Abstract
Objectives
We conducted a workup of a previously published systematic review and aimed to analyse why most of the identified non-randomised controlled clinical trials with patient-reported outcomes did not match a set of basic quality criteria.
Setting
There were no limits on the level of care and the geographical location.
Participants
The review evaluated permanent interstitial low-dose rate brachytherapy in patients with localised prostate cancer and compared that intervention with alternative procedures such as external beam radiotherapy, radical prostatectomy and no primary therapy.
Primary outcome measure
Fulfilment of basic inclusion criteria according to a Participants, Interventions, Comparisons, Outcomes (PICO) framework and accomplishment of requirements to contain superimposed risk of bias.
Results
We found that 21 of 50 excluded non-randomised controlled trials did not meet the PICO inclusion criteria. The remaining 29 studies showed a lack in the quality of reporting. The resulting flaws included attrition bias due to loss of follow-up, lack of reporting baseline data, potential confounding due to unadjusted data and lack of statistical comparison between groups.
Conclusions
With respect to the reporting of patient-reported outcomes, active efforts are required to improve the quality of reporting in non-randomised controlled trials concerning permanent interstitial low-dose rate brachytherapy in patients with localised prostate cancer.
Strengths and limitations of this study.
We conducted a comprehensive literature search and strictly adhered to the projected methodology.
We identified a lack of quality in non-randomised controlled clinical trials reporting patient-reported outcomes, analysed the cause and suggested possible improvements in designing studies in the future.
The analysis is confined to a single disease and a specific treatment and conclusions drawn from its results may not be generalisable to other diseases and treatments.
The limits for the inclusion of studies are arbitrarily set.
Introduction
The present paper reports a workup of a previously published systematic review.1 It may be regarded as a methodological supplement adding information on a subset of excluded studies. We have compared permanent interstitial low-dose rate brachytherapy, with radical prostatectomy, external beam radiotherapy and ‘no primary therapy’ in patients with localised prostate cancer categorised as T1 to T2. We used the term ‘no primary therapy’ to accommodate different types of observation including active surveillance, watchful waiting and observing without a distinctive management. As a result, we included one randomised controlled trial (RCT) and 30 non-randomised controlled clinical trials (CCT). The primary outcome was overall survival. The secondary outcomes were clinically defined disease-free survival, biochemical recurrence-free survival, physician-reported severe adverse events and patient-reported outcomes (PROs) such as function and bother scores as well as generic and disease-related health-related quality of life. We concluded that the current evidence is insufficient to allow a definitive conclusion about overall survival. Radical prostatectomy and external beam radiotherapy can severely affect the structural integrity of neighbouring organs and their functions and can also cause considerable long-term impairment of health-related quality of life. With a view of expecting similar survival but a tremendous difference of adverse events between treatment alternatives, valid data on health-related quality of life could tip the balance. At the least, we assume that shared decision-making and consideration of patients’ preferences in searching for the best individual treatment would rely on information on the health-related quality-of-life data. Of the 30 included non-randomised studies, 13 studies reported PROs, that is, only the patients provided the information.2 During the study selection process, we experienced that we excluded another 50 non-randomised PRO studies. We found it a pity that we could not use the data. We had the impression that a considerable number of studies were excluded because of a lack in the quality of reporting. Therefore, we wanted to summarise the reasons for excluding those PRO studies and make the authors of PRO studies aware of some basic requirements for reporting of comparative PRO data to achieve higher acceptance in the scientific community. The importance of reporting PRO has been addressed by the Consolidated Standards of Reporting Trials (CONSORT) group3 which recently published a PRO extension to their acclaimed previous statement.4 It may be wise to build a PRO extension to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement5 that addresses specific issues of observational studies.
The first aim of this study was to assess whether the excluded studies met the basic inclusion criteria using the PICO framework. The second aim was to ensure whether the excluded studies met the requirements to contain high risk of bias.
Materials and methods
Study inclusion criteria
We defined the inclusion criteria according to the PICO framework that should include four essential constituents, that is, the type of participants (P), intervention (I), comparator (C) and outcome (O).6 The four PICO items can be supplemented by timing (T) and setting (S), two other important features of a systematic review, to create the so-called PICOTS typology.7 A further extension embraces the study design (SD) to complete all major items of a search strategy (PICOTS-SD).8
Population
Initial and present publication
Localised prostate cancer is defined by the categories T1 to T2 of the tumour-node-metastasis staging system9 if combined with the absence of regional lymph node metastasis and distant metastasis.
Intervention
Initial and present publication
Brachytherapy10 is short-distance radiotherapy placing radiation sources with different duration and rates of dose delivery in or near tumours.11 Permanent interstitial low-dose rate brachytherapy means implanting of low-energy radioactive sources emitting radiation, which are contained in titanium pellets of the size of rice grains called seeds.12
Comparator
Initial and present publication
The European Association of Urology suggested three different treatment concepts for localised prostate cancer in addition to permanent interstitial low-dose rate brachytherapy10: radical prostatectomy, external beam radiotherapy and different types of observation including active surveillance, watchful waiting and observing without distinctive management.
Outcome
Initial publication
Overall survival, cancer-specific survival, disease-free survival, biochemical recurrence-free survival, severe adverse events and PROs. PROs comprised function and bother scores as well as generic and disease-related health-related quality of life.
Present publication
Fulfilment of basic inclusion criteria according to a PICO framework by the excluded CCT. Accomplishment of requirements to contain superimposed risk of bias in addition to the high risk of bias caused by the lack of randomisation framework by the excluded CCT.
Timing
Initial and present publication
We did not set limits on the length of the observation period.
Setting
Initial and present publication
We did not set limits on the setting such as type of country, year of recruitment or level of healthcare.
Study design
Initial publication
We included RCT and CCT evaluating permanent interstitial low-dose rate brachytherapy as monotherapy in patients with localised prostate cancer. The proportion of relevant patients was required to be at least 80% of the study population and the response rate of questionnaires was expected to be at least 70%. For CCT to be included, comparable baseline characteristics between treatment groups or adjustment for imbalances of these data were required. Limits on year of publication or language were not applied.
Present publication
We included specifically the CCT that were excluded in the initial publication.
Search strategy
The search strategy was reported previously.1
Study selection
In the present study, we selected only those 50 non-randomised studies on PRO that were excluded from the evaluation in the initial publication. In the study selection process, two reviewers independently judged whether a study was included or excluded. Differences were resolved by discussion without the need for a third opinion.
Data collection and analysis
The reasons for exclusion were extracted independently by two reviewers. We sought for the following data: the inclusion criteria using the PICO framework, the proportion of response of participants to questionnaires, which was required to be at least 70%, the reporting of separate baseline characteristics for each treatment group, the reporting of comparable baseline characteristics or adjustment for imbalances of these data such as the use of a Cox proportional hazard model and the reporting of statistics comparing treatment groups. Sufficient comparability was defined as a difference between baseline values that were not statistically significant. If a statistical test was not reported, we assumed two comparable values if the greater of the two values was less than 10% above the smaller one. We also required that authors reported effect measures and statistics testing the difference between treatment groups, for example, p values or effect measures including 95% CIs. Reporting of within group comparisons or before-and-after analyses was not deemed sufficient for inclusion. We did not apply a principal summary measure as we aimed to synthesise the information in a qualitative way.
Assessment of risk of bias and quality of reporting
Two reviewers independently assessed the quality of reporting of CCT according to the criteria specified in the previous paragraph. We did not specifically assess the risk of bias because we decided to exclude all papers with regard to a lack of reporting essential data.
Results
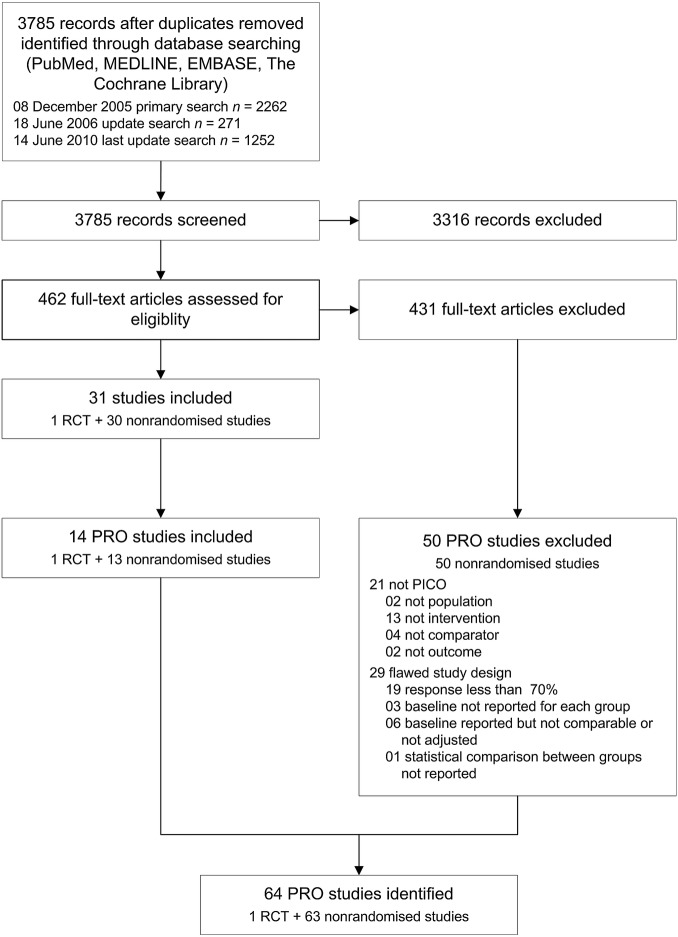
Of a total of 462 full-text articles assessed for eligibility in the previously published systematic review, 31 studies were included and 431 studies were excluded. Among the 431 excluded articles, we identified 50 non-randomised studies that were reporting on PRO (figure 1). We evaluated the reasons for exclusion of those 50 studies and documented the results in table 1. In 42% (21 of 50) studies, the essential PICO framework was simply not met. In the majority of 58% (29 of 50) studies, the predefined requirement to apply measures to contain high risk of bias was not met. Of these 29 studies, 19 reported a proportion of patients responding to questionnaires of less than 70% or did not address this item. Baseline characteristics were not presented for treatment groups in three studies. In another six studies, baseline characteristics were not comparable between treatment groups or there was no confounder control in the analysis adjusting for important different factors such as mean age. The statistical comparison between treatment groups was deemed not appropriate in one study.
Figure 1.
Study flow. PICO: population, intervention, comparator, outcome; PRO: patient-reported outcomes; RCT: randomised controlled trial.
Table 1.
Reasons for excluding PRO articles
| Non-randomised studies | Inclusion criteria |
Requirements to contain high risk of bias |
Comments | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P | I | C | O | Response ≥70% | Baseline each group | Baseline comparable/or adjusted | Statistical comparison between groups | ||
| Bacon et al29 | Yes | Yes | No | – | – | – | – | – | No concurrent group |
| Ball et al30 | Yes | Yes | No | – | – | – | – | – | Cryotherapy |
| Befort et al31 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Bergman et al32 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No appropriate test |
| Bergman et al33 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Brandeis et al34 | Yes | No | – | – | – | – | – | – | 29% LDR-BT+EBRT |
| Brown et al35 | Yes | No | – | – | – | – | – | – | EBRT |
| Burnett et al36 | Yes | Yes | Yes | Yes | No | – | – | – | Response not reported |
| Chaikin et al37 | No | – | – | – | – | – | – | – | Staging not reported |
| Chen et al38 | Yes | Yes | Yes | Yes | Yes | Yes | No | – | No confounder control |
| Choo et al39 | Yes | Yes | Yes | Yes | Yes | No | – | – | Baseline not reported |
| Clark et al40 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Downs et al41 | Yes | Yes | Yes | Yes | Yes | Yes | No | – | No confounder control |
| Eton et al42 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Frank et al43 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Fulmer et al44 | Yes | Yes | Yes | Yes | No | – | – | – | Response not reported |
| Gore et al45 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Guedea et al46 | Yes | Yes | Yes | Yes | Yes | Yes | No | – | No confounder control |
| Hashine et al47 | Yes | Yes | Yes | Yes | Yes | Yes | No | – | No confounder control |
| Hashine et al48 | Yes | Yes | Yes | Yes | Yes | Yes | No | – | No confounder control |
| Hervouet et al49 | No | – | – | – | – | – | – | – | ≥20% T3–T4 in control groups |
| Hollenbeck et al50 | Yes | No | – | – | – | – | – | – | LDR-BT+EBRT |
| Jo et al51 | Yes | No | – | – | – | – | – | – | High-dose rate brachytherapy |
| Johnstone et al52 | Yes | No | – | – | – | – | – | – | EBRT |
| Joly et al53 | Yes | No | – | – | – | – | – | – | LDR-BT+EBRT |
| Kakehi et al54 | Yes | Yes | Yes | Yes | Yes | No | – | – | Baseline not reported |
| Lev et al55 | Yes | No | – | – | – | – | – | – | LDR-BT+EBRT |
| Lilleby et al56 | Yes | No | – | – | – | – | – | – | EBRT |
| Litwin et al57 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Litwin et al58 | Yes | No | – | – | – | – | – | – | 25% LDR-BT+EBRT |
| Mehta et al59 | Yes | Yes | Yes | No | – | – | – | – | “Fear of cancer”* |
| Miller et al60 | Yes | No | – | – | – | – | – | – | 44% LDR-BT+EBRT |
| Miller et al61 | Yes | Yes | Yes | Yes | Yes | No | – | – | Baseline not reported |
| Monahan et al62 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Namiki et al63 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Namiki et al64 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Ohashi et al65 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Pinkawa et al66 | Yes | Yes | No | – | – | – | – | – | LDR-BT+hormones† |
| Roach et al67 | Yes | No | – | – | – | – | – | – | EBRT, single-arm trial |
| Sanda et al68 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Schover et al69 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Soderdahl et al70 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Speight et al71 | Yes | Yes | Yes | Yes | No | – | – | – | Response not reported |
| Stone et al72 | Yes | Yes | No | – | – | – | – | – | LDR-BT+hormones† |
| Trojan et al73 | Yes | Yes | Yes | Yes | No | – | – | – | Low response |
| Tward et al74 | Yes | Yes | Yes | No | – | – | – | – | Mortality differs§ |
| Valicenti et al75 | Yes | Yes | Yes | Yes | No | – | – | – | Response not reported |
| Van de Poll- Franse et al76 | Yes | No | – | – | – | – | – | – | LDR-BT+EBRT |
| Wyler et al77 | Yes | Yes | Yes | Yes | Yes | Yes | No | – | No confounder control |
| Zagar et al78 | Yes | No | – | – | – | – | – | – | LDR-BT+EBRT |
| ‘NO’ counts | 2 | 13 | 4 | 2 | 19 | 3 | 6 | 1 | Total: 50 studies |
| PICO not met: 21 | High risk of bias: 29 | ||||||||
–: not appropriate.
*Mehta et al59: no appropriate endpoint.
§Tward et al74: non-disease-related mortality differs greatly.C, comparison of interest is radical prostatectomy, external beam radiotherapy, or no primary therapy; EBRT, external beam radiotherapy; I, intervention of interest is low-dose rate brachytherapy as monotherapy; LDR-BT, permanent interstitial low-dose rate brachytherapy; O, outcome of interest is function, bother, or generic health-related quality of life; P, patients with localised prostate cancer; PRO, patient-reported outcomes.
Discussion
Main results
In summary, we found that roughly 4 of 10 excluded PRO studies did not meet the essential inclusion criteria using the PICO framework. This result is consistent with the problem of information retrieval aiming at a high recall and ending up with a low precision. The papers were obviously not relevant to the research question and we did not further examine the quality of reporting. We also found that roughly 6 of 10 excluded PRO studies met the PICO framework but did not provide the predefined requirements to care sufficiently enough for a low response of patients to questionnaires, for reporting baseline characteristics between treatment groups, for adjusting differences in those baseline characteristics between treatment groups and to use appropriate statistics to compare the outcome between treatment groups.
Quality of reporting of PROs
We identified a lack in the quality of reporting in many excluded CCT and wish to stress the importance of considering a series of requirements while conducting a study on PRO. Other authors have reported recently that, concerning disease-specific mortality or disease-free survival, the available studies did not show significant differences between treatment groups.13 14 In the view of unknown or small differences in survival measures, the results of PRO studies could have a noticeable impact on medical decision-making.15 16 None of the 50 excluded studies reported a non-responder analysis, although it is known that non-responders may have different attitudes than responders. Etter and Perneger17 concluded that low response rates may be associated with overestimating an effect and that the strength and direction of a non-response bias may depend on the mechanism of non-response. Therefore, results may be confounded if the proportion of included data not available for analysis such as data from non-responders or due to loss to follow-up is considerable. We believe that a value of 30% or more can be denoted as considerable. Lowering this threshold, for example, to 20%, would have resulted in less included studies. However, others suggested that 20% or more loss would be sufficient for a high risk of bias threatening the validity of results.18 Concerning questionnaires, we recommend taking measures that are known to improve response rates.19 20 Edwards21 conducted a systematic review to identify effective strategies to increase the response to postal and electronic questionnaires. The authors found several strategies to increase the response, for example, prenotification, follow-up contact, shorter questionnaires, mentioning an obligation to respond, university sponsorship, non-monetary incentives, a statement that others had responded, an offer of survey results, giving a deadline. We did not use a strict algorithm to differentiate between comparable and not comparable baseline values between treatment groups. A statistically significant difference was judged as not comparable. Non-significant differences were also regarded as not comparable if the difference was at least 10% of the lower of two values. Using this approach we tried to reduce subjective decisions. We are not aware of published strict algorithms in this matter.
High risk of bias inherent in non-RCTs
With a view to include only one RCT, the initial publication was based almost exclusively on CCT. However, the lack of randomisation poses a very large challenge to the authors who are advised to deal with essential problems such as selection bias and confounding. Otherwise, the findings may not be valid and of limited usefulness and the many efforts may be in vain. We wish to stress that the non-randomised design is associated with a high risk of bias because known and unknown characteristics may be distributed unequally between groups.22 Certain study characteristics, such as prospective design, concurrent control group, adjustment of results with respect to different baseline values and confounder control, can limit additional bias. For example, Ioannidis et al23 reported that discrepancies between RCT and CCT were less common when only CCT with a prospective design were considered. The Cochrane Collaboration offers a guide for inclusion of non-randomised studies24 and it has developed a tool for assessing the risk of bias in RCT and CCT.25 Guidelines for reporting observational studies have been published to improve their quality.5 Cox regression analysis, propensity-score-based analysis and instrumental variable analysis are methods that have been used for correction of confounding bias in non-randomised studies.26 Different values of various outcome measures between groups may be simply caused by different baseline data in lieu of absent significant treatment effects. We accepted any type of method adjusting or stratifying for one or more known differences in baseline characteristics. Nevertheless, it should be kept in mind that methods of adjustment do not guarantee removal of bias and that residual confounding may remain high.22 Concerning the non-randomised design, we strongly recommend the use of methods for adjusting the results for confounders to aim for a less-biased estimation of the treatment effect27 and the adoption of guidelines for the reporting of observational studies.5
Strengths and limitations
The strengths of the present study are a comprehensive literature search, strict adherence to the projected methodology, the identification of a lack of quality in PRO studies and addressing the specific problems of PRO studies. We should consider some limitations: the study is confined to a single disease, so conclusions drawn from its results may not be generalisable to other diseases. The arbitrary limits set for inclusion of studies are responsible for the extent of excluded studies. These limits may be questioned by other investigators. During the re-evaluation of study quality, we found that one study fulfilled all criteria, although, this study was excluded in previous reports.28 The minimum follow-up of 70% for inclusion was set arbitrarily and others might find this threshold too low. We did not endorse the recently published reporting of PRO in randomised trials, an extension of the CONSORT statement.4 All included studies in the present review are non-randomised. We think that the lack of randomisation is the prevailing issue. We did not endorse the CONSORT PRO extension for another reason. The included studies were published many years before this extension was published. There might be a need to develop an extension of the STROBE statement5 with the aim of improving the reporting of PRO in non-randomised studies. This extension could emphasise the specific challenges of reporting PRO with respect to lack of randomisation.
Conclusions
We found that a considerable number of non-randomised controlled reporting PROs were excluded from a systematic review because of a lack of predefined reporting requirements. The assumed overall risk of bias was regarded as too high to consider the data of these studies for inclusion in the systematic review. With respect to the reporting of PROs, active efforts are required to improve the quality of reporting in non-randomised controlled trials and to increase the number of randomised controlled trials.
Supplementary Material
Footnotes
Contributors: FP conceived, designed and performed the experiments. FP and MP analysed the data. FP, AML, CT and MP wrote the manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Peinemann F, Grouven U, Bartel C, et al. Permanent interstitial low-dose rate brachytherapy for patients with localized prostate cancer—a systematic review of randomized and non-randomized controlled clinical trials. Eur Urol 2011;60:881–93 [DOI] [PubMed] [Google Scholar]
- 2.Patrick D, Guyatt GH, Acquadro C. Chapter 17: Patient-reported outcomes. In: Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions Version 510 [updated March 2011]. Chichester: The Cochrane Collaboration, 2011. www.cochrane-handbook.org [Google Scholar]
- 3.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 2010;7:e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvert M, Blazeby J, Altman DG, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814–22 [DOI] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007;4:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Connor D, Green S, Higgins JPT. Chapter 5: Defining the review question and developing criteria for including studies. In: Higgins JPT, Green S. eds. Cochrane handbook for systematic reviews of interventions Version 510 [updated March 2011] Chichester: The Cochrane Collaboration, 2011. www.cochrane-handbook.org [Google Scholar]
- 7.Chang SM, Matchar DB, Smetana GW, Umscheid, CA. eds. Methods guide for medical test reviews. Rockville: Agency for Healthcare Research and Quality; 2012, AHRQ Publication No: 12-EC017 [PubMed] [Google Scholar]
- 8.White CM, Ip S, McPheeters M, et al. Using existing systematic reviews to replace de novo processes in conducting comparative effectiveness reviews. Methods guide for comparative effectiveness reviews. Rockville: Agency for Healthcare Research and Quality, 2009 [PubMed] [Google Scholar]
- 9.Ebele JN, Sauter G, Epstein JI, et al. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press, 2004 [Google Scholar]
- 10.Heidenreich A, Bolla M, Joniau S, et al. Guidelines on prostate cancer. Arnhem: European Association of Urology, 2011 [Google Scholar]
- 11.Thompson I, Thrasher JB, Aus G, et al. Prostate cancer. Guideline for the management of clinically localized prostate cancer: 2007 update. Linthicum: American Urological Association, 2007 [Google Scholar]
- 12.Nath R. Overview of brachytherapy physics. In: Thomadsen BR, Rivard MR, Butler W. eds. Brachytherapy physics. 2nd edn. Madison: Medical Physics Publishing, 2005 [Google Scholar]
- 13.Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med 2008;148:435–48 [DOI] [PubMed] [Google Scholar]
- 14.Koukourakis G, Kelekis N, Armonis V, et al. Brachytherapy for prostate cancer: a systematic review. Adv Urol 2009;2009:327945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal SA, Bittner NH, Beyer DC, et al. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys 2011;79:335–41 [DOI] [PubMed] [Google Scholar]
- 16.Crook JM, Gomez-Iturriaga A, Wallace K, et al. Comparison of health-related quality of life 5 years after SPIRIT (Surgical Prostatectomy [RP] versus Interstitial Radiation [BT] Intervention trial ACOSOG Z0070). Int J Radiat Oncol Biol Phys 2010;78(3 Suppl 1):S76. [DOI] [PubMed] [Google Scholar]
- 17.Etter JF, Perneger TV. Analysis of non-response bias in a mailed health survey. J Clin Epidemiol 1997;50:1123–8 [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359:781–5 [DOI] [PubMed] [Google Scholar]
- 19.Smeeth L, Fletcher AE. Improving the response rates to questionnaires. BMJ 2002;324:1168–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brealey SD, Atwell C, Bryan S, et al. Improving response rates using a monetary incentive for patient completion of questionnaires: an observational study. BMC Med Res Methodol 2007;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;(3):MR000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:iii–x, 1–173 [DOI] [PubMed] [Google Scholar]
- 23.Ioannidis JP, Haidich AB, Pappa M, et al. Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001;286:821–30 [DOI] [PubMed] [Google Scholar]
- 24.Reeves BC, Deeks JJ, Higgins JPT, et al. Chapter 13: Including non-randomized studies. In: Higgins JPT, Green S. eds. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011] Chichester: The Cochrane Collaboration, 2011. www.cochrane-handbook.org [Google Scholar]
- 25.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S. eds. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011] Oxford: The Cochrane Collaboration, 2011. www.cochrane-handbook.org [Google Scholar]
- 26.Schmoor C, Gall C, Stampf S, et al. Correction of confounding bias in non-randomized studies by appropriate weighting. Biom J 2011;53:369–87 [DOI] [PubMed] [Google Scholar]
- 27.Cox E, Martin BC, Van Staa T, et al. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report—Part II. Value Health 2009;12:1053–61 [DOI] [PubMed] [Google Scholar]
- 28.Pinkawa M, Asadpour B, Piroth MD, et al. Health-related quality of life after permanent I-125 brachytherapy and conformal external beam radiotherapy for prostate cancer: a matched-pair comparison. Radiother Oncol 2009;91:225–31 [DOI] [PubMed] [Google Scholar]
- 29.Bacon CG, Giovannucci E, Testa M, et al. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol 2001;166:1804–10 [PubMed] [Google Scholar]
- 30.Ball AJ, Gambill B, Fabrizio MD, et al. Prospective longitudinal comparative study of early health-related quality-of-life outcomes in patients undergoing surgical treatment for localized prostate cancer: a short-term evaluation of five approaches from a single institution. J Endourol 2006;20:723–31 [DOI] [PubMed] [Google Scholar]
- 31.Befort CA, Zelefsky MJ, Scardino PT, et al. A measure of health-related quality of life among patients with localized prostate cancer: results from ongoing scale development. Clin Prostate Cancer 2005;4:100–8 [DOI] [PubMed] [Google Scholar]
- 32.Bergman J, Gore JL, Penson DF, et al. Erectile aid use by men treated for localized prostate cancer. J Urol 2009;182:649–54 [DOI] [PubMed] [Google Scholar]
- 33.Bergman J, Kwan L, Litwin MS. Improving decisions for men with prostate cancer: translational outcomes research. J Urol 2010;183:2186–92 [DOI] [PubMed] [Google Scholar]
- 34.Brandeis JM, Litwin MS, Burnison CM, et al. Quality of life outcomes after brachytherapy for early stage prostate cancer. J Urol 2000;163:851–7 [PubMed] [Google Scholar]
- 35.Brown MW, Brooks JP, Albert PS, et al. An analysis of erectile function after intensity modulated radiation therapy for localized prostate carcinoma. Prostate Cancer Prostatic Dis 2007;10:189–93 [DOI] [PubMed] [Google Scholar]
- 36.Burnett AL, Aus G, Canby-Hagino ED, et al. Erectile function outcome reporting after clinically localized prostate cancer treatment. J Urol 2007;178:597–601 [DOI] [PubMed] [Google Scholar]
- 37.Chaikin DC, Broderick GA, Malloy TR, et al. Erectile dysfunction following minimally invasive treatments for prostate cancer. Urology 1996;48:100–4 [DOI] [PubMed] [Google Scholar]
- 38.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol 2009;27:3916–22 [DOI] [PubMed] [Google Scholar]
- 39.Choo R, Long J, Gray R, et al. Prospective survey of sexual function among patients with clinically localized prostate cancer referred for definitive radiotherapy and the impact of radiotherapy on sexual function. Support Care Cancer 2010;18:715–22 [DOI] [PubMed] [Google Scholar]
- 40.Clark JA, Inui TS, Silliman RA, et al. Patients’ perceptions of quality of life after treatment for early prostate cancer. J Clin Oncol 2003;21:3777–84 [DOI] [PubMed] [Google Scholar]
- 41.Downs TM, Sadetsky N, Pasta DJ, et al. Health related quality of life patterns in patients treated with interstitial prostate brachytherapy for localized prostate cancer—data from CaPSURE. J Urol 2003;170:1822–7 [DOI] [PubMed] [Google Scholar]
- 42.Eton DT, Lepore SJ, Helgeson VS. Early quality of life in patients with localized prostate carcinoma: an examination of treatment-related, demographic, and psychosocial factors. Cancer 2001;92:1451–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank SJ, Pisters LL, Davis J, et al. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol 2007;177:2151–6 [DOI] [PubMed] [Google Scholar]
- 44.Fulmer BR, Bissonette EA, Petroni GR, et al. Prospective assessment of voiding and sexual function after treatment for localized prostate carcinoma: comparison of radical prostatectomy to hormonobrachytherapy with and without external beam radiotherapy. Cancer 2001;91:2046–55 [DOI] [PubMed] [Google Scholar]
- 45.Gore JL, Kwan L, Lee SP, et al. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst 2009;101:888–92 [DOI] [PubMed] [Google Scholar]
- 46.Guedea F, Ferrer M, Pera J, et al. Quality of life two years after radical prostatectomy, prostate brachytherapy or external beam radiotherapy for clinically localised prostate cancer: the Catalan Institute of Oncology/Bellvitge Hospital experience. Clin Transl Oncol 2009;11:470–8 [DOI] [PubMed] [Google Scholar]
- 47.Hashine K, Kusuhara Y, Miura N, et al. A prospective longitudinal study comparing a radical retropubic prostatectomy and permanent prostate brachytherapy regarding the health-related quality of life for localized prostate cancer. Jpn J Clin Oncol 2008;38:480–5 [DOI] [PubMed] [Google Scholar]
- 48.Hashine K, Kusuhara Y, Miura N, et al. Health-related quality of life using SF-8 and EPIC questionnaires after treatment with radical retropubic prostatectomy and permanent prostate brachytherapy. Jpn J Clin Oncol 2009;39:502–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hervouet S, Savard J, Simard S, et al. Psychological functioning associated with prostate cancer: cross-sectional comparison of patients treated with radiotherapy, brachytherapy, or surgery. J Pain Symptom Manage 2005;30:474–84 [DOI] [PubMed] [Google Scholar]
- 50.Hollenbeck BK, Dunn RL, Wei JT, et al. Neoadjuvant hormonal therapy and older age are associated with adverse sexual health-related quality-of-life outcome after prostate brachytherapy. Urology 2002;59:480–4 [DOI] [PubMed] [Google Scholar]
- 51.Jo Y, Junichi H, Tomohiro F, et al. Radical prostatectomy versus high-dose rate brachytherapy for prostate cancer: effects on health-related quality of life. BJU Int 2005;96:43–7 [DOI] [PubMed] [Google Scholar]
- 52.Johnstone PA, Gray C, Powell CR. Quality of life in T1–3N0 prostate cancer patients treated with radiation therapy with minimum 10-year follow-up. Int J Radiat Oncol Biol Phys 2000;46:833–8 [DOI] [PubMed] [Google Scholar]
- 53.Joly F, Brune D, Couette JE, et al. Health-related quality of life and sequelae in patients treated with brachytherapy and external beam irradiation for localized prostate cancer. Ann Oncol 1998;9:751–7 [DOI] [PubMed] [Google Scholar]
- 54.Kakehi Y, Takegami M, Suzukamo Y, et al. Health related quality of life in Japanese men with localized prostate cancer treated with current multiple modalities assessed by a newly developed Japanese version of the Expanded Prostate Cancer Index Composite. J Urol 2007;177:1856–61 [DOI] [PubMed] [Google Scholar]
- 55.Lev EL, Eller LS, Gejerman G, et al. Quality of life of men treated for localized prostate cancer: outcomes at 6 and 12 months. Support Care Cancer 2009;17:509–17 [DOI] [PubMed] [Google Scholar]
- 56.Lilleby W, Fossa SD, Waehre HR, et al. Long-term morbidity and quality of life in patients with localized prostate cancer undergoing definitive radiotherapy or radical prostatectomy. Int J Radiat Oncol Biol Phys 1999;43:735–43 [DOI] [PubMed] [Google Scholar]
- 57.Litwin MS, Sadetsky N, Pasta DJ, et al. Bowel function and bother after treatment for early stage prostate cancer: a longitudinal quality of life analysis from CaPSURE. J Urol 2004;172:515–19 [DOI] [PubMed] [Google Scholar]
- 58.Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer 2007;109:2239–47 [DOI] [PubMed] [Google Scholar]
- 59.Mehta SS, Lubeck DP, Pasta DJ, et al. Fear of cancer recurrence in patients undergoing definitive treatment for prostate cancer: results from CaPSURE. J Urol 2003;170:1931–3 [DOI] [PubMed] [Google Scholar]
- 60.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol 2005;23:2772–80 [DOI] [PubMed] [Google Scholar]
- 61.Miller DC, Wei JT, Dunn RL, et al. Use of medications or devices for erectile dysfunction among long-term prostate cancer treatment survivors: potential influence of sexual motivation and/or indifference. Urology 2006;68:166–71 [DOI] [PubMed] [Google Scholar]
- 62.Monahan PO, Champion V, Rawl S, et al. What contributes more strongly to predicting QOL during 1-year recovery from treatment for clinically localized prostate cancer: 4-weeks-post-treatment depressive symptoms or type of treatment? Qual Life Res 2007;16:399–411 [DOI] [PubMed] [Google Scholar]
- 63.Namiki S, Satoh T, Baba S, et al. Quality of life after brachytherapy or radical prostatectomy for localized prostate cancer: a prospective longitudinal study. Urology 2006;68:1230–6 [DOI] [PubMed] [Google Scholar]
- 64.Namiki S, Kwan L, Kagawa-Singer M, et al. Distress and social dysfunction following prostate cancer treatment: a longitudinal cross-cultural comparison of Japanese and American men. Prostate Cancer Prostatic Dis 2009;12:67–71 [DOI] [PubMed] [Google Scholar]
- 65.Ohashi T, Yorozu A, Toya K, et al. Serial changes of international prostate symptom score following I-125 prostate brachytherapy. Int J Clin Oncol 2006;11:320–5 [DOI] [PubMed] [Google Scholar]
- 66.Pinkawa M, Fischedick K, Gagel B, et al. Association of neoadjuvant hormonal therapy with adverse health-related quality of life after permanent iodine-125 brachytherapy for localized prostate cancer. Urology 2006;68:104–9 [DOI] [PubMed] [Google Scholar]
- 67.Roach M, III, Chinn DM, Holland J, et al. A pilot survey of sexual function and quality of life following 3D conformal radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 1996;35:869–74 [DOI] [PubMed] [Google Scholar]
- 68.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med 2008;358:1250–61 [DOI] [PubMed] [Google Scholar]
- 69.Schover LR, Fouladi RT, Warneke CL, et al. Defining sexual outcomes after treatment for localized prostate carcinoma. Cancer 2002;95:1773–85 [DOI] [PubMed] [Google Scholar]
- 70.Soderdahl DW, Davis JW, Schellhammer PF, et al. Prospective longitudinal comparative study of health-related quality of life in patients undergoing invasive treatments for localized prostate cancer. J Endourol 2005;19:318–26 [DOI] [PubMed] [Google Scholar]
- 71.Speight JL, Elkin EP, Pasta DJ, et al. Longitudinal assessment of changes in sexual function and bother in patients treated with external beam radiotherapy or brachytherapy, with and without neoadjuvant androgen ablation: data from CaPSURE. Int J Radiat Oncol Biol Phys 2004;60:1066–75 [DOI] [PubMed] [Google Scholar]
- 72.Stone NN, Marshall DT, Stone JJ, et al. Does neoadjuvant hormonal therapy improve urinary function when given to men with large prostates undergoing prostate brachytherapy? J Urol 2010;183:634–9 [DOI] [PubMed] [Google Scholar]
- 73.Trojan L, Harrer K, Schafer J, et al. [Complications and side effects of low dose rate brachytherapy for the treatment of prostate cancer: data on a 13year follow-up study from Mannheim]. Urologe A 2007;46:1542–7 [DOI] [PubMed] [Google Scholar]
- 74.Tward JD, Lee CM, Pappas LM, et al. Survival of men with clinically localized prostate cancer treated with prostatectomy, brachytherapy, or no definitive treatment: impact of age at diagnosis. Cancer 2006;107:2392–400 [DOI] [PubMed] [Google Scholar]
- 75.Valicenti RK, Bissondtte EA, Chen C, et al. Longitudinal comparison of sexual function after 3-dimensional conformal radiation therapy or prostate brachytherapy. J Urol 2002;168:2499–504 [DOI] [PubMed] [Google Scholar]
- 76.Van de Poll-Franse LV, Sadetsky N, Kwan L, et al. Severity of cardiovascular disease and health-related quality of life in men with prostate cancer: a longitudinal analysis from CaPSURE. Qual Life Res 2008;17:845–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wyler SF, Engeler DS, Seelentag W, et al. Health-related quality of life after radical prostatectomy and low-dose-rate brachytherapy for localized prostate cancer. Urol Int 2009;82:17–23 [DOI] [PubMed] [Google Scholar]
- 78.Zagar TM, Stock RG, Cesaretti JA, et al. Assessment of postbrachytherapy sexual function: a comparison of the IIEF-5 and the MSEFS. Brachytherapy 2007;6:26–33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.