Summary
When telomeres become critically short DNA damage response factors are recruited at chromosome ends initiating a cellular response to DNA damage. We performed Proteomic Isolation of Chromatin fragments (PICh) to define changes in chromatin composition that occur upon onset of acute telomere dysfunction triggered by depletion of the telomere-associated factor TRF2. This unbiased purification of telomere-associated proteins in functional or dysfunctional conditions revealed the dynamic changes in chromatin composition that take place at telomeres upon DNA damage induction. Based on our results, we describe a critical role for the polycomb group protein Ring1b in NHEJ-mediated end-to-end chromosome fusions. We show that cells with reduced levels of Ring1b have a reduced ability to repair uncapped telomeric chromatin. Our data represent the first unbiased isolation of chromatin undergoing DNA damage and are a valuable resource to map the changes in chromatin composition in response to DNA damage activation.
Introduction
Most human cancer cells have short telomeres when compared to normal surrounding tissues (Hastie et al., 1990; Meeker et al., 2004). This is due to the relatively late reactivation of telomerase during tumorigenesis, resulting in the proliferation of cells for an extended period of time in the absence of a telomere elongation mechanism (Maser and DePinho, 2002; Shay and Wright, 2001). Loss of end-protection is frequently detected in human cancers such as colorectal carcinomas (Rudolph et al., 2001), oral squamous cell carcinomas (Gordon et al., 2003) and chronic lymphocytic leukemia (Augereau et al., 2011; Chin et al., 2004; Lin et al., 2010; Suram et al., 2012). Mounting evidence suggests that telomere dysfunction plays a crucial role in promoting tumor development (Ramsay et al., 2013). For example, the induction of telomere dysfunction in mice results in epithelial cancers with a complex karyotype, indicative of rampant genomic instability (Artandi et al., 2000; Chin et al., 1999). The most striking illustration of the role of end-protection in human health comes from patients affected by the rare inherited disorder, Dyskeratosis congenita (DC). This disease is caused by mutations affecting either telomere elongation factors (dyskerin and hTERT) or telomere associated proteins (Dokal, 2000; Kirwan and Dokal, 2009; Mitchell et al., 1999; Sarper et al.; Shay and Wright, 1999; Touzot et al.; Vulliamy et al., 2004; Walne et al., 2008). Amongst other symptoms, DC patients have critically short telomeres, show signs of telomere dysfunction and display an increased incidence of cancer.
When telomeres become critically short they fail to recruit sufficient levels of protective shelterin complex resulting in failure to protect chromosome ends and leading to the activation of a DNA damage response (DDR) pathway (d’Adda di Fagagna et al., 2003; Takai et al., 2003). Indeed, to date, the following DNA damage proteins have been shown to play a role in the response to dysfunctional telomeres; MRE11, ATM, γH2AX, MDC1, RNF8, RNF168, 53BP1, RIF1 and PTIP1 (Attwooll et al., 2009; Chapman et al., 2013; Denchi and de Lange, 2007; Di Virgilio et al., 2013; Dimitrova et al., 2008; Dimitrova and de Lange, 2006, 2009; Peuscher and Jacobs, 2011; Zimmermann et al., 2013). However, a global approach to systematically determine which factors relocalize to or from dysfunctional telomeres is currently lacking.
Here, we applied a proteomic approach to comprehensively characterize the proteins whose localization change upon telomere dysfunction. Using this approach we isolated a series of previously characterized DDR factors as telomere dysfunction recruited factors, in addition to a set of novel factors that are recruited to dysfunctional telomeres. In addition, our data revealed a critical role for the polycomb protein Ring1b for the efficient NHEJ-mediated fusion of dysfunctional telomeres.
Results
Changes in chromatin composition following telomere dysfunction
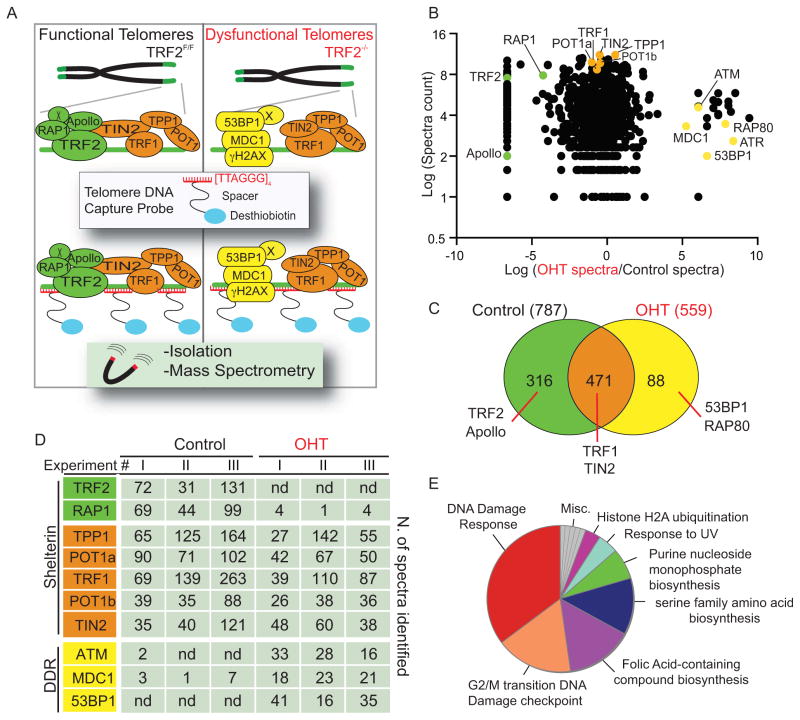
We hypothesized that upon acute telomere dysfunction, relocalization of DDR factors at telomeric repeats [TTAGGG]n would lead to a drastic change in chromatin composition at chromosome ends, and that this could be isolated and identified using an unbiased proteomic approach. To verify this hypothesis we adapted a technique termed PICh (Proteomics of Isolated Chromatin segments) that allows the isolation of chromatin regions using a biotinylated oligonucleotide complementary to a DNA sequence of interest (Dejardin and Kingston, 2009). PICh was originally designed to isolate telomeric chromatin derived from human cells and employed LNA-modified oligos. We optimized several steps in the PICh protocol in order to efficiently isolate telomeric chromatin from murine cells including the use of PNA-modified oligonucleotides as capture probes. In our experiments the use of PNA probes resulted in more efficient isolation of telomeric chromatin when compared to LNA-modified oligonucleotides (data not shown). To achieve synchronous and homogenous telomere dysfunction we used TRF2 conditional knockout MEFs (Celli and de Lange, 2005) harboring an inducible CRE recombinase (Rosa26-CRE-ER) (previously described in (Okamoto et al., 2013)). 4-hydroxytamoxifen (OHT) treatment of these cells allows for efficient and synchronous deletion of TRF2 resulting in DNA damage activation at every chromosome end (Okamoto et al., 2013). Telomeric chromatin was isolated using a PNA capture probe complementary to the telomeric repeat sequence [TTAGGG]3 conjugated with a desthiobiotin moiety (see Figure 1A for schematics). Telomeric chromatin was isolated in three independent experiments from TRF2 depleted (OHT) and control cells. The resulting chromatin-associated proteins were identified using liquid chromatography-tandem mass spectrometry (LC MS/MS) and only proteins identified in at least 2 independent experiments were further analyzed. In total we identified 787 unique proteins in samples derived from TRF2-proficient cells and 559 in samples derived from TRF2-deficient cells (Figure 1 and Supplemental Table1). Importantly, despite the different cellular systems used, there is 60% of overlap between the proteins that we identified in TRF2-proficient MEFs and the ones isolated in Hela cells (Dejardin and Kingston, 2009). In support of our initial hypothesis we found significant differences in the composition of telomeric chromatin in the presence or absence of TRF2, with 316 proteins found only at TRF2 proficient telomeres, 88 proteins only at TRF2 deficient telomeres and 471 proteins found in both conditions (Figure 1C). Amongst the proteins that were only found at TRF2 proficient telomeres we found TRF2 and its binding partners RAP1 and Apollo (Lenain et al., 2006; van Overbeek and de Lange, 2006, Li, 2000 #3057) (Figure 1B–D and Supplemental Table 2). In contrast, from TRF2-deficient telomeres we isolated several DNA damage factors that have been previously shown to localize to dysfunctional telomeres such as ATM, the MRE11 complex, MDC1 and 53BP1 (Celli and de Lange, 2005; Dimitrova and de Lange, 2006) (Figure 1B–D and Supplemental Table 3). Finally, proteins that are known to bind to telomeres in a TRF2-independent manner were not significantly enriched in either samples such as the shelterin components (TRF1, TIN2, TPP1, POT1a and POT1b) and other known telomere associated proteins such as members of the STN1 complex and of the THO complex, and the chromatin remodeling factors ATRX and Daxx (Figure 1B–D and Supplemental Table 1) (Buscemi et al., 2004; de Lange, 2005; Lewis et al., 2010; Pfeiffer et al., 2013; Wong et al., 2010). Annotation of the proteins found only in the TRF2-depleted setting revealed a significant enrichment for DDR genes (Figure1E). Collectively, our data show that using a PICh-based approach we were able to detect the changes that occur at chromatin upon removal of the telomeric factor TRF2 and the consequent induction of a DNA damage response at uncapped chromosome ends.
Figure 1. Proteomics of isolated chromatin segments (PICh) of functional and dysfunctional mouse telomeres.
A. Schematic representation of the PICh analysis employed to define the chromatin changes that occur at telomeres upon TRF2 depletion. B. Identified proteins were plotted based on the Log2 Intensity (Y axis) calculated based on the total spectra counts identified and ratio between TRF2 proficient and deficient settings (X axis) calculated as Log2 (OHT spectra/control spectra). Only proteins identified in at least 2 experiments are plotted. Green and orange dots represent proteins recruited at telomeres in a TRF2-dependent or independent manner, respectively. Yellow dots represent known DNA damage factors recruited to dysfunctional telomeres. C. Venn diagrams depicting the total number of proteins identified in at least 2 out of 3 PICh experiments from the control and OHT samples and the degree of overlap between the datasets from the two samples. D. Table listing shelterin components and some of the DNA damage response (DDR) factors identified with the corresponding spectra counts isolated in each individual experiment (nd= not detected). E. Pie chart illustrating the distribution of proteins identified in the TRF2-depleted samples (OHT), classified according to Gene Ontology (GO). Note the enrichment for DNA damage response proteins
Isolation of proteins that are displaced from dysfunctional telomeres
To define the critical changes that occur at telomeric chromatin upon removal of TRF2 we first focused on the proteins that were lost upon OHT treatment. To this end, proteins with significant telomeric enrichment in TRF2-proficient cells relative to OHT-treated controls were ranked based on their absolute expression in mouse cells using the online database PaxDB (Wang et al., 2012). In addition, the resulting lists were run against the Contaminant Repository for Affinity Purification (www.crapome.org) (Mellacheruvu et al., 2013) and common contaminants were excluded from further analysis. Significantly, the list of proteins that are lost upon TRF2 depletion contained at top ranking positions RAP1 and Apollo, two proteins that have been shown to be recruited at telomeres in a TRF2-dependent manner (Celli and de Lange, 2005; Lenain et al., 2006; Li et al., 2000; van Overbeek and de Lange, 2006), as well as TRF2 (Supplemental Table 2). To validate additional factors that are lost from telomeres upon TRF2 depletion we chose two proteins amongst the top 5% of the factors enriched at TRF2-proficient telomeres: LRWD1 and CDCA8. Localization of these proteins at telomeres was tested in the presence or absence of TRF2 using tagged alleles expressed from retroviral vectors in TRF2F/F Rosa26 CRE-ER MEFs. As positive control we used a myc-tagged RAP1 allele. Our results indicate that CDCA8 and LRWD1 showed frequent co-localization with telomeric DNA (Supplemental Figure 1A and B). In agreement with our PICh results we found that upon TRF2 depletion the localization of CDCA8 with telomeres was reduced by approximately 50% (Supplemental Figure 1B). On the contrary, LRWD1 localization to telomeres was not significantly affected by the loss of TRF2 suggesting that its recruitment to telomeres is independent from TRF2 (Supplemental Figure 1B). These data therefore suggest that our analysis can detect both known as well as novel telomere associated factors that are affected by TRF2 depletion. To address whether CDCA8 and/or LRWD1 play a critical role in telomere protection, we performed shRNA depletion of these proteins. Efficient down-regulation of either CDCA8 or LRWD1 did not result in accumulation of the DNA damage factors γH2AX and 53BP1 at telomeres (Supplemental Figure 1C–E). In contrast, shRNA-mediated downregulation of TRF2 resulted in frequent localization of γH2AX and 53BP1 foci at chromosome ends (Supplemental Figure 1C–E). CDCA8 is part of the chromosome passenger complex (CPC), which is involved in proper mitotic segregation and cytokinesis (reviewed in (Carmena et al., 2012)). LRWD1 mediates association of the Origin Recognition Complex (ORC) to chromatin and was shown to be required for maintenance of pericentric heterochromatin silencing (Chan and Zhang, 2012; Shen et al., 2010). Although depletion of these proteins did not result in activation of a DNA damage response at TRF2-depleted telomeres, we could not exclude that CDCA8 and/or LRWD1 have a role at telomeres that was not detected by our analyses, and further functional characterization is currently undergoing and will be reported in a future study.
Isolation of proteins that are recruited to dysfunctional telomeres
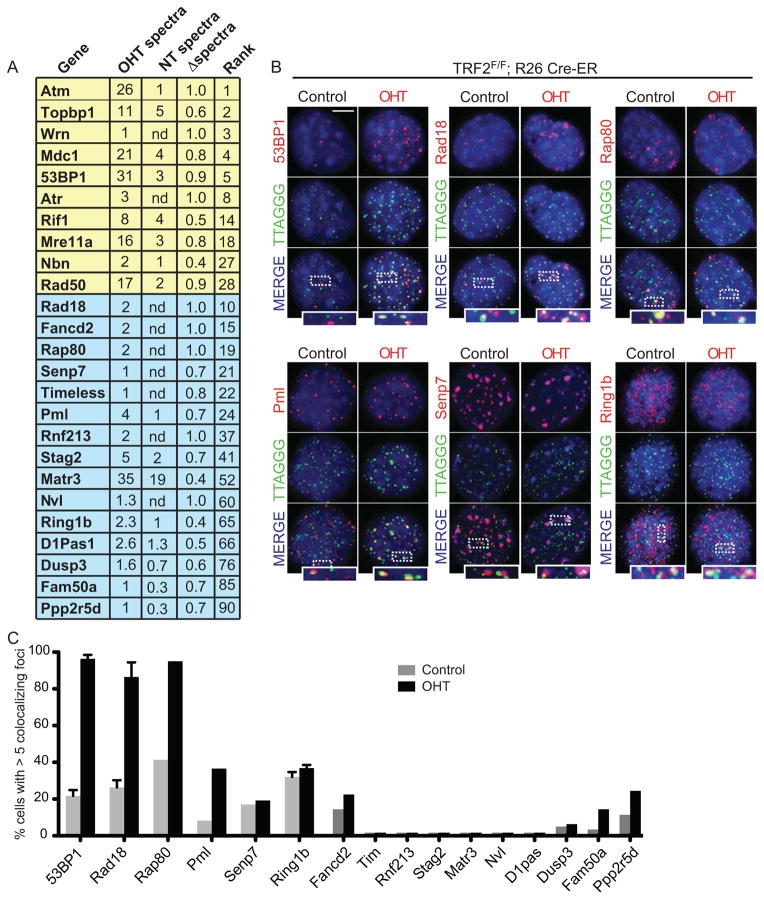
Next, we focused on factors that are recruited to telomeres following TRF2 loss. Proteins were ranked based on their relative enrichment at dysfunctional telomeres and normalized to their absolute expression in mouse cells (Wang et al., 2012). This analysis revealed 114 proteins that are enriched at TRF2-depleted telomeres. Amongst these proteins we found 24 known DDR proteins of which 10 were previously reported to be associated with dysfunctional telomeres (Figure 2A and Supplemental Table 3). To validate whether our approach identified novel factors that relocalize to dysfunctional telomeres we selected 15 proteins ranked at different positions within this list and tested their localization in the presence or absence of TRF2 (Figure 2). The selected factors represented both proteins that were previously shown to interact with DNA damage sites, as well as proteins with no reported link to the DDR pathway. TRF2F/F Rosa26 CRE-ER MEFs expressing a tagged version of the selected proteins were treated with tamoxifen (OHT) or left untreated (control) and the localization of the ectopically expressed proteins to telomeric DNA was assessed by indirect immunoflourescence. As a positive control we used a 53BP1 allele that has been previously shown to localize to dysfunctional telomeres (Dimitrova et al., 2008). As expected, ectopically expressed 53BP1 co-localized with telomeres in the vast majority of TRF2-deficient cells (Figure 2B and C). Similarly, 8 of the selected proteins (Rad18, Rap80, Senp7, Ring1b, Pml, Fam50a, Fancd2 and Ppp2r5d) localized at telomeres upon TRF2 depletion, while the remaining 7 showed either no detectable co-localization or a diffuse nuclear staining (Figure 2B and C). Nucleoplasmic extraction experiments revealed tight association of Rad18, Rap80, Pml, Senp7, Ring1b and Fancd2 with dysfunctional telomeres (Supplemental Figure 2).
Figure 2. Validation of novel factors that localize at dysfunctional telomeres.
A. List of proteins identified by PICh as enriched at dysfunctional telomeres. Average number of spectra identified at TRF2 deficient (OHT) or proficient (NT) telomeres is indicated. Proteins were ranked based on their relative enrichment at TRF2 depleted telomeres and normalized to absolute expression of the protein in mouse cells (PaxDB database). Proteins highlighted in yellow represent DNA damage response factors known to localize at dysfunctional telomeres. Proteins highlighted in blue represent proteins selected for validation. B. Localization of ectopically expressed tagged proteins (myc/GFP) (stained in red) to telomere repeats (TTAGGG, stained in green). TRF2F/F Rosa26 CRE-ER MEFs infected with the indicated constructs were treated with OHT and harvested 3 days later. Scale bar 5 μm. C. Quantification of data shown in B: cells with 5 or more foci co-localizing with telomeres were counted as positive. Error bars represent standard deviation (SD) of average of three independent experiments.
See also Figure S2.
A role for Ring1b in the onset of end-to-end chromosome fusions
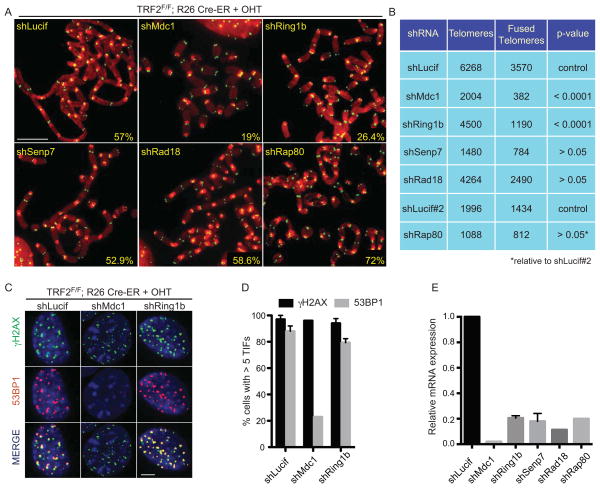
TRF2-depleted cells undergo dramatic end-to-end chromosome fusions (Celli and de Lange, 2005). To test whether any of the factors identified at TRF2-depleted telomeres plays a role in this process we infected cells with specific shRNAs and tested the frequency of end-to-end chromosome fusions on metaphase spreads (Figure 3A). As a positive control, we used an shRNA directed against MDC1 that results in reduced levels of NHEJ-mediated telomere fusions (Dimitrova and de Lange, 2006). Strikingly, this analysis revealed that cells with reduced Ring1b expression showed approximately a 50% reduction in the rate of NHEJ-mediated telomere fusions (Figure 3A, B and E). In contrast efficient knockdown of Rad18, Rap80 or Senp7 did not show a significant effect on the rate of telomere fusions. The effect of Ring1b on telomere fusion was further confirmed using telomere restriction fragment (TRF) analysis on southern blots (Supplemental Figure 3A). Importantly, we exclude that the observed defect in NHEJ efficiency reflects an alteration in cell cycle progression caused by reduced Ring1b levels based on FACS analysis (Supplemental Figure 3B). To test whether the NHEJ defects observed upon Ring1b depletion could be due to altered DNA damage signaling at TRF2-deficient telomeres, we tested the induction of phosphorylated H2AX (γH2AX) and recruitment of 53BP1 in the TRF2F/F Rosa26 CRE-ER cells treated with tamoxifen and infected with the shRNA construct against Ring1b. As a control, we used a previously validated shRNA against MDC1 that affects the recruitment of 53BP1 at TRF2 depleted telomeres (Dimitrova and de Lange). Knockdown of Ring1b had no significant effect on the dynamics of γH2AX or 53BP1 to TRF2-depleted telomeres, while depletion of MDC1 resulted in impaired recruitment of 53BP1 as previously described (Figure 3C, D and E). In addition, Ring1b depletion did not impair recruitment of the 53BP1 downstream effector RIF1 (Chapman et al., 2013; Escribano-Diaz et al., 2013; Zimmermann et al., 2013) to dysfunctional telomeres, excluding that the NHEJ defects observed in Ring1b depleted cells are due to impaired Rif1 recruitment (Supplemental Figure 3C and D). These experiments suggest that Ring1b might play a novel role at dysfunctional telomeres independent of the dynamics of γH2AXand 53BP1 at these sites.
Figure 3. Ring1b plays a critical role in the response to dysfunctional telomeres.
A. Metaphase spreads were harvested from TRF2F/F Rosa26 CRE-ER MEFs infected with a control lentiviral construct (shLucif) or with the indicated shRNA constructs and treated with OHT for 96 hours. Chromosomes were stained with a PNA probe complementary to telomeric repeats (green) and DAPI (red). Percentages of fused chromosome ends are indicated. Scale bar 5 μm. B. Quantification of data shown in panel A. C. TRF2F/F Rosa26 CRE-ER MEFs infected with the indicated shRNA constructs were treated with OHT for 72 hours, fixed and stained for γH2AX (green), 53BP1 (red) and DAPI (blue). Scale bar 5 μm. D. Quantification of the data shown in C (cells with 5 or more γH2AX/53BP1 foci were counted as positive). Error bars represent SD of average of three independent experiments. E. Quantification of relative mRNA levels of the indicated genes in cells infected with the indicated shRNA constructs, normalized to shLucif as control.
See also Figure S3.
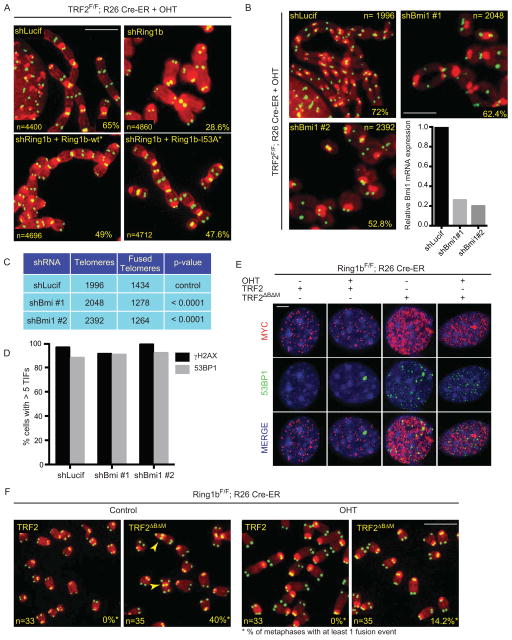
To exclude the possibility that the observed phenotype is caused by an off-target effect, we generated an shRNA-resistant allele of Ring1b (Ring1b-wt*). Expression of Ring1b-wt* in TRF2 deficient cells infected with shRing1b rescued the NHEJ defects, thus excluding potential off target effects (Figure 4A and Supplemental Figure 4A). Ring1b is a RING Finger E3 ubiquitin ligase of the Polycomb Repressive Complex 1 (PRC1) that acts in concert with Bmi1 to ubiquitinate histone H2A on K119 (H2AK119ub), via its RING Finger domain, and to promote gene silencing and chromatin compaction (Cao et al., 2005; Stock et al., 2007; Vidal, 2009; Wang et al., 2004). In agreement with our observation that Ring1b localizes to chromosome ends, we observed an enrichment of the H2AK119ub mark at telomeres compared to other genomic loci by ChIP (Supplemental Figure 4B–D). To test whether the enzymatic activity of Ring1b is required to promote NHEJ at TRF2 depleted cells we complemented Ring1b depleted cells with an sh-RNA-resistant, catalytically inactive mutant allele of Ring1b, carrying a mutation in the RING domain (Ring1b-I53A*) (Buchwald et al., 2006). Expression of Ring1b-I53A* in TRF2 deficient cells rescues the NHEJ defect observed in the absence of Ring1b to levels that are comparable to ones observed in cells expressing the wild type Ring1b-wt* allele (Figure 4A and Supplemental Figure 4A). These data indicate that the catalytic activity of Ring1b is dispensable for its role in promoting NHEJ at dysfunctional telomeres. Next, we tested whether inhibition of Ring1b binding partner Bmi1 by shRNA would affect NHEJ-mediated telomere fusions. Indeed, expression of two independent shRNA constructs directed against Bmi1 resulted in a significant reduction in the rate of telomere fusions in TRF2 null cells (Figure 4B and C). Bmi1 inhibition did not affect phosphorylation of H2AX or recruitment of 53BP1 at TRF2 depleted telomeres (Figure 4D), similarly to what was observed for the inhibition of Ring1b. To further confirm our findings we tested whether cells lacking Ring1b would exhibit defects in NHEJ-mediated repair of telomeres. Expression of a dominant negative allele of TRF2 in Ring1bF/F; Rosa26 CRE ER MEFs resulted in DNA damage activation at telomeres both in Ring1b deficient or Ring1b proficient cells (Figure 4E and Supplemental Figure 4E–G). In contrast, the occurrence of end-to-end chromosome fusions upon expression of the TRF2 dominant negative allele was severely reduced in Ring1b deficient cells (Figure 4F).
Figure 4. The Ring1b/Bmi1 heterodimer acts on the chromatin at dysfunctional telomeres.
A. Metaphase spreads of TRF2F/F Rosa26 CRE-ER MEFs infected with shLuciferase or shRing1b and Ring1b-wt* or Ring1b-I53A* and treated with OHT for 96 hours. Percentages of fused chromosome ends are indicated. Scale bar 5 μm. B. Metaphase spreads of TRF2F/F Rosa26 CRE-ER MEFs infected with shRNA constructs directed against Bmi1 and treated with OHT for 96 hours. Percentages of fused chromosome ends are indicated. Scale bar 5 μm. Graph shows quantification of relative Bmi1 mRNA levels measured by qPCR, normalized to shLucif as control. C. Quantification of end-to-end chromosome fusions in B. D. Quantification of γH2AX- and 53BP1-positive TRF2F/F Rosa26 CRE-ER MEFs infected as described in B, treated with OHT for 72 hours, fixed and stained for γH2AX and 53BP1 (cells with 5 or more γH2AX/53BP1 were counted as positive).
E. Ring1bF/F Rosa26 CRE-ER MEFs (−/+ OHT, 96 hours) were infected with wild type TRF2 or a TRF2 dominant negative allele (TRF2ΔBΔM) as indicated, fixed and stained for myc (red), 53BP1 (green) and DAPI (blue). Scale bar 5 μm. F. Metaphase spreads of Ring1bF/F Rosa26 CRE-ER MEFs treated as in E were stained for telomeric DNA (Green) to detect end-to-end chromosome fusions (arrowheads). Number of metaphases analyzed and percentage of metaphases with at least 1 fusion event are indicated. Scale bar 5 μm.
See also Figure S4.
Our results suggest that the presence of the heterodimer Ring1b-Bmi1 is required for efficient NHEJ at chromosome ends.
Ring1b plays a critical role for efficient DNA repair of heterochromatin loci
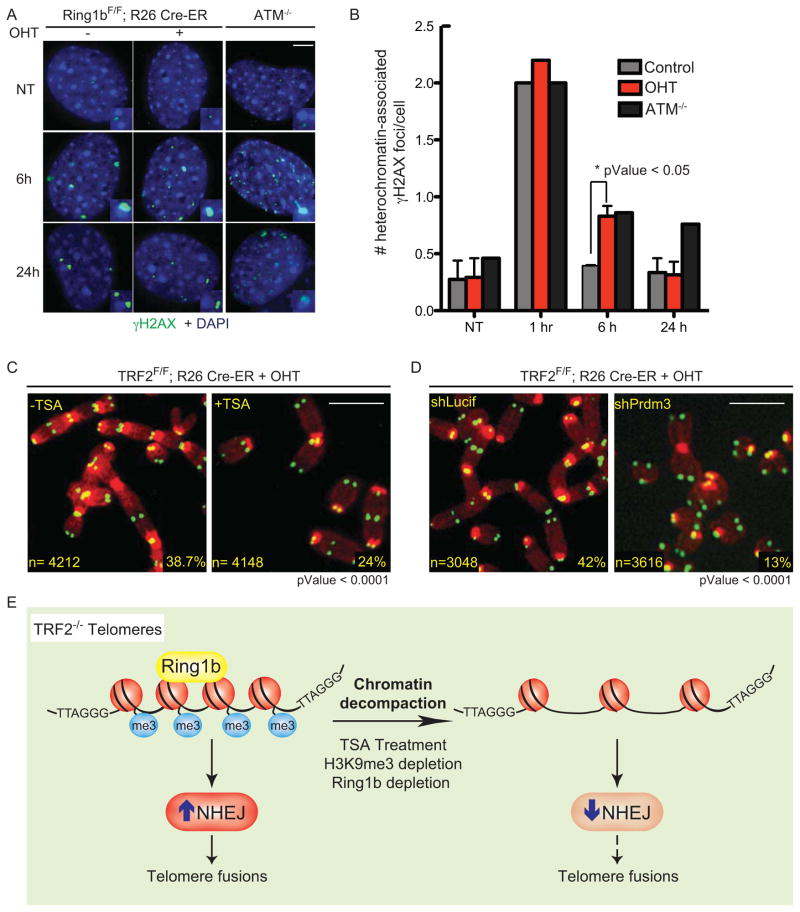
Next, we asked whether Ring1b deficient cells show defective DNA repair throughout the genome. Ring1b null cells were treated with gamma irradiation to induce random DNA damage. As controls, we used wild type cells and NHEJ-defective cells (Lig4-null) (Kuhne et al., 2004). DNA repair efficiency was assayed by Fraction of Activity Released (FAR) assay (Gulston et al., 2002) (Supplemental Figure 5A and B). Our results show that Ring1b-null cells do not show any significant defect in DNA repair when compared to wild type cells. In contrast, Lig4-null cells displayed the expected defect in DNA repair (Supplemental Figure 5A and B). Next, we asked whether Ring1b activity is required for the repair of DNA damage occurring at other heterochromatin loci. To test this hypothesis we irradiated Ring1bF/F; Rosa26 CRE ER MEFs and assayed cells for the persistence of the DNA damage marker γH2AX over time at “chromocenters”, cytological distinct structures that correspond to pericentric and centromeric heterochromatin (Guenatri et al., 2004). As a control we included ATM−/− cells that were previously shown to have a defect in DNA repair at heterochromatin loci (Goodarzi et al., 2008). Our results show that Ring1b null cells, similarly to ATM−/− cells, show a significant persistence of heterochromatin-associated γH2AX foci at 6 hours after irradiation (Figure 5A and B). These data suggest that Ring1b activity is required for efficient repair of DNA damage occurring at heterochromatin loci. Notably at 24 hours post irradiation Ring1b−/− cells repaired most DNA damage lesions as assessed by γH2AX foci dissipation. In agreement with the FAR assay data, Ring1b−/− cells did not show a defect in DNA repair at non-heterochromatin loci, as shown by the dissipation of γH2AX foci throughout the nuclei (Supplemental Figure 5C). In conclusion our data suggest that the role of Ring1b in DNA repair is specific for heterochromatin loci.
Figure 5. Compact chromatin favors NHEJ-mediated repair of dysfunctional telomeres.
A. Confluent, stationary-phase Ring1bF/F Rosa26 CRE-ER MEFs untreated or treated with tamoxifen (−/+ OHT) were left untreated (NT) or irradiated (2 Gy) and harvested at the indicated time points post irradiation. ATM−/− MEFs were used as control. Cells were fixed and stained for γH2AX (green) and DAPI (blue). Scale bar 5 μm. B. Quantification of the total number of heterochromatin-associated γH2AX foci in cells treated as described in A. Error bars represent SD of average of three independent experiments. C. Metaphase spreads of TRF2F/F Rosa26 CRE-ER MEFs treated with OHT for 72 hours and when indicated with TSA (18 hours). Percentages of fused chromosome ends are indicated. Scale bar 5 μm. D. Metaphase spreads of TRF2F/F Rosa26 CRE-ER MEFs infected with a shRNA against Prdm3 (shPrdm3) or a control shRNA (shLucif) and treated with OHT for 72 hours. Scale bar 5 μm. E. Model for the role of chromatin compaction in NHEJ-mediated repair of dysfunctional telomeres.
See also Figure S5.
Chromatin compaction activity plays an important role for efficient DNA repair of dysfunctional telomeres
Ring1b is associated to chromatin compaction and gene silencing (Vidal, 2009) and has been shown to be required for the maintenance of a compact chromatin state at Hox loci in embryonic stem cells (ESCs) (Eskeland et al., 2010). The chromatin context in which DNA damage occurs has a major impact on the pathway and the efficiency of DNA repair activities, with closed chromatin favoring the NHEJ pathway (Chiolo et al., 2011; Miller et al., 2010; Soria et al., 2012). We therefore hypothesized that reduced levels of Ring1b could lead to reduced levels of DNA repair at telomeres due to changes in the heterochromatic state. To test this hypothesis we treated TRF2F/F Rosa CRE ER MEFs with the histone deacetylase inhibitor Trichostatin A (TSA) to decompact chromatin. TSA treatment did not have a significant effect on the recruitment of 53BP1 to TRF2 depleted telomeres (Supplemental Figure 5D). However, TSA treatment significantly reduced the percentage of telomere fusions (Figure 5C). FACS analysis excluded that TSA treatment affects NHEJ indirectly by altering cell cycle progression (Supplemental Figure 5E).
To further validate our hypothesis that loss of chromatin compaction would result in reduction of NHEJ at dysfunctional telomeres we used Suv39 double null (dn) MEFs (Peters et al., 2001). The lysine methyltransferases (KMTs) Suv39h1 and Suv39h2 govern H3K9 trimethylation at pericentric heterochromatin (Peters et al., 2001) and were shown to be important for H3K9me3 deposition at telomeres (Garcia-Cao et al., 2004). The presence of trimethylated histone H3 (H3K9me3) is a hallmark of mammalian heterochromatin (Bannister et al., 2001; Lachner et al., 2001; Rea et al., 2000). Suv39dn cells display loss of heterochromatic features such as loss of heterochromatic association of HP1 proteins and partial decondensation of heterochromatic foci (Lachner et al., 2001; Pinheiro et al., 2012). Expression of a dominant negative allele of TRF2 in Suv39 dn MEFs also resulted in a lower occurrence of end-to-end chromosome fusions relative to the wild type control (Supplemental Figure 5F and G). Moreover, shRNA-mediated depletion of the KMT Prdm3, that was shown to prevent Suv39h-mediated H3K9 trimethylation resulting in chromatin decompaction at mouse chromocenters (Pinheiro et al., 2012), significantly reduced the percentage of fused chromosomes in TRF2 deficient cells relative to control cells (Figure 5D and Supplemental Figure 5H). Collectively these results suggest that chromatin compaction is a critical determinant for the frequent occurrence of NHEJ-mediated fusions at dysfunctional telomeres (See schematic model in Figure 5E).
Discussion
Our data reveal the dynamic changes that occur at the level of chromatin upon the induction of DNA damage at mammalian telomeres. We identified 345 factors that are significantly lost upon TRF2 depletion at telomeric chromatin. These included TRF2 and its associated proteins RAP1 and Apollo. In addition we identified one novel factor, CDCA8 that is significantly affected by the removal of TRF2. We identified 471 factors that are present at telomeres regardless of the TRF2 status. These factors included DNA metabolism genes, such as Histones, DNA polymerases, and telomere associated proteins that can be recruited to telomeric repeats independently of TRF2 such as the shelterin components TRF1, TIN2, TPP1, POT1 and the telomere associated factors Atrx and Daxx (Lewis et al., 2010; Wong et al., 2010). Finally, our isolation identified several known DNA damage response factors that relocalize to telomeres following TRF2 deletion and several additional proteins that were not previously associated with the response to telomere dysfunction. Our validation identified 8 novel proteins that relocalize to dysfunctional telomeres. However, it is important to note that a number of known DNA damage proteins that have been shown to play a crucial role in the response to telomere dysfunction were not identified in our analysis. Notably, RNF8 and RNF168, which are required for the recruitment of 53BP1, are missing from our isolations. This could be explained by the transient nature of the interaction between these ubiquitin ligases at their DNA damage target sites. Nevertheless, the absence of such critical factors represents a limitation of our approach that most likely indicates the lack of other critical factors recruited to dysfunctional telomeres. An additional limitation of the PICh technique for this type of analysis is the requirement for a very large amount of starting material for each individual experiment (over 5*10^8 cells) which limits the number of conditions and time points that can be tested. In addition, we found a large number of contaminants in our isolations that overlap with the commonly found contaminants in MS experiments (Mellacheruvu et al., 2013).
Despite these limitations, this approach represents the first unbiased identification of the dynamic changes of chromatin composition upon the onset of DNA damage. As such, the lists of proteins that we identified will represent a valuable resource not only to the telomere biology field but also to researchers studying general DNA repair reactions and genome stability pathways that act throughout the genome.
Finally, we have found a significant role for the polycomb protein Ring1b in the onset of NHEJ at telomeres. These observations further substantiate previous observations of a role for these factors in the response to DNA damage (Chagraoui et al., 2011; Chou et al., 2010; Facchino et al., 2010; Ginjala et al., 2011; Ismail et al., 2010). Strikingly, however, our data suggest that in the absence of Ring1b the signaling events downstream of ATM activation such as γH2AX and 53BP1 recruitment are not affected. These data are in agreement with the observation that recruitment of 53BP1 is independent from Ring1b-mediated H2A ubiquitylation (Gatti et al., 2012; Mattiroli et al., 2012). Our data suggest that Ring1b plays a critical role to maintain a compact chromatin status that is permissive for NHEJ. Indeed previous reports have shown that compact chromatin is more prone to undergo NHEJ-mediated DNA repair (Chiolo et al., 2011; Miller et al., 2010). Ring1b activity in chromatin compaction has been well-established in the context of gene expression at Hox loci (Eskeland et al., 2010). Notably, it has been reported that the E3 activity of Ring1b is not required for chromatin compaction, similarly to what we found in the context of promotion of NHEJ at dysfunctional telomeres. We found that treatment of cells with the HDAC inhibitor TSA resulted in reduced levels of NHEJ at telomeres, thus further suggesting that chromatin compaction is critical for NHEJ at dysfunctional telomeres. Similarly, we found that reducing H3K9me3 levels in cells depleted of TRF2, either by depletion of Prdm3 or by the use of Suv39dn MEFs, impaired the formation of NHEJ-mediated end-to-end chromosome fusions. These results are in agreement with previous results showing a role for HDAC1 and HDAC2 in NHEJ-dependent DNA repair at genome-wide sites of DNA lesion (Miller et al., 2010) as well as with the observation that TSA treatment shifts the repair choice towards the homologous recombination pathway (Tang et al., 2013). The heterochromatic status of telomeres would therefore explain why telomere dysfunction preferentially triggers NHEJ-mediated DNA repair and suggests that alterations in chromatin status could be responsible for the increased homologous recombination observed at telomeres in tumors that engage the alternative telomere-lengthening (ALT) pathway (Cesare and Reddel, 2008). In line with this hypothesis, increased rates of telomere sister chromatid exchange (T-SCE) were recently reported in ALT tumor cells subjected to TSA treatment (Jung et al., 2013).
Experimental Procedures
Telomeric Chromatin isolation by PICh
Proteomics of isolated chromatin segments (PICh) was carried out as previously described (Dejardin and Kingston, 2009) with the following modifications: 5 × 108 MEFs were used per sample; two sonication cycles were performed using a Misonix S-4000. First cycle (Power 70%), second Cycle (Power 40%). Each cycle consisted of 15 seconds on and 45 seconds off for a total process time of 2 minutes. Hybridization with telomeric probe: A PNA probe (0.6 μM) was used (Desthiobiotin-112 atoms spacer-TTAGGGTTAGGGTTAGGGTTAGGG) using the conditions described before with the exception that denaturation was carried out at 65°C for 6 minutes.
Mass Spectrometry Analysis
For mass spectrometry analysis samples were denatured, reduced and alkylated prior to an overnight digestion with trypsin. Peptide mixtures were analyzed by nanoflow liquid chromatography mass spectrometry using an Eksigent nanopump (Dublin, CA) and LTQ-Orbitrap mass spectrometer (Thermo Scientific, Bremen, Germany) using a 7 step MudPIT separation. MS/MS spectra were collected in a data dependent fashion and resulting spectra were extracted using RawXtract. Protein identification was done with Integrated Proteomics Pipeline (IP2) by searching against UniProt Human database and filtering to 1% false positive at the spectrum level using DTASelect.
MEFs and shRNA infections
TRF2F/F Rosa26 CRE-ER MEFs were previously described (Okamoto et al., 2013). Ring1bF/F Rosa CRE ER MEFs were derived from a cross between Ring1b conditional mice (de Napoles et al., 2004) and Rosa26 CRE-ER mice (Ventura et al., 2007). CRE activation was obtained treating cells with 4-hydroxytamoxifen (OHT) at a final concentration of 0.6 μM. Suv39dn iMEFs and wild type control iMEFs were a kind gift from T. Jenuwein.
shRNAs were cloned into the pLKO-puromycin lentiviral vector (for sequences see Supplemental Experimental Procedures). shRNA infections were performed as previously described (Okamoto et al., 2013). Knockdown efficiency was analyzed by qPCR using Roche Sybr Green Mastermix according to manufacturer’s instructions on a LightCycler 480 II (Roche).
Immunofluorescence (IF), IF-FISH, Chromatin IP (ChIP) and immunoblotting
Immunoblots and IF were performed using the protocols described previously (Celli and de Lange, 2005). For Ring1b detection by immunoblot extracts were prepared according to (Vella et al., 2013). For IF, where indicated cells were permeabilized with Triton X-100 buffer (for details see Supplemental Experimental Procedures). FISH-IF staining was performed using the protocol developed by Sedivy and colleagues (Herbig et al., 2004). For quantification at least 100 cells were counted following FISH-IF analysis. Cells with at least 5 foci co-localizing with telomere DNA or TRF1 were scored as positive. Error bars indicate standard deviations and derive from averages of three independent experiments. P values were calculated using Student’s t test.
ChIP experiments were performed as described (Loayza and De Lange, 2003; O’Sullivan et al.; Ye and de Lange, 2004).
Full details of all other experimental procedures are given in Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Characterization of the changes in telomeric chromatin upon DNA damage induction
Ring1b promotes DNA repair at telomeres and other heterochromatic regions
Compact chromatin is a favorable environment for NHEJ-mediated DNA repair
Acknowledgments
We thank Michael Nicholas Boddy, Sara Buonomo, Titia de Lange, Ronald Hay, Thomas Jenuwein, Akio Koizumi, Yoko Koseki, Agnel Sfeir and Bertrand Tan for providing critical reagents. We thank Jerome Dejardin for suggestions on PICh purification. We are grateful to Claire Attwooll, Daniele Fachinetti, Kyle Miller and Agnel Sfeir for critical reading of the manuscript. This work was supported by a Pew Scholars Award (E.L.D.), the Novartis Advanced Discovery Institute (E.L.D.), NIH AG038677 (E.L.D.), P41 GM103533 (J.R.Y.), the Italian Association for Cancer Research and the Italian Ministry of Health (D.P.) and a fellowship from FIRC (A.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- Attwooll CL, Akpinar M, Petrini JH. The mre11 complex and the response to dysfunctional telomeres. Molecular and cellular biology. 2009;29:5540–5551. doi: 10.1128/MCB.00479-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augereau A, T’Kint de Roodenbeke C, Simonet T, Bauwens S, Horard B, Callanan M, Leroux D, Jallades L, Salles G, Gilson E, et al. Telomeric damage in early stage of chronic lymphocytic leukemia correlates with shelterin dysregulation. Blood. 2011;118:1316–1322. doi: 10.1182/blood-2010-07-295774. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. doi: 10.1038/35065138. [DOI] [PubMed] [Google Scholar]
- Buchwald G, van der Stoop P, Weichenrieder O, Perrakis A, van Lohuizen M, Sixma TK. Structure and E3-ligase activity of the Ring-Ring complex of polycomb proteins Bmi1 and Ring1b. The EMBO journal. 2006;25:2465–2474. doi: 10.1038/sj.emboj.7601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi G, Perego P, Carenini N, Nakanishi M, Chessa L, Chen J, Khanna K, Delia D. Activation of ATM and Chk2 kinases in relation to the amount of DNA strand breaks. Oncogene. 2004;23:7691–7700. doi: 10.1038/sj.onc.1207986. [DOI] [PubMed] [Google Scholar]
- Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Molecular cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol. 2012;13:789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol. 2005;7:712–718. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- Cesare AJ, Reddel RR. Telomere uncapping and alternative lengthening of telomeres. Mech Ageing Dev. 2008;129:99–108. doi: 10.1016/j.mad.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Chagraoui J, Hebert J, Girard S, Sauvageau G. An anticlastogenic function for the Polycomb Group gene Bmi1. Proc Natl Acad Sci U S A. 2011;108:5284–5289. doi: 10.1073/pnas.1014263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KM, Zhang Z. Leucine-rich repeat and WD repeat-containing protein 1 is recruited to pericentric heterochromatin by trimethylated lysine 9 of histone H3 and maintains heterochromatin silencing. J Biol Chem. 2012;287:15024–15033. doi: 10.1074/jbc.M111.337980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Barral P, Vannier JB, Borel V, Steger M, Tomas-Loba A, Sartori AA, Adams IR, Batista FD, Boulton SJ. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Molecular cell. 2013;49:858–871. doi: 10.1016/j.molcel.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, de Solorzano CO, Knowles D, Jones A, Chou W, Rodriguez EG, Kuo WL, Ljung BM, Chew K, Myambo K, et al. In situ analyses of genome instability in breast cancer. Nat Genet. 2004;36:984–988. doi: 10.1038/ng1409. [DOI] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Chiolo I, Minoda A, Colmenares SU, Polyzos A, Costes SV, Karpen GH. Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell. 2011;144:732–744. doi: 10.1016/j.cell.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DM, Adamson B, Dephoure NE, Tan X, Nottke AC, Hurov KE, Gygi SP, Colaiacovo MP, Elledge SJ. A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci U S A. 2010;107:18475–18480. doi: 10.1073/pnas.1012946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R, Nesterova TB, Silva J, Otte AP, Vidal M, et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell. 2004;7:663–676. doi: 10.1016/j.devcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Dejardin J, Kingston RE. Purification of proteins associated with specific genomic Loci. Cell. 2009;136:175–186. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denchi EL, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature. 2007;448:1068–1071. doi: 10.1038/nature06065. [DOI] [PubMed] [Google Scholar]
- Di Virgilio M, Callen E, Yamane A, Zhang W, Jankovic M, Gitlin AD, Feldhahn N, Resch W, Oliveira TY, Chait BT, et al. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science. 2013;339:711–715. doi: 10.1126/science.1230624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, Chen YC, Spector DL, de Lange T. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature. 2008;456:524–528. doi: 10.1038/nature07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, de Lange T. MDC1 accelerates nonhomologous end-joining of dysfunctional telomeres. Genes Dev. 2006;20:3238–3243. doi: 10.1101/gad.1496606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, de Lange T. Cell cycle-dependent role of MRN at dysfunctional telomeres: ATM signaling-dependent induction of nonhomologous end joining (NHEJ) in G1 and resection-mediated inhibition of NHEJ in G2. Molecular and cellular biology. 2009;29:5552–5563. doi: 10.1128/MCB.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- Escribano-Diaz C, Orthwein A, Fradet-Turcotte A, Xing M, Young JT, Tkac J, Cook MA, Rosebrock AP, Munro M, Canny MD, et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Molecular cell. 2013;49:872–883. doi: 10.1016/j.molcel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Eskeland R, Leeb M, Grimes GR, Kress C, Boyle S, Sproul D, Gilbert N, Fan Y, Skoultchi AI, Wutz A, et al. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Molecular cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchino S, Abdouh M, Chatoo W, Bernier G. BMI1 confers radioresistance to normal and cancerous neural stem cells through recruitment of the DNA damage response machinery. J Neurosci. 2010;30:10096–10111. doi: 10.1523/JNEUROSCI.1634-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao M, O’Sullivan R, Peters AH, Jenuwein T, Blasco MA. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nat Genet. 2004;36:94–99. doi: 10.1038/ng1278. [DOI] [PubMed] [Google Scholar]
- Gatti M, Pinato S, Maspero E, Soffientini P, Polo S, Penengo L. A novel ubiquitin mark at the N-terminal tail of histone H2As targeted by RNF168 ubiquitin ligase. Cell Cycle. 2012;11:2538–2544. doi: 10.4161/cc.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginjala V, Nacerddine K, Kulkarni A, Oza J, Hill SJ, Yao M, Citterio E, van Lohuizen M, Ganesan S. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Molecular and cellular biology. 2011;31:1972–1982. doi: 10.1128/MCB.00981-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Molecular cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Gordon KE, Ireland H, Roberts M, Steeghs K, McCaul JA, MacDonald DG, Parkinson EK. High levels of telomere dysfunction bestow a selective disadvantage during the progression of human oral squamous cell carcinoma. Cancer research. 2003;63:458–467. [PubMed] [Google Scholar]
- Guenatri M, Bailly D, Maison C, Almouzni G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol. 2004;166:493–505. doi: 10.1083/jcb.200403109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulston M, Fulford J, Jenner T, de Lara C, O’Neill P. Clustered DNA damage induced by gamma radiation in human fibroblasts (HF19), hamster (V79-4) cells and plasmid DNA is revealed as Fpg and Nth sensitive sites. Nucleic Acids Res. 2002;30:3464–3472. doi: 10.1093/nar/gkf467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Molecular cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Ismail IH, Andrin C, McDonald D, Hendzel MJ. BMI1-mediated histone ubiquitylation promotes DNA double-strand break repair. J Cell Biol. 2010;191:45–60. doi: 10.1083/jcb.201003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung AR, Yoo JE, Shim YH, Choi YN, Jeung HC, Chung HC, Rha SY, Oh BK. Increased alternative lengthening of telomere phenotypes of telomerase-negative immortal cells upon trichostatin--a treatment. Anticancer research. 2013;33:821–829. [PubMed] [Google Scholar]
- Kirwan M, Dokal I. Dyskeratosis congenita, stem cells and telomeres. Biochim Biophys Acta. 2009;1792:371–379. doi: 10.1016/j.bbadis.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhne M, Riballo E, Rief N, Rothkamm K, Jeggo PA, Lobrich M. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer research. 2004;64:500–508. doi: 10.1158/0008-5472.can-03-2384. [DOI] [PubMed] [Google Scholar]
- Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410:116–120. doi: 10.1038/35065132. [DOI] [PubMed] [Google Scholar]
- Lenain C, Bauwens S, Amiard S, Brunori M, Giraud-Panis MJ, Gilson E. The Apollo 5′ exonuclease functions together with TRF2 to protect telomeres from DNA repair. Current biology: CB. 2006;16:1303–1310. doi: 10.1016/j.cub.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci U S A. 2010;107:14075–14080. doi: 10.1073/pnas.1008850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Oestreich S, de Lange T. Identification of human Rap1: implications for telomere evolution. Cell. 2000;101:471–483. doi: 10.1016/s0092-8674(00)80858-2. [DOI] [PubMed] [Google Scholar]
- Lin TT, Letsolo BT, Jones RE, Rowson J, Pratt G, Hewamana S, Fegan C, Pepper C, Baird DM. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: evidence for a telomere crisis. Blood. 2010;116:1899–1907. doi: 10.1182/blood-2010-02-272104. [DOI] [PubMed] [Google Scholar]
- Loayza D, De Lange T. POT1 as a terminal transducer of TRF1 telomere length control. Nature. 2003;423:1013–1018. doi: 10.1038/nature01688. [DOI] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. Keeping telomerase in its place. Nat Med. 2002;8:934–936. doi: 10.1038/nm0902-934. [DOI] [PubMed] [Google Scholar]
- Mattiroli F, Vissers JH, van Dijk WJ, Ikpa P, Citterio E, Vermeulen W, Marteijn JA, Sixma TK. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell. 2012;150:1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Iacobuzio-Donahue CA, Montgomery EA, Westra WH, Chan TY, Ronnett BM, De Marzo AM. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, Miteva YV, Hauri S, Sardiu ME, Low TY, et al. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nature methods. 2013;10:730–736. doi: 10.1038/nmeth.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, Jackson SP. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nature structural & molecular biology. 2010;17:1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- O’Sullivan RJ, Kubicek S, Schreiber SL, Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nature structural & molecular biology. 17:1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Bartocci C, Ouzounov I, Diedrich JK, Yates JR, Iii, Denchi EL. A two-step mechanism for TRF2-mediated chromosome-end protection. Nature. 2013 doi: 10.1038/nature11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Peuscher MH, Jacobs JJ. DNA-damage response and repair activities at uncapped telomeres depend on RNF8. Nat Cell Biol. 2011;13:1139–1145. doi: 10.1038/ncb2326. [DOI] [PubMed] [Google Scholar]
- Pfeiffer V, Crittin J, Grolimund L, Lingner J. The THO complex component Thp2 counteracts telomeric R-loops and telomere shortening. The EMBO journal. 2013 doi: 10.1038/emboj.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro I, Margueron R, Shukeir N, Eisold M, Fritzsch C, Richter FM, Mittler G, Genoud C, Goyama S, Kurokawa M, et al. Prdm3 and Prdm16 are H3K9me1 methyltransferases required for mammalian heterochromatin integrity. Cell. 2012;150:948–960. doi: 10.1016/j.cell.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Ramsay AJ, Quesada V, Foronda M, Conde L, Martinez-Trillos A, Villamor N, Rodriguez D, Kwarciak A, Garabaya C, Gallardo M, et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nat Genet. 2013;45:526–530. doi: 10.1038/ng.2584. [DOI] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28:155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- Sarper N, Zengin E, Kilic SC. A child with severe form of dyskeratosis congenita and TINF2 mutation of shelterin complex. Pediatric blood & cancer. 2010;55:1185–1186. doi: 10.1002/pbc.22624. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Mutant dyskerin ends relationship with telomerase. Science. 1999;286:2284–2285. doi: 10.1126/science.286.5448.2284. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE. Telomeres and telomerase: implications for cancer and aging. Radiat Res. 2001;155:188–193. doi: 10.1667/0033-7587(2001)155[0188:tatifc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shen Z, Sathyan KM, Geng Y, Zheng R, Chakraborty A, Freeman B, Wang F, Prasanth KV, Prasanth SG. A WD-repeat protein stabilizes ORC binding to chromatin. Molecular cell. 2010;40:99–111. doi: 10.1016/j.molcel.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Polo SE, Almouzni G. Prime, repair, restore: the active role of chromatin in the DNA damage response. Molecular cell. 2012;46:722–734. doi: 10.1016/j.molcel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, Brockdorff N, Fisher AG, Pombo A. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat Cell Biol. 2007;9:1428–1435. doi: 10.1038/ncb1663. [DOI] [PubMed] [Google Scholar]
- Suram A, Kaplunov J, Patel PL, Ruan H, Cerutti A, Boccardi V, Fumagalli M, Di Micco R, Mirani N, Gurung RL, et al. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. The EMBO journal. 2012;31:2839–2851. doi: 10.1038/emboj.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Current biology: CB. 2003;13:1549–1556. doi: 10.1016/s0960-9822(03)00542-6. [DOI] [PubMed] [Google Scholar]
- Tang J, Cho NW, Cui G, Manion EM, Shanbhag NM, Botuyan MV, Mer G, Greenberg RA. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nature structural & molecular biology. 2013;20:317–325. doi: 10.1038/nsmb.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzot F, Callebaut I, Soulier J, Gaillard L, Azerrad C, Durandy A, Fischer A, de Villartay JP, Revy P. Function of Apollo (SNM1B) at telomere highlighted by a splice variant identified in a patient with Hoyeraal-Hreidarsson syndrome. Proc Natl Acad Sci U S A. 107:10097–10102. doi: 10.1073/pnas.0914918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overbeek M, de Lange T. Apollo, an Artemis-related nuclease, interacts with TRF2 and protects human telomeres in S phase. Current biology: CB. 2006;16:1295–1302. doi: 10.1016/j.cub.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Vella P, Scelfo A, Jammula S, Chiacchiera F, Williams K, Cuomo A, Roberto A, Christensen J, Bonaldi T, Helin K, et al. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Molecular cell. 2013;49:645–656. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Vidal M. Role of polycomb proteins Ring1A and Ring1B in the epigenetic regulation of gene expression. Int J Dev Biol. 2009;53:355–370. doi: 10.1387/ijdb.082690mv. [DOI] [PubMed] [Google Scholar]
- Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wang M, Weiss M, Simonovic M, Haertinger G, Schrimpf SP, Hengartner MO, von Mering C. PaxDb, a database of protein abundance averages across all three domains of life. Molecular & cellular proteomics: MCP. 2012;11:492–500. doi: 10.1074/mcp.O111.014704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LH, McGhie JD, Sim M, Anderson MA, Ahn S, Hannan RD, George AJ, Morgan KA, Mann JR, Choo KH. ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res. 2010;20:351–360. doi: 10.1101/gr.101477.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JZ, de Lange T. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nat Genet. 2004;36:618–623. doi: 10.1038/ng1360. [DOI] [PubMed] [Google Scholar]
- Zimmermann M, Lottersberger F, Buonomo SB, Sfeir A, de Lange T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science. 2013;339:700–704. doi: 10.1126/science.1231573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.