Abstract
Objective
To assess the outcome of pediatric patients supported by concomitant extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT).
Design, Setting, and Patients
Acute kidney injury (AKI) is associated with mortality in ECMO patients. CRRT on ECMO provides an efficient and potentially beneficial method of AKI management. Concern that concomitant CRRT use increases the risk of developing anuria and chronic renal failure (CRF) limits its use in some centers. We hypothesized that development of CRF is rare with concurrent ECMO and CRRT. Outcomes of 154 ECMO/CRRT patients cared for over 10 years at a referral pediatric medical center were evaluated.
Measurements and Main Results
Among 68 ECMO/CRRT survivors (44%), 45 were assigned a pRIFLE score at CRRT initiation. Seventeen patients (38%) met criteria for Risk, 15 (33%) for Injury, and 10 (22%) for Failure. Two Failure patients later met End stage criteria. Of all survivors, 18 (26%) required ongoing RRT (15 CVVH, 2 peritoneal dialysis, 1 intermittent hemodialysis) following ECMO discontinuation. Renal recovery occurred in 65 of 68 (96%) before discharge. One neonatal patient had sepsis-induced renal injury on transfer, but normal creatinine one month later. Two pediatric patients with vasculitis and primary renal disease at presentation (both meeting Failure criteria) developed end-stage renal disease. One received peritoneal dialysis and subsequent renal transplant. The other has diminished function without need for renal replacement therapy.
Conclusion
In the absence of primary renal disease, CRF did not occur following concurrent use of CRRT with ECMO. Concern for precipitating CRF by using CRRT during ECMO is not substantiated by this large single center experience. Consistent with previous reports, mortality is higher in patients receiving concomitant CRRT and ECMO compared to those receiving ECMO alone. Mortality is similar to patients requiring CRRT who are not on ECMO. Additional studies are warranted to determine the optimal role of CRRT use in ECMO patients.
Keywords: ECMO, ECLS, CVVH, CRRT, Outcome, Chronic Renal Failure
Introduction
Extracorporeal membrane oxygenation (ECMO) provides temporary life-saving support to critically ill neonates and children with cardiac and respiratory failure when conventional therapies prove inadequate(1). Coexistence or development of renal failure has been identified as an associated risk factor for death in patients receiving ECMO(2,3), likely as an element of progression to multi-system organ failure in nonsurvivors. Acute kidney injury often complicates care of these critically ill children on ECMO, leading to accumulation of fluid and volume overload that can worsen heart and lung disease. The institution of ECMO is itself often followed by a period of oliguria or anuria which can worsen fluid overload (4).
Consensus does not exist regarding the optimal method of treatment of fluid overload and acute kidney injury during ECMO. Center-specific trends range from fluid restriction and diuretic therapy to early institution of continuous renal replacement therapy (CRRT) concomitant with institution of ECMO, even in patients without acute kidney injury. Renal replacement therapies such as peritoneal dialysis, intermittent hemodialysis, and continuous veno-venous hemofiltration (CVVH) have all been used concomitantly with ECMO to treat the metabolic and fluid derangements resulting from acute kidney injury. Each of these modalities has benefits and disadvantages in critically ill patients that have been reviewed elsewhere(5,6,7).
A commonly used method of CRRT in ECMO patients is continuous veno-venous hemofiltration (CVVH) with or without dialysate flow (CVVHDF). CVVH delivered via an ECMO circuit can avoid potential problems seen with other dialytic modalities, including hemodynamic instability common with acute hemodialysis, and complications of emergent catheter placement, including peri-catheter bleeding or leakage and poor catheter drainage that are common with peritoneal dialysis. In contrast, CVVH provides an easily initiated and efficient method of renal replacement and fluid management utilizing catheters already in place on ECMO. However, concern exists that premature CVVH use while on ECMO could increase survivor risk of developing chronic renal failure and may limit its use in some centers(8). Renal recovery outcome data are limited in children who have received ECMO and CVVH. We evaluated our large single center experience with CVVH use in ECMO. We hypothesized that prolonged renal insufficiency or persistent requirement for renal replacement therapy are rare in children treated with concomitant ECMO and CVVH.
Materials and Methods
Children's Healthcare of Atlanta institutional review board approval was obtained for review. Data were collected from individual medical records as well as the Children's Healthcare of Atlanta at Egleston ECMO patient database. Data on all patients receiving ECMO from May 1997 to May 2007 were reviewed. Neonatal and pediatric patients receiving ECMO for respiratory and/or cardiac failure were included. Neonatal patients were defined as children less than 28 days at the initiation of ECMO. Pediatric patients were defined as children greater than 28 days old but less than nineteen years old at time of ECMO. Adult patients were excluded from this analysis. Survival was defined as survival to hospital discharge or to transfer back to the referring facility.
Data collected included patient age, indication for ECMO, type of ECMO, primary diagnosis, renal replacement therapy prior to ECMO, renal replacement therapy after ECMO, need and type of renal replacement therapy at time of discharge from the intensive care unit/hospital, serum creatinine immediately prior to CRRT initiation, serum creatinine at discharge from the ICU, and last serum creatinine prior to hospital discharge. Indication for use of CRRT on ECMO was determined by review of the attending nephrologist's consultation note and was generally classified as fluid overload, acute kidney injury, ECMO circuit change, or sepsis. Specific percentage of fluid overload was not retrospectively calculated. To better classify the degree of acute kidney injury, we calculated retrospectively a pediatric risk, injury, failure, loss and end-stage (pRIFLE) classification (9) at initiation of CRRT. Based on previous data demonstrating acute reduction in urine output in children initiated on ECMO support (2), even absent kidney injury, we elected to use only serum creatinine criteria to determine pRIFLE scoring. For determination of baseline estimated creatinine clearance, the lowest measured value of serum creatinine in the 3 months prior to hospital admission for ECMO was used, if known. If no previous creatinine value was available, patients were assumed to have normal renal function and assigned a creatinine clearance of 120 mL/min/1.73m2 (10) or actual discharge estimated creatinine clearance, whichever was higher. Criteria for pRIFLE categories (9) are defined as decrease in estimated creatinine clearance by 25% for Risk, 50% for Injury, and 75% for Failure. Loss is defined as persistent failure for greater than four weeks. End stage is defined as persistent failure for greater than three months. Renal outcome was defined as death, recovery of renal function at hospital discharge, or by type of chronic renal replacement therapy required.
Recovery of renal function in children over 1 year of age was defined as the lack of need for renal replacement therapy and estimated creatinine clearance (CrCl) of greater than 60 ml/min/1.73m2 at hospital discharge. Although multiple renal function scoring systems exist, they have contradictory definitions of renal injury/failure. In none of the currently used scoring systems for renal injury is a creatinine clearance of > 60 mL/min/1.73m2 indicative of moderate or severe kidney disease. For children less than one year of age at the time of discharge, a creatinine less than 0.6 mg/dL and lack of need of renal replacement therapy was defined as renal recovery. For the patients between 31 days and 18 years of age, pRIFLE loss and end stage categories were calculated at the appropriate time points. Creatinine clearance was calculated at the time of discharge or transfer using the Schwartz equation (11).
CVVH on ECMO was provided using a standardized hemofilter insertion procedure as previously described (12). Briefly, we have configured a simplified CVVH arrangement by positioning a pre-oxygenator blood line running to a hemofilter with return proximal to the ECMO bladder. In this setup, blood flow is driven by the ECMO pump flow rather than requiring an additional hemofilter pump. Ultrafiltrate production is controlled by a standard IV pump and measured by a urometer. Replacement fluid is delivered immediately after the hemofilter. Replacement fluid rate is adjusted hourly to effect a predetermined overall net fluid balance, permitting precise volume management with minimal variation. All patients received CVVH and no patients received solely ultrafiltration. In cases when ultrafiltration rate exceeded two liters an hour, hemofiltration was provided by inline use of a stand-alone CRRT delivery device (Diapact, B. Braun Medical Inc., Bethlehem, PA).
Statistical analysis was performed (Sigma Stat v. 3.11, Systat Software Inc, San Jose, CA). Groups were compared by Chi-square analysis for discrete variables and by multiple analysis of variance for continuous variables. A p value of < 0.05 was considered statistically significant.
Results
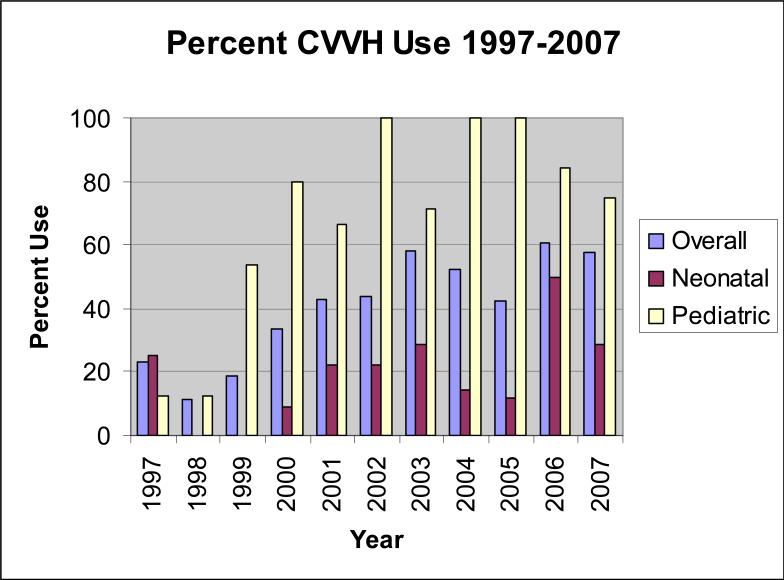
Three hundred seventy eight pediatric and neonatal patients received ECMO during this time period and 250 (66%) survived to hospital discharge. Of the survivors, one hundred and eighty one received ECMO alone without any renal replacement therapy. No patient receiving ECMO alone developed chronic renal failure. One hundred fifty four patients (41% of total ECMO patients) received concomitant CVVH while on ECMO (Table 1), and were reviewed further. Indications for ECMO in ECMO/CVVH patients included neonatal respiratory failure in 30 patients, pediatric respiratory failure in 77, cardiac failure in 29, and ECMO cardiopulmonary resuscitation (CPR) in 18. Fifty percent of the patients were male. There were no significant differences between patient ages in neonatal or pediatric patients who received CVVH versus those who did not. Neonates who received CVVH had significantly longer ECMO duration (248 hours) compared to those who did not (97 hours, p<0.0001), as did pediatric patients who received CVVH (211 hours) compared to those who did not (173 hours, p=0.0267). CVVH use on ECMO increased over the last decade (Figure 1), from 17% in 1997-1999 to 59% in 2005-2007. This trend was more prominent in pediatric patients, in whom CVVH was used significantly more frequently (71%) compared to neonatal patients (18%) (p <0.0001).
Table 1.
All ECMO patients demographic characteristics
| All Patients | ECMO/CVVH | Neonatal Respiratory | Pediatric Respiratory | Cardiac | ECPR | |
|---|---|---|---|---|---|---|
| Number | 378 | 154 | 170 | 108 | 54 | 46 |
| Median Age | 8 days | 114 days | 1 day | 43 months | 21 days | 70 days |
| Median weight (kg) | 3.8 | 5.3 | 3.3 | 15 | 4.1 | 4.3 |
| ECMO Hours | 125 | 208 | 113 | 197 | 135 | 68 |
| Percent Survival | 66 | 44 | 81 | 62 | 33 | 57 |
Figure 1.
CVVH use at Children's Healthcare of Atlanta at Egleston1997-2007 as percent of ECMO cases
Sixty eight patients (44%) who received concomitant ECMO and CVVH survived to discharge (Table 2). Overall, ECMO patients treated with concomitant CVVH had a lower survival rate than ECMO patients not receiving CVVH (Table 2; p=<0.0001). ECMO/CVVH survivors were evaluated in further detail for renal outcomes. The indication for CVVH could be retrospectively determined in 62 of the 68 survivors (91%). The most prevalent indications noted were fluid overload (52%) and acute kidney injury (37%). (Table 3) Forty-five of 50 patients of appropriate age could be stratified by pRIFLE at initiation of CVVH. (Figure 2) Five did not have adequate clinical information for scoring. Three patients did not meet criteria for acute kidney injury by the pRIFLE score having a median reduction in creatinine clearance of 17.5%. Seventeen patients (38%) met criteria for Risk, while 15 (33%) and 10 (22%) met criteria for Injury and Failure, respectively. Eighteen of the 68 survivors (26%) required ongoing renal replacement therapy (15 CVVH, 2 peritoneal dialysis,1 intermittent hemodialysis) at the time of discontinuation of ECMO. However, recovery of renal function and discontinuation of renal replacement occurred in 65 of 68 patients (96%) prior to hospital discharge. Two patients (both of whom initially met Failure criteria) met End stage criteria 3 months after discharge.
Table 2.
Survival rate to discharge by therapy received
| All ECMO | Neonatal | Pediatric | Cardiac | ECPR | |
|---|---|---|---|---|---|
| Number | 378 | 170 | 108 | 54 | 46 |
| Percent Survival | 66 | 81 | 62 | 33 | 57 |
| 1997-2007 ELSO registry percent survival | 76 | 55 | 42 | 38 | |
| ECMO/CVVH | |||||
| Number | 154 | 30 | 77 | 29 | 18 |
| Percent Survival | 44 | 50 | 55 | 14 | 33 |
| ECMO/No CVVH | |||||
| Number | 224 | 140 | 31 | 25 | 28 |
| Percent Survival | 81 | 88 | 81 | 56 | 68 |
| P value comparing CVVH vs. No CVVH Survival | p<0.0001 | p<0.0001 | p=0.0112 | p=0.0007 | p=0.0215 |
Table 3.
Outcome by age and indication for RRT in ECMO/CVVH survivors. Normal renal outcome was defined as CrCl >60 mL/min/1.73m2 in patients greater than 1 year of age and not requiring RRT. In children <1 year, normal is defined as serum creatinine ≤0.5 mg/dL. Patients with abnormal renal function are described in the text.
| Age | Indication for RRT | Number Evaluated | Outcome |
|---|---|---|---|
| 13-18 years | 11 | ||
| Renal Failure | 8 | 2 with CrCl <60 6 with CrCl >60 |
|
| Fluid Overload | 2 | 1 with CrCl <60 1 with CrCl >60 |
|
| Sepsis | 1 | >60 CrCl | |
| 1-12 years | 18 | ||
| Renal Failure | 6 | All >60 CrCl | |
| Fluid Overload | 11 | All >60 CrCl | |
| Circuit Change | 1 | All >60 CrCl | |
| Less than 1 year old | 33 | ||
| Renal Failure | 9 | 1 with Cr of 1.3 1 with Cr of 0.6 7 with Cr <0.5 |
|
| Fluid Overload | 19 | All with Cr ≤0.5 | |
| Circuit Change | 2 | All with Cr ≤0.5 | |
| Not listed | 3 | All with Cr ≤0.5 |
Figure 2.
Patients stratified by pRIFLE Criteria
Thirty of the 31 patients whose primary indication for CVVH was fluid overload achieved renal recovery at discharge or transfer. The one patient who had an elevated creatinine at transfer was a 14 year old who had been off of ECMO and CVVH for 1 week with no need for renal replacement therapy and a creatinine of 1.1 mg/dL. He then developed hospital-acquired enterococcal sepsis and was treated with vancomycin with a subsequent rise in creatinine to a maximum of 3.8 mg/dL. He required no renal replacement therapy during this episode, and was transferred back to his referring facility with a falling creatinine of 3 mg/dL. He was subsequently lost to followup. Due to age, only eighteen of 31 patients with fluid overload were eligible for pRIFLE scoring, among whom 11 met criteria for Risk, 6 for Injury, and 1 for Failure.
In twenty three patients, the indication for CVVH was acute kidney injury (AKI), among whom seventeen were eligible to have a pRIFLE score assigned (2 Risk, 6 Injury, 9 Failure). Complete recovery of renal function occurred in 20 of the 23 patients at the time of discharge. Courses of the 3 AKI patients with variable recovery are noted as follows:
Patient 1: A 15 year old male developed pulmonary hemorrhage, acute kidney injury (initial serum creatinine 13.7 mg/dL), and respiratory failure unresponsive to conventional and high frequency oscillatory ventilation. He was cannulated for ECMO and CVVH was immediately initiated. Weaning from ECMO support was possible after a complicated run of 284 hours. He continued to receive CVVH for oliguric renal failure. Attempts to wean from CVVH resulted in elevated blood urea nitrogen (118 mg/dL) and creatinine (5.2 mg/dL) and in electrolyte abnormalities requiring resumption of renal replacement therapy. With continued improvement and hemodynamic stability, he was converted to therapy with chronic peritoneal dialysis (PD). At hospital discharge his blood urea nitrogen was 76 mg/dL and creatinine 4.6 mg/dL while receiving PD. Renal biopsy demonstrated microscopic polyangiitis. One year later, with his primary disease under good control, he received a successful deceased donor renal transplant.
Patient 2: A 15 year old male presented with hemoptysis, respiratory distress, and acute kidney injury (initial serum creatinine 6.3 mg/dL). Worsening respiratory failure unresponsive to conventional and high frequency oscillatory ventilation resulted in cannulation for ECMO support approximately one day after admission. He was maintained on CVVH throughout his 228 hour ECMO course. At discontinuation of ECMO, renal failure persisted with a rise in blood urea nitrogen to 133 mg/dL, creatinine to 3.5 mg/dL, and electrolyte abnormalities that could not be controlled medically. He was restarted on CVVH. As he improved, a peritoneal dialysis catheter was placed and he received PD while in the hospital. While convalescing in the hospital, he was weaned off PD and did not require chronic renal replacement therapy at discharge. However, diminished renal function persisted at discharge with serum creatinine of 4.1 mg/dL. P-ANCA positive, pauci-immune crescentic glomerulonephritis was diagnosed by renal biopsy. Currently, his disease is managed conservatively by the pediatric nephrology service and requires no renal replacement therapy.
Patient 3: A neonatal patient was placed on ECMO on the first day of life for respiratory failure and pulmonary hypertension from overwhelming streptococcal sepsis. Serum creatinine was 1.4 mg/dL immediately before going onto ECMO, and he was treated with CVVH during his ECMO course. After a 145 hour ECMO run, the patient stabilized and was able to be transferred back to the referring facility with a creatinine of 1.3 mg/dL. He required no additional renal replacement therapy. One month later, the patient was transferred back to our rehabilitation facility and had a serum creatinine of 0.3 mg/dL.
Discussion
A large body of experience has demonstrated the adverse impact of acute kidney injury on survival of critically ill neonates, older children, and adults receiving ECMO, and thus evaluation of any associations of CRRT use and ECMO survival must recognize the independent effect of renal failure alone. Acute kidney injury is an independent risk factor for death in critically ill neonates or children with or without ECMO (9,13,14,15), and is associated with mortality. Similarly, in adult ECMO patients, renal failure is a risk factor for mortality. Kolla et al reported elevated serum creatinine values in 53 of 100 adult ECMO patients, with 16 (30%) survivors (2). Elevated creatinine was associated with death in multivariate analysis. Clearly AKI negatively impacts outcome in ECMO patients, and intervention with CRRT must be viewed in that light.
Reported experience with concomitant use of CRRT on ECMO remains limited in children. Weber et al. described 135 neonates treated with ECMO for respiratory failure(16). Forty-three patients (32%) had acute renal failure (defined as a serum creatinine greater than 2 mg/dL, or requiring ultrafiltration/dialysis). Only 8 of the 43 (19%) survived compared to 92% survival in patients without acute renal failure. Outcome data reporting the recovery of renal function in survivors were not presented. Sell et al (17) described experience with treatment of renal failure in 6 neonatal and pediatric ECMO patients with low volume hemofiltration (5-10 mL/kg/hour); two patients survived, both with renal recovery. Weber et al reported 38% survival to discharge in 55 non-neonatal ECMO patients, of whom 38 were treated with ultrafiltration or hemodialysis(18). The largest previous series of neonatal and pediatric survivors (15 of 35, 43%) receiving concomitant ECMO and CRRT was reported by Meyer et al(8). The most common indications for CRRT in their study were renal failure (51%) and fluid overload (46%), similar to the current experience. Renal recovery was present in 14 of the15 survivors (93%). The lone survivor who did not recover function in that series was later diagnosed with Wegener's granulomatosis and subsequently underwent renal transplantation. Outcomes in Meyer's series mirror those of our broader institutional experience. Interestingly, these survival rates are similar to those in pediatric reports of patients receiving CVVH alone. Data from the pediatric prospective continuous renal replacement therapy registry demonstrated 51% survival in 116 non-ECMO patients with multiple organ dysfunction syndrome treated with CRRT(19).
Data on use of CRRT in ECMO patients with congenital heart disease are even more limited(15). Kolovos et al described 74 post-operative congenital heart disease patients requiring ECMO support(13). Twenty six patients (35%) received hemofiltration and use of hemofiltration was associated with a five-fold increase in mortality risk. Patients in this series who received hemofiltration had longer ECMO support time, suggesting that the increased mortality risk was related to factors associated with multi-organ failure. Renal recovery in survivors was not reported. In contrast, Shah et al found temporary renal insufficiency in 48% of 84 congenital heart disease patients receiving ECMO(20). Development of renal insufficiency on ECMO and the use of concurrent hemofiltration was not associated with ability to wean off ECMO support or with survival to discharge.
Experience from our institution, comprising the largest and most diverse pediatric CRRT/ECMO series to date, confirms the higher risk of mortality with CRRT compared to ECMO use alone. It is likely that this difference is related to the increased risk of death conferred by the existence of renal failure rather than the introduction of CVVH per se. Provider-specific differences in CRRT use on ECMO complicate the analysis of the impact of CRRT use itself on outcome. A common practice is to institute CRRT only after the development of renal failure failing medical management. In such cases, the institution of CRRT may be a surrogate marker for patients declining into multiple organ failure syndrome, which is associated with worse ECMO survival. Alternatively, CVVH use may be a marker for patients who are at higher risk for death because of more severe illness, fluid overload, or a prolonged duration of ECMO therapy. For example, our neonatal data demonstrate that the duration of ECMO was 2.5 times as long in patients who received concomitant CVVH (248 vs. 97 hours). We used pRIFLE criteria to determine severity of renal illness at outset. Lin et al found the Risk, Injury, Failure, Loss, and End-Stage Kidney disease (RIFLE) criteria to be a satisfactory method to stratify patients treated with ECMO to determine hospital mortality risk (21). Forty-two of the 45 survivors who could be scored demonstrated evidence of renal injury or risk of injury. From previous ECMO experience, survival would be expected to be much lower in this group, mitigating the possibility of an adverse impact on survival of CVVH itself.
One important finding of this series was that, in the absence of primary renal disease at presentation, chronic renal failure did not occur in ECMO patients treated concomitantly with CVVH. Confirmation of this finding, also suggested by Meyer et al. (8), should encourage less reticence in the utilization of CRRT. Strict fluid management of pediatric ECMO patients, including early institution of CVVH, has evolved in our pediatric intensive care unit. Multiple reports in critically ill pediatric patients not receiving ECMO have identified fluid overload as a risk factor predictive for mortality (22-25). In ECMO patients, data on effects of fluid overload are limited. Swaniker et al. found significant fluid overload (greater than 10% above dry body weight) in 41% of pediatric ECMO patients at time of cannulation (26). Degree of fluid overload was associated with survival; non-survivors were a median 25% above dry weight at ECMO initiation compared to 9% excess in survivors. Reduction in volume overload has also been associated with ECMO patient improvement. Weber (18) observed that ECMO weaning for non-neonatal patients “frequently occurred after significant diuresis was achieved, either pharmacologically (furosemide) or with ultrafiltration or dialysis.” Degree of fluid overload may also correlate with duration of ECMO support required for neonates (27). Additional studies are needed to clarify the role of fluid overload and its management on the duration of ECMO support. CVVH may offer certain advantages in the effort to maintain optimal fluid status in patients receiving ECMO. At our center, a retrospective case control study found children on ECMO who received CVVH had improved daily fluid balance, shorter time to goal nutritional support, and decreased daily diuretic dose compared to matched ECMO patients not receiving CVVH (12). While it is clear that mortality risk increases when an ECMO patient has renal failure, our findings suggest that the institution of CVVH itself does not contribute to an increased risk of mortality. With early use of concomitant CVVH, we have maintained survival rates for pediatric ECMO patients equivalent to those obtained by other ELSO centers (Table 2).
This study has several important limitations. While this is to our knowledge the largest reported outcome study of children receiving concomitant ECMO and CVVH, the sample size remains small. When assigning pRIFLE categories to these patients, serum creatinine values alone were used rather than including definition by urine output. Thus patients with renal failure criteria may have been underestimated. However, in the validation description of pRIFLE scoring (9), only 6 of 150 patients attained a pRIFLE classification based on urine output that was higher than the classification that they would have received if stratified by creatinine, and none of those patients received renal replacement or died. Additionally, this study is restricted to a single center, is retrospective, and represents a heterogeneous ECMO population. In our center, ECMO is managed by three separate physician groups in our pediatric, cardiac, and neonatal intensive care units, and variations in physician practice between these units may have influenced both the use of and outcome of these therapies. While it is possible that the mode of ECMO could impact the development of AKI due to the effects of pulsatile/nonpulsatile flow (28), data from our study do not allow meaningful conclusions in this regard due to our preferential use of VV ECMO and the likelihood that those patients receiving VA ECMO had worse organ failure prior to institution of ECMO. To better address many of these concerns, a prospective multi-center study would be beneficial to compare outcomes with routine use of CVVH compared to standard fluid management alone on ECMO.
Conclusions
In this large single center experience, development of chronic renal failure in ECMO patients receiving CVVH did not occur in the absence of primary renal disease. Mortality was higher in patients receiving concomitant CVVH and ECMO compared to those receiving ECMO alone. However, mortality with ECMO/CVVH was similar to that in critically ill pediatric patients requiring CVVH who are not on ECMO, while overall ECMO mortality was similar to that of other ELSO centers. Additional studies are warranted to determine the role of early or routine CVVH use in patients on ECMO.
Footnotes
Financial support: None
Financial Disclosure: Drs. Paden and Fortenberry have a patent pending on a device to improve safety and delivery of continuous renal replacement therapy in ECMO patients.
References
- 1.Van Meurs K, Lally KP, Peek G, Zwischenberger JB, editors. ECMO – Extracorporeal cardiopulmonary support in critical care. Extracorporeal Life Support Organization. (3rd Edition.) 2005:1–5. [Google Scholar]
- 2.Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226(4):544–66. doi: 10.1097/00000658-199710000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swaniker F, Kolla S, Moler F, Custer J, Grams R, Bartlett RH, Hirschl RB. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35(2):197–202. doi: 10.1016/s0022-3468(00)90009-5. [DOI] [PubMed] [Google Scholar]
- 4.Roy BJ, Cornish JD, Clark RH. Venovenous extracorporeal membrane oxygenation affects renal function. Pediatrics. 1995;95:573–578. [PubMed] [Google Scholar]
- 5.Ronco C, Bellomo R, Ricci Z. Continuous renal replacement therapy in critically ill patients. Nephrol Dial Transplant. 2001;16(Suppl 5):67–72. doi: 10.1093/ndt/16.suppl_5.67. [DOI] [PubMed] [Google Scholar]
- 6.Maxvold NJ, Bunchman TE. Renal failure and renal replacement therapy. Crit Care Clinics. 2003;19:563–75. doi: 10.1016/s0749-0704(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein SL. Overview of pediatric renal replacement therapy in acute renal failure. Artif Org. 2003;27(9):781–5. doi: 10.1046/j.1525-1594.2003.07281.x. [DOI] [PubMed] [Google Scholar]
- 8.Meyer RJ, Brophy PD, Bunchman TE, et al. Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med. 2001;2:238–242. doi: 10.1097/00130478-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 10.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–54. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Haycock GB, Edelmann CM., Jr Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 12.Hoover NG, Heard M, Reid C, Wagoner S, Rogers K, Foland J, Paden ML, Fortenberry JD. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving ECMO support. Intensive Care Medicine. 2008;34(12):2241–7. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]
- 13.Kovolos NS, Bratton SL, Moler FW, Bove EL, Ohye RG, Bartlett RH, Kulik TJ. Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Ann Thorac Surg. 2003;76:1435–42. doi: 10.1016/s0003-4975(03)00898-1. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett RH, Toomasian J, Roloff D, Gazzaniga AB, Corwin AG, Rucker R. Extracorporeal membrane oxygenation for neonatal respiratory failure – 100 cases. Ann Surg. 1986;204(3):236–44. doi: 10.1097/00000658-198609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AH, Hardison DC, Worden CR, Fleming GM, Taylor MB. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. 2009 Jul-Aug;55(4):412–6. doi: 10.1097/MAT.0b013e31819ca3d0. [DOI] [PubMed] [Google Scholar]
- 16.Weber TR, Connors RH, Tracy TF, Jr, et al. Prognostic determinants in extracorporeal membrane oxygenation for respiratory failure in newborns. Ann Thorac Surg. 1990 Nov;50(5):720–3. doi: 10.1016/0003-4975(90)90669-w. [DOI] [PubMed] [Google Scholar]
- 17.Sell LS, Cullen ML, Whittlesey GC, et al. Experience with renal failure during extracorporeal membrane oxygenation: Treatment with continuous hemofiltration. J Pediatr Surg. 1987;22:600–602. doi: 10.1016/s0022-3468(87)80107-0. [DOI] [PubMed] [Google Scholar]
- 18.Weber TR, Kountzman B. Extracorporeal membrane oxygenation for nonneonatal pulmonary and multiple organ failure. J Pediatr Surg. 1998;33:1605–1609. doi: 10.1016/s0022-3468(98)90590-5. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multiple organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–8. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 20.Shah SA, et al. Clinical outcomes of 84 children with congenital heart disease managed with extracorporeal membrane oxygenation after cardiac surgery. ASAIO J. 2005;51:504–7. doi: 10.1097/01.mat.0000171595.67127.74. [DOI] [PubMed] [Google Scholar]
- 21.Lin CY, Chen YC, Tsai FC, Tian YC, Jenq CC, Fang JT, Yang CW. RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant. 2006;21:2867–2873. doi: 10.1093/ndt/gfl326. [DOI] [PubMed] [Google Scholar]
- 22.Yap HJ, Chen YC, Fang JT, Huang CC. Combination of continuous renal replacement therapies (CRRT) and extracorporeal membrane oxygenation (ECMO) for advanced cardiac patients. Ren Fail. 2003;25(2):183–93. doi: 10.1081/jdi-120018719. [DOI] [PubMed] [Google Scholar]
- 23.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload prior to continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–76. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein SL, Currier H, Graf JM, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie RS, Seidel K, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;19:1394–9. doi: 10.1007/s00467-004-1655-1. [DOI] [PubMed] [Google Scholar]
- 26.Swaniker F, Kolla S, Moler FW, Custer J, Grams R, Bartlett RH, Hirschl R. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Ped Surg. 2000;35(2):197–202. doi: 10.1016/s0022-3468(00)90009-5. [DOI] [PubMed] [Google Scholar]
- 27.Kelly RE, Phillips JD, Foglia RP, et al. Pulmonary edema and fluid mobilization as determinants of the duration of ECMO support. J Pediatr Surg. 1991;26:1016–1022. doi: 10.1016/0022-3468(91)90665-g. [DOI] [PubMed] [Google Scholar]
- 28.Undar A, Masai T, Beyer EA, Goddard-Finegold J, McGarry MC, Fraser CD., Jr Pediatric physiologic pulsatile pump enhances cerebral and renal blood flow during and after cardiopulmonary bypass. Artif Organs. 2002 Nov;26(11):919–23. doi: 10.1046/j.1525-1594.2002.07127.x. [DOI] [PubMed] [Google Scholar]