Abstract
Perivascular epithelioid cell tumour (PEComa) of the liver is very uncommon and may be overlooked in the clinical and histological differential diagnosis of a liver tumour. We report the case of an incidentally discovered liver mass suspicious for hepatocellular carcinoma, which on biopsy was suggestive of a pseudocyst but after resection was found to be hepatic PEComa with some of the usual characteristics of this neoplasm as well as several less familiar features. We have also reviewed cases of hepatic PEComa from the literature in order to provide insight into recognising possible PEComa preoperatively and assessing its risk of malignancy after diagnosis.
Background
A very rare presentation of perivascular epithelioid cell tumour (PEComa) in the liver. Only a few case reports in the literature of primary liver PEComa have been reported. This was important as it can be potentially confused with primary liver tumours.
Case presentation
Introduction
PEComas belong to a family of tumours including angiomyolipoma (AML), lymphangioleiomyomatosis (LAM) of the lung, clear-cell sugar tumour of the lung (CCSTL), clear-cell myomelanocytic tumour of the falciform ligament and ligamentum teres (CCTFL) and rare clear-cell tumours of other sites,1 which contain a common cell type, the perivascular epithelioid cell (PEC), recognised in these neoplasms by its unique expression of markers of both myoid and melanocytic differentiation.1 2 Recently, PEComas have been found to have genetic alterations, in common with the tuberous sclerosis complex (TSC), in which this family of tumours is more prevalent.2 It has been postulated that the histological diversity of this group is attributable to the morphological variability of the PEC, which may take the form of spindle-shaped cells resembling smooth muscle cells or vacuolated cells resembling adipocytes in AML or epithelioid cells with clear to granular cytoplasm, which predominate in the monomorphic members of the PEComa family.
The liver is said to be second only to the kidney as the most common visceral site of PEComa,2 but since first described in 1976,3 about 200 cases of hepatic PEComa have been reported,4 most of which have been classic AML with only 16 cases identified as having monotypic epithelioid morphology,4–9 as in our patient. Herein, we report the case of a man who, after an inconclusive fine-needle aspiration and core needle biopsy, underwent partial liver resection for a possible primary liver tumour, which on final pathological examination presented a broad differential diagnosis of primary and secondary epithelioid and clear cell neoplasms and was found by immunohistochemical evaluation to be PEComa.
Case report
A liver mass was discovered incidentally on ultrasonography being performed for evaluation of kidney stones in a 61-year-old man. The patient had no gastrointestinal symptoms, and there were no other pertinent findings on history or physical examination apart from morbid obesity (body mass index 47 kg/m2) and associated comorbidities; there was nothing to suggest that he might have tuberous sclerosis. All laboratory findings including liver function tests, hepatitis panel and tumour markers, including α-fetoprotein (AFP), carcinoembryonic antigen (CEA) and CA 19-9 were within normal limits. He subsequently had an MRI that demonstrated a 7 cm, complex heterogeneous cystic lesion in hepatic segment 7/8, which was hypointense on T1-weighted images (figure 1A). On the T2-weighted images it appeared to be mildly heterogeneous with high signal intensity and well demarcated margins (figure 1B). Contrast-enhanced CT revealed a 6.7×6.5×6.4 cm, complex cystic and solid mass in segment 7. The mass appeared to be subcapsular and exophytic with intrinsic septation and partial enhancement in the arterial phase (figure 2A). It showed decreased enhancement and washout in the venous phase (figure 2B).
Figure 1.
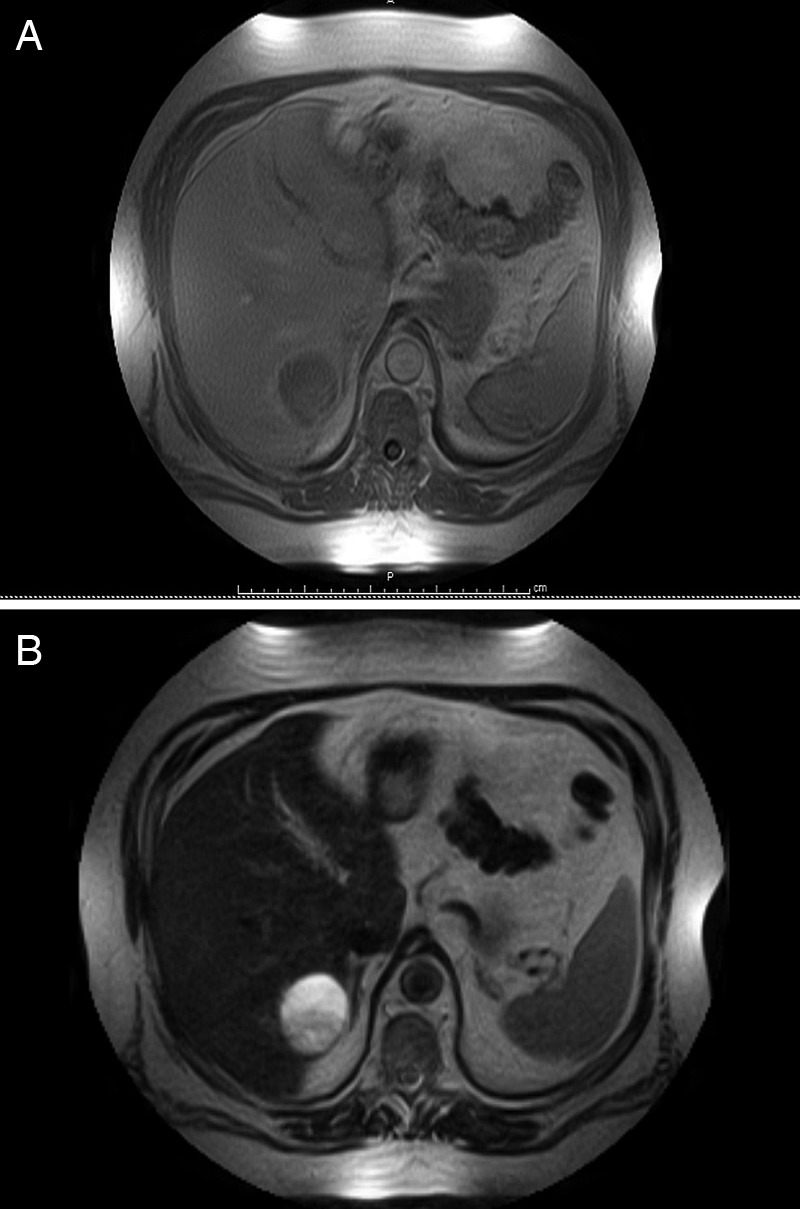
(A) MRI T1-weighted image showing hypointense, complex heterogeneous mass in segment 7/8. (B) MRI T2-weighted image showing mildly heterogeneous high signal intensity with well demarcated margins.
Figure 2.
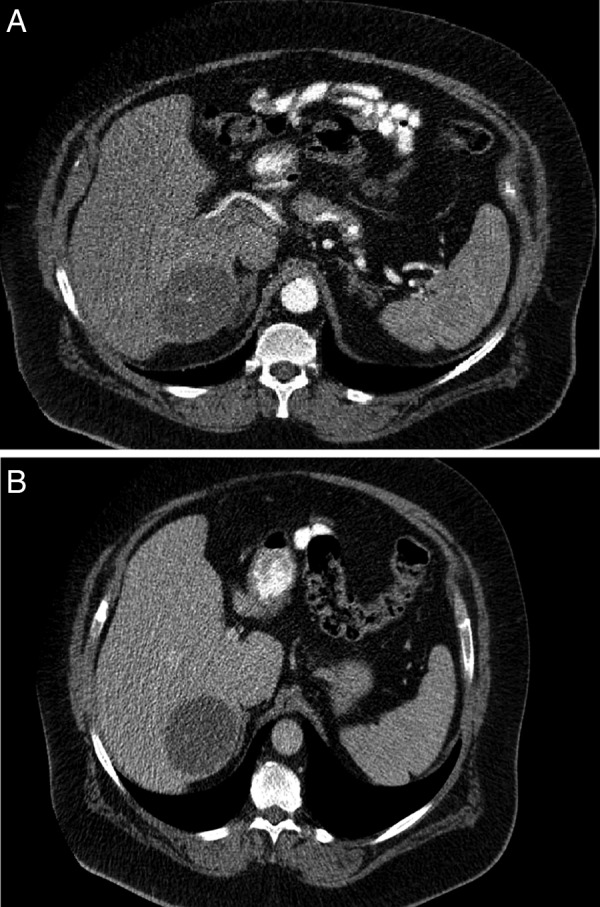
(A) CT scan (arterial phase) demonstrating partially enhancing mass in segment 7/8. (B) CT scan (venous phase) demonstrating decreased enhancement in the segment 7/8 mass.
Prior to our evaluation, ultrasound-guided fine-needle aspiration of the mass demonstrated foamy macrophages and lymphocytes, and core needle demonstrated findings suggestive of a possible pseudocyst, but neoplastic cells were not identified in either of these specimens on initial examination.
Owing to the complexity and septation of the mass on imaging, the possibility of malignancy could not be excluded and a laparoscopic-assisted partial right hepatectomy was performed. After placement of three 5 mm ports, a hand port was inserted to facilitate mobilisation of the right lobe of the liver in this morbidly obese patient. Intraoperative ultrasound demonstrated no other lesion in the liver. No evidence of any infiltration into the surrounding liver parenchyma was appreciated. Segments 7 and 8 were resected with a margin of 1.5 cm measured intraoperatively.
Investigations
Pathological findings
Gross examination of the resected liver specimen revealed a cavitary lesion measuring 4.5×4.0×3.0 cm extending to the posterior edge of the lobe. The cavity had a ragged red-brown lining and was surrounded by a fibrous wall averaging 3 mm in thickness. A solid nodule was found at one end of the cavity measuring 1.5×0.6×0.6 cm.
Histological examination revealed a neoplasm with a cavity containing only old blood and histiocytes surrounded by epithelioid cells with abundant granular, eosinophilic-to-clear cytoplasm arranged in trabeculae and acini within a rich capillary network. The cells had large, round-to-oval, pleomorphic, vesicular nuclei with distinct large nucleoli, but no mitoses were observed (figure 3). The tumour was contained within a fibrous capsule with no invasion of the surrounding liver parenchyma. The complete immunohistochemical profile of the tumour is presented in table 1. Most significantly, the neoplastic cells showed strong reactivity for melanoma antigen recognised by T cell-1 (MART-1) and human melanoma black (HMB-45; figure 4) but were negative for S-100 protein and cytokeratin. Staining for muscle-specific actin was equivocal or absent, and desmin stain was negative. Positive PAS staining of the tumour cells was extinguished with diastase consistent with the presence of intracytoplasmic glycogen.
Figure 3.
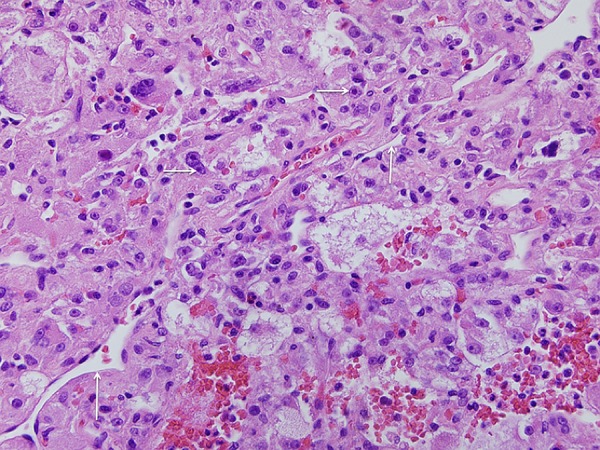
Appearance of the solid portion of the tumour demonstrating trabecular arrangement of large cells with abundant pale to clear cytoplasm, large vesicular pleomorphic nuclei and prominent nucleoli in a vascular network resembling clear-cell tumours of several different types. H&E ×40.
Table 1.
Immunohistochemical stain results
| Antibody/clone | Source | Result |
|---|---|---|
| Actin, smooth muscle/1A4 | Cell Marque, Rocklin, CA | Weak in some tumour cells |
| ALK-1/ALK01 | Ventana, Tucson, AZ | Negative in tumour cells |
| α-fetoprotein/polyclonal | Cell Marque | Negative in tumour cells |
| β-catenin/14 | Cell Marque | Negative in tumour cells |
| Calretinin/SP65 | Ventana | Negative in tumour cells |
| CD1a/EP3622 | Cell Marque | Negative in tumour cells |
| CD21/EP3093 | Cell Marque | Negative in tumour cells |
| CD23/SP23 | Ventana | Negative in tumour cells |
| CD30/Ber-H2 | Ventana | Negative in tumour cells |
| CD31/JC70 | Cell Marque | Negative in tumour cells |
| CD34/QBEnd/10 | Ventana | Negative in tumour cells |
| CD68/KP-1 | Ventana | Negative in tumour cells |
| CD163/MRQ-26 | Cell Marque | Negative in tumour cells |
| CEA/polyclonal | Cell Marque | Negative in tumour cells |
| Chromogranin/LK2H10 | Cell Marque | Negative in tumour cells |
| Cytokeratin | ||
| AE1/AE3/PCK26 | Ventana | Negative in tumour cells |
| CAM 5.2 | Ventana | Negative in tumour cells |
| CK7/SP52 | Ventana | Negative in tumour cells |
| CK20/SP33 | Ventana | Negative in tumour cells |
| Desmin/DE-R-11 | Ventana | Negative in tumour cells |
| Glypican-3/GC33 | Ventana | Negative in tumour cells |
| HSA (hepatocyte)/OCH1E5 | Cell Marque | Negative in tumour cells |
| Inhibin/R1 | Cell Marque | Negative in tumour cells |
| Ki-67/30–9 | Ventana | Positive in 5–10% of tumour cells |
| MART-1 (melan-A)/A103 | Cell Marque | Positive in tumour cells |
| Melanosome/HMB-45 | Ventana | Positive in tumour cells |
| PAX-8/MRQ-50 | Cell Marque | Negative in tumour cells |
| Renal cell carcinoma/PN-15 | Cell Marque | Negative in tumour cells |
| SOX-10/polyclonal | Cell Marque | Negative in tumour cells |
| Synaptophysin/MRQ-40 | Cell Marque | Negative in tumour cells |
| TTF-1/V9 | Ventana | Negative in tumour cells |
ALK-1: anaplastic lymphoma kinase-1, a tyrosine receptor kinase expressed by neoplastic cells in anaplastic large cell lymphoma, inflammatory myofibroblastic tumor and other neoplasms; AE1: Clone name of antibody to acidic cytokeratins 10, 14, 15, 16 and 19; AE3: Clone name of antibody to basic cytokeratins 1, 2, 3, 4, 5, 6, 7 and 8; CAM 5.2: Clone name of antibody to cytokeratins 8 and 18; CEA: carcinoembryonic antigen; HMB-45, human melanoma black-45; PCK26: Clone name of antibody to cytokeratins 5 and 8; MART-1, melanoma antigen recognised by T Cell-1; MRQ-26: Clone name of antibody to CD163, scavenger protein expressed by cells of monocytes/macrophage lineage; MRQ-40: Clone name of antibody to synaptophysin; MRQ-50: Clone name of antibody to PAX-8 protein; EP3622: Clone name of antibody to CD1a; EP3093: Clone name of antibody to CD21; SP23: Clone name of antibody to CD23; SP33: Clone name of antibody to cytokeratin 20; SP52: Monoclonal antibody to cytokeratin 7; SP65: Clone name of antibody to calretinin, a protein expressed by normal and neoplastic mesothelial cells; Ber-H2: Clone name of antibody to CD30; HSA: Hepatocyte specific antigen present in normal and neoplastic hepatocytes; OCH 1E5: Clone name of antibody to HAS; PAX-8: Transcription factor encoded by the PAX8 (paired box 8) gene, expressed in neoplasms of the kidney, upper urinary tract, thyroid and Müllerian system; SOX-10: Transcription factor encoded by the SRY (sex determining region Y) box 10 gene, involved in the regulation of embryonic development and cell fate, expressed in melanoma cells; TTF-1: Thyroid transcription factor, expressed in normal and neoplastic cells of the thyroid and lung.
Figure 4.
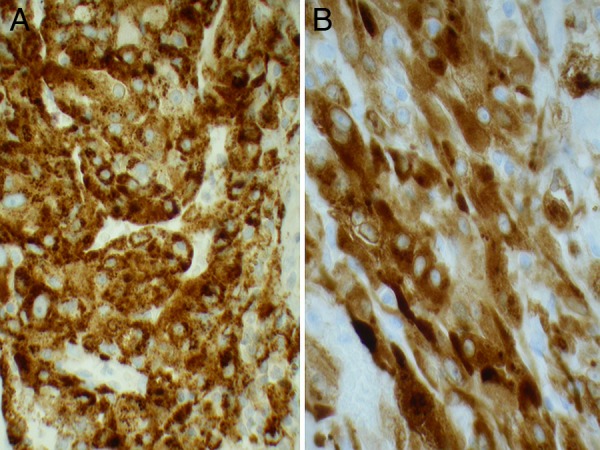
Strong uniform staining of the tumour cells for human melanoma black-45 (A) and similar staining for melanoma antigen recognised by T Cell-1(B). IHC, ×40.
Review of the original H&E-stained slides of the patient's previous liver biopsy demonstrated old and recent haemorrhage with reactive hepatocytes, which on initial examination had been interpreted as a possible pseudocyst. One or two small collections of cells resembling degenerative hepatocytes or histiocytes were found, in retrospect, to be positive for HMB-45 and MART-1 as were the neoplastic cells in the liver resection specimen (figure 5).
Figure 5.
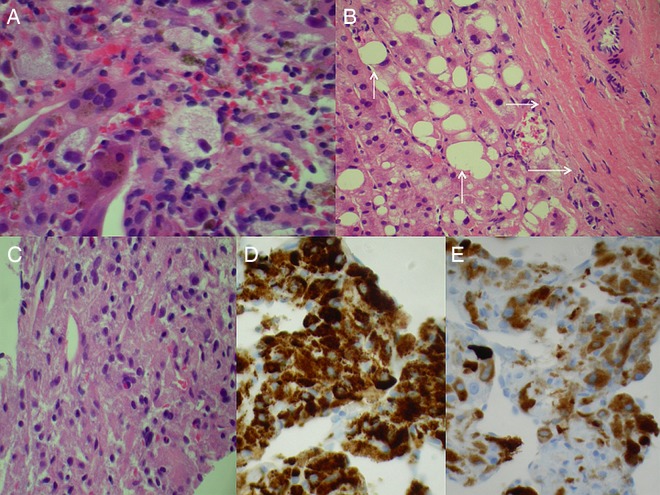
Core needle biopsy of hepatic tumour taken prior to referral showing (A) histiocytes and inflammatory cells suggesting pseudocyst, (B) reactive hepatocytes with steatosis (vertical arrows) adjacent to fibrous tissue (horizontal arrows) possibly representing the tumour capsule, (C) cells resembling degenerative histiocytes or hepatocytes in H&E-stained sections, (D) same cells with strong uniform staining for human melanoma black-45 and (E) same cells staining for melanoma antigen recognised by T Cell-1 on IHC performed retrospectively for both markers. Original magnification ×40.
Discussion
With their characteristic sites of occurrence and histological features, the individual types of the PEComa have long been recognised, but recognition of a common cell type and lineage among these apparently distinct tumours and the existence of a more generic monomorphic epithelioid cell variant has occurred only recently, with the PEComa family formally recognised as such by the WHO in 2002.1 While certain types have a predilection for specific locations, such as AML of the kidney or CCSTL and LAM of the lung, PEComas with either AML or monomorphic epithelioid morphology may be found in various soft tissue and visceral sites including the uterus, skin, pancreas, colon and liver.
Tumours of the PEComa family are more common in women in their fourth and fifth decade of life1 10 with a median age at presentation of 43 years.11 They are primarily benign tumours, but they may rarely behave malignantly.12 Clinical manifestations of PEComa in general, including hepatic PEComa, are uncommon. As in our case, most are found incidentally on imaging studies performed for other reasons. However, there has been a previously published case report by Priola et al of a case of hepatic PEComa presenting as an acute abdomen. They reported a haemorrhagic mass in the left lobe of the liver for which the patient underwent emergency surgery and the final diagnosis turned out to be hepatic PEComa.13
On imaging, hepatic PEComas may exhibit a wide spectrum of findings. They may resemble hepatocellular carcinoma, focal nodular hyperplasia, metastatic neoplasm or haemangioma depending on the consistency and fat content of the lesion. In some of the reported cases, hepatic PEComa demonstrates mixed echogenicity on ultrasound; however, most are hyperechoic.6–8 As the name implies, PEComas have abundant vascularity in or around the tumours, and CT scan with contrast is more sensitive than ultrasound in detecting them. On arterial phase, these tumours enhance inhomogeneously.14 Contrast-enhanced CT scan has also shown this to be circular peripheral enhancement in the arterial phase, with decreased enhancement in the portal and delayed phases of the CT scan.15 Most of the reported hepatic PEComas show high intensity on T1-weighted and low intensity on T2-weighted MRI. This was not true in our patient because of the large cystic component and small solid component in his tumour. Hypervascularity of the lesion was consistently demonstrated in a majority of the previously reported cases by means of enhancement in the arterial phase.
AML may be readily recognised histologically by its smooth muscle, adipose tissue and vascular components, but the expression of melanocytic markers HMB-45, Melan A/MART-1 and microphthalmia transcription factor (MITF)—but not S-100 protein or SOX-10—as well as the presence of smooth muscle marker actin, in at least some of the tumour cells defines the PEComa family of tumours.16 A predominant or monomorphic population of tumour cells may render a PEComa unrecognisable if not for this immunohistochemical phenotype. Moreover, a monomorphic phenotype in a PEComa poses a broad differential diagnosis of epithelioid and clear cell neoplasms, including primary hepatocellular, renal cell and adrenal neoplasms, which, as in our case, may require the application of an exhaustive immunohistochemical (IHC) panel before the diagnostic immunohistochemical profile of PEComa is ultimately discovered. The absence of actin expression which may be lost in epithelioid monomorphic PEComa,2 and a high degree of cytological pleomorphism by the tumour cells in this case also presented diagnostic difficulties, raising the possibility of metastatic melanoma, the exclusion of which was aided by the absence of significant cytological atypia, lack of mitotic activity and absence of staining for S-100 protein and SOX-10, as well as the lack of a history of melanoma.
FNA has been found to be useful in establishing an early diagnosis of hepatic PEComa.9 In this case, however, the neoplastic cells of PEComa were not recognised as such in the FNA or core needle biopsy due to the unusual amount of obscuring haemorrhage, the small number of tumour cells present and their resemblance to degenerative or reactive hepatocytes.
As useful as its immunohistochemical profile is in the identification of PEComa, the apparent absence of a normally occurring cell with the myomelanocytic phenotype, that is the PEC, has perhaps thwarted a more complete understanding of the pathogenesis of these lesions. However, the known association of AML as well as other PEComa variants with TSC and the discovery of similar alterations in the TSC1 (9q34) and TSC2 (16p13.3) genes in TSC-associated as well as sporadic PEComas has provided new insight into the relationship and development of these tumours; these genes apparently have a role in the regulation of the Rheb/mTOR/p70S6 K) pathway.2
While histological examination with IHC establishes a diagnosis of PEComa, recognition or prediction of malignant potential in a PEComa on histological or immunohistochemical grounds is problematic. LAM may pursue an aggressive course with local and distant spread, but involvement of lymph nodes and other regional structures in other variants such as CCTFL has been considered by some to represent multifocality rather than metastasis.2 It is clear, however, that some PEComas demonstrate malignant behaviour, but at present there are no uniformly accepted morphological criteria for malignancy. A tumour size greater than 5 cm, the presence of any mitotic activity, tumour infiltration, necrosis and high nuclear grade have been common characteristics in the reported examples of malignant PEComa.17
In summary, we have reported another example of the rare monotypic variant of PEComa of the liver. The case demonstrates clinical and histological features characteristic of epithelioid PEComa but is notable for its nuclear pleomorphism, prominent nucleoli, extensive haemorrhage, cystic cavitation and absence of actin expression by IHC, which may lead one to consider a number of alternative, morphologically similar neoplasms in the differential diagnosis. Whether any of these unusual features are indicative of a more aggressive clinical course remains to be clarified.
Learning points.
Recognition of perivascular epithelioid cell tumour (PEComa) as an entity.
Role of imaging and surgery in diagnostic confirmation.
PEComa can present as an isolated liver lesion primarily.
Importance of pathological diagnosis and differentiation.
Footnotes
Contributors: All authors contributed to this case report. The surgical team was involved in the clinical aspects and writing of the clinical scenario and the pathology team was for evaluation of the specimens/slides and discussion. Planning: SCK MD, HMK MD. Conduct: PSS MD Report of work: N PL MD. Overall Content: PSS MD, HK MD, N. PL MD.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Folpe AL. Neoplasms with perivascular epithelioid cell differentiation (PEComas). In: Flectcher CDM. ed World Health Organization classification of tumours: pathology and genetics of soft tissue and bone. Lyon, France: IARC Press, 2002:221–2 [Google Scholar]
- 2.Martignoni G, Pea M, Reghellin D, et al. PEComas: the past, the present and the future. Virchows Arch 2008;452:119–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishak KG. Mesenchymal tumors of the liver. In: Okuda K, Peters RL. eds Hepatocellular carcinoma. New York: John Wiley & Sons, 1976:247–307 [Google Scholar]
- 4.Tsui WM, Colombari R, Portmann BC, et al. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol 1999;23:34–48 [DOI] [PubMed] [Google Scholar]
- 5.Szekely E, Schaff Z, Madaras L, et al. Trabecular angiomyolipoma mimicking hepatic cell carcinoma. Pathol Oncol Res 2000;6:224–6 [DOI] [PubMed] [Google Scholar]
- 6.Yamasaki S, Tanaka S, Fujii H, et al. Monotypic epithelioid angiomyolipoma of the liver. Histopathology 2000;36:451–6 [DOI] [PubMed] [Google Scholar]
- 7.Akitake R, Kimura H, Sekoguchi S, et al. Perivascular epithelioid cell tumor (PEComa) of the liver diagnosed by contrast enhanced ultrasonography. Intern Med 2009;48:2083–6 [DOI] [PubMed] [Google Scholar]
- 8.Tan Y, Xiao EH. Hepatic perivascular epithelioid cell tumor (PEComa): dynamic CT, MRI, ultrasonography, and pathologic features: analysis of 7 cases and review of the literature. Abdom Imaging 2012;37:781–7 [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Jessurun J, Manivel JC, et al. Hepatic epithelioid angiomyolipoma with trabecular growth pattern: a mimic of hepatocellular carcinoma on fine needle aspiration cytology. Diagn Cytopathol 2012;40:639–50 [DOI] [PubMed] [Google Scholar]
- 10.Pea M, Bonetti F, Martignoni G, et al. Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol 1998;22:180–7 [DOI] [PubMed] [Google Scholar]
- 11.Chaplin A, Conrad DM, Tatlidil C, et al. Primary cutaneous PEComa. Am J Dermatopathol 2012;32:310–12 [DOI] [PubMed] [Google Scholar]
- 12.Selvaggi F, Risio D, Claudi R, et al. Malignant PEComa: a case report with emphasis on clinical and morphological criteria. BMC Surg 2011;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Priola AM, Priola SM, Cataldi A, et al. Acute abdomen as an unusual presentation of hepatic PEComa. A case report . Tumori 2009;95:123–8 [DOI] [PubMed] [Google Scholar]
- 14.Yu D, Tang S. Hepatic perivascular epithelioid cell tumor: a case report and review of literature. Intern Med 2013;52:1333–6 [DOI] [PubMed] [Google Scholar]
- 15.Yan F, Zeng M, Zhou K, et al. Hepatic angiomyolipoma: various appearances on two phase contrast scanning of spiral CT. Eur J Radiol 2002;14:12–18 [DOI] [PubMed] [Google Scholar]
- 16.Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of literature. Am J Surg Path 2005;29:1558–75 [DOI] [PubMed] [Google Scholar]
- 17.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol 2010;41:1–15 [DOI] [PubMed] [Google Scholar]