) | 270 (40) | 388 (55) | t=-7.41 | <0.001 |
| Mean (sd) reaction time for correct answers (ms) | 294 (42) | 416(50) | t=-7.87 | <0.001 |
a Z-value from Mann-Whitney rank test
3.2. Comparison of amplitudes and latencies of the crude and standardized ERN waves
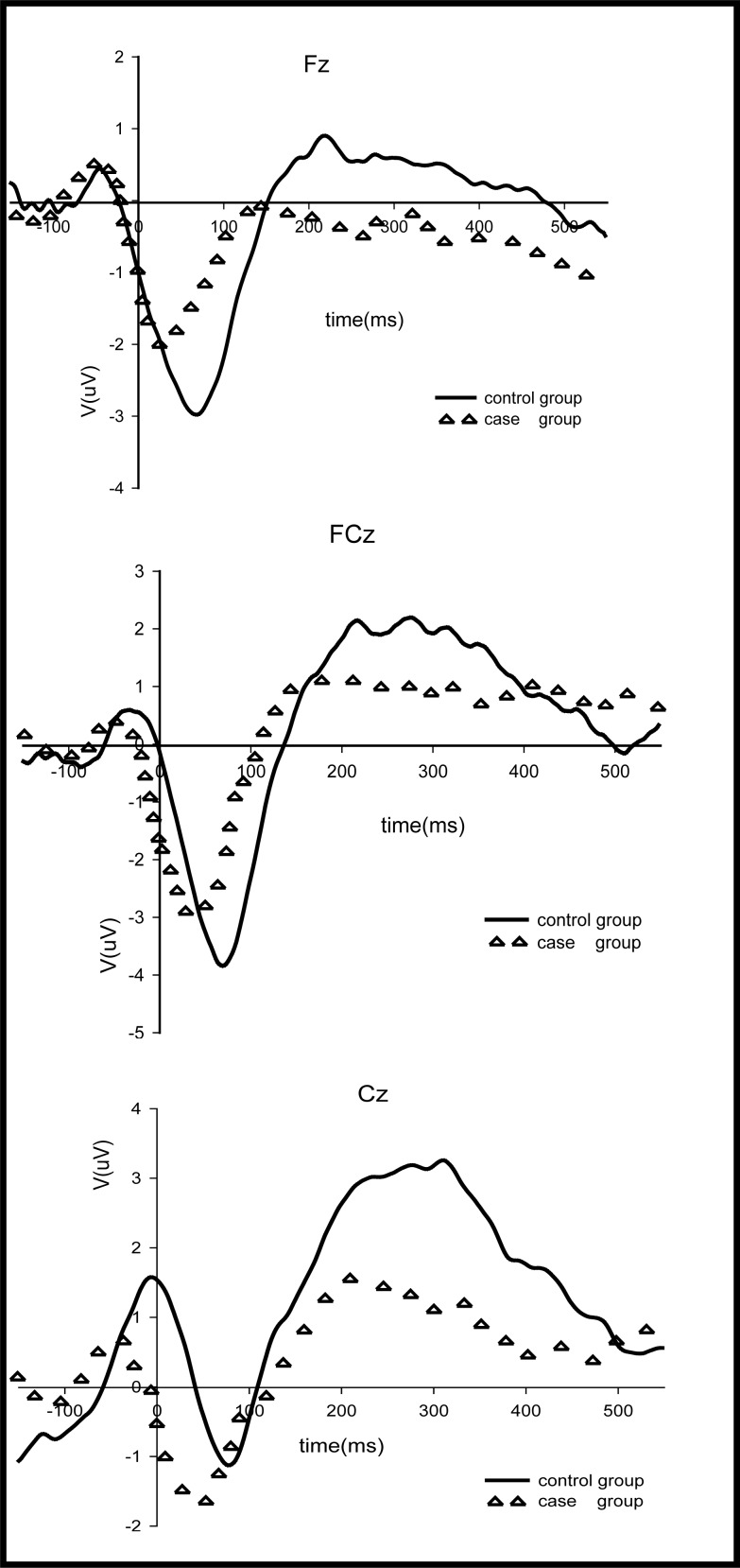
After eliminating three participants from the patient group who had less than 20 erroneous responses, ERN data from the EEG results of three frontal electrodes (Fz, FCz and Cz) were compared between 17 patients and 15 controls.
The crude ERN waves at the three electrodes for the patient and control groups are shown in Figure 2 and the univariate comparison of the amplitudes and latencies of these waves is shown in Table 2. Repeated measures ANOVA found no significant difference between patients and controls in the amplitude of the crude ERN wave (F=1.32, p=0.260) but there was a statistically significant difference in amplitude between the three electrodes (F=12.86, p<0.001). The interaction between group and electrode location was not statistically significant (F=3.07, p=0.064). The significant difference in crude ERN amplitude by electrode site disappeared after adjustment for the level of education (F=0.80, p=0.435). The latency of the crude ERN waves was not significantly different at the three electrode sites (F=1.01, p=0.341), but it was significantly shorter in the patient group than in the control group (F=17.63, p<0.001), and this difference in latency remained significant after adjustment for education (F=15.12, p<0.001).
Figure 2. ERN waves of the case and control groups.
Table 2. Comparisons of mean (sd) amplitudes and latencies of crude and standardized ERN waves between patients and controls.
| region of the brain | heroin- dependent patients (n=17) | controls (n=15) | t-test | p | |
| Amplitude (µV) | |||||
| Crude ERN wave | Fz | -2.4 (1.5) | -3.9 (1.5) | 2.73 | 0.011 |
| FCz | -3.4 (2.6) | -4.6 (2.4) | 1.38 | 0.178 | |
| Cz | -2.3 (2.7) | -2.0 (2.9) | 0.23 | 0.816 | |
| Standardized ERN wave | Fz | -2.3 (2.1) | -3.4 (2.4) | 1.42 | 0.167 |
| FCz | -3.7 (3.0) | -6.1 (1.9) | 2.62 | 0.014 | |
| Cz | -3.8 (3.2) | -5.7 (2.2) | 1.95 | 0.061 | |
| Latency (ms) | |||||
| Crude ERN wave | Fz | 31.4 (15.2) | 64.0 (26.0) | 4.39 | <0.001 |
| FCz | 37.5 (21.7) | 65.2 (18.8) | 3.84 | 0.001 | |
| Cz | 43.9 (29.4) | 64.5 (28.7) | 2.00 | 0.054 | |
| Standardized ERN wave | Fz | 49.7 (31.1) | 64.7 (28.8) | 1.42 | 0.166 |
| FCz | 47.3 (27.6) | 71.1 (20.9) | 2.81 | 0.009 | |
| Cza | 44.6 (30.4) | 73.3 (15.7) | 3.41 | 0.002 |
a This value was not normally distributed. Median (IQR) for patient and control groups were 40.0ms (23.0-65.0) and 72.0ms (59.0-88.0), respectively; Z-value for Mann-Whitney test is 2.91, (p=0.004).
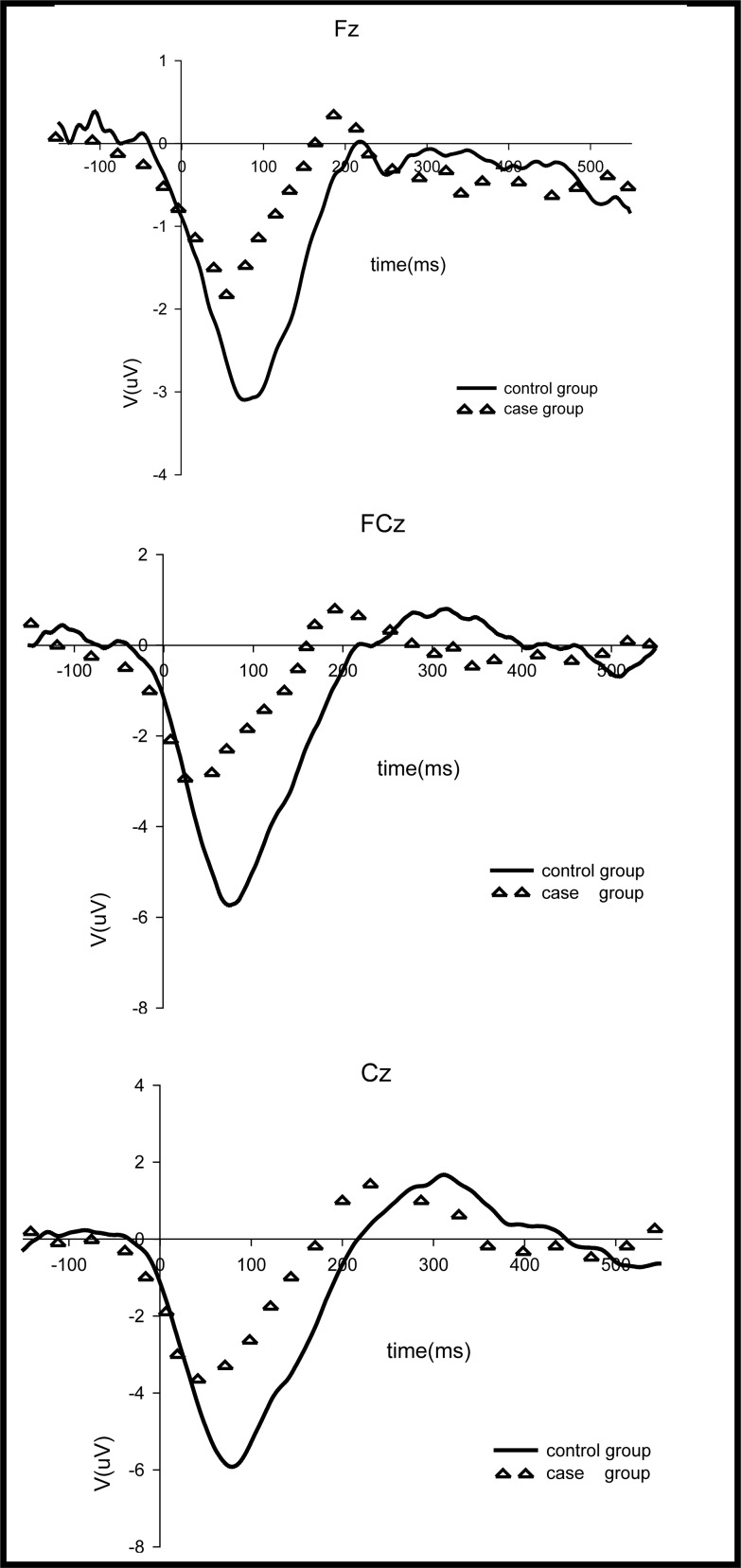
The standardized ERN waves for the patient and control groups are shown in Figure 3 and the amplitudes and latencies of these waves are shown in Table 2. Repeated measures ANOVA found statistically significant differences in the amplitude of the standardized ERN wave between the two groups (F=4.46, p=0.021) and between the three electrode locations (F=13.81, p<0.001). No significant interaction effects between group and electrode locations were found (F=1.01, p=0.341). After adjustment for educational differences between the groups, the differences in amplitude by group (F=3.53, p=0.070) and by electrode location (F=1.79, p=0.191) were no longer statistically significant. The latency of the standardized ERN waves was significantly shorter in patients than in controls, and this difference remained significant after adjustment for educational level (F=4.69, p=0.039). However, the latency of standardized ERN waves was not significantly different at the three electrode placements (F=0.14, p=0.871), and the interaction term between group and electrode location was not significant (F=1.27, p=0.288).
Figure 3. Standardized ERN waves of the case and control groups.
3.3. Correlations between ERN and variables related to heroin use in the heroin-dependence group
Duration of heroin use was significantly correlated with the amplitude of the standardized ERN wave at the FCz area (Spearman correlation coefficient, rs=0.52, p=0.032) and at the Cz area (rs=0.53, p=0.029) but not at the Fz area. However, duration of heroin use was not significantly correlated with the latency of the standardized ERN wave at any of the three electrodes. The standardized ERN amplitude at the FCz area among patients was also significantly correlated with age (rs=0.62, p=0.008); a correlation that was not observed in the control group. No statistically significant correlations were found between the amplitude or latency of the standardized ERN wave and the level of education, age at first heroin use, or number of relapses.
4. Discussion
4.1. Main findings
This study is the first ERP study on error-monitoring in individuals with heroin dependence. We applied the widely used Erikson flanker tasks to provoke ERN waves on the EEG when erroneous responses are induced by presenting incongruent flankers around the target stimulus. We found no significant differences in the error rates for patients with a history of heroin addiction compared to controls who had no history of substance abuse or other mental disorder, but the response times for both correct and incorrect responses were shorter in the heroin-dependent patients than in the controls. The amplitude of the crude and standardized ERN waves was lower in patients than controls, but this difference became non-significant after adjustment for the lower educational level in the patient group. Consistent with the reaction time results, the latency of both crude and standardized ERN waves was significantly shorter in the patient group and this difference remained significant after adjustment for educational level. The more rapid reaction times and shorter latencies in our patients suggest that persons with a history of substance abuse are more impulsive than those without such a history, a finding that is in line with studies that have reported neurophysiological indicators of impaired impulse control in heroin-dependent patients.[1]
Overall, our results suggest – but do not prove – abnormalities in error-monitoring among individuals who have a history of heroin dependence. These results are largely consistent with other studies of error-monitoring among individuals with addictive disorders and other types of mental disorders. Previous studies reported decreased ERN amplitude in individuals with alcohol dependence,[13] cocaine dependence,[14] cannabis dependence,[15] and internet addiction.[16] Other studies have also reported decreased ERN amplitude in individuals with schizophrenia.[9] In contrast, studies of individuals with anxiety disorders report higher ERN amplitude.[10]
In the patient group we also found a significant correlation between the negative amplitude of the standardized ERN wave and the duration of heroin use, suggesting that the damage to error-monitoring functions may be progressive. Previous studies about the relationship of duration of abuse and severity of cognitive impairments have been inconsistent: Hill and colleagues[17] found a positive association between the degree of cognitive dysfunction and the duration of heroin abuse, while Guerra and colleagues[18] found no such relationship.
4.2. Limitations
This study had a relatively small sample size and was limited to male subjects who had been in a compulsory rehabilitation facility for several months. Thus the sample is not representative of all heroin-dependent individuals in China and some of the non-significant results may be due to Type II errors. The failure to match patients and controls on educational status had a substantial effect on the results; after adjustment for educational level several of the statistically significant differences disappeared. Future studies in this are clearly need to match cases and controls by education. The cross-sectional nature of the study made it impossible to determine whether the changes in ERN identified in the patient group are state phenomena, trait phenomena, or have both state and trait components. It is essential to conduct longitudinal studies to distinguish these different potential etiologies for ERN abnormalities. Such studies would help determine how best to employ these measures: if the ERN differences in individuals with a history of heroin addiction are traits, then they can be used to identify persons at high risk of developing addictive conditions; if, on the other hand, the ERN abnormalities are state variables, it may be possible to use them as biological markers of the severity of disease or of the effectiveness of interventions.
4.3. Significance
This study is the first to report on the characteristics of ERN among patients with a history of heroin dependence. Compared to control subjects, heroin-dependent patients had clear evidence of shortened reaction times and ERN latency. Consistent with ERN studies in other addictive conditions, we also found a non-significant reduction in ERN amplitude and a significant correlation between duration of heroin use and the negativity of the standardized ERN wave. These findings suggest abnormal error-monitoring functions in persons with heroin dependence and add to the growing literature on the neurological mechanisms related to cognitive dysfunction in individuals with addictive disorders. Further research with larger samples that integrate the neuropsycholgical tests with neuroimaging analyses are needed to move this important line of inquiry forward.
Acknowledgments
Authors are grateful for the support and help from staff at the Shanghai First Compulsory Rehabilitation Center during this project.
Biography

Dr. Hong Chen graduated from Jining Medical School in June 2009 with a major of Clinical Medicine. In September 2010, she was enrolled in the master's program in Psychiatry and Mental Health at the Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine. Her research interest is in drug dependence.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Funding: The current study was funded by the National Science Foundation of China (81271468, 81130020, 81171267), the Pillar Projects of the Twelfth 5-year Plan of China (2012BAI01B07), the New 100-People Plan of the health system in the city of Shanghai, the National Key Specialized Clinical Medicine-Shanghai Mental Health Center (Medical Administrative Department of the Ministry of Health, 2011-873), and the Shanghai Health Bureau Foundation (2011Y097).
References
- 1.Committee on Illicit Drug Use . 2012 Report on Illicit Drugs. Beijing: Committee on Illicit Drug Use; 2012. (in Chinese) [Google Scholar]
- 2.Lin B, Qian RB, Fu XM, Hu WF, Yin T, Niu CS, et al. Impulsive behavior in heroin addicts: a P300 ERP study. Chinese Journal of Behavioral Medicine and Brain Science. 2012;21(3):235–237. (in Chinese) [Google Scholar]
- 3.Li W, Chen DY, Hu CF, Hao W, Wu DX, Yao SQ. A study on attention and auditory P300 in male patients with heroin dependence in a stage of rehabilitation. Journal of Psychiatry. 2008;21(2):81–83. (in Chinese) [Google Scholar]
- 4.Jiang YP, Xu P, Wang Y, Lu GH. Effects of electroacupuncture on event-related potentials in male heroin addicts' emotion attention. Shanghai Journal of Traditional Chinese Medicine. 2007;41(5):60–62. (in Chinese) [Google Scholar]
- 5.Yang WM, Xiao ZP, Zhang M, Li H, Tang YY, Wang JJ. A comparative study of error-related negativity (ERN) between obsessive-compulsive disorder and of schizophrenia. Chinese Journal of Nervous and Mental Diseases. 2011;37(6):370–373. (in Chinese) [Google Scholar]
- 6.Schefers MK, Coles MG, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: an analysis of incorrect responses to go and no-go stimuli. Psychophysiology. 1996;33(1):42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 7.Miller AE, Watson JM, Strayer DL. Individual differences in working memory capacity predict action monitoring and the error-related negativity. J Exp Psychol Learn. 2012;38(3):757–763. doi: 10.1037/a0026595. [DOI] [PubMed] [Google Scholar]
- 8.Themanson JR, Rosen PJ, Pontifex MB, Hillman CH, McAuley E. Alteration in error-related brain activity and post-error behavior over time. Brain Cognition. 2012;80(2):257–265. doi: 10.1016/j.bandc.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen XS, Xu YF, Tang YX, Wang Y, Zhang MD, Lou FY, et al. Preliminary study on variations and neural generators of error-related negativity in first episode schizophrenics. National Medical Journal of China. 2011;91(43):3040–3043. (in Chinese) [PubMed] [Google Scholar]
- 10.Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, et al. Error-related negativity abnormalities in generalized anxiety disorder and obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):265–272. doi: 10.1016/j.pnpbp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: American Psychiatric Assoation; 1994. [Google Scholar]
- 12.Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a non-search task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 13.Easdona C, Izenberga A, Armilioa ML, Yu H, Alaina C. Alcohol consumption impairs stimulus- and error-related processing during a Go/No-Go Task. Brain Res Cogn Brain Res. 2005;25(3):873–883. doi: 10.1016/j.cogbrainres.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Franken IH, van Strien JW, Franzek EJ, van de Wetering BJ. Error-processing deficits in patients with cocaine dependence. Biol Psychol. 2007;75(1):45–51. doi: 10.1016/j.biopsycho.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulated cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34(11):2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhai TY, Sheng WB, Shao YC, Zhang Y, Ma HC, et al. Electrophysiological evidence for abnormal error monitoring in people with internet addiction disorder. Chinese Journal of Drug Dependence. 2012;21(3):197–199. (in Chinese) [Google Scholar]
- 17.Hill SY, Mikhael MA. Computerized transaxial tomographic and neuropsychol evalutions in chronic alcoholics and heroin abusers. Am J Psychiatry. 1979;136(4-B):598–602. [PubMed] [Google Scholar]
- 18.Guerra D, Sole A, Cami J, Tobena A. Neuropsychological performance in opiate addicts after rapid detoxification. Drug Alcohol Depend. 1987;20(3):261–270. doi: 10.1016/0376-8716(87)90036-6. [DOI] [PubMed] [Google Scholar]