Abstract
Background
Analysis of genetic polymorphisms in short tandem repeats (STRs) is an accepted method for detecting associations between genotype and phenotype but it has not previously been used in the study of the genetics of impulsive violent behavior.
Objective
Compare the prevalence of different polymorphisms in 15 STR loci (D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, D5S818 and FGA) between men with a history of impulsive violence and male control subjects without a history of impulsive violence.
Methods
The distributions of the alleles of the 15 STR loci were compared between 407 cases with impulsive violent behavior and 415 controls using AmpFlSTR® Identifiler™ kits.
Results
Compared to controls, the average frequencies of the following alleles were significantly lower in individuals with a history of violent behavior: allele 10 of TH01 (OR=0.29, 95%CI=0.16-0.52, p<0.0001,), allele 8 of TPOX (OR=0.71, 95%CI=0.58-0.86, p=0.0005), allele 9 of TPOX (OR=0.65, 95%CI=0.47-0.89, p=0.0072) and allele 14 of CSF1PO (OR=0.27, 95%CI=0.11-0.68, p=0.0035). One allele was significantly higher in cases than controls: allele 11 of TPOX (OR=1.79, 95%CI=1.45-2.22, p<0.0001).
Conclusions
To the best of our knowledge, this is the first behavioral genetic study that clearly demonstrates a close relationship between specific genetic markers and impulsive aggression in non-psychiatric offenders. Further prospective work will be needed to determine whether or not the alleles identified can be considered risk factors for impulsive aggression and, if so, the underlying mechanisms that result in this relationship.
Abstract
背景
短串联重复序列基因多态性(short tandem repeats, STRs)分析是用于检测基因型和表型之间关联的公认方法,但它以前没有在冲动暴力行为的遗传学研究中使用。
目的
在有冲动暴力行为历史的男性和无冲动暴力行为男性对照组之间,比较15个STR基因位点(D8S1179,D21S11,D7S820,CSF1PO,D3S1358,TH01,D13S317,D16S539,D2S1338,D19S433,VWA,TPOX,D18S51,D5S818和FGA)不同多态性的发生率。
方法
应用AmpFlSTR®Identifiler™试剂盒比较407个有冲动暴力行为的病例和415个对照的15个STR基因位点等位基因的分布。
结果
有冲动攻击行为历史的人以下等位基因的平均频率显著低于对照组:TH01的等位基因10(OR=0.29,95%CI=0.16-0.52,p<0.0001),TPOX的等位基因8(OR=0.71,95%CI=0.58-0.86,p=0.0005),TPOX的等位基因9(OR=0.65,95%CI=0.47-0.89,p=0.0072),和CSF1PO的等位基因14(OR=0.27,95%CI=0.11-0.68,p=0.0035)。而患者组有一个等位基因频率显著高于对照组:TPOX的等位基因11(OR=1.79,95% CI=1.45-2.22,p<0.0001)。
结论
据我们所知,本项行为遗传学研究最先清楚表明了特定的遗传标记与非精神病罪犯的冲动暴力行为之间的密切关系。进一步的前瞻性工作将需要确定已辨识出的等位基因是否可以被认为是冲动暴力行为的危险因素以及导致这种关系的基本机制。
1. Introduction
Impulsive violent behavior is a non-premeditated aggressive act that an individual engages in hastily without any consideration of the consequences. It usually occurs as an exaggerated response to emotion- provoking events and often leads to undesirable consequences.[1] Individuals with impulsive violent behavior often have difficulty controlling their behavior (i.e., they have behavioral disinhibition). Most of them feel regret after their violent acts.[2]
Compared to individuals who have premeditative violent behavior, those with impulsive violent behavior have a relatively low 5-hydroxyindoleacetic acid (5HIAA) concentration in their cerebrospinal fluid (CSF); this suggests an underlying biological basis for impulsive violent behavior.[3] Previous studies have found that postsynaptic 5-serotonergic (5-HT1B) receptors are involved in the modulation of impulsivity and aggression: the 5-HT1B receptor agonists inhibit ‘aggression or impulsivity’ modulating neurons (including dopaminergic, cholinergic and GABA-ergic neurons) and, thus, facilitate various behaviors related to impulsivity, hyperactivity and aggression.[4]
Accumulating evidence suggests that impulsive violent behavior is partially determined by genetic factors.[5] A previous study found that dopamine D3 receptor (DRD3) polymorphism is highly associated with impulsivity.[6] Retz and colleagues revealed a significant role of the central serotonin transporter promoter gene (5-HTT gene-linked polymorphic region, 5-HTTLPR) in impulsive violent behavior in their study of 153 male Caucasians referred for a forensic psychiatric examination.[7] A systematic review of 109 papers concluded that the functional variable number of tandem repeats (VNTR) polymorphism of the promoter region of the monoamine oxidase A (MAOA) gene plays a role in impulsivity and aggressive behavior.[8] Moreover, the catechol-O-methyltransferase (COMT) gene, which codes for a principle enzyme that catalyzes the metabolism of dopamine, was also considered one of the candidate genes that contributes to the risk of impulsive violent behavior. Some studies report an association between the COMT gene and impulsive violence among patients with schizophrenia, [9]-[11] but other studies have not confirmed this finding.[12],[13] Vevera and colleagues[14] found that male non-psychotic violent offenders who were repeatedly sentenced for impulsive attacks and diagnosed with Antisocial Personality Disorder (APD) had higher frequencies than control subjects of the Ala146Val polymorphism of catecol-O-methyltransferase (COMT). Prichard and colleagues[15] assessed 15 simple sequence repeat polymorphisms in 10 candidate genes in 2097 young Caucasian adults who were arrested; they found significant associations of antisocial behavior in men with androgen receptor (AR) and estrogen receptor 1 (ESR1) polymorphisms, and significant associations of antisocial behavior in women with the nuclear receptor subfamily 4 group A member 2 (NR4A2) and transcription factor AP-2 beta (TFAP2B). In summary, although it is certain that genetics play a role in impulsive violent behavior, the specific genes or genotypes have not yet been identified.
In a previous study our group compared 15 short tandem repeat (STR) loci in individuals with and without impulsive violent behavior and found statistically significant differences in the TH01 and TPOX loci.[16] A short tandem repeat (STR) is a type of VNTR that is less than 400 base pairs in length and that includes 10 to 30 repetitions of sequences of 2 to 6 base pairs. STRs were selected from the non-coding regions of DNA, commonly termed ‘junk DNA’. Markers from such chromosomal regions do not provide specific genetic information,[17] but these regions do include relatively long DNA sequences that exhibit high levels of linkage disequilibrium (LD) with adjacent ‘recombination hotspots’[18],[19] that are called genomic or haplotype blocks. These STR loci vary across different ethnic groups and may even occur uniquely within particular populations.[20] Thus, genetic polymorphism analysis of STR loci has been widely used in a number of fields including forensic biology, genetic mapping, linkage analysis of disease mechanisms, paternity tests, species polymorphism analysis, tumor biology, population genetics, evolutionary biology and so on.
The AmpFlSTR® Identifiler™ kit contains 15 STR loci of euchromosome and one sex-chromosome loci. As shown in Table 1, the 15 STR loci are tetranucleotide repeats distributed in 13 chromosomes (2, 3, 4, 5, 7, 8, 11, 12, 13, 16, 18, 19 and 21). It is currently believed that the heredity of each STR is independent, that is, unrelated to the heredity of other STRs. The 15 STR loci are inherited in a Medellin fashion with a low mutation rate in the population. These STR loci are characterized by easy amplification, accurate electrophoresis classification, good repeatability and consistent genotype in different tissues.[21]
Table 1. The chromosomal location of the 15 short tandem repeats (STRs) loci.
| STR Loci | Location on chromosome |
| D8S1179 | 8 |
| D21S11 | 21q11.2-q21 |
| D7S820 | 7q11 |
| CSF1PO | 5q33.3-34 |
| D3S1358 | 3p |
| D2S1338 | 2q35-37.1 |
| D19S433 | 19q12-13.1 |
| vWA | 12p12-peter |
| TPOX | 2p23-2per |
| D18S51 | 18q21.3 |
| D5S818 | 5q21-23 |
| FGA | 4q28 |
| D13S317 | 13q22-31 |
| TH01 | 11p15.5 |
| D16S539 | 16q24-qter |
Schizophrenia, a disorder that may include symp-toms of increased aggressive behavior, has been associated with genetic abnormalities in regions on several human chromosomes: 1q21-22, 5q21-23, 6p22-24, 6q21-25, 8p12-21, 10pl1-15, 10q25.3-q26.3, 13q32-34, 22ql1.[22] The 15 STR loci in the AmpFlSTR® Identifiler™ kit include adjacent or overlapping areas with these regions that have been associated with schizophrenia. And some of the 15 STR loci are adjacent to the identified impulsivity-related genes mentioned earlier: the DAD3 receptor gene and D3S1358 are both on chromosome 3; the DAD4 receptor gene and TH01 are both located on chromosome 3p; and the 5-HT2A receptor gene on chromosome q14-21 is adjacent to chromosome 13q22-31 where D13S317 is located. Therefore, confirming the association between impulsive violence and these 15 STR loci has the potential for identifying genetic markers that could be used to screen for impulsive offenders.
Built upon our previous study with 203 violent offender subjects,[16] the current study seeks to further examine the association between the 15 STR loci and impulsive violent behavior by conducting genetic polymorphism analysis in a significantly enlarged sample of Han male offenders in Jiangsu province, China.
2. Methods
2.1. Sample
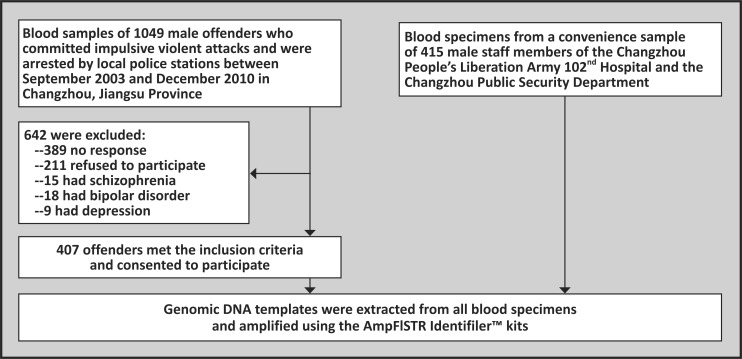
Figure 1 shows the enrollment of participants. The records and blood samples of all male offenders arrested from September 2003 to December 2010 were obtained from local police stations in Changzhou, Jiangsu Province. The aggressive behaviors of these offenders resulted in either wounding (i.e. worse than minor injuries) or death to the victims. By reviewing the documents, staff members at the Changzhou Bureau of Public Security determined that the acts of 1049 offenders were in response to spontaneous anger-provoking stimuli without premeditation (i.e. categorized as having impulsive violent behavior), and the acts of the other 946 were planned and intentional (i.e. categorized as premeditated aggression).
Figure 1. Flow chart of the study.
All the 1049 offenders with impulsive violent behavior were contacted by telephone; 389 did not respond and 211 refused to participate. Each participant who provided oral informed consent was interviewed by a psychiatrist over the telephone; this interview, which included an unstructured diagnostic exam, was subsequently presented to another psychiatrist and the two psychiatrists would then collectively determine whether or not the individual had a mental disorder and, if so, the probable diagnosis. Interviewers also spoke to at least one relative of each offender by telephone and recorded standardized information about the health and behavior of the individual. Records in the China National Crime Information System were checked to determine whether or not biological relatives of the enrolled offenders had a history of criminal behavior. Individuals who met any of the following criteria were excluded from the sample: (a) a serious mental disorder based on the telephone interview or based on a history of a mental disorder that had previously been diagnosed by a psychiatrist; (b) a history of premeditated aggressive behavior; (c) having a biological relative with a criminal record; (d) history of a head injury; (e) an obvious physical illness; or (f) a history of substance abuse problems. Following this screening procedure, 407 (38.7%) individuals with impulsive violent behavior were enrolled in the current study.
For the control group, blood specimens from a convenience sample of 415 males were obtained from among the male staff members of the People's Liberation Army (PLA) 102nd hospital in Changzhou and the Public Security Bureau of Changzhou who were undergoing a routine annual physical examination. Potential participants in the control group were screened (typically in a face-to-face interview), and those with any of the following conditions were excluded: (a) self-report of a history of a mental disorder in the individual or his family members; (b) having criminal records or a history of violent behavior; (c) having family members with a history of violent behavior or criminal records; (d) a biological relationship with any of the other individuals in the control sample. All 415 of the identified individuals were eligible to participate and provided written informed consent.
All participants in this study were Chinese males of Han ethnicity and had no kinship with each other. The study was approved by the Ethics Committee for the Protection of Human Subjects of the PLA 102nd Hospital.
2.2. Genetic testing
2.2.1. Sample preparation
The specimens of whole ethylene diamine tetraacetic acid (EDTA) blood were collected from each participant. Genomic DNA was extracted from white blood cell fractions using the chelex-100 protocol.[23]
2.2.2. PCR amplifications
AmpFlSTR® Identifiler™ kits (patent no. 5364759, Applied Biosystems, UK) with reaction mix and primer mix was used in this study. The amplification was performed following the manufacturer's instructions.[24] Five U of AmpliTaq Gold® DNA Polymerase were used for the amplification of STR loci. AmpFlSTR® control DNA (9947A) was used as a positive PCR control in all amplifications. Sterile deionized water was used as a negative control in all PCR batches. Extracted DNA was amplified in a total reaction volume of 10 µL, containing 1 µL of genomic DNA template, 4.2 µL of AmpFlSTR® PCR Reaction Mix, 2.2 µL of AmpFlSTR® Identifiler Primer Set, 0.2 U of AmpliTaq Gold® DNA Polymerase and 3.3 µL of ddH2O. Without using mineral oil, the amplification was performed on a 9700 thermal cycler (Applied Biosystems GeneAmp® PCR system 9700) using the following cycling parameters: 95°C for 11 min; 28 cycles of 94°C for 60 seconds, 59°C for 60 seconds, 72°C for 60 seconds; 60°C extension for 45 min; holding at 4°C.
2.2.3. Detection of PCR product
1 µL of each PCR product and 14 µl of 37:1 Hi-Di™ formamide (Applied Biosystems, USA): GeneScan™-500 LIZ™ size standard (Applied Biosystems, UK, part no. 4322682) was added to each well in a 96-well micro-titre plate. Wells containing 1 µL AmpFlSTR® allelic ladder were included on the plate, containing amplified alleles for all of the STR loci included in the Identifiler™ kit. Following the manufacturer's protocol,[24] samples were run on a capillary electrophoresis (CE) sequencer (Applied Biosystems, ABI Prism® 3100 Genetic Analyser) using Collection software V1.0 (Applied Biosystems). Sample data from the 3100 CE instrument was analyzed using ABI Prism™ GeneMapper™ ID v3.2. The LIZ™ size standard peaks were used to determine the size (bp) of peaks, and allele designation was determined by comparing it to the allelic ladder.
2.3. Statistical analysis
The PowerStats software (Promega Corporation) was used to obtain allele gene and genotype frequencies of the 15 STR loci, and tests of Hardy-Weinberg equilibrium were performed using an online calculator (http://www.oege.org/software/hwe-mr-calc.shtml). SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA) was used to perform statistical comparisons between the two groups. The significance level was set at 0.05. T-tests were used to compare the distributions of age in offenders and controls. R×C chi-squared tests were employed to compare the distributions of genotypes and alleles in each STR. When the R×C chi-squared test was significant at the 0.05 level, the allele or genotype frequencies of the STR was subsequently compared using 2×2 chi-squared tests or, if there were insufficient numbers in any of the cells, by Fisher exact tests. To adjust for the multiple testing issue, the level of statistical significance for these pairwise tests was set at 0.05/N, where N was the number of pairwise comparisons in each STR. In addition, univariate odds ratios (ORs) with 95% confidence intervals (CI) of the different allele or genotype frequencies were determined as a measure of the strength of association.
3. Results
The mean (sd) age of the case group (27.4 [6.9] years, range=20-56) was not statistically different from that of the control group (28.3 [7.1] years, range=18-60) (t=1.84, p=0.076).
All genotype frequencies of the 15 STRs were consistent with the Hardy-Weinberg equilibrium in both groups (p>0.05).
Table 3. Comparison of TPOX locus allele frequencies among men with impulsive violent behavior (cases) and men without impulsive violent behavior (controls) in a Han Chinese population.
| alleles | allele frequenciesa |
statistic | p-valueb | odds ratio | 95%CI | |
| cases (n=407) | controls (n=415) | |||||
| 7 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| 8 | 400 (49.1%) | 479 (57.7%) | χ2=12.13 | 0.0005 | 0.71 | 0.58-0.86 |
| 9 | 72 (8.9%) | 108 (13.0%) | χ2=7.32 | 0.0072 | 0.65 | 0.47-0.89 |
| 10 | 22 (2.7%) | 20 (2.4%) | χ2=0.14 | 0.7065 | 1.13 | 0.61-2.08 |
| 11 | 294 (36.1%) | 199 (24.0%) | χ2=28.86 | <0.0001 | 1.79 | 1.45-2.22 |
| 12 | 24 (3.0%) | 24 (2.9%) | χ2=0.01 | 0.9454 | 1.02 | 0.57-1.81 |
| Total | 814 (100%) | 830 (100%) | ---- | ---- | ---- | ---- |
a 2x6 χ2=34.6, p<0.001
b p<0.0083 was considered statistically significant
Table 4. Comparison of CSF1PO locus allele frequencies among men with impulsive violent behavior (cases) and men without impulsive violent behavior (controls) in a Han Chinese population.
| alleles | allele frequenciesa |
statistic | p-valueb | odds ratio | 95%CI | |
| cases (n=407) | controls (n=415) | |||||
| 7 | 0 (0.0%) | 2 (0.2%) | exact test | 0.4997 | ---- | ---- |
| 8 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| 9 | 26 (3.2%) | 30 (3.6%) | χ2=0.22 | 0.6385 | 0.88 | 0.52-1.50 |
| 10 | 162 (19.9%) | 208 (25.1%) | χ2=6.27 | 0.0123 | 0.74 | 0.59-0.94 |
| 11 | 218 (26.8%) | 227 (27.4%) | χ2=0.07 | 0.7955 | 0.97 | 0.78-1.21 |
| 12 | 332 (40.8%) | 291 (35.1%) | χ2=5.73 | 0.0167 | 1.28 | 1.04-1.56 |
| 13 | 66 (8.1%) | 50 (6.0%) | χ2=2.72 | 0.0990 | 1.38 | 0.94-2.01 |
| 14 | 6 (0.7%) | 22 (2.7%) | χ2=8.99 | 0.0035 | 0.27 | 0.11-0.68 |
| 15 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| Total | 814 (100%) | 830 (100%) | ---- | ---- | ---- | ---- |
a 2x9 χ2=24.04, p=0.001
b p<0.0055 was considered statistically significant
Table 5. Comparison of D3S1358 locus allele frequencies among men with impulsive violent behavior (cases) and men without impulsive violent behavior (controls) in a Han Chinese population.
| alleles | allele frequenciesa |
statistic | p-valueb | odds ratio | 95%CI | |
| cases (n=407) | controls (n=415) | |||||
| 12 | 6 (0.7%) | 0 (0.0%) | exact test | 0.0146 | ---- | ---- |
| 14 | 37 (4.6%) | 30 (3.6%) | χ2=0.91 | 0.3398 | 1.27 | 0.78-2.08 |
| 15 | 286 (35.1%) | 310 (37.4%) | χ2=0.87 | 0.3504 | 0.91 | 0.74-1.11 |
| 16 | 256 (31.5%) | 246 (29.6%) | χ2=0.64 | 0.4253 | 1.09 | 0.88-1.34 |
| 17 | 177 (21.7%) | 178 (21.5%) | χ2=0.02 | 0.8830 | 1.02 | 0.80-1.29 |
| 18 | 52 (6.4%) | 56 (6.8%) | χ2=0.09 | 0.7691 | 0.94 | 0.64-1.39 |
| 19 | 1 (0.1%) | 10 (1.2%) | exact test | 0.0115 | 0.10 | 0.01-0.79 |
| Total | 814 (100%) | 830 (100%) | ---- | ---- | ---- | ---- |
a 2x7 χ2=15.34, p=0.0178
b p<0.0071 was considered statistically significant
Table 6. Comparison of D13S317 locus allele frequencies among men with impulsive violent behavior (cases) and men without impulsive violent behavior (controls) in a Han Chinese population.
| alleles | allele frequenciesa |
statistic | p-valueb | odds ratio | 95%CI | |
| cases (n=407) | controls (n=415) | |||||
| 7 | 0 (0.0%) | 6 (0.7%) | exact test | 0.0310 | ---- | ---- |
| 8 | 206 (25.3%) | 257 (31.0%) | χ2=6.50 | 0.0108 | 0.76 | 0.61-0.94 |
| 9 | 118 (14.5%) | 104 (12.5%) | χ2=1.36 | 0.2435 | 1.18 | 0.89-1.57 |
| 10 | 118 (14.5%) | 124 (14.9%) | χ2=0.06 | 0.7997 | 0.97 | 0.73-1.29 |
| 11 | 182 (22.4%) | 188 (22.7%) | χ2=0.02 | 0.8873 | 0.98 | 0.78-1.24 |
| 12 | 138 (17.0%) | 108 (13.0%) | χ2=5.02 | 0.0251 | 1.36 | 1.04-1.79 |
| 13 | 42 (5.2%) | 35 (4.2%) | χ2=0.82 | 0.3657 | 1.24 | 0.78-1.96 |
| 14 | 10 (1.2%) | 8 (1.0%) | χ2=0.27 | 0.6062 | 1.28 | 0.50-3.25 |
| Total | 814 (100%) | 830 (100%) | ---- | ---- | ---- | ---- |
a 2x8 χ2=17.11, p=0.0167
b p<0.0062 was considered statistically significant
Table 7. Comparison of D18S51 locus allele frequencies among men with impulsive violent behavior (cases) and men without impulsive violent behavior (controls) in a Han Chinese population.
| alleles | allele frequenciesa |
statistic | p-valueb | odds ratio | 95%CI | |
| cases (n=407) | controls (n=415) | |||||
| 9 | 0 (0.0%) | 2 (0.2%) | exact test | 0.4997 | ---- | ---- |
| 11 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| 12 | 34 (4.2%) | 22 (2.7%) | χ2=2.91 | 0.0880 | 1.60 | 0.92-2.76 |
| 13 | 156 (19.2%) | 174 (21.0%) | χ2=0.83 | 0.3625 | 0.89 | 0.70-1.14 |
| 13.2 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | 1.00 | 0.99-1.00 |
| 14 | 172 (21.1%) | 149 (18.0%) | χ2=2.64 | 0.1041 | 1.22 | 0.96-1.56 |
| 15 | 137 (16.8%) | 144 (17.4%) | χ2=0.08 | 0.7799 | 0.96 | 0.75-1.25 |
| 16 | 133 (16.3%) | 104 (12.5%) | χ2=4.83 | 0.0279 | 1.36 | 1.03-1.80 |
| 17 | 54 (6.6%) | 72 (8.7%) | χ2=2.42 | 0.1199 | 0.75 | 0.52-1.08 |
| 18 | 36 (4.4%) | 44 (5.3%) | χ2=0.69 | 0.4078 | 0.83 | 0.53-1.30 |
| 19 | 28 (3.4%) | 54 (6.5%) | χ2=8.15 | 0.0043 | 0.51 | 0.32-.82 |
| 20 | 28 (3.4%) | 21 (2.5%) | χ2=1.18 | 0.2782 | 1.3723 | 0.77-2.44 |
| 21 | 12 (1.5%) | 22 (2.7%) | χ2=2.81 | 0.0938 | 0.55 | 0.27-1.12 |
| 22 | 10 (1.2%) | 16 (1.9%) | χ2=1.29 | 0.2559 | 0.63 | 0.29-1.40 |
| 23 | 6 (0.7%) | 6 (0.7%) | χ2=0.00 | 0.9730 | 1.02 | 0.38-3.18 |
| 24 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| 25 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| Total | 814 (100%) | 830 (100%) | ---- | ---- | ---- | ---- |
a 2x17 χ2=35.71, p=0.0032
b p<0.0029 was considered statistically significant
The main results are shown in Tables 2 to 8. The distributions of specific alleles of TH01, TPOX, CSF1PO, D3S1358, D13S317, D18S51, and FGA in cases with impulsive violent behavior were significantly different from those of the controls. The frequencies of the following alleles were significantly lower among offenders compared to the controls: allele 10 of TH01 (OR=0.29, 95%CI=0.16-0.52), allele 8 of TPOX (OR=0.71, 95%CI=0.58-0.86), allele 9 of TPOX (OR=0.65, 95%CI=0.47-0.89), and allele 14 of CSF1PO (OR=0.27, 95% CI = 0.11-0.68). On the other hand, allele 11 of TPOX was significantly more prevalent in violent offenders than in controls (OR=1.79, 95%CI=1.45-2.22).
Table 2. Comparison of TH01 locus allele frequencies among men with impulsive violent behavior (cases) and men without impulsive violent behavior (controls) in a Han Chinese population.
| alleles | allele frequenciesa |
statistic | p-valueb | odds ratio | 95%CI | |
| cases (n=407) | controls (n=415) | |||||
| 6 | 100 (12.3%) | 76 (9.2%) | χ2=4.21 | 0.0402 | 1.39 | 1.01-1.90 |
| 7 | 198 (24.3%) | 206 (24.8%) | χ2=0.05 | 0.8190 | 0.97 | 0.78-1.22 |
| 8 | 36 (4.4%) | 35 (4.2%) | χ2=0.04 | 0.9036 | 1.05 | 0.65-1.69 |
| 9 | 430 (52.8%) | 445 (53.6%) | χ2=0.10 | 0.7669 | 0.97 | 0.80-1.18 |
| 9.3 | 36 (4.4%) | 20 (2.4%) | χ2=5.06 | 0.0245 | 1.87 | 1.08-3.27 |
| 10 | 14 (1.7%) | 48 (5.8%) | χ2=18.70 | <0.0001 | 0.29 | 0.16-0.52 |
| Total | 814 (100%) | 830 (100%) | ---- | ---- | ---- | ---- |
a 2x6 χ2=26.7, p<0.001
b p<0.0083 was considered statistically significant
Table 8. Comparison of FGA locus allele frequencies among men with impulsive violent behavior (cases) and men without impulsive violent behavior (controls) in a Han Chinese population.
| alleles | allele frequenciesa |
statistic | p-valueb | odds ratio | 95%CI | |
| cases (n=407) | controls (n=415) | |||||
| 15.2 | 0 (0.0%) | 2 (0.2%) | exact test | 0.4997 | ---- | ---- |
| 17 | 4 (0.5%) | 0 (0.0%) | exact test | 0.0599 | ---- | ---- |
| 18 | 30 (3.7%) | 16 (1.9%) | χ2=4.67 | 0.0307 | 1.95 | 1.05-3.60 |
| 19 | 38 (4.7%) | 28 (3.4%) | χ2=1.79 | 0.1812 | 1.40 | 0.85-2.31 |
| 19.2 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| 20 | 34 (4.2%) | 44 (5.3%) | χ2=1.15 | 0.2837 | 0.78 | 0.49-1.23 |
| 21 | 75 (9.2%) | 100 (12.3%) | χ2=3.47 | 0.0624 | 0.74 | 0.54-1.02 |
| 21.2 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| 22 | 146 (17.9%) | 136 (16.4%) | χ2=0.70 | 0.4044 | 1.11 | 0.86-1.44 |
| 22.2 | 6 (0.7%) | 4 (0.5%) | exact test | 0.5059 | 1.53 | 0.43-5.45 |
| 23 | 176 (21.6%) | 223 (16.9%) | χ2=6.15 | 0.0131 | 0.75 | 0.60-0.94 |
| 23.2 | 4 (0.5%) | 6 (0.7%) | exact test | 0.5461 | 0.68 | 0.19-2.41 |
| 24 | 141 (17.3%) | 140 (16.9%) | χ2=0.06 | 0.8067 | 1.03 | 0.80-1.33 |
| 24.2 | 6 (0.7%) | 8 (1.0%) | χ2=0.25 | 0.6169 | 0.76 | 0.26-2.21 |
| 25 | 98 (12.0%) | 76 (9.2%) | χ2=3.61 | 0.0575 | 1.38 | 0.99-1.86 |
| 25.2 | 2 (0.3%) | 0 (0.0%) | exact test | 0.2450 | ---- | ---- |
| 26 | 38 (4.7) | 40 (4.8%) | χ2=0.02 | 0.8855 | 0.97 | 0.61-1.52 |
| 27 | 8 (1.0%) | 5 (0.6%) | χ2=0.76 | 0.3840 | 1.64 | 0.53-5.03 |
| 28 | 4 (0.5%) | 0 (0.0%) | exact test | 0.0599 | ---- | ---- |
| 29 | 0 (0.0%) | 2 (0.2%) | exact test | 0.4997 | ---- | ---- |
| Total | 814 (100%) | 830 (100%) | ---- | ---- | ---- | ---- |
a 2x20 χ2=38.98, p=0.0044
b p<0.0025 was considered statistically significant
The frequencies of some other alleles were different between the two groups but only at a trend level (i.e., they did not reach the specified level to be considered statistically significant): alleles 12 and 19 of D3S1358; alleles 7, 8 and 12 of D13S317; alleles 16 and 19 of D18S51; and alleles 18 and 23 of FGA.
We did not find any statistically significant difference when comparing specific genotypes between individuals with and without impulsive violent behavior.
4. Discussion
4.1. Main findings
By comparing genetic polymorphism of 15 STR loci between cases with impulsive violent behavior and controls, we found that certain alleles on loci TH01, TPOX and CSF1PO were associated with impulsive aggression among Chinese males of Han ethnicity. The frequencies of TH01 allele 10, TPOX allele 8 and 9, and CSF1PO allele 14 were significantly lower in cases than in controls and the frequency of TOPX allele 11 was significantly higher in cases than in controls. These results are similar to our previous study that used a smaller sample size (n=203),[16] with the exception that the previous study did not identify a statistically significant difference between cases and controls in allele 14 of the CSF1PO locus.
The underlying mechanism for the relationship between these allelic differences and impulsive aggression remain unclear, but there are several possible pathways that deserve further investigation. TH01, also known as TC11, is a tetrameric STR locus located in the first intron of the tyrosine hydroxylase (TH) gene. It has an AATG repeat and its chromosomal location is 11p15.5p. TH is the enzyme that regulates the synthesis of catecholamine neurotransmitters, including epinephrine, norepinephrine and dopamine.[25] TPOX, also known as hTPO, is the tenth intron of thyroid peroxidase (TP) gene. It has an AATG repeat and its chromosomal location is 2p23-1pter. TP is a glycoprotein of thyroid cell membrane; it is an enzyme expressed mainly in thyroid hormones.[26] CSF1PO locus is a c-fms proto-oncogene for colony stimulating factor 1 receptor (CSF1R). It has an AGAT repeat and its chromosomal location is 5q33.5-q34. CSF1R is encoded by the c-fms proto-oncogene, and is a member of a family of growth factor receptors that exhibits an intrinsic tyrosine-specific protein kinase activity.[27] Thus the allelic differences we identified in men with impulsive aggression suggest that they may have abnormalities in the syntheses and metabolism of catecholamine neurotransmitters and thyroid hormones, changes that could be associated with an increase in impulsive aggression.[28]-[29]
4.2. Limitations
The current study is associative in nature, so we were unable to identify a causal relationship between the identified alleles and impulsive aggression. It is possible that the observed association between TH01, TPOX and CSF1PO loci and impulsive aggression may be attributed to adjacent genes that are expressed jointly with the loci under study. Further studies are needed to explore the mechanisms underlying the associations we have identified. There is still a long way to go before it will be possible to use genetic markers such as these loci to predict violent behavior or to identify individuals at high risk of violent behavior. There are, moreover, several ethical issues that would need to be addressed before the genetic results could be used to do this.
Given the complexity of violent behavior, our assessment of whether or not an individual had ‘impulsive aggression’ was based on limited information obtained by a telephone interview so there may have been some level of misclassification. More detailed, standardized measures need to be developed that can more precisely classify the phenotypes of aggression, though there will always be some cases in whom it will be impossible to definitively distinguish premeditated from non-premeditated (i.e., impulsive) aggression.
The present study was restricted to male offenders, so the results may not be relevant for females. Only 38.7% of the offenders with impulsive aggression were included in the study and many individuals with impulsive aggression do not become criminal offenders, so our results may not be representative of all men with impulsive aggression. And despite the relatively large sample, the frequencies of some of the alleles were very low (or zero), so even larger studies will be needed to assess the potential relationship of these alleles to impulsive aggression.
4.3. Implications
To the best of our knowledge, this is the first behavioral genetic study that clearly demonstrates a close relationship between specific genetic markers and impulsive aggression in non-psychiatric offenders. Further prospective work will be needed to determine whether or not the alleles identified can be added to the list of risk factors that are already known to predict violent behavior: a prior history of violence,[30] antisocial personality[31] and substance abuse.[32] Gu and colleagues[11] have suggested using the COMT gene and the haplotypes A-A-G and G-G-A as a bio-marker for predicting violent behavior among people with schizophrenia; more work will need to determine whether or not similar genetic biomarkers will be of value in predicting impulsive violence among people without mental illnesses.
Given the multiplicity of factors that influence complex behaviors such as violence, it is probable that a variety of genetic mechanisms are involved in increasing or decreasing the likelihood of such behavior. Our study has shown that testing genetic polymorphism of STR loci – a technology that is now relatively mature and widely available – is an effective method of simultaneously considering a number of genetic pathways that could be involved in such complex behaviors.
Biography

Chun Yang obtained her bachelor's degree in medicine from Xinxiang Medical School in 2002. She has been enrolled in the master's program in psychiatry at the Second Military Medical University since 2011. Currently, she is also working as an attending doctor in the Psychiatry Center of Chinese People's Liberation Army, the PLA 102nd Hospital. Her research interest is in the association between STR loci, abnormal behavior and mental diseases.
Footnotes
Conflict of interest: The authors report no conflict of interest related to this manuscript.
Funding: This study was not financed by any grant.
References
- 1.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8(11):1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 2.Chen CY, Tien YM, Juan CH, Tzeng OJ, Hung DL. Neural correlates of impulsive-violent behavior: an event-related potential study. Neuroreport. 2005;16(11):1213–1216. doi: 10.1097/00001756-200508010-00016. [DOI] [PubMed] [Google Scholar]
- 3.Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci. 1983;33(26):2609–2614. doi: 10.1016/0024-3205(83)90344-2. [DOI] [PubMed] [Google Scholar]
- 4.Olivier B, van Oorschot R. 5-HT1B receptors and aggression: a review. Eur J Pharmacol. 2005;526(1–3):207–217. doi: 10.1016/j.ejphar.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 5.Eley TC, Lichtenstein P, Stevenson J. Sex differences in the etiology of aggressive and nonaggressive antisocial behavior: results from two twin studies. Child Dev. 1999;70(1):155–168. doi: 10.1111/1467-8624.00012. [DOI] [PubMed] [Google Scholar]
- 6.Retz W, Rösler M, Supprian T, Retz-Junginger P, Thome J. Dopamine D3 receptor gene polymorphism and violent behavior: relation to impulsiveness and ADHD-related psychopathology. J Neural Transm. 2003;110(5):561–572. doi: 10.1007/s00702-002-0805-5. [DOI] [PubMed] [Google Scholar]
- 7.Retz W, Retz-Junginger P, Supprian T, Thome J, Rösler M. Association of serotonin transporter promoter gene polymorphism with violence: relation with personality disorders, impulsivity, and childhood ADHD psychopathology. Behav Sci Law. 2004;22(3):415–425. doi: 10.1002/bsl.589. [DOI] [PubMed] [Google Scholar]
- 8.Nishioka SA, Perin EA, Sampaio AS, Cordeiro Q, Cappi C, Mastrorosa RS, et al. The role of the VNTR functional polymorphism of the promoter region of the MAOA gene on psychiatric disorders. Rev Psiq Clín. 2011;38(1):34–42. [Google Scholar]
- 9.Kim YR, Kim JH, Kim SJ, Lee D, Min SK. Catechol-O-methyltransferase Val158Met polymorphism in relation to aggressive schizophrenia in a Korean population. Eur Neuropsychopharmacol. 2008;18(11):820–825. doi: 10.1016/j.euroneuro.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Hong JP, Lee JS, Chung S, Jung J, Yoo HK, Chang SM. New functional single nucleotide polymorphism (Ala72Ser) in the COMT gene is associated with aggressive behavior in male schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(5):658–660. doi: 10.1002/ajmg.b.30649. [DOI] [PubMed] [Google Scholar]
- 11.Gu Y, Yun L, Tian Y, Hu Z. Association between COMT gene and Chinese male schizophrenic patients with violent behavior. Med Sci Monit. 2009;15(9):CR484–489. [PubMed] [Google Scholar]
- 12.Zammit S, Jones G, Jones SJ, Norton N, Sanders RD, Milham C, et al. Polymorphisms in the MAOA, MAOB, and COMT genes and aggressive behavior in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;128B(1):19–20. doi: 10.1002/ajmg.b.30021. [DOI] [PubMed] [Google Scholar]
- 13.Avramopoulos D, Stefanis NC, Hantoumi I, Smyrnis N, Evdokimidis I, Stefanis CN. Higher scores of self reported schizotypy in healthy young males carrying the COMT high activity allele. Mol Psychiatry. 2002;7(7):706–711. doi: 10.1038/sj.mp.4001070. [DOI] [PubMed] [Google Scholar]
- 14.Vevera J, Stopkova R, Bes M, Albrecht T, Papezova H, Zukov I, et al. COMT polymorphisms in impulsively violent offenders with antisocial personality disorder. Neuro Endocrinol Lett. 2009;30(6):753–756. [PubMed] [Google Scholar]
- 15.Prichard ZM, Jorm AF, Mackinnon A, Easteal S. Association analysis of 15 polymorphisms within 10 candidate genes for antisocial behavioural traits. Psychiatr Genet. 2007;17(5):299–303. doi: 10.1097/YPG.0b013e32816ebc9e. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Ba HJ, Gao ZQ, Zhao HJ, Yu HJ, Shi JA, et al. Association study of the genetic polymorphism of fifteen short tandem repeats loci and the aggressive violent behavior. Chin J Psychiatry. 2012;45(2):85–88. (in Chinese) [Google Scholar]
- 17.Schneider PM. Basic issues in forensic DNA typing. Forensic Sci Int. 1997;88(1):17–22. doi: 10.1016/s0379-0738(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DB, Hirschhorn JN. In genetic control of disease, does ‘race’ matter? Nat Genet. 2004;36(12):1243–1244. doi: 10.1038/ng1204-1243. [DOI] [PubMed] [Google Scholar]
- 19.Phillips MS, Lawrence R, Sachidanandam R, Morris AP, Balding DJ, Donaldson MA, et al. Chromosome-wide distribution of haplotype blocks and the role of recombination hot spots. Nat Genet. 2003;33(3):382–387. doi: 10.1038/ng1100. [DOI] [PubMed] [Google Scholar]
- 20.Yunis EJ, Larsen CE, Fernandez-Viña M, Awdeh ZL, Romero T, Hansen JA, et al. Inheritable variable sizes of DNA stretches in the human MHC: conserved extended haplotypes and their fragments or blocks. Tissue Antigens. 2003;62(1):1–20. doi: 10.1034/j.1399-0039.2003.00098.x. [DOI] [PubMed] [Google Scholar]
- 21.Bär W, Brinkmann B, Budowle B, Carracedo A, Gill P, Lincoln P, et al. DNA recommendations. Further report of the DNA Commission of the ISFH regarding the use of short tandem repeat systems. International Society for Forensic Haemogenetics. Int J Legal Med. 1997;110(4):175–176. doi: 10.1007/s004140050061. [DOI] [PubMed] [Google Scholar]
- 22.Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh PS, Metzgar DA, Higuchi R. Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991;10(4):506–513. [PubMed] [Google Scholar]
- 24.USA: Life Technologies Corporation; 2012. AmpFISTER Identifiler PCR amplification kit user guide. [cited Nov 2013]. Available from: http://www3.appliedbiosystems.com/cms/groups/applied_markets_support/documents/generaldocuments/cms_041201.pdf. [Google Scholar]
- 25.Vadasz C, Kobor G, Lajtha A, Sziraki I, Fleischer A. L-Tyrosine-3-hydroxylase regulation in the brain: genetic aspects. Amino Acids. 1992;3(3):229–234. doi: 10.1007/BF00805997. [DOI] [PubMed] [Google Scholar]
- 26.De Deken X, Wang D, Dumont JE, Miot F. Characterization of ThOX proteins as components of the thyroid H(2)O(2)-generating system. Exp Cell Res. 2002;273(2):187–196. doi: 10.1006/excr.2001.5444. [DOI] [PubMed] [Google Scholar]
- 27.Sherr CJ, Roussel MF, Rettenmier CW. Colony-stimulating factor-1 receptor (c-fms) J Cell Biochem. 1988;38(3):179–187. doi: 10.1002/jcb.240380305. [DOI] [PubMed] [Google Scholar]
- 28.Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. 2011;75:151–169. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- 29.Sinai C, Hirvikoski T, Vansvik ED, Nordström AL, Linder J, Nordström P, et al. Thyroid hormones and personality traits in attempted suicide. Psychoneuroendocrinology. 2009;34(10):1526–1532. doi: 10.1016/j.psyneuen.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Mullen PE. Forensic mental health. Br J Psychiatry. 2000;176:307–311. doi: 10.1192/bjp.176.4.307. [DOI] [PubMed] [Google Scholar]
- 31.Petras H, Kellam SG, Brown CH, Muthén BO, Ialongo NS, Poduska JM. Developmental epidemiological courses leading to antisocial personality disorder and violent and criminal behavior: effects by young adulthood of a universal preventive intervention in first- and second-grade classrooms. Drug Alcohol Depend. 2008;95(Suppl. 1):S45–59. doi: 10.1016/j.drugalcdep.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks BM, Vaidyanathan U, Patrick CJ. Validating female psychopathy subtypes: differences in personality, antisocial and violent behavior, substance abuse, trauma, and mental health. Personal Disord. 2010;1(1):38–57. doi: 10.1037/a0018135. [DOI] [PMC free article] [PubMed] [Google Scholar]