Abstract
Background & Aims
Oltipraz (4-methyl-5(pyrazinyl-2)-1-2-dithiole-3-thione), a promising cancer preventive agent, has an anti-oxidative activity and ability to enhance glutathione biosynthesis, phase II detoxification enzymes and multidrug resistance-associated protein-mediated efflux transporters. Oltipraz can protect against hepatotoxicity caused by carbon tetrachloride, acetaminophen and alpha-naphthylisothiocyanate. Whether oltipraz has hepato-protective effects on obstructive cholestasis is unknown.
Methods
We administered oltipraz to mice for 5 days prior to bile duct ligation (BDL) for 3 days. Liver histology, liver function markers, bile flow rates and hepatic expression of profibrogenic genes were evaluated.
Results
Mice pretreated with oltipraz prior to BDL demonstrated higher levels of serum aminotransferases and more severe liver damage than in control mice. Higher bile flow and glutathione secretion rates were observed in unoperated mice treated with oltipraz than in control mice, suggesting that liver necrosis in oltipraz-treated BDL mice may be related partially to increased bile-acid independent flow and biliary pressure. Oltipraz treatment in BDL mice enhanced -smooth muscle actin expression, consistent with activation of hepatic stellate cells and portal fibroblasts. Matrix metalloproteinases (MMP) 9 and 13 and tissue inhibitors of metalloproteinases (TIMP) 1 and 2 levels were increased in the oltipraz -treated BDL group, suggesting that the secondary phase of liver injury induced by oltipraz might be due to excessive MMP and TIMP secretions which induce remodeling of the extracellular matrix.
Conclusions
Oltipraz treatment exacerbates the severity of liver injury following BDL and should be avoided as therapy for extrahepatic cholestatic disorders due to bile duct obstruction.
Keywords: oltipraz, Nrf2, obstructive cholestasis, bile secretion, hepatic stellate cells
1. INTRODUCTION
Cholestasis is associated with reductions in bile flow, hepatic accumulation of bile acids, and hepatocellular injury and fibrosis. Exposure of hepatocytes to these increasing levels of toxic bile acids can result in production of reactive oxidative species, leading to oxidative stress and progressive liver damage [1]. Increased levels of the by-products of oxidative stress have been detected in obstructive cholestasis in rodents and humans [2, 3]. In an attempt to restore redox balance between radical-generating and radical-scavenging capacity, specific pathways can be activated in order to prevent further oxidative injury in the liver [1]. This adaptive stress response involves enhancing the expressions of antioxidant genes, including NAD(P)H quinone oxidoreductase (NqoI), heme oxygenase-1, glutathione-S-transferase and UDP-glucuronosyltransferase1A6 [4].
Ursodeoxycholic acid (UDCA), the only drug approved by the FDA specifically for the treatment of primary biliary cirrhosis (PBC), has both direct and indirect antioxidant properties [5]. It acts directly by scavenging hydroxyl radicals and indirectly through the induction of endogenous antioxidant defenses, including increasing the expression of -glutamylcysteine synthetase regulatory subunit and increasing the rate of glutathione (GSH) synthesis. Recently, a novel therapeutic mechanism of UDCA action via Nrf2 activation has been suggested and may represent another drug target in cholestatic liver diseases [5, 6]. Nonetheless, the effectiveness of UDCA is limited to the early stages of PBC [7]. Thus, alternative therapeutic interventions are clearly needed for patients with cholestatic liver diseases.
Oltipraz is a known antioxidant and a promising chemo-preventive agent [8]. To date, oltipraz has proved effective as anti-carcinogenesis in experimental models for breast, bladder, liver, forestomach, colon, tracheal, lung, and skin cancer [9]. Oltipraz is undergoing clinical trial evaluation in Qidong, China, as a possible chemoprotective agent against aflatoxin B1 in humans. The anti-tumorigenic effects of oltipraz in rodents include inhibition of certain cytochrome P450 (CYP1A2) and induction of phase II detoxifying enzymes, including microsomal epoxide hydrolase, UDP-glucuronyltransferase and glutathione-S-transferase. Moreover, oltipraz has the ability to modulate liver regeneration [10], and to inhibit hepatitis B virus [11] and human immunodeficiency virus replication [12]. Furthermore, oltipraz can protect against hepatotoxicity caused by carbon tetrachloride [13], acetaminophen [14] and -naphthylisothiocyanate, a cause of intrahepatic cholestasis in an animal disease model [15]. In addition, oltipraz can up-regulate the gene expression of canalicular efflux transporters, including the multidrug resistance-associated protein (Mrp) 2 [16] and bile salt efflux pump (BSEP) [17], as well as the alternative basolateral efflux transporters, Mrp3 and Mrp4 having an essential role in the adaptive response to obstructive cholestatic liver injury [18, 19]. Considering its antioxidant and many favorable pharmacological effects on the liver, oltipraz may be an attractive candidate drug for the treatment of cholestatic liver diseases.
In this study we determined if oltipraz has hepato-protective effects in a murine model of obstructive cholestasis. Unexpectedly, our findings demonstrate that oltipraz treatment significantly exacerbates the severity of liver injury following bile duct ligation (BDL). We conclude that prospective clinical studies with oltipraz require caution and that oltipraz should be avoided as therapy for cholestatic disorders related to bile duct obstruction.
2. MATERIALS AND METHODS
2.1 Materials
All reagents were obtained from Sigma-Aldrich (St Louis, MO). Oltipraz was purchased from Axxora (San Diego, CA). Polyclonal antibody to Mrp2 was a gift from Dr. Bruno Stieger (University of Zurich). Mrp4 and Bsep antibodies were purchased from Everest Biotech (Oxfordshire, UK), and Kamiya Biomedical (Seattle, WA), respectively. Mrp3 antibody was developed in our laboratory [20]. SH-PTP1 antibody was obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). DAB peroxidase substrate kit was supplied from Vector Laboratories (Burlingame, CA).
2.2 Animals and Surgical procedures
Male C57BL/6J mice (8-9 weeks old) were obtained from the Jackson Laboratory, Bar Harbor, ME. Mice were housed in the Yale animal facility, and were kept in controlled light (12-h light: 12-h dark cycle) and temperature (22°C), and provided with food and water ad libitum. The study protocol was approved by Yale Animal Care and Use Committee, and was accordance with National Institutes of Health guidelines (protocol no. 2009-07458). Mice were randomized and pretreated orally with oltipraz at a dose of 150 mg/kg body weight that is known to be the safety dose (LD50 > 5000 mg/kg) or the solvent control (carboxymethylcellulose, CMC) for 5 days prior to bile duct ligation [21] for 3 days. Total duration for oltipraz treatment was 8 days. Animals were fasted overnight prior to sacrifice. Plasma, bile, urine and liver tissue were collected and stored at −80°C or fixed in 4% neutral buffered formaldehyde for blinded histological evaluation by J.L.B.
2.3 Immunohistochemical analysis
Immunohistochemistry for cytokeratin 19 was performed on paraffin sections to quantify bile duct proliferation. Antigen retrieval using 1 mM EDTA, pH 8.0, in a steamer was done prior to overnight incubation with the monoclonal rat anti-CK19 (Troma III) as previously described [22].
2.4 Hepatocellular function
Plasma ALT and total bilirubin levels were measured as indicators of hepatic injury using standard diagnostic kits (Thermo Scientific, Middletown, VA). Bile acid concentrations in the plasma, gallbladder, and liver were determined using a commercial total bile acid kit (Diazyme Laboratories, Poway, CA) as previously described [22].
2.5 Quantitative real-time polymerase chain reaction
RNA was isolated from frozen liver samples using Trizol reagent according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) synthesis and subsequent quantitative real-time PCR using TaqMan technology were performed as described previously [17]. The gene-specific primers used were provided by Applied Biosystems and are listed in Supplementary Table 1. Gapdh, a house keeping gene, was run for each sample to normalize expression.
2.6 Western blot analysis for bile acid transporter expression
Membrane enriched liver proteins were isolated as previously described [23]. Western blot analysis was performed as previously described with some modifications [17]. Briefly, samples were loaded onto 4-20% Bis-Tris gradient gel and were electrophoresed with MOPS running buffer (Invitrogen). Proteins were transferred onto nitrocellulose membrane and incubated with the antibodies listed above. IRDye680 or IRDye800 conjugated to rabbit or goat IgG were used as secondary antibodies (Li-Cor, Lincoln NE). The relative quantities of protein expression were analyzed using the Odyssey infrared image system (Li-Cor).
2.7 Measurement of bile flow
Bile flow was determined in a separate group of mice which were treated with either CMC or oltipraz for 8 days. Bile was collected every 5 min for 60 min in pre-weighed eppendorf tubes containing water or 6% sulfosalicylic acid for bile acid and glutathione determination, respectively, as previously described [24].
2.8 Glutathione content determination
Glutathione content in the liver homogenate or in bile samples was determined using a glutathione assay kit (Sigma).
2.9 Statistical Analysis
All data are expressed as the mean ± standard deviation (S.D). Statistical analyses were performed using analysis of variance with Bonferroni post-testing (SigmaStat program, Jandel Scientific). Differences were considered statistical significant at p < 0.05.
3. RESULTS
3.1. Oltipraz exacerbates BDL-induced liver damage
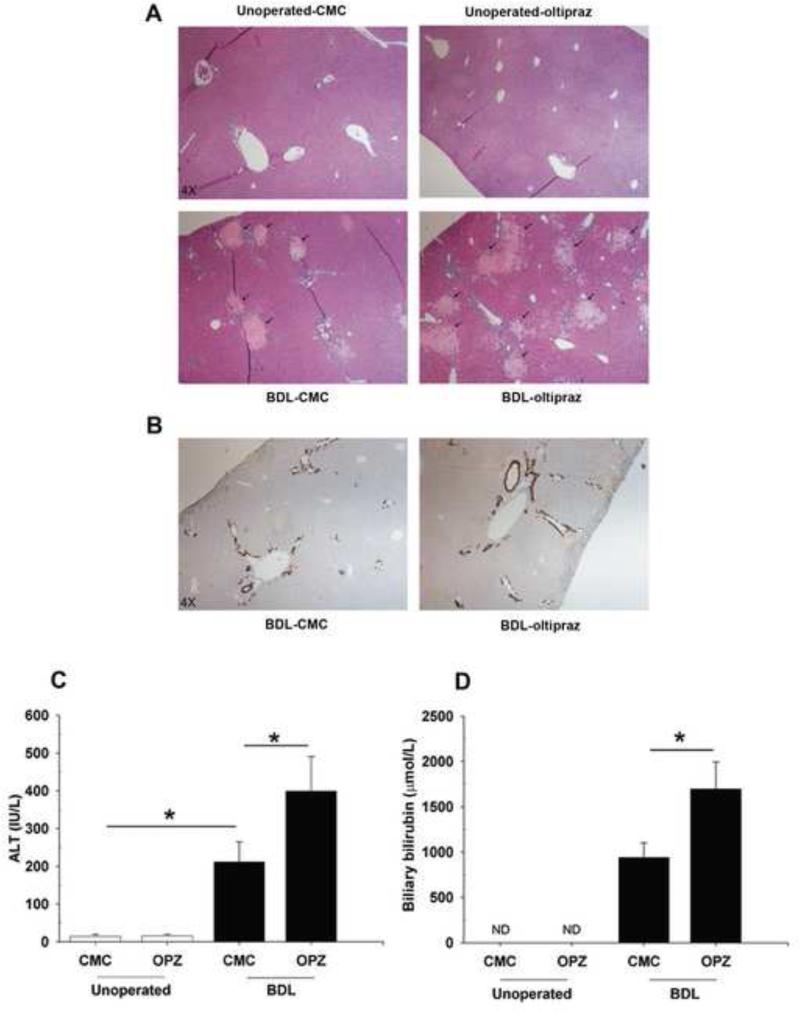
Oltipraz treatment had no effect on body weight either in normal animals or when given prior to BDL (Supplementary Fig. S1A). However, oltipraz treatment significantly increased the liver weight in BDL animals compared to CMC-BDL mice (Supplementary Fig. S1B). Liver histology was normal in unoperated mice receiving the control CMC or oltipraz (Fig. 1A). However, oltipraz treatment in BDL mice resulted in extensive centrilobular necroinflammation, as indicated by greater hepatocellular ballooning, larger foci of hepatocyte necrosis in the periportal regions, and lobular inflammation (Fig. 1A). Furthermore, oltipraz significantly increased the extent of “bile infarcts” in the parenchyma in the oltipraz-BDL mice compared to CMC-BDL mice (Fig. 1A). TUNEL positive hepatocytes were not increased in the bile infarcts in oltipraz-BDL mice (data not shown), suggesting that necrosis seems to be primary cause of the cell death rather than apoptosis. Immunohistochemical staining for CK19 showed more, but not statistically significant, bile duct proliferation in oltipraz-BDL mice (Fig. 1B). Oltipraz did not alter plasma bile acids, bilirubin or ALT in unoperated mice, but the elevated plasma ALT levels due to BDL were further increased after oltipraz treatment in BDL mice (Fig. 1C). As quantified by morphometric analysis of bile infarct areas, we also observed significantly increased liver injury in BDL mice treated with oltipraz compared to BDL mice treated with CMC (4.1 ± 3.7% in BDL-CMC and 23.6 ± 9.5% in BDL-oltipraz, p<0.05), thus recapitulating and substantiating the increased ALT serum. Although there was no change in serum bilirubin between CMC-and oltipraz-treated BDL mice (data not shown), oltipraz-BDL mice had increased levels of bilirubin in bile (Fig. 1D). Collectively, these findings indicate that oltipraz potentiates liver damage in BDL mice.
Fig. 1. Effect of oltipraz on BDL-induced liver injury.
(A) Representative hematoxylin and eosin images of liver sections from unoperated and BDL mice treated with either CMC or oltipraz (OPZ). Oltipraz treated livers from BDL mice showed extensive hepatic necrosis as indicated by arrows. (B) Immunohistochemical staining for CK19 in livers from CMC-BDL and oltipraz-BDL mice, (original magnification, 4X). (C) Plasma ALT and (D) Biliary bilirubin levels were significantly higher in oltipraz-BDL mice compared to CMC-BDL mice. * p < 0.05; between indicated groups.
3.2. Oltipraz increases bile flow rates
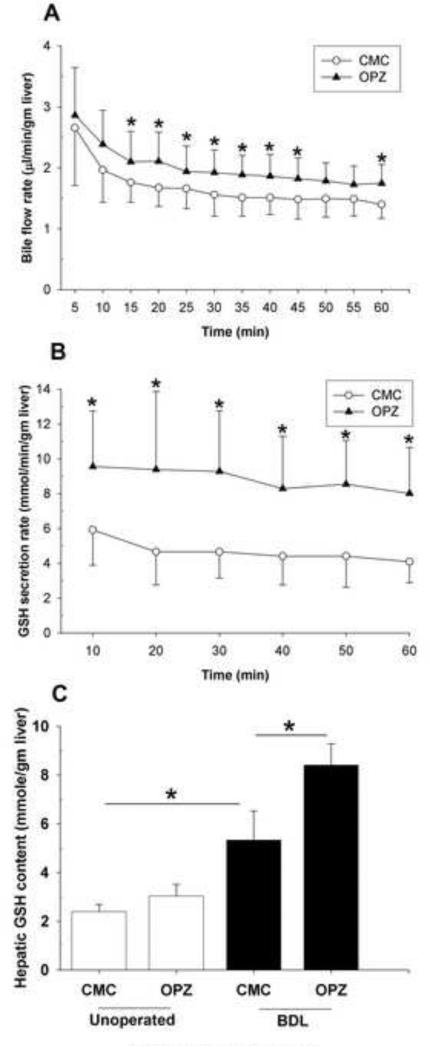
Oltipraz is a microsomal enzyme inducer and increases Mrp2 expression in rats [16] and BSEP expression in humans [17]. Therefore, we determined whether stimulation of bile acid -dependent or -independent bile flow by oltipraz might be responsible for the additional liver injury, as has been reported in BDL Fxr +/+ mice [25, 26]. Indeed, when we treated unoperated mice with either CMC or oltipraz for 8 days, bile flow was significantly greater in the oltipraz treated group (Fig. 2A). This suggests that the development of liver necrosis in oltipraz-treated BDL mice could be due in part to increased bile flow and biliary pressure.
Fig. 2. Oltipraz increases hepatic GSH content and bile flow rates.
(A) Bile flow rate and (B) GSH secretion rate in unoperated mice treated with CMC or oltipraz. (C) Hepatic glutathione contents of unoperated and BDL mice treated with CMC or oltipraz. * p < 0.05; between indicated groups.
3.3. Oltipraz enhances hepatic GSH content and GSH secretion
To ascertain whether the increased bile flow was due to changes in the bile acid -dependent or -independent component of bile flow, we examined biliary secretion of reduced GSH, a key determinant of bile acid -independent bile flow [27], and bile acids in unoperated mice treated with CMC or oltipraz. Oltipraz treatment did not induce changes in the bile acid secretion rate (data not shown); however, it substantially increased the rate of GSH secretion. Figure 2B illustrates that higher GSH rates were sustained over 60 min in oltipraz treatment compared with CMC treatment. We found that hepatic GSH content was also significantly higher in the oltipraz-treated unoperated mice compared to the CMC-treated group (Fig. 2C), implying that oltipraz stimulates hepatic GSH synthesis. Similarly, a more profound induction in hepatic levels of GSH was seen in oltipraz-treated BDL mice than in the CMC-BDL mice (Fig. 2C). Also, the mRNA and protein expression of the rate-limiting enzyme of GSH synthesis, so called glutamate-cysteine ligase catalytic subunit (Gclc) or also known as -glutamylcysteine synthetase (GCS), further significant increased approximately 2-fold in BDL mice treated with oltipraz (Supplementary Fig. S5). Taken together, these results indicate that oltipraz increases the bile acid-independent bile flow via excretion of GSH, a finding that could contribute to more severe liver injury in BDL mice.
3.4. Expression of major basolateral and canalicular bile acid efflux transporters in oltipraz-treated BDL mice
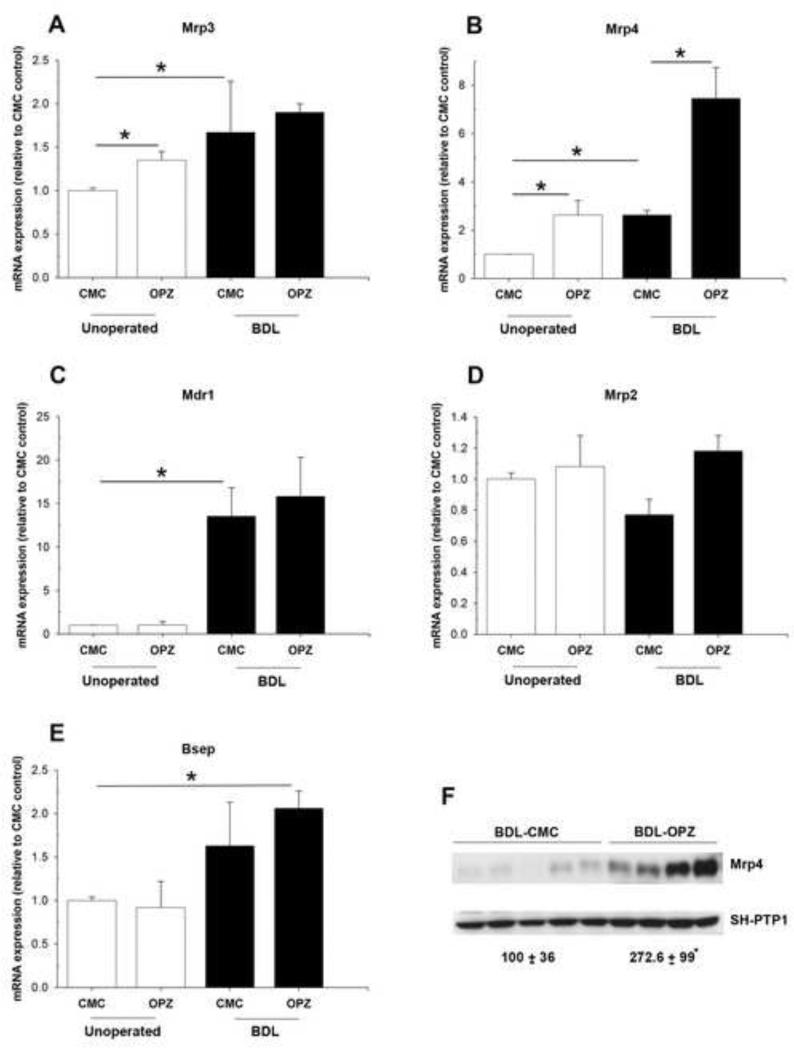
Because adaptive regulation of liver membrane transporters has been shown to limit hepatotoxicity in obstructive cholestasis, we determined whether there was a difference in the expression of hepatic efflux transporters in these mice. mRNA levels of two key basolateral transporters, Mrp3 and Mrp4, were up-regulated significantly by the oltipraz treatment alone (Fig. 3A, 3B), consistent with a previous report [18]. Mrp3 and Mrp4 were further up-regulated in CMC and oltipraz-BDL mice (Fig. 3A, 3B). The oltipraz-BDL mice demonstrated a greater increase in Mrp4 mRNA than in Mrp3 mRNA. Gene expression of canalicular Mdr1a was equally increased after BDL in the CMC and oltipraz treated groups (Fig. 3C). Following BDL in CMC-treated mice, Mrp2 mRNA levels tended to decrease but remained unchanged in oltipraz-treated mice (Fig. 3D). The mRNA expression of Bsep was increased in both the CMC- and oltipraz-BDL mice, but was only statistically significant after oltipraz treatment (Fig. 3E). Western blotting of liver membrane proteins also confirmed that oltipraz further increased protein expression of Mrp4 compared to CMC-BDL mice (Fig. 3F). No difference in Mrp2, Bsep or Mrp3 protein expression was seen between CMC- and oltipraz-BDL groups (data not shown), thereby suggesting that Mrp2 and Bsep continue to be expressed and able to extrude bile acids into the obstructed biliary tree as evidenced by unchanged levels of bile acid concentrations in the bile of both CMC- and oltipraz-BDL mice (data not shown).
Fig. 3. Effect of oltipraz on hepatic expression of genes associated with bile acid transport.
Relative mRNA expression for (A) Mrp3, (B) Mrp4, (C) Mdr1, (D) Mrp2 and (E) Bsep. Data are expressed as the fold change relative to CMC-control mice. (F) Representative blots and densitometry data of Mrp4 normalized to SH-PTP1 in BDL mouse liver with and without oltipraz treatment. Data are expressed in arbitrary units. * p < 0.05; between indicated groups.
3.5. Mechanism of the deleterious effect of oltipraz in BDL mice
Hepatocellular injury activates the innate immune system, thus leading to release of growth factors and cytokines that stimulate extracellular matrix (ECM) synthesis. To further determine if other mechanisms contribute to the aggravated liver injury in oltipraz-BDL mice, we next evaluated if oltipraz treatment could affect the hepatic expression of proinflammatory and profibrogenic genes. While oltipraz did not increase the expression of genes involved in hepatic inflammation, including IL-1, IL-6 and TNF- (data not shown), oltipraz significantly increased the mRNA expression of key genes involved in hepatic fibrogenesis, including TGF- 1 (Supplementary Fig. S2A), -SMA (Supplementary Fig. S2B) and procollagen 1 (I) (Supplementary Fig. S2C) compared to CMC-BDL mice. This data suggest that oltipraz treatment is associated with activation of hepatic stellate cells (HSCs).
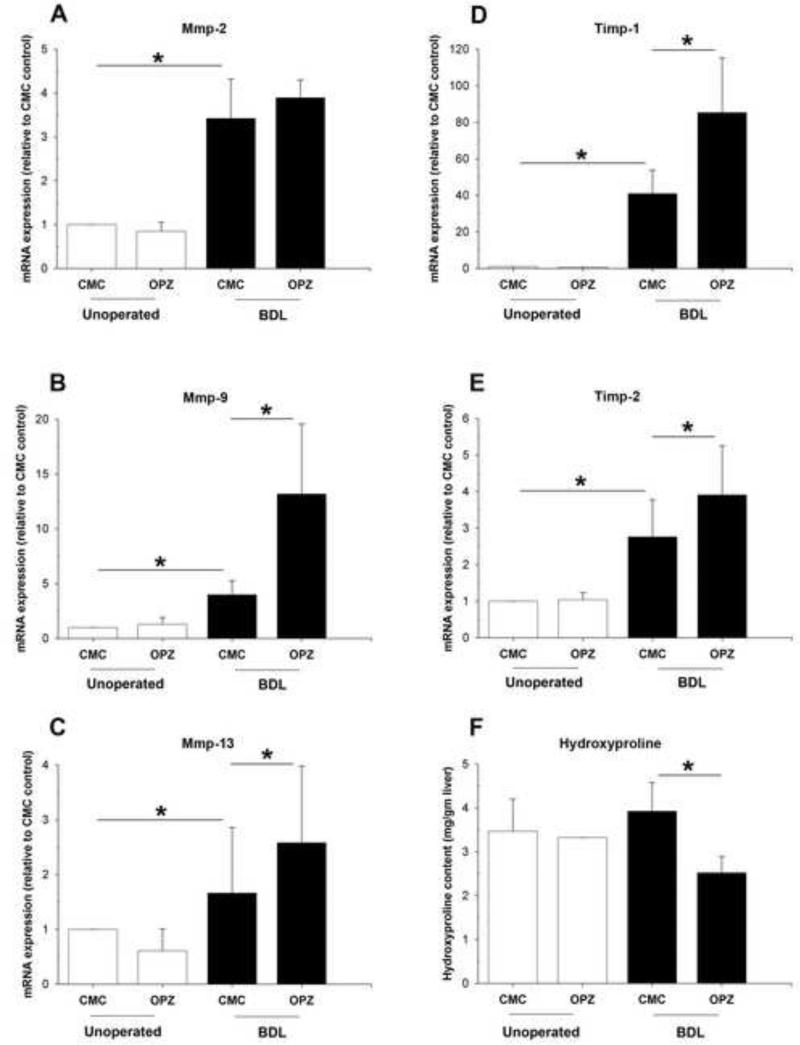
Activated HSCs increase production and accumulation of ECM constituents through the actions of hepatic fibrolytic matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) [28]. Quantitative PCR demonstrated that hepatic expression of MMP-2, MMP-9, and MMP-13 mRNA were all significantly up-regulated in BDL mice compared to unoperated mice (Fig. 4A-C). Oltipraz pretreatment of the BDL group did not affect the mRNA level of MMP-2 (Fig. 4A). However, hepatic transcript levels of MMP-9 (Fig. 4B) and MMP-13 (Fig. 4C) were 3.3-fold and 1.6-fold increased, respectively, compared to CMC treated BDL mice. TIMP-1 and TIMP-2 were also increased in both CMC-and oltipraz-treated BDL groups (Fig. 4D-E), with a more profound increase of both seen in the oltipraz-treated BDL mice. Quantification of hydroxyproline revealed a decrease in collagen content in oltipraz-treated BDL mice compared to CMC-treated BDL mice (Fig. 4F). However, morphometric analysis of Sirius Red stained liver sections did not show differences in the area of fibrosis (data not shown). Collectively, these data suggest that the secondary phase of liver injury induced by oltipraz might be due to excessive and prolonged MMP and TIMP secretions which induce remodeling of the ECM deposition and further contribute to the liver injury following BDL.
Fig. 4. Effect of oltipraz on hepatic expression of genes involved in fibrosis and tissue remodeling.
Relative mRNA expression of (A) MMP2, (B) MMP9, (C) MMP13, (D) TIMP-1, and (E) TIMP-2 in livers of unoperated and BDL mice treated with CMC or oltipraz. All five genes were significantly increased by the bile duct ligation and, in addition, MMP9, MMP13, TIMP-1 and TIMP-2 mRNA levels were further increased after treatment with oltipraz. (F) Livers from oltipraz treated BDL mice exhibited decreased hepatic collagen deposition as determined by relative hydroxyproline content. * p < 0.05; between indicated groups.
3.6. Oltipraz leads to up-regulation of PDGF receptor in BDL mice
Both TGF- 1 and PDGF are mediators implicated in liver injury. Quantitative PCR demonstrated increases in both PDGF and PDGF receptor mRNA after BDL in the CMC and oltipraz groups (Supplementary Fig. S3A, B). However, only PDGF receptor mRNA was significantly increased compared to the CMC-BDL control (Supplementary Fig. S3B), suggesting the involvement of PDGF-signaling in oltipraz-enhanced hepatic injury in BDL mice. mRNA levels for signal transducer and activator of transcription 3 (Stat3), a downstream pathway of PDGF/PDGFR, were equally increased after BDL in the CMC-BDL and oltipraz-BDL mouse livers (Supplementary Fig. S3C). This suggests that the additional liver injury induced by oltipraz is likely to be independent of Stat3 signaling.
4. DISCUSSION
In this study we expected that oltipraz would have hepato-protective effects following BDL in the mouse presumably by up-regulating detoxifying enzymes and hepatic efflux transporters. In contrast, oltipraz aggravated rather than improved liver injury in this BDL model. These findings raise the question of why this accepted hepato-protective and anti-hepatocarcinogenesis agent enhanced liver injury in this cholestatic model.
Clearly, the increased liver injury in oltipraz-BDL mice was not a result of altered bile acid synthesis since there was no difference in the expression of bile acid synthesis enzymes or in hepatic, plasma and biliary bile acid concentrations between CMC- and oltipraz-BDL mice (data not shown). Rather, increased bile flow rates and increased expression of canalicular membrane efflux transporters most likely explain these findings. This finding is also consistent with observations in Fxr −/− mice which are protected from developing bile infarcts following BDL due to reduced bile acid-dependent bile flow (BADF) and intra biliary pressure as a result of decreased expression of Bsep, an Fxr regulated bile acid transporter [25, 26]. In contrast, oltipraz, while maintaining the expression of Mrp2, stimulated the biliary excretion of GSH and through its osmotic activity, increased bile acid-independent bile flow (BAIF). Furthermore, the expression of Bsep was increased, which maintained the excretion of bile acids. These results suggest that drugs that stimulate either BAIF or BADF may be detrimental in patients whose bile ducts are obstructed and support the concept that increased flow rates in an obstructed biliary tree may be detrimental. The adverse effects of high doses of UDCA seen recently in patients with primary sclerosing cholangitis may be one example of this phenomenon [29].
Could other mechanisms contributed to the deleterious effects of oltipraz in BDL mice? Hepatocyte injury during cholestasis usually leads to an inflammatory response and the release of pro-inflammatory mediators, resulting in stimulation of ECM synthesis by activation of quiescent hepatic stellate cells (HSC) and portal fibroblasts (PF) [30]. In our study, oltipraz treatment in BDL mice stimulated the activation of HSC and PF as seen by a marked increase in mRNA expression of TGF- 1 and -SMA, two phenotypic markers of HSC and PF activation. Based on a previous study [31], cathepsin B, which is also implicated in liver fibrogenesis, contributes to the degradation of PDFG receptor (PDFG R), leading to the inability of PDGF to induce HSC proliferation and activation [32]. PDFG R was increased in oltipraz-BDL mice, suggesting that oltipraz may induce HSC proliferation through this mechanism. In contrast, there was no difference in Stat3 mRNA, a downstream pathway of PDFG /PDFG R, making it unlikely that Stat3 is implicated in the accelerated liver injury induced by oltipraz. Pathways responding to PDFG /PDFG R can activate Ras and sequentially stimulate the phosphorylation of mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) and the phosphatidylinositol 3-kinase (PI3K)/Akt/70-kDa ribosomal S6 kinase signaling pathway. These two pathways regulate the transcription of profibrogenic genes and proliferation and apoptosis in HSC [33], suggesting additional pathways by which oltipraz might accelerate liver injury in BDL mice. These possibilities will require further investigations.
TGF- stimulates HSC activation and differentiation of portal fibroblasts into myofibroblasts. TGF- also up-regulates the production and deposition of ECM constituents, but down-regulates fibrolytic matrix metalloproteinases (MMPs) [34]. Oltipraz-BDL mice have higher levels of TGF- compared to control BDL mice. However, rather than down-regulation of MMPs, MMP-9 and MMP-13 were increased after BDL and was further stimulated by oltipraz (Fig. 4), even though mRNA expression of procollagen1 (I) and the MMP-inhibitors TIMP-1 and TIMP-2 were elevated. It is the balance between MMPs and TIMPs that regulate ECM synthesis and degradation [35]. In hepatic injury the excessive synthesis of ECM proteins prevails over their degradation and liver fibrosis results. Thus, we speculate that the relatively greater increase in TIMP-1 expression compared to MMP9 and MMP13 may tilt the balance towards net ECM deposition, ultimately leading to the formation of scar tissue. However, oltipraz also resulted in a significant decrease in hydroxyproline content in BDL mice (which reflects collagen content and the degree of fibrosis), possibly due to the two-fold induction in procollagen1 (I) and TIMP-1 and TIMP-2 transcriptions (Supplementary Fig. S2 and Fig. 4). While these effects should decrease the rate of collagen formation, it may be that 3 days of BDL were insufficient to fully develop collagen deposition and liver fibrosis. We were not able to study the effects of longer exposures to BDL and oltipraz because of increases in mice mortality.
Oltipraz is neither a potent nor typical Nrf2 activator and also has off-target effects, including activating AhR [36] and CAR [37]. Therefore, oltipraz's effects might not be directly due to Nrf2 activation. However, if AhR was involved, similar deleterious effects might occur since the AhR ligand 2, 3, 7, 8-tetrachorobenzo-p-dioxin also enhances cholestatic liver damage caused by BDL [38]. Paradoxically, oltipraz protects the liver from ANIT-induced cholestatic liver injury presumably by an Nrf2-independent mechanism as NqoI, the prototype Nrf2 target gene, was not induced [15]. In contrast, NqoI mRNA expression increased both in unoperated mice and further in BDL mice when treated with oltipraz (Supplementary Fig. S4). Thus, Nrf2 would seem to be relevant to the detrimental effects we observed. However, Yamamoto's group showed that Nrf2 protected BDL-induced liver injury in Keap1-knockout mice (Keap1 −/−), an animal model for Nrf2-activation, albeit only after 2 days BDL in mice [39]. Thus, the precise role of Nrf2 following BDL remains uncertain.
Oltipraz has been used to treat patients with schistosomiasis and residents of Qidong, China, exposed to dietary aflatoxin in phase ll oncologic clinical trials [8]. Marked photosensitivity was noted when oltipraz was used at high doses as an antischistosomal drug [9]. However, harmful effects have never been reported in obstructive cholestasis. Whether oltipraz would protect if given at a lower dose is uncertain. Lower doses of oltipraz were not used in our study since they have not had beneficial therapeutic effects in other studies [40-44]. Nevertheless, it is possible that oltipraz might be beneficial in non-obstructive causes of cholestasis, including hereditary transporter defects, reduced transporter expression in sepsis-induced cholestasis, or functional impairment of transporter activity by estrogen.
In summary, this study shows that oltipraz administration in a mouse model of bile-duct ligation exacerbates liver injury in part by increasing bile acid-independent bile flow as well as TGF- activation and signaling. Our findings suggest that oltipraz should be avoided as therapy for extrahepatic cholestatic disorders due to bile duct obstruction and prospective clinical studies should be aware of these adverse effects of oltipraz.
Supplementary Material
Acknowledgments
Financial support: This study was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grants R37 DK-25636 (to J.L.B.), the Yale Liver Center P30 DK-34989 (Yale Liver Center), and Mahidol University.
List of abbreviations in the order of appearance
- BDL
bile duct ligation
- MMP
matrix metalloproteinases
- TIMP
tissue inhibitors of metalloproteinase
- NqoI
NAD(P)H quinone oxidoreductase
- UDCA
ursodeoxycholic acid
- PBC
primary biliary cirrhosis
- GSH
glutathione multidrug resistance-associated protein
- BSEP
bile salt efflux pump
- LD50
lethal dosage
- CMC
carboxymethylcellulose
- EDTA
ethylenediaminetetraacetic acid
- CK19
cytokeratin 19
- ALT
alanine aminotransferase
- FXR
farnesoid X receptor
- ECM
extracellular matrix
- IL-1
interleukin-1 beta
- IL-6
interleukin-6
- TNF-
tumor necrosis factor-alpha
- TGF- 1
transforming growth factor-beta 1
- -SMA
alpha smooth muscle actin
- HSC
hepatic stellate cells
- PDGF
platelet-derived growth factor-beta
- BADF
bile acid-dependent bile flow
- BAIF
bile acid-independent bile flow
- PF
portal fibroblasts
- AhR
aryl hydrocarbon receptor
- CAR
constitutive androstane receptor
- ANIT
alpha-naphthylisothiocyanate
- Keap1
kelch-like ECH-associated protein 1
- OPZ
oltipraz
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: No conflict of interest
REFERENCES
- 1.Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Seminars in liver disease. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- 2.Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free radical biology & medicine. 1996;20:351–359. doi: 10.1016/0891-5849(96)02055-2. [DOI] [PubMed] [Google Scholar]
- 3.Sokol RJ, Devereaux M, Khandwala RA. Effect of dietary lipid and vitamin E on mitochondrial lipid peroxidation and hepatic injury in the bile duct-ligated rat. Journal of lipid research. 1991;32:1349–1357. [PubMed] [Google Scholar]
- 4.Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicology and applied pharmacology. 2010;244:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawata K, Kobayashi Y, Souda K, Kawamura K, Sumiyoshi S, Takahashi Y, et al. Enhanced hepatic Nrf2 activation after ursodeoxycholic acid treatment in patients with primary biliary cirrhosis. Antioxidants & redox signaling. 2010;13:259–268. doi: 10.1089/ars.2009.2903. [DOI] [PubMed] [Google Scholar]
- 6.Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. American journal of physiology Gastrointestinal and liver physiology. 2008;295:G735–747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- 7.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ, et al. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 8.Kensler TW, Qian GS, Chen JG, Groopman JD. Translational strategies for cancer prevention in liver. Nature reviews Cancer. 2003;3:321–329. doi: 10.1038/nrc1076. [DOI] [PubMed] [Google Scholar]
- 9.Helzlsouer KJ, Kensler TW. Cancer chemoprotection by oltipraz: experimental and clinical considerations. Preventive medicine. 1993;22:783–795. doi: 10.1006/pmed.1993.1072. [DOI] [PubMed] [Google Scholar]
- 10.Cho IJ, Sung DK, Kang KW, Kim SG. Oltipraz promotion of liver regeneration after partial hepatectomy: The role of PI3-kinase-dependent C/EBPbeta and cyclin E regulation. Archives of pharmacal research. 2009;32:625–635. doi: 10.1007/s12272-009-1419-3. [DOI] [PubMed] [Google Scholar]
- 11.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prochaska HJ, Yeh Y, Baron P, Polsky B. Oltipraz, an inhibitor of human immunodeficiency virus type 1 replication. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3953–3957. doi: 10.1073/pnas.90.9.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu W, Hellerbrand C, Kohler UA, Bugnon P, Kan YW, Werner S, et al. The Nrf2 transcription factor protects from toxin-induced liver injury and fibrosis. Laboratory investigation; a journal of technical methods and pathology. 2008;88:1068–1078. doi: 10.1038/labinvest.2008.75. [DOI] [PubMed] [Google Scholar]
- 14.Ansher SS, Dolan P, Bueding E. Chemoprotective effects of two dithiolthiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983;3:932–935. doi: 10.1002/hep.1840030608. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicological sciences : an official journal of the Society of Toxicology. 2009;108:247–257. doi: 10.1093/toxsci/kfp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson DR, Habeebu SS, Klaassen CD. Increase in bile flow and biliary excretion of glutathione-derived sulfhydryls in rats by drug-metabolizing enzyme inducers is mediated by multidrug resistance protein 2. Toxicological sciences : an official journal of the Society of Toxicology. 2002;66:16–26. doi: 10.1093/toxsci/66.1.16. [DOI] [PubMed] [Google Scholar]
- 17.Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression. Hepatology. 2009;50:1588–1596. doi: 10.1002/hep.23151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 19.Xu S, Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Aryl hydrocarbon receptor and NF-E2-related factor 2 are key regulators of human MRP4 expression. American journal of physiology Gastrointestinal and liver physiology. 2010;299:G126–135. doi: 10.1152/ajpgi.00522.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, et al. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, et al. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43:1013–1021. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 22.Soroka CJ, Ballatori N, Boyer JL. Organic solute transporter, OSTalpha-OSTbeta: its role in bile acid transport and cholestasis. Seminars in liver disease. 2010;30:178–185. doi: 10.1055/s-0030-1253226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, et al. OSTalpha-OSTbeta: a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Wang W, Soroka CJ, Mennone A, Harry K, Weinman EJ, et al. NHERF-1 binds to Mrp2 and regulates hepatic Mrp2 expression and function. The Journal of biological chemistry. 2010;285:19299–19307. doi: 10.1074/jbc.M109.096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 26.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 27.Ballatori N, Truong AT, Ma AK, Boyer JL. Determinants of glutathione efflux and biliary GSH/GSSG ratio in perfused rat liver. The American journal of physiology. 1989;256:G482–490. doi: 10.1152/ajpgi.1989.256.3.G482. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Friedman SL. Hepatic fibrogenesis. Seminars in liver disease. 2007;27:413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 29.Sinakos E, Lindor K. Treatment options for primary sclerosing cholangitis. Expert review of gastroenterology & hepatology. 2010;4:473–488. doi: 10.1586/egh.10.33. [DOI] [PubMed] [Google Scholar]
- 30.Bataller R, Brenner DA. Liver fibrosis. The Journal of clinical investigation. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moles A, Tarrats N, Fernandez-Checa JC, Mari M. Cathepsins B and D drive hepatic stellate cell proliferation and promote their fibrogenic potential. Hepatology. 2009;49:1297–1307. doi: 10.1002/hep.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuyama H, Shimahara Y, Kawada N, Seki S, Kristensen DB, Yoshizato K, et al. Regulation of cell growth by redox-mediated extracellular proteolysis of platelet-derived growth factor receptor beta. The Journal of biological chemistry. 2001;276:28274–28280. doi: 10.1074/jbc.M102995200. [DOI] [PubMed] [Google Scholar]
- 33.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Seminars in liver disease. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 34.Wells RG. Fibrogenesis. V. TGF-beta signaling pathways. American journal of physiology Gastrointestinal and liver physiology. 2000;279:G845–850. doi: 10.1152/ajpgi.2000.279.5.G845. [DOI] [PubMed] [Google Scholar]
- 35.Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. Journal of hepatology. 2007;46:955–975. doi: 10.1016/j.jhep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Le Ferrec E, Lagadic-Gossmann D, Rauch C, Bardiau C, Maheo K, Massiere F, et al. Transcriptional induction of CYP1A1 by oltipraz in human Caco-2 cells is aryl hydrocarbon receptor- and calcium-dependent. The Journal of biological chemistry. 2002;277:24780–24787. doi: 10.1074/jbc.M111319200. [DOI] [PubMed] [Google Scholar]
- 37.Merrell MD, Jackson JP, Augustine LM, Fisher CD, Slitt AL, Maher JM, et al. The Nrf2 activator oltipraz also activates the constitutive androstane receptor. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:1716–1721. doi: 10.1124/dmd.108.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozeki J, Uno S, Ogura M, Choi M, Maeda T, Sakurai K, et al. Aryl hydrocarbon receptor ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin enhances liver damage in bile duct-ligated mice. Toxicology. 2011;280:10–17. doi: 10.1016/j.tox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochemical and biophysical research communications. 2009;389:431–436. doi: 10.1016/j.bbrc.2009.08.156. [DOI] [PubMed] [Google Scholar]
- 40.Ansher SS, Dolan P, Bueding E. Biochemical effects of dithiolthiones. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1986;24:405–415. doi: 10.1016/0278-6915(86)90205-x. [DOI] [PubMed] [Google Scholar]
- 41.Clapper ML, Wood M, Leahy K, Lang D, Miknyoczki S, Ruggeri BA. Chemopreventive activity of Oltipraz against N-nitrosobis(2-oxopropyl)amine (BOP)-induced ductal pancreatic carcinoma development and effects on survival of Syrian golden hamsters. Carcinogenesis. 1995;16:2159–2165. doi: 10.1093/carcin/16.9.2159. [DOI] [PubMed] [Google Scholar]
- 42.Kwak MK, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, et al. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutation research. 2001:480–481. 305–315. doi: 10.1016/s0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 43.Ruggeri BA, Robinson C, Angeles T, Wilkinson Jt, Clapper ML. The chemopreventive agent oltipraz possesses potent antiangiogenic activity in vitro, ex vivo, and in vivo and inhibits tumor xenograft growth. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:267–274. [PubMed] [Google Scholar]
- 44.Wattenberg LW, Bueding E. Inhibitory effects of 5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione (Oltipraz) on carcinogenesis induced by benzo[a]pyrene, diethylnitrosamine and uracil mustard. Carcinogenesis. 1986;7:1379–1381. doi: 10.1093/carcin/7.8.1379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.