Abstract
This study used a stress biomarker, diurnal cortisol, to identify how elevated stress in mothers of children and adults with autism and other disabilities relates to their health and mental health. Based on semi-parametric, group-based trajectory analysis of 91 mothers, two distinctive cortisol trajectories emerged: blunted (63%) or steep (37%). Mothers in the blunted (versus steep) trajectory had higher stress levels, lower health ratings, and 89% of mothers of children with autism, and 53% with other disabilities, belonged to this trajectory. Atypical cortisol awakening responses and evening rises were differentially associated with anxiety, depression, health problems and employment status. Stress-reducing interventions are needed for parents of children with autism and other disabilities that include biomarkers as indices of risk or treatment outcome.
Keywords: diurnal cortisol, mothers of children with disabilities, health, mental health
Compared to parents of typically developing children, parents of children with developmental disabilities are at higher risk for elevated stress over the lifespan of their child. Many studies describe elevated stress in mothers of offspring with autism spectrum disorders (ASDs), Down syndrome and other disabilities (see Hodapp & Li, 2005, for a review), and attribute this stress to multiple, interacting factors. These include: distress upon learning of the child’s disability; family routines that center on the child’s medical or therapeutic needs; more restricted social or employment opportunities; financial burden due to specialized therapies or care; possible stigma or changes in relationships with family or friends; and feeling isolated and without adequate supports (for reviews see Bailey, Golden, Roberts, & Ford, 2007; Emerson, 2007). Parental stress is also robustly associated with child emotional or behavior problems, including those seen in children with ASD. Indeed, mothers of children with autism appear especially prone to negative psychological or economic outcomes (Kogan et al., 2008).
Only a handful of researchers have examined biomarkers of stress in these high-risk parents. Epel et al. (2004) found that compared to controls, mothers of 39 children with chronic medical illnesses or developmental disabilities had greater oxidative stress and accelerated shortening of their telomeres (the tips of chromosomes that protect against cellular damage.) Compared to mothers of typically developing children, mothers of children with chronic illness or disabilities had cellular aging that was accelerated by 9 to 17 years, which may lead to earlier onsets of age-related diseases. Gallagher et al. (2009) found that compared to mothers of typically developing children, mothers of children with ASDs and other developmental disabilities mounted a poor antibody response to pneumococcal vaccinations, indicating a reduced capacity to ward off infections.
Seltzer et al. (2009) examined cortisol, a glucocorticoid and marker of hypothalamic-pituitary-adrenal (HPA) axis activity, in 82 mothers of children with psychiatric or developmental disorders and well-matched controls. Cortisol typically shows a robust daily pattern, rising shortly after awakening (the cortisol awakening response, CAR), and then gradually declining over the course of the day. Mothers of children with psychiatric or developmental disabilities, however, had less pronounced daily declines in their diurnal patterns of cortisol, especially on days when they had increased contact with their children. Examining mothers of adults, Seltzer et al. (2010) found that relative to controls, mothers of adults with ASD had lower levels of cortisol throughout the day. Maternal CARs were predicted by acute versus chronic behavior problems in their offspring with autism. Following a “bad behavior day”, mothers of adults without chronic behavior problems had a sharp increase in CAR, while mothers who were accustomed to such problems had a blunted CAR.
The present study related diurnal salivary cortisol in 91 mothers of children and adults with disabilities to their stress, health and psychological functioning. This study also took into account individual differences often seen in maternal stress and psychological outcomes. Thus, even though mothers are at risk for negative outcomes, some mothers report being positively transformed by raising children with disabilities. These mothers cite new growth and insights related to their parenting role, including enhanced empathy, patience, social advocacy, gratitude and wisdom (Dykens, 2005; Hastings, Allen, McDermott & Still, 2002). Other mothers are not necessarily transformed, but are “hanging in” and doing their best to meet the extra demands of raising offspring with disabilities. Still others are adversely affected, showing very high stress, poor mental health, and negative effects on their health, marriage, friendships, employment and economic status (Bailey et al., 2007; Emerson, 2007).
Consistent with these diverse outcomes, mothers of children with disabilities may not necessarily have a homogeneous or typical pattern of diurnal cortisol. Atypical diurnal patterns include hypercortisolism, or high cortisol activity that stays high over time and is often associated with acute stress, as well as hypocortolism, or lower cortisol activity that is blunted over time and often associated with exposure to chronic stress. Dysregulated cortisol is also reflected in excessively steep or blunted CARs, and an evening rise instead of a decline in cortisol at days end.
As we expect heterogeneity or dysregulated cortisol in mothers of children with disabilities, this study used group-based trajectory modeling, which takes sample heterogeneity into account (Nagin, 2005). This approach identifies common trajectories in samples of individual growth curves, with no a priori specification of the number or type of trajectories. Other widely used techniques for analyzing cortisol data (e.g., area under the curve, analysis of variance) require homogeneity in the study population, and assume that individuals vary in their cortisol levels but cluster around the same diurnal pattern. In contrast, group-based trajectory modeling identifies common patterns and trajectories in individuals with presumed heterogeneity.
Van Ryzin, Chatham, Kryzer, Kertes, and Gunnar (2009) used group-based trajectory modeling to identify three distinctive cortisol trajectories in preschool children. Further, child members of these cortisol trajectories had significantly different behavioral profiles, as did their parents. Similarly, in the present study we first identified trajectories and then made comparisons between them in maternal health and mental health, as well as child etiology and behavior problems. In doing so, we provide a novel, objective assessment of diurnal cortisol patterns and what they signify for this at-risk group of mothers.
Methods
Participants
The sample consisted of 91 mothers of offspring with several different types of developmental disabilities, including Down syndrome (n = 11), Prader-Willi syndrome (n = 25), autism spectrum disorder (n = 30), and Williams syndrome (n = 25). Each of these groups has a distinctive phenotype and varying profiles of behavior or emotional problems (Dykens, 2000), providing ample diversity for analyses relating child problems to cortisol trajectories. All children resided with their mothers.
Mothers ranged in age from 21 to 62 years (M = 43.87, SD = 7.78), and were relatively well educated, with 32% completing high school or 2 years of college, and 68% completing 4 years of college or professional training. On average, mothers had 2.45 children, and most, 77%, were Caucasian, 6% were African American, 5% Asian, 8% Hispanic, and 4% other. The mean income level of participants was between $50,000 to $70,000 annually. Regarding employment, 24% of mothers did not work outside the home, 12% worked an average of 9 hours weekly (up to 19 hours), 29% worked between 20 and 39 hours (M = 25.00 hours), and 35% worked 40 or more hours weekly (M = 42.89 hours). No differences were found in maternal demographics or child etiology across these work categories. However, compared to employed women, mothers who did not work outside the home or worked scant hours had children with significantly higher externalizing behavior problems on the Child Behavior Checklist (M = 16.50, SD = 8.74 versus M = 11.23, SD = 7.47, respectively; F (85) = 6.78, p < .01; ES = .65).
Mothers were recruited through disorder-specific parent organizations and university-based recreation groups for children or adults with disabilities. No differences in maternal or child characteristics were found across recruitment sources.
We obtained diagnostic confirmation on offspring with genetic syndromes via previous laboratory test results, and for those with ASDs, from previous evaluations that used well-accepted diagnostic procedures (i.e., ADOS-R, ADI-R plus clinical evaluations). Offspring averaged 12.94 years of age (SD = 7.71), with a range from 4 to 32 years. Most offspring with ASD were males (74%), consistent with the predominance of males with this disorder; males comprised 53% of remaining offspring. Aside from gender, diagnostic groups did not otherwise differ in child variables, or in maternal age, income, education, or marital or employment status.
Procedures
Consistent with Vanderbilt University IRB, written informed consent was obtained from all participants. Mothers then completed questionnaires either during their visits to the lab or at home. Saliva samples for cortisol testing were collected on the same day, or within a one-week window of completing the following measures:
Child Behaviors
Child Behavior Checklist (CBCL, Achenbach, 2001)
The CBCL asks parents to rate 112 problem behaviors on a three-point scale, and contains an Internalizing Domain (anxiety/depression, withdrawal, somatic complaints), Externalizing Domain (aggression, noncompliance), and additional subdomains (thought, social and attention problems). Data analyses used Internalizing and Externalizing Domain raw scores.
Maternal and Family Functioning
Demographics
This questionnaire identified family composition, marital status, parental education, income, employment, and diagnostic information about the child.
Parenting Stress Index (PSI)
In the short form of the PSI (Abidin, 1995), parents rate how much they agree with 36 items using a 5-point scale. We used a robust 2-factor scoring solution from Haskett, Ahern, Ward, & Allaire (2006): Personal Stress (e.g., “I have given up more of my life to child than expected”) and Childrearing Stress (e.g., “Things my child does bother me a lot”.) These two factors show adequate construct and predictive validity, and test- retest stability. The PSI also includes a checklist (yes/no) of specific stressors that occurred in the last year.
Mindful Attention Awareness Scale
This psychometrically sound, 15-item scale (Brown & Ryan, 2003) assesses dispositional mindfulness, or the state of being aware of and attuned to what is taking place in the present moment. Respondents use a 6-point scale to assess the frequency of such experiences as “I rush through activities without being really attentive to them”, and higher scores reflect increased mindfulness.
Beck Depression and Anxiety Inventories
The Beck Depression Inventory-II (BDI; Beck, Steer & Brown, 1996) is a widely used, 21-item inventory of depressive symptoms, rated on a 0 to 3 point scale. The BDI taps affective, mood and somatic symptoms of depression over the last two weeks, and scores of 20 and higher are considered clinically elevated. The Beck Anxiety Inventory (BAI; Beck & Steer, 1990) also uses 21 items on a 0 to 3 scale. The BAI assesses physical and cognitive dimensions of anxiety over the last two weeks, and scores of 16 and higher are clinically elevated.
Health
A brief medical and psychiatric history of mothers was obtained, as was their current height, weight, blood pressure, and a self-perception item that has been widely used in epidemiological studies (e.g., Idler & Benyamini, 1997; “Would you say your health in general is: poor, fair, good, excellent?”).
Cortisol
Saliva samples were collected for diurnal cortisol analyses in 103 participants, however 12 were excluded due to inadequate samples or incorrect sampling times, yielding adequate cortisol data from 91 participants. Mothers were instructed to provide samples using Salivette collection tubes (Sarstedt, Newton NC) upon awakening, 30 minutes later, before lunch, and at approximately 4:00, 6:00, and before bedtime by chewing on a cotton swab for approximately two minutes until the cotton was saturated. Only one cycle of diurnal cortisol was obtained. Samples were processed at Vanderbilt’s Hormone Assay and Analytic Core using RIA procedures, and cortisol values were reported as nanograms per milliliter (ng/ml).
Statistical Approach
We used a group-based semiparametric growth curve mixture model (Nagin, 2005; Nagin & Tremblay, 2001). It is “group-based” because it sorts participants into groups that share common trajectories. PROC TRAJ (Jones, Nagin, & Roeder, 2001) discovers common patterns like a cluster analysis of cases, except that it sorts individual growth curves into clusters with a common trajectory. PROC TRAJ, downloaded from the authors (Jones, 2004), is “semi-parametric” because it models nonlinear growth curves with a simple polynomial function.
For the modest sample size in this study, we kept the model simple to avoid over-fitting. The final model had 12 parameters and 91 subjects, or 7.6 subjects per parameter, less than the recommended 10:1 or 20:1 ratio for ordinary regression (Harrell, 2001). The TRAJ model takes each person’s 6 cortisol values and estimates an individual timeline model. Polynomials up to fifth degree may be chosen, but we limited the model to quadratic time: Cortisol level = β0 + β1t+ β2t2 where t is time in hours, β0 is the time zero intercept, the cortisol level upon awakening, β1 is linear slope of constant change, and β2 is a quadratic slope indicating a curve of acceleration or deceleration. Because the mode of these cortisol levels is often near zero, with a long positive tail, we used the zero-inflated Poisson distribution in PROC TRAJ.
A statistician, blind to study context, ran PROC TRAJ six times to produce solutions with 2–7 trajectory clusters. Each potential solution was evaluated by two objective statistical criteria: (a) whether the number of cases in each trajectory group was significantly greater than zero; and (b) the model misfit given by the Bayesian Information Criterion (BIC). The BIC has been likened to an adjusted R2 because it penalizes more complex models. Solutions with 4–7 classes all contained one or more nonsignificant clusters. These models were rejected leaving solutions with either 2 or 3 clusters. We inspected the “scree plot” of the BIC model fit. There was a large improvement from 2 to 3 cluster solutions, with quickly diminishing returns after 3 solutions. According to a likelihood ratio test, the 3-cluster model had significantly better fit than the 2-cluster model; χ2(3) = 206, p < .001. The 3-cluster solution, however, contained one group with only 2 people, both with extremely high cortisol levels and an erratic pattern across time. The 2-cluster solution sorted these outliers into the higher group, which explains why the 2-group fit was significantly worse than the 3-group fit. We chose the better fitting 3-cluster model and treated the two high-scoring, erratic cases in cluster 3 as atypical outliers related to assay problems or other measurement error. T-tests or chi-squares were then used to compare the characteristics of the 89 participants in Trajectory 1 versus 2.
Analyses also assessed the cortisol awakening response (CAR) and changes in evening cortisol levels. Comparisons were made between women who did or did not show the typical, expected CAR, which is defined in most studies as an increase of at least .09 ng/ml between awakening and 30 minute post awakening responses (Wust et al., 2000). Similar analyses compared women who did or did not show an atypical rise in evening cortisol of at least .09 ng/ml.
Although we conducted three sets of comparative analyses, we retained a conventional p < .05. As this is the first study to use biomarkers instead of child diagnosis to examine maternal health and mental health, we were equally worried about false negatives as false positive results. As such, we calculated effect sizes to guide our interpretations of group differences. For mean differences we used d = (M1 − M2)/SDpooled Cohen (1992). For Chi-square analyses effect sizes were Phi coefficients (2 × 2) or Cramer’s V, a generalization of Phi to larger tables.
Results
Cortisol Trajectories
Figure 1 shows the two distinct trajectories identified in growth curve mixture model analyses. Approximately 63% of the sample was categorized within the first trajectory type. These mothers had significantly lower mean cortisol values than their counterparts at every time point, and a markedly blunted pattern of cortisol values throughout the day. The second trajectory consisted of 37% of the sample. Figure 1 shows that these mothers had higher cortisol values and larger and steeper cortisol changes.
Figure 1.
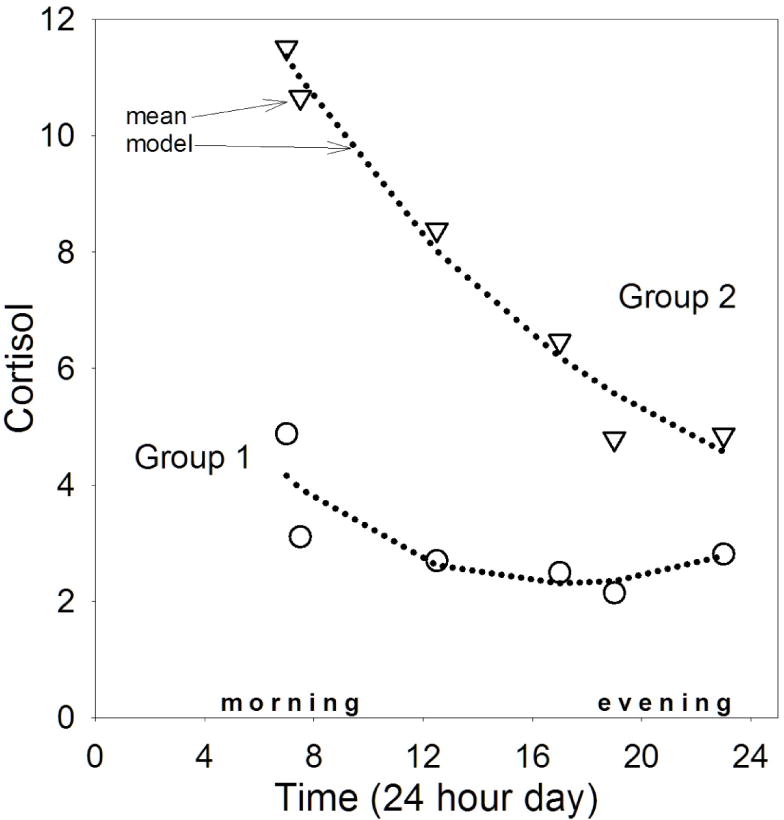
Trajectories of diurnal cortisol based on group-based trajectory modeling in 91 mothers of children with ASD and other disabilities.
Notes. Circles and triangles show observed means. Dotted lines show TRAJ polynomial models (intercept + linear + quadratic).
Although Trajectories 1 and 2 are, by definition, significantly different, follow-up analyses were run with a mixed model to identify coefficients and magnitude of change. The significant coefficients (all p < .001) indicate that Trajectory 2 started at hypothetical time 0 at 12.14 ng/ml and declined 0.36 units per hour. In contrast, Trajectory 1 started at 4.17 ng/ml, and had a shallower decline of 0.25 cortisol units per hour.
Trajectory 1 versus 2 Comparisons
Child Characteristics
Relative to Trajectory 2, children of mothers in Trajectory 1 had significantly higher CBCL Internalizing scores, a difference that was primarily attributed to higher scores in the anxiety/depression subdomain (see Table 1). Strikingly, the majority of mothers of children with ASD (89%) were assigned to Trajectory 1, the blunted type. In contrast, mothers in remaining diagnostic groups were more evenly divided across trajectories, with 53% in the blunted trajectory group χ2 (1) = 11.32, p < .001, ES = .92.
Table 1.
Comparison of participant characteristics across cortisol trajectories.
| Trajectory 1 | Trajectory 2 | ||
|---|---|---|---|
| M (SD) | M (SD) | t or χ2, d | |
| Child Factors | |||
| Age | 12.48 (7.47) | 15.51 (8.81) | −.99 |
| Externalizing problems | 13.97 (8.84) | 11.21 (8.31) | 1.23 |
| Internalizing problems | 12.46 (8.27) | 8.56 (5.42) | 2.31*, d =.57 |
| Diagnostic group | 89% ASD | 11% ASD | 10.73***, d=.78 |
| 53% IDD | 47% IDD | ||
| Maternal Factors | |||
| PSI Childrearing Stress | 50.17 (10.57) | 47.69 (11.11) | 1.10 |
| PSI Personal Stress | 44.98 (11.40) | 36.18 (12.36) | 3.08***, d = .74 |
| # Stressors last year | 1.76 (1.35) | 2.11 (1.98) | −.89 |
| Beck Depression | 12.85 (9.39) | 10.64 (8.15) | 1.00 |
| Beck Anxiety | 6.61 (5.52) | 6.44 (7.57) | .11 |
| Mindfulness | 53.40 (10.55) | 59.66 (12.13) | −2.31*, d =.55 |
| Health Self-Perception | 2.17 (.78) | 1.78 (.60) | 2.35*, d =.57 |
| # Health Problems | 2.13 (1.74) | 2.33 (1.64) | −.42 |
| Body Mass Index | 26.54 (5.95) | 27.22 (3.77) | −.49 |
| Demographics | |||
| Maternal Age | 43.85 (7.74) | 45.38 (7.66) | −.88 |
| Median Income | 50–70K | 50–70K | 1.98 |
| Maternal Education | 2–4 yrs college | 2–4yrs college | .05 |
| # Hours mother works | 26.54 (5.95) | 27.22 (3.77) | −.49 |
| Married | 76% | 85% | 2.06*, d =.34 |
Note:
p < .05;
p < .01;
p < .001.
Effect sizes are shown only for results significant at p<.05 or better.
Maternal and Family Characteristics
Table 1 shows that mothers in the more blunted trajectory had significantly higher PSI Personal Stress scores than Trajectory 2 mothers, with a large effect size (d =.76). Trajectory 1 mothers also had lower Mindfulness and Perceived Health Status, (d = .57, .55), and although they were also more likely to be divorced, the effect size was modest (d = .34). Trajectories were not associated with income or marital status.
Within Trajectory 1: ASD versus other Disabilities
As the majority of mothers of offspring with ASD were members of Trajectory 1, we explored possible differences between these mothers and their Trajectory 1 peers who were raising children with other developmental disabilities. No significant differences were found in maternal functioning.
Cortisol Awakening Response (CAR) and Evening Decline
CAR
Across trajectory types, 60% of mothers did not show the expected CAR. Compared to those with adequate CARs, women with blunted CARs had significantly lower Body Mass Indices (BMI’s, see Table 2). As well, their medical histories were more apt to include anxiety disorders, and although relatively infrequent, eating disorders and hypoglycemia were reported exclusively in mothers with blunted CARs. Women who worked between 20 to 35 hours weekly were more apt to show an adequate CAR compared to women who were not employed or to women who worked 40+ hours a week. No other differences were found.
Table 2.
Areas of significant difference across mothers with adequate versus blunted cortisol awakening responses (CAR).
| Adequate CAR | Blunted CAR | ||
|---|---|---|---|
| M (SD) | M (SD) | t or χ2, d or ES | |
| Body Mass Index | 29.94 (5.03) | 24.94 (5.73) | −3.08**, d =.93 |
| % Eating Disorder Dx | 0 | 15% | 4.03*, ES =.43 |
| % Anxiety Disorder Dx | 10% | 33% | 4.46*. ES =.45 |
| Hypoglycemia | 0 | 13% | 4.72*, ES = .47 |
| # Hours work/week | 6.29*, ES = .54 | ||
| 0 to 19 hours | 35% | 65% | |
| 20 to 39 hours | 64% | 36% | |
| 40+ hours | 26% | 74% |
Note:
p < .05;
p < .01.
Evening Cortisol Response
Approximately 48% of mothers across trajectories manifested an atypical nighttime rise in cortisol. Table 3 shows that women with evening rises were older and had more children; not surprisingly, these were related; r = .27, p < .01. Women with nighttime rises versus declines had significantly lower scores on the Mindfulness Questionnaire, higher scores on the depression (BDI) and anxiety (BAI) inventories, and a higher percentage of them exceeded the BDI clinical cutoff. Nighttime risers reported more troubles with constipation and high blood pressure. Trajectories 1 and 2 had similar proportions of women with evening upticks in cortisol (43% and 50%, respectively). No other differences were found.
Table 3.
Areas of significant difference across mothers with evening declines versus rises in cortisol.
| Evening Decline | Evening Rise | ||
|---|---|---|---|
| M (SD) | M (SD) | t or χ2, d or ES | |
| Age | 42.57 (5.91) | 46.18 (7.67) | −1.96*, d = .54 |
| Depression (BDI) | 9.81 (6.99) | 14.08 (9.50) | −1.96*, d = .59 |
| BDI Clinical Cutoff | 7% | 29% | 5.76**, ES = .59 |
| Anxiety (BAI) | 5.38 (5.24) | 9.17 (7.30) | −2.22*, ES = .72 |
| Mindfulness | 60.34 (10.05) | 52.25 (10.69) | 2.92**, ES = .80 |
| Constipation | 3% | 26% | 5.46**, ES = .57 |
| High Blood Pressure | 8% | 24% | 3.10+, ES = .42 |
| # Children | 2.17 (1.15) | 2.73 (.92) | 2.13*, ES = .56 |
Note:
p < .05;
p < .01.
Examined another way, most women (70%) had either an abnormal CAR or evening rise, while 18% had both an abnormal CAR and evening rise. Just 11% had a typical CAR and evening decline, or what is considered a normal diurnal cortisol pattern.
Morning to Night Change
Correlations were conducted between maternal variables and both the highest morning cortisol value, and the magnitude of change in cortisol from the morning peak to the evening nadir. Significant correlations emerged on the PSI only. PSI Personal Stress was correlated with peak cortisol values (r = .34, p < .01) and difference scores (r = .35, p < .001). The number of stressors in the last year was also associated with peak and difference cortisol values (r’s = .32, .33, respectively, p’s < .01).
Discussion
Group-based trajectory modeling provided an objective evaluation of maternal diurnal cortisol, without taking child diagnoses into account. Using a biomarker of stress to classify groups is a novel approach, and yielded significant differences in maternal health, stress and child diagnoses across cortisol trajectories. Further, 45 to 48% of mothers showed aberrant CARs or evening cortisol activity, and these patterns were also associated with important differences in maternal functioning. Taken together, findings demonstrate that stress and aberrant HPA functioning are associated with health and mental health problems in mothers of children with ASD and other disabilities.
The majority of mothers (63%) fell into a blunted cortisol trajectory, showing lower mean cortisol values at every time point than their counterparts in Trajectory 2. Relative to Trajectory 2, mothers in Trajectory 1 had significantly higher levels of PSI Personal Stress, and lower levels mindfulness and perceived health. They were also less likely to be married, which may lead to additional financial or childrearing burdens, although the effect size for marital status was relatively low. Blunted diurnal cortisol is typically associated with hypoactivation of HPA functioning and exposure to chronic as opposed to acute stress. Diminished cortisol activity has, for example, been identified in such groups as women living in poverty (Ranjit, Young & Kaplan, 2009), parents of pediatric cancer patients (Miller, Cohen, & Ritchey, 2002), adults with Post-Traumatic Stress Disorder related to war or other trauma (Meewisse et al., 2007), blue-collar working women (Daniel et al, 2006), and patients with somatic disorders (Heim, Ehlert, & Hellhammer, 2000).
In addition to altered diurnal cortisol, exposure to chronic stress can also reduce immune responses to vaccines, trigger production of proinflammatory cytokines, increase susceptibility to infection, accelerate telomere shortening, and dampen telomerase activity— processes associated with premature aging and adverse health outcomes (Epel, 2009; Kiecolt-Glaser, 2009). These neuroendocrine risk factors are found in groups that share common characteristics with mothers of children with disabilities, including adults with depression or anxiety, and elderly caregivers of spouses with dementia (Gouin et al., 2008). Unlike these elderly spousal caregivers, however, caring for offspring with disabilities is a lifelong commitment, and most adults with intellectual disabilities reside with their aging parents. Mothers may thus accumulate decades of stress and altered HPA activity, which does not bode well for their healthy aging over time. Indeed, Seltzer et al. (2011) followed mothers of children with disabilities from middle age into their mid-sixties, and found increased health and mental health concerns primarily in the older women.
One advantage of trajectory analysis is that it identifies consistent patterns in longitudinal data, with no a priori knowledge of groups. It is thus quite striking that the majority of mothers of children with ASD were discovered to be members of the blunted cortisol trajectory. This finding is consistent with previous work showing lower levels of cortisol in mothers of adults with ASD relative to controls (Seltzer et al., 2010).
Belonging to the blunted cortisol trajectory in the present study, however, was not unique to mothers of children with ASD, as a full 53% of mothers of non-ASD children were also members of Trajectory 1. Surprisingly, Trajectory 1 mothers of ASD versus non-ASD children did not differ on any measure of maternal functioning. This finding contrasts with previous studies showing that mothers of children with ASD versus other disabilities or typical children invariably show higher levels of stress, health problems, anxiety and depression (e.g., Bailey et al., 2007). By using a biomarker instead of child diagnosis to form groups, however, we identified a group of chronically stressed mothers who, irrespective of their child’s diagnosis, shared a blunted cortisol trajectory and remarkably similar scores on health and mental health measures.
Beyond diurnal trajectories, 60% of women had aberrant CARs. The morning rise in cortisol is generally thought to represent how people prepare for their day, how they get activated, oriented and “up” for the day’s activities (Fries, Dettenborn & Kirschbaum, 2009). Relative to those with adequate CARs, women with diminished CARS had lower BMIs, higher rates of anxiety disorders, and although relatively infrequent, eating disorders and hypoglycemia were seen exclusively in this group. Perhaps these mothers have disrupted sleep, or awaken and “hit the ground running” – their HPA is primed and ready to go. Their blunted CAR was predominantly associated with anxiety and somatic effects involving the gastrointestinal system, which is consistent with previous work linking hypocortisolism to stress-related bodily disorders (Heim et al., 2000).
Blunted CARs in mothers may be associated with specific demands of their days. A diminished CAR was more frequent in women who did not work outside the home, and this same group had children with significantly more externalizing behaviors. Upon arising, these women may thus immediately anticipate, or be involved with, the demands of caring for these more behaviorally challenging children. Similarly, Seltzer et al (2010) found that chronicity of child behavior problems was associated with hypocortisolism in mothers of adults with ASD.
Beyond child problems, women who were employed for 40+ hours a week outside the home also had a diminished CAR, perhaps reflecting the juggle between work and family demands. Using a normative sample of mothers, Adams and Gunnar (2001) also reported a blunted CAR and diurnal cortisol in women who worked full-time. On the flip side, women in the present study with adequate CARS were apt to work on a part-time basis, for an average of 25 hours weekly. Perhaps these women worked just enough take a break from childrearing demands while also engaging in an appropriate amount of other meaningful, adult activities. Interestingly, Parks et al. (2011) found that women who worked full-time or overtime had shorter telomeres relative to women who worked part-time, again suggesting beneficial effects of part-time schedules on stress biomarkers.
Aberrant diurnal patterns were also seen at day’s end, with 48% of mothers showing an upward rise in evening cortisol instead of the usual decline. Compared to those with expected declines, nighttime rising women were older, and the increased constipation and high blood pressure noted in this group may simply relate to typical aging. Beyond being older, however, nighttime risers versus decliners also had higher Beck depression and anxiety scores, and more of them had clinically elevated levels of depression. They also scored lower on dispositional mindfulness, and the combination of anxiety, depression and being less “in the moment” sets the stage for rumination and worry at days end. Rumination and worry, in turn, are robustly associated with HPA dysregulation (Zoccola, Dickerson, & Zaldivar, 2008). Nighttime risers were thus not disengaging physiologically from the day and such disruptions to the rest and restorative function of the HPA may cause them to be at even higher risks for medical or psychiatric problems over the course of time.
Maternal dysregulation of evening cortisol may be associated with a build-up of stress throughout the day, or with specific challenges of evening dinner or bedtime routines. Nighttime risers versus decliners had more children in the home to manage, feed, and get to bed. Such daily routines can be quite difficult, as many children with ASD or other disabilities are “picky” eaters or on special diets (e.g., Nadon, Feldman, Dunn & Gisel, 2010), and many have significant sleep disturbances (e.g., Lenjavi, Ahua, Touchette, & Sandman, 2010). Future studies are needed on the impact of children’s feeding or sleep problems on maternal stress and sleep quality.
This study has several limitations. First, the sample size was relatively small, although effect sizes for all but one difference were moderate to large. As well, we only sampled one day’s cycle of diurnal cortisol, and two or more days are often needed to reduce assay variability. Third, analyses did not take medication status or menstrual cycle phase into account, and both can alter diurnal cortisol. Finally, even though this study focused on differences within at-risk mothers, future work needs to benchmark cortisol levels and health outcomes in a control group of mothers of typically developing children. Cortisol levels in our sample, for example, were high relative to other studies of females (e.g., Seltzer et al., 2010; Stalder et al., in press). Even so, our peak cortisol and peak/nadir difference scores behaved as expected and were associated with perceived stress. Similarly, compared to population estimates, the rate of eating disorders in our sample was relatively high (Hudson et al., 2007; 5.9% versus 13%), again suggesting a need for control mothers.
Despite these limitations, this study is the first to use biomarker analyses and group-based trajectory modeling in lieu of traditional approaches based on child diagnosis to identify mothers at high risk for negative outcomes. Mothers of children with ASD and other disabilities who shared blunted diurnal cortisol had remarkably similar levels of stress and psychological functioning. Across disability groups, biomarkers of stress may be important treatment targets in future interventions aimed at reducing distress and enhancing well being in these at-risk parents.
Acknowledgments
The authors thank the mothers who graciously participated in this research, as well as Elizabeth Roof, MA, Senior Research Scientist, for her expertise in working with families, and Research Assistants Grace Kulbaba and Lauren Deisenroth. We are also grateful to Robert Hodapp, PhD for his feedback on an earlier draft of this manuscript. This work was supported by NICHD Grants R01HD035684 and P30HD015052 and NCRR/NIH Grant UL1RR024975-01.
References
- Achenbach T. Manual for the Child Behavior Checklist 4/18 and 2001 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 2001. [Google Scholar]
- Abidin RR. Parent Stress Index Professional Manual. 3. Odessa, FL: Psychological Assessment resources; 1995. [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in woman. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Golden RN, Roberts J, Ford A. Maternal depression and developmental disability: Research critique. Mental Retardation and Developmental Disability Research Reviews. 2007;13:321–329. doi: 10.1002/mrdd.20172. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. San Antonio, TX: Psychological Corporation; 1990. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Brown KW, Ryan R. The benefits of being present: Mindfulness and its role in psychological well being. Journal of Personality and Social Psychology. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Daniel M, Moore DS, Decker S, Belton L, DeVellis B, Doolen A, Campbell MK. Associations among education, cortisol rhythm, and BMI in blue-collar women. Obesity. 2006;14:327–335. doi: 10.1038/oby.2006.42. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Psychopathology in children with intellectual disabilities. Journal of Child Psychology and Psychiatry. 2000;41:407–417. [PubMed] [Google Scholar]
- Dykens EM. Happiness, well-being, and character strengths: Outcomes for families of persons with mental retardation. Mental Retardation. 2005;43:360–364. doi: 10.1352/0047-6765(2005)43[360:HWACSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Emerson E. Poverty and people with intellectual disabilities. Mental Retardation and Developmental Disability Research Reviews. 2007;13:107–113. doi: 10.1002/mrdd.20144. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. PNAS. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Telomeres in a life-span perspective: A new “psychobiomarker”? Current Directions in Psychological Science. 2009;18:6–10. [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gallagher A, Phillpis AC, Drayson MT, Carroll D. Parental caregivers of children with developmental disabilities mount a poor antibody response to pneumococcal vaccination. Brain, Behavior, and Immunity. 2009;23:338–346. doi: 10.1016/j.bbi.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Gouin JG, Hantsoo L, Kiecolt-Glaser JK. Immune dysregulation and chronic stress among older adults: A review. Neuroimmunomodulation. 2008;15:251–259. doi: 10.1159/000156468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE. Regression Modeling Strategies. New York: Springer-Verlag; 2001. [Google Scholar]
- Haskett ME, Ahern LS, Ward CS, Allaire JC. Factor structure and validity of the Parenting Stress Index-Short Form. Journal of Clinical and Adolescent Psychology. 2006;35:302–312. doi: 10.1207/s15374424jccp3502_14. [DOI] [PubMed] [Google Scholar]
- Hastings RP, Allen R, McDermott K, Still D. Factors related to positive perceptions in mothers of children with intellectual disabilities. Journal of Applied Research in Intellectual Disabilities. 2002;15:269–275. [Google Scholar]
- Heim C, Ehlert U, Hellmammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hodapp RM, Ly T. Parenting children with developmental disabilities. In: Luster T, Okagaki L, editors. Parenting: An Ecological Perspective. Mahwah, NJ: Erlbaum; 2005. pp. 177–207. [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Jones BL. SAS PROC TRAJ: Group Based Modeling of Longitudinal Data. 2004 5/10/2004. from http://www.ncovr.org/docs/Special_Project/Trajectory/index.htm.
- Jones BL, Nagin DS, Roeder K. A SAS Procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Kiecolt-Glaser J. Psychoneuroimmuniology: Psychology’s gateway to the biomedical future. Perspectives on Clinical Science. 2009;4:367–369. doi: 10.1111/j.1745-6924.2009.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Glaser R. Psychological stress, telomeres, and telomerase. Brain, Behavior, and Immunityy. 2010;24:529–530. doi: 10.1016/j.bbi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan M, Strickland BB, Blumberg S, Singh G, Perin JM, van Dyck PC. A national profile of the health care experiences and family impact of autism spectrum disorder among families in the United States 2005–2006. Pediatrics. 2008;122:e1149–1158. doi: 10.1542/peds.2008-1057. [DOI] [PubMed] [Google Scholar]
- Lenjavi MR, Ahuja MA, Touchette PE, Sandman CA. Maladaptive behaviors are linked to inefficient sleep in individuals with developmental disabilities. Journal of Neurodevelopmental Disorders. 2010;2:174–180. doi: 10.1007/s11689-010-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, DeVries GJ, Dersons BPR, Olff M. Cortisol and post-traumatic stress disorder in adults-Systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Nadon G, Feldman DE, Dunn W, Gisel E. Mealtime problems in children with autism spectrum disordes and their typically developing siblings. Autism. 2010;15:98–113. doi: 10.1177/1362361309348943. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-based modeling of development. Cambridge, Mass.: Harvard University Press; 2005. [Google Scholar]
- Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: A group-based method. Psychological Methods. 2001;6(1):18–34. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Parks CG, DeRoo LA, Miller DB, McCanlies EC, Cawthon RM, Sandler DP. Employment and work schedule are related to telomere length in women. Occupational Environmental Medicine. 2011;68:582–589. doi: 10.1136/oem.2010.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. International Journal of Epidemiology. 2005;34:1138–1143. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Almeida DM, Greenberg JS, Savla J, Stawski RS, Hong J, Taylor JL. Psychosocial and biological markers of daily lives of midlife parents of children with disabilities. Journal of Health and Social Behavior. 2009;50:1–15. doi: 10.1177/002214650905000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MS, Floyd F, Song J, Greenberg J, Hong J. Midlife and aging parents of adults with intellectual and developmental disabilities: Impact of lifelong parenting. American Journal of Intellectual and Developmental Disabilities. 2011;116:479–499. doi: 10.1352/1944-7558-116.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, Stawski R. Maternal cortisol levels and behavior problems in adolescents and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40:457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T, Evans P, Hucklebridge F, Clow A. State associations with the cortisol awakening response in healthy females. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.02.014. (in press) [DOI] [PubMed] [Google Scholar]
- Van Ryzin MJ, Chatham M, Kryzer E, Kertes DA, Gunnar MR. Identifying atypical cortisol patterns in young children: The benefits of group-based trajectory modeling. Psychoneuroendocrinology. 2009;34:50–61. doi: 10.1016/j.psyneuen.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response-normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, Zaldivar PP. Rumination and cortisol response to laboratory stressors. Psychosomatic Medicine. 2008;70:661–667. doi: 10.1097/PSY.0b013e31817bbc77. [DOI] [PubMed] [Google Scholar]


