Abstract
Background
Keloids are benign dermal scars characterized by enhanced growth factor signaling, hyperproliferation activity and reduced extracellular matrix (ECM) deposition of hyaluronic acid. Our hypothesis is that high molecular weight HA can be used to replenish HA deposition in keloids thereby normalizing the keloid fibroblast phenotype.
Methods
One normal (NF1) fibroblast culture and five keloid (KF1, KF2, KF3, KF4, KF5) fibroblast cultures were analyzed for changes in hyperproliferation, growth factor production and extracellular matrix deposition following 72 hour treatment with or without 10 μg/ml HA.
Results
Proliferation activity decreased significantly in KF3 following HA treatment. Pro-collagen I expression in KF2 was decreased following HA treatment in association with changes in fiber arrangement to more parallel collagen bundles. In addition, HA demonstrated a downregulation on TGF-b1 growth factor expression in KF3 and KF4 and a decrease in active TGF-b1 release in KF2 and KF5 using ELISA.
Conclusion
Our data demonstrates that HA has the potential to normalize keloid fibroblast characteristic features such as hyperproliferation, growth factor production and ECM deposition depending on the specific genotype of the keloid fibroblast cell line. This study suggests that high molecular weight HA can be used to replenish HA deposition in keloid fibroblasts thereby decreasing fibrosis and ultimately decreasing keloid manifestation.
Keywords: Keloid, Scarring, Hyaluronic acid
Introduction
Excessive keloid scarring following dermal injury and its therapy continues to challenge the medical community. Keloids appear frequently (15–20%) in the darker skinned population including African Americans, Hispanics and Asians.1,2 Keloids are characterized by the extension of the developing scar tissue over the boundaries of the original injury.1 The benign characteristics of keloids can cause cosmetic disfigurement, functional impairment and can be associated with pruritus and pain. Current treatment options include excision of the keloid scar followed by a variety of therapies such as radiation and laser therapy, steroid injection, wound compression, cryotherapy and application of topical silicone or other dressings with anti-tumorigenic potential.3–6 Thus, a 50% recurrence is likely following most of these therapies better treatment options are desired.1,5,7 Keloid formation is characterized by an extensive fibroblast proliferation during and after the granulation phase accompanied by aberrant deposition of extracellular matrix (ECM) components such as fibronectin and collagen types I and III and enhanced microvascular occlusion.8–15 These keloid characteristic aberrations are thought to originate from alterations in growth factor production, e.g., TGF-β1 and FGF-1 as well as changes in cytokine signaling.15–17 In addition, keloid fibroblasts are deficient in the production of the ECM component hyaluronic acid (HA) that plays a crucial role in ECM organization and transmission of extracellular signals.18–21 High molecular weight HA > 107 Da that is synthesized during tissue proliferation, regeneration and during embryogenesis demonstrates anti-angiogenic, anti-inflammatory and immuno-suppressive characteristics and is thought to play a role in fetal scarless wound repair.22–25 In contrast, HA fragments of 6–20 kDa are characterized by their pro-angiogenic and pro-inflammatory actions.26–28 During wound healing, the hydroscopic characteristics of HA support hydration and the structural integrity of the ECM, thereby contributing to migration, adhesion and proliferation of cells within the granulation tissue. In addition to the regeneration of the cellular components within the wound bed, HA production is also important for the ECM reorganization, e.g., collagen deposition, cytokine and growth factor adhesion as well as recruitment of matrix metalloproteinases (MMPs) such as collagenases and gelatinases.6,29 Thus, decreased ECM deposition of HA is a major characteristic of keloid scarring, the replenishment of HA by external administration of high molecular weight HA is thought to represent a beneficial therapy for the prevention of scar formation.20,21 This study is designed to determine the ability of HA to reverse the keloid fibroblast deficiencies such as hyperproliferation activity and extensive ECM deposition to a more normal fibroblast phenotype.
Methods
Drug Treatment
Cells at seventy percent confluency were treated with serum-free medium (negative control) or serum-free medium supplemented with 10 μg/ml of high molecular weight hyaluronic acid (HA; molecular weight ∼107 Da), comparable to the in vivo HA concentration range found in fetal rabbit wounds.30 To avoid possible confounding by factors contained in fetal bovine serum that disturb the experimental setup, serum-free medium was used for all treatment conditions. The seventy percent fibroblast confluency at the onset of treatment was consistent with previously used experimental conditions and represents a state where cells are still able to proliferate and produce ECM components.31,32 To evaluate time-dependent changes in ECM deposition and mitogenic activities of keloid fibroblast cells in presence of HA, the exposure time of 72 hour HA treatment was used for all experiments.
Fibroblast Culture
Primary keloid fibroblast cultures were derived from the earlobes from pathological specimen of three male (KF1, KF3, and KF5) and two female (KF2 and KF4) black adult individuals (ages 21–40) following surgical keloid excision conforming to the NIH regulations for the use of human subjects.3 Biopsies were rinsed with PBS, minced into small fragment and samples were incubated for 16 hour at 4 °C in Dispase II (2.5 mg/ml in DMEM) followed by separation of the dermis from the epidermis using forceps. The dermal pieces were incubated with Collagenase D (3 mg/ml) for 3–4 hour at 37 °C under shaking. Any cells and pieces were collected by centrifugation and resuspended in trypsin/EDTA (0.5 mg/ml trypsin and 0.25 mm EDTA in PBS) for an additional 15–30 min at 37 °C with occasional shaking. Cells were washed in PBS followed by cultivation at 37 °C, 5% CO2 in DMEM (10% FBS; 1X Pen-Strep, Fungizone). As a control, one normal skin fibroblast cell line of a female individual with similar age to the keloid individuals (NF1; normal skin fibroblast culture) was purchased from the cell culture bank ATCC (CRL-2617 Manassas, VA). To avoid passage-related phenotypical changes, we only used cells from early passage (3–7). Cells were cultivated at 37 °C, 5% CO2 in DMEM (10% FBS; 1X Pen-Strep, Fungizone).
Microscopy
For microscopy, cells were grown on coverslips in 12-well plates. Following drug treatment, cells were fixed in 2% paraformaldehyde/0.1% Triton-X100. For immunocytochemical staining, samples were blocked for 1 hour with PBS supplemented with 2% bovine serum albumin (BSA) followed by incubation for 3 hour with primary polyclonal antibodies, i.e., collagen type I (L-19), collagen type III (C-15) (Santa Cruz Biotechnology, Santa Cruz, CA) in PBS/2% BSA. Following 3× washing, samples were incubated with the corresponding secondary antibodies consisting of F(ab′)2 fragments (host species donkey) conjugated to the fluorescent dye Cy2TM (Jackson ImmunoResearch, West Grove, PA) for 1 hour in PBS/2% BSA. After additional 3× washing, coverslips were mounted on slides. Images were acquired at 63X with an Optronics Magnafire CCD camera using a Leica DMR fluorescent microscope or at 63X using a Leica TCS SP2 confocal microscope. Volocity software (Improvision, Lexington, MA) was used to assess changes in expression of collagen.
CellTiter 96® Cell Viability/Proliferation Assay
The CellTiter 96® Cell Viability/Proliferation Assay (Molecular Probes, Eugene, OR) is a colorimetric assay for determining the number of proliferating viable cells. The assay is based on conversion of the colorimetric compound (3-(4,5-dimethyl-2yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; MTS) by metabolically active cells. Cells were grown in 12-well plates and exposed to the corresponding drug treatment. In the last hour of drug treatment, the medium was supplemented with 70 μl CellTiter 96® Aqueous One Solution Reagent per 1 ml of culture medium. Following 1 hour incubation, the absorbance at 490 nm was recorded using a Fusion™ Microplate Analyzer (Packard).
Protein Extraction
For Western blotting, cellular proteins were extracted according to the CelLytic™ NuCLEAR™ Extraction Kit (Sigma, St. Louis, MO). In brief, cells were lysed for 15 min at 4 °C in 150 μl hypotonic lysis buffer containing protease inhibitors and 1 μm DTT. After addition of Igepal CA-630 (final concentration 0.6% v/v), lysates were vortexed and centrifuged for 2 minute at 11,000 × g. The cytoplasmic supernatant was separated from the nuclear pellet. The nuclear pellet was dissolved in 50 μl extraction buffer, vortexed for 30 minute and centrifuged at 16,000 × g for 5 minute. The supernatant containing the nuclear fraction was pooled with the cytosolic fraction to obtain total cellular protein extracts. Protein concentrations of cell extracts were determined using the Bradford method.33
SDS-PAGE and Western blotting
Protein extracts (50 μg total) were separated on 10% or 15% SDS-polyacrylamide gels and transferred to polyvinylidenedifluoride (PVDF) membranes. PVDF membranes were incubated with the primary polyclonal antibodies that recognize both, pro- and active forms of ECM components and growth factors: collagen type I (L-19), collagen type III (C-15) (Santa Cruz Biotechnology, Santa Cruz, CA) and TGF-β1 (R&D Systems Minneapolis, MN) as well as the primary polyclonal antibody actin (I-19) that stains all forms of actin (loading control, Santa Cruz Biotechnology, Santa Cruz, CA). Staining with primary antibodies was followed by fluorescein-linked secondary antibody at 1:600 dilution and tertiary antibody anti-fluorescein AP conjugate 1:1000 according to the enhanced chemifluorescence (ECF) Western Blotting Kit (Amersham, Piscataway, NY). Following membrane scanning with a FUJIFLA2000 Fluorescence Imager, the antibody staining intensities/area of the immunostained protein bands were normalized to the housekeeping gene actin using densitometric analysis by ImageGauge 4.1 (Fuji Photo Film Co, Ltd).
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA for active TGF-β1 was prepared according to the Quantikine (R&D Systems, Minneapolis, MN) user manual. Samples were measured in triplicate and averaged. The ELISA setup included standard dilutions for TGF-β1 protein standards to determine the exact sample concentration. The ELISA is based on monoclonal primary antibodies specific for TGF-β1. Addition of sample to the ELISA plate will result in binding of existing TGF-β1 to the corresponding matrix-bound antibodies. Following several washing steps, bound TGF-β1 was detected by addition of polyclonal primary antibodies for TGF-β1 coupled to horseradish peroxidase for conversion of tetramethylbenzidine into a colorimetric product that was detected at 450 nm using a Fusion™ Universal Microplate Analyzer (Packard).
Statistical Analysis
A minimum of three independently grown samples of each normal fibroblast: NF1 and keloid fibroblast: KF1, KF2, KF3, KF4, and KF5 cultures were used for all experiments. The cell proliferation assay included three independently grown samples and three independent experiments. Due to the tremendous phenotypic differences of primary keloid fibroblast cultures observed in the preliminary data, fibroblast cultures were analyzed separately without combining KF1, KF2, KF3, KF4, and KF5 into a keloid fibroblast group. For volocity densitometric measurements, three-dimensional confocal images were taken of ten cells/independently grown samples with a total of three independently grown samples per primary culture. The data is presented as mean ± SEM. Prism 4TM Software for Macintosh (Graph Pad Software, Inc.) was used for two-tailed unpaired student's t-test. p < 0.05 was used as a criterion for significance.
Results
One normal (NF1) fibroblast culture from the abdominal skin of a black female adult was purchased from ATCC (CRL-2617). Five keloid fibroblast (KF1, KF2, KF3, KF4, and KF5) cultures were generated from the pathological specimens of three male (KF1, KF3, and KF5) and two female (KF2 and KF4) black adult individuals following surgical keloid excision from the earlobe (KF1-5; keloid fibroblast culture) in compliance with the NIH regulations for the use of human subjects.2
To determine HA-dependent effects on normal and keloid fibroblasts, cells were incubated for 72 hour with or without (control) high-molecular weight HA of μ107 Da at a concentration of 10 μg/ml in serum-free medium.
Effect of Hyaluronic Acid on Proliferation
Cell proliferation was determined using the CellTiter 96® proliferation assay that is based on metabolic conversion of a colorimetric dye representative for active proliferating cells. The proliferation assay demonstrated no changes in NF1 proliferation activity following HA treatment with 98 ± 5.81% of the non-treated control. In contrast, KF3 keloid fibroblasts demonstrated a significant decrease (p < 0.01) in proliferation activity following HA treatment (68.59 ± 2.06%) compared to the KF3 control (79.06 ± 2.8%). All other keloid cultures, e.g., KF1, KF2, KF4 and KF5 showed no significant changes in percent proliferation following HA treatment (Figure 1). The results suggest that HA has the ability to decrease hyperproliferation activity in selective keloid tissues.
Figure 1.
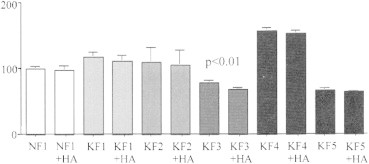
Proliferation activity of normal and keloid fibroblasts. Graph represents percent cell proliferation measure by CellTiter 96® proliferation assay in normal (NF1) and keloid (KF1, KF2, KF3, KF4, KF5) fibroblasts with (+HA) or without 72 hour hyaluronic acid treatment. The data is presented as mean ± SEM. Statistical analysis was done using a two-tailed unpaired student's t-test. p <0.05 was used as a criterion for significance.
Effect of Hyaluronic Acid on Collagen Production
To confirm the previous findings of other groups that demonstrated alterations in collagen I expression and fiber formation in keloid fibroblasts compared to normal fibroblasts, pro-collagen type I expression in normal and keloid fibroblasts was analyzed using immunocytochemical staining with a pro-collagen I primary antibody that also stains mature collagen fibers (collagen type I (L-19); Santa Cruz Biotechnology, Santa Cruz, CA). After staining, volumetric analysis of three-dimensional confocal images using Volocity software demonstrated an approximate 6 fold increase in percent pro-collagen I volume in keloid fibroblasts (13.53 ± 4.81%) compared to normal fibroblasts (2.07 ± 0.32%) (Figure 2). This data validates increased pro-collagen I expression in keloid fibroblasts compared to normal skin fibroblasts that are correlated with an extensive spreading of pro-collagen clusters into the whole cell body.34
Figure 2.
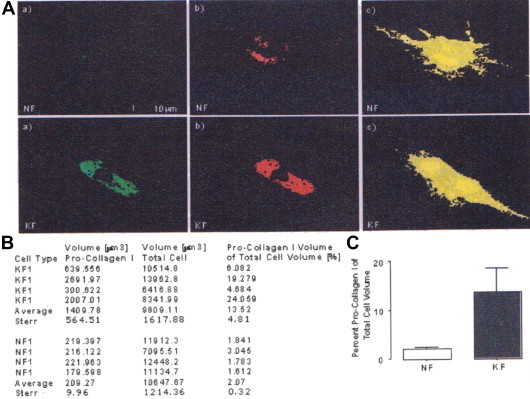
Pro-collagen I expression in normal and keloid fibroblasts analyzed by Volocity software. A. Representative xy-planes of original three-dimensional confocal images: a) original confocal image, b) volumetric measurement of intensely stained collagen clusters by threshold exclusion, and c) volumetric measurement of total cell volume using complete threshold. B. Summary table of volumetric Volocity data, four representative normal (NF) and keloid (KF) cells were analyzed. C. Bar graph represents mean percent pro-collagen I volume of four analyzed NF and KF cells compared to total cell volume with standard error shown. Statistical analysis was done using a two-tailed unpaired student's t-test. p <0.05 was used as a criterion for significance.
To determine the HA-dependent effects on pro-collagen I expression, normal and keloid fibroblast cell lines were analyzed by Western blotting following 72 hour HA treatment. HA demonstrated no changes in pro-collagen I expression in NF1 compared to the non-treated control (Figure 3). In contrast, the KF2 keloid fibroblast cell line showed a decreasing trend in percent pro-collagen I expression following 72 hour HA treatment (107.7 ± 0.03% KF2; 98.87 ± 4.7 KF2 + HA; p = 0.2). However, KF1, KF3 and KF4 showed no significant changes in pro-collagen I expression, thus, there exists a tendency of decreased expression.
Figure 3.
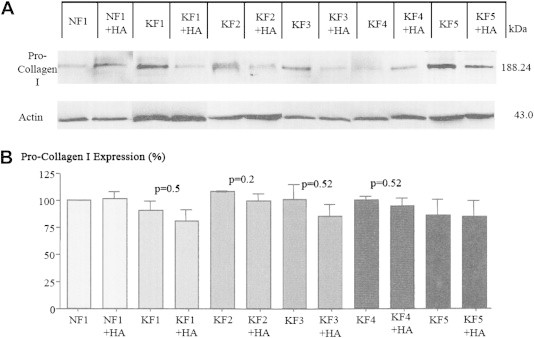
Pro-collagen I expression in normal and keloid fibroblasts. A. Western blot demonstrating expression of pro-collagen I in normal (NF1) and keloid (KF1, KF2, KF3, KF4, KF5) fibroblasts with (+HA) or without 72 hour hyaluronic acid treatment. Actin staining was used as a loading control. Molecular weights in kDa are given in numbers. B. Graph represents densitometric analysis of percent pro-collagen I expression normalized to the actin control. Statistical analysis was done using a two-tailed unpaired student's t-test. p <0.05 was used as a criterion for significance.
To determine HA-dependent effects on collagen-I fiber formation, normal and keloid fibroblasts were treated for 72 hour with HA and analyzed by immunocytochemistry using fluorescence microscopy. Similarly to previous observations, the normal fibroblast cells showed organization of collagen fibers into parallel bundles compared to keloid fibroblast deposited fibers that were less organized with multiple twists resembling an interwoven pattern35 (Fig. 4). In addition, both, normal and keloid fibroblasts showed pro-collagen I distribution in perinuclear clusters. Furthermore, keloid fibroblasts demonstrated larger ovoid nuclear shapes compared to normal fibroblasts that might be correlated with higher proliferation activity.36 Following 72 hour HA treatment the keloid fibroblasts demonstrated arrangement of collagen fibers into higher organized parallel bundles similar to the morphology of normal fibroblasts suggesting a positive effect of HA on collagen fiber formation. In addition, the nuclear shapes of HA-treated keloids changed to smaller rounder nuclei similar to less proliferating normal fibroblasts.36 No differences in the collagen staining intensities were observed following HA treatment suggesting that HA-dependent changes in collagen expression are minimal to non-existent. In addition, HA demonstrated no changes in collagen III expression of normal and keloid fibroblasts analyzed by immunocytochemistry and Western blotting regarding fiber organization, collagen proliferation, or morphology (data not shown).
Figure 4.
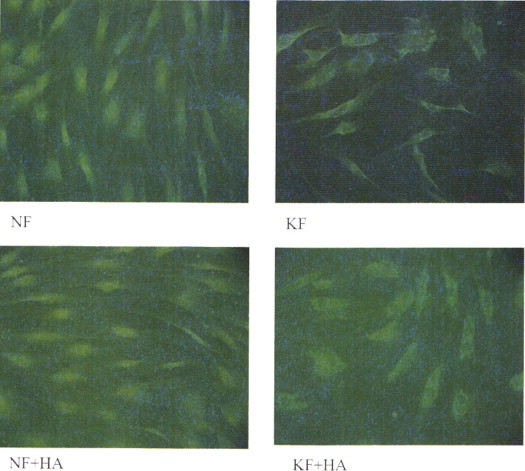
Collagen I fiber distribution in normal and keloid fibroblasts. Representative immunocytochemistry images of normal (NF) and keloid (KF) fibroblasts of pro-collagen I with (+HA) or without 72 hour hyaluronic acid treatment.
Effect of Hyaluronic Acid on TGF-β1 Growth Factor Production
To determine the effect of HA on TGF-β1 Growth Factor expression, normal and keloid fibroblasts were analyzed by Western blotting following 72 hour HA treatment. Regarding TGF-β1 growth factor expression, HA demonstrated no changes in normal NF1 fibroblast cells. In contrast, HA showed a decreasing trend in latent TGF-β1 expression of KF3 and KF4 keloid fibroblast cell lines compared to the non-treated controls (e.g., KF3: 115.3 ± 4.64% and KF3 + HA: 101.2 ± 10.64%; p = 0.29; KF4: 114.9 ± 0.45 and KF4 + HA: 98.26 ± 10.52; p = 0.18) (Figure 5). No significant changes were detected in keloid fibroblast cell lines KF1, KF2, and KF5.
Figure 5.
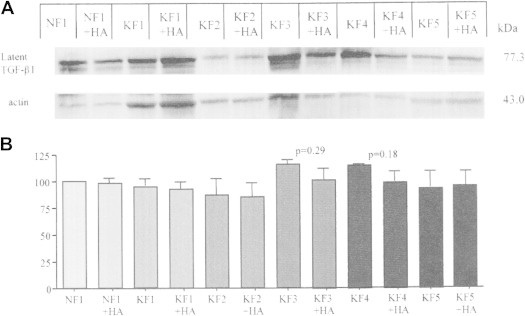
Latent TGF-b1 expression in normal and keloid fibroblasts. A. Western blot demonstrating expression of latent TGF-β1 in normal (NF1) and keloid (KF1, KF2, KF3, KF4, KF5) fibroblasts with (+HA) or without 72 hour hyaluronic acid treatment. Actin staining was used as a loading control. Molecular weights in kDa are given in numbers. B. Graph represents densitometric analysis of percent TGF-b1 expression normalized to the actin control. Statistical analysis was done using a two-tailed unpaired student's t-test. p <0.05 was used as a criterion for significance.
The concentration of active TGF-β1 within the cell culture media of normal and keloid fibroblasts was additionally determined using Quantikine© ELISA analysis. Cell culture media demonstrated similar TGF-β1 secretion levels in NF1 following 72 hour HA (276.5 ± 30.37 pg/ml) compared to the control (211.4 ± 64.65 pg/ml) (Figure 6). Thus, KF1, KF3 and KF4 showed no significant changes, the keloid fibroblast cell lines KF2 and KF5 demonstrated a decreasing trend in TGF-β1 secretion levels following HA treatment (e.g., KF2: 501.8 ± 112.6 pg/ml vs. KF2 + HA: 420.3 ± 73.53 pg/ml; p = 0.56 and KF5: 111.8 ± 51.33 pg/ml vs. KF5 + HA: 70.63 ± 6.5 pg/ml; p = 0.57).
Figure 6.
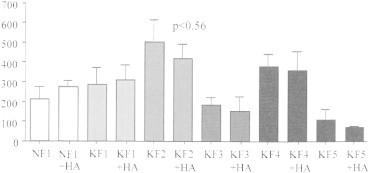
TGF-b1 secretion in normal and keloid fibroblasts. Quantikine© ELISA analysis of active TGF-b1 release into the cell culture media. Graph represents concentration [pg/ml] of active TGF-β1 into cell culture media of normal (NF1) and keloid (KF1, KF2, KF3, KF4, KF5) fibroblasts with (+HA) or without 72 hour hyaluronic acid treatment. Statistical analysis was done using a two-tailed unpaired student's t-test. p <0.05 was used as a criterion for significance.
Discussion
Keloids are benign dermal scars that extend beyond the boundaries of the original wound injury and commonly recur after treatment. They are characterized by enhanced growth factor signaling, excessive proliferation activity, and altered ECM properties i.e., increased collagen levels and decreased HA deposition. According to literature, the replenishment of high molecular weight HA by external administration is thought to represent a beneficial therapy for the prevention of scar formation.20,21 This study was designed to determine if the administration of high molecular weight HA is able to reverse keloid deficiencies such as hyperproliferation and aberrant ECM deposition to a more normal phenotype. Our study demonstrated that HA has the potential to normalize some of the characteristic features of keloid fibroblasts such as hyperproliferation activity, growth factor production, and extracellular matrix deposition depending on the specific genotype of the keloid fibroblast cell line.
A major effect of HA receptor signaling is due to the modulation of mitogenic signaling responses to reduce fibroblast proliferation activity.19,37–39 Based upon this concept, the analysis of changes in proliferation following HA treatment demonstrated a significant reduction of proliferation activity in the KF3 keloid fibroblast cell line. Thus, the failure of the proliferation assay to efficiently detect changes in proliferation activity in all keloid cell lines might be explained by the assay conditions that measure metabolic conversion of a colorimetric dye by NADH/NADPH-dependent dehydrogenases. Thus, changes in NADH/NADPH or dehydrogenase levels that are not directly correlated with the proliferation activity may falsify results. Additionally, differences in passage numbers among the keloid cell lines may impact the outcome of the proliferation assay. Further evidence of HA-dependent changes in proliferation activity was given by the corresponding light-microscopic observations of pro-collagen I stained immunocytochemistries. The immunocytochemistries demonstrated changes in nuclear shape from a larger ovoid nucleus in active proliferating keloid fibroblasts to smaller rounder nucleus in less proliferating normal fibroblasts.40 In addition, keloid fibroblast morphology demonstrated changes to a less fibrous phenotype with reduced ECM fiber thickness and higher organized collagen I fiber pattern in response to HA treatment. These observations are consistent with the HA-dependent decrease in cell proliferation that might be directly linked to a reduction in the amount of collagen I production. Confirming the light-microscopic observations, keloid fibroblasts demonstrated a tendency toward reduction of pro-collagen I expression following HA administration with a decreasing trend in the KF2 keloid fibroblast cell line. Consistent with other studies, collagen III levels were less abundant than collagen I in normal and keloid fibroblast cell lines and no significant changes following HA treatment could be detected in Western blotting or immunocytochemical staining.10,11 This suggests that the changes in fiber arrangement mostly correspond with changes in collagen I expression. In addition to their direct impact on ECM deposition, HA was demonstrated to act on enzymes, e.g., matrix metalloproteinases (MMPs) that process TGF-b1 growth factor proforms for activation and release from the extracellular matrix.19,41–49 In addition to other studies and in correlation with the above results, ELISA analysis demonstrated a decreasing trend in TGF-b1 secretion in the KF2 and KF5 keloid fibroblast cell lines. This was further supported by the Western blot that demonstrated a decreasing trend in latent TGF-β1 expression of KF3 and KF4 keloid fibroblast cell lines.
In summary, our study demonstrated that HA has the potential to normalize some of the characteristic features of keloid fibroblasts such as hyperproliferation activity, TGF-b1 growth factor production, and regulation of extracellular matrix deposition depending on the specific genotype of the keloid cell line. Some of the genotypic inconsistencies between keloid fibroblast cell lines might also be explained by errors that occurred due to dosage or time exposure of HA. Thus, the concentration of 10 μg/ml HA may not have been high enough, and the exposure time of 72 hour may not have been long enough to achieve significant results among all keloid lines.
Currently, HA is used in liquids, dressings, and matrices of artificial skin substitutes for wound healing in chronic wounds, e.g., ulcers, burns and mucous tissue healing.50–55 Due to the benign characteristics of keloids, HA has not yet been used in the field of keloid therapy.1 The major aspects of our study resemble previous studies that demonstrated beneficial effects of HA in normal wound healing. For instance, related to previous studies that used external HA administration in dermal models, our study demonstrated a decreasing effect on pro-collagen type I expression in two of the keloid fibroblast cell lines. Also, similar to previous studies of normal fibroblasts, we demonstrated that high molecular weight HA reduces the keloid characteristic extensive fibroblast proliferation activity in one of the keloid fibroblast cell lines.37 In addition, our study demonstrated similar effects of HA to other glycosaminoglycans such as heparin by reducing TGF-β1 expression in four of the keloid fibroblast cell lines.56–58 In summary, our study demonstrates for the first time that HA may have beneficial effects in the prevention of keloid scarring. Clinical trials are needed to confirm our findings.
Footnotes
Financial disclosure and products: The content of this paper does not contain any products and does not require any financial disclosure statement.
Conflicts of interest: All authors have none to declare.
References
- 1.Tuan T., Nichter L. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- 2.Brown J.J., Ollier W., Arscott G. Genetic susceptibility to keloid scarring: SMAD gene SNP frequencies in Afro-Caribbeans. Exp Dermatol. 2008;17(7):610–613. doi: 10.1111/j.1600-0625.2007.00654.x. [DOI] [PubMed] [Google Scholar]
- 3.Simman R., Alani H., Williams F. Effect of mitomycin C on keloid fibroblasts: an in vitro study. Ann Plast Surg. 2003;50:71–76. doi: 10.1097/00000637-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Baier Leach J., Bivens K., Patrick C., Schmidt C. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 5.Alster T., Tanzi E. Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol. 2003;4:235–243. doi: 10.2165/00128071-200304040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Wolfram D., Tzankov A., Pülzl P., Piza-katzer H. Hypertrophic scars and keloids – a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35(2):171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 7.Berman B., Bieley H. Adjunct therapies to surgical management of keloids. Dermatol Surg. 1996;22:126–130. doi: 10.1111/j.1524-4725.1996.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 8.Diegelmann R., Cohen I., McCoy B. Growth kinetics and collagen synthesis of normal skin, normal scar and keloid fibroblasts in vitro. J Cell Physiol. 1979;98:341–346. doi: 10.1002/jcp.1040980210. [DOI] [PubMed] [Google Scholar]
- 9.Abergel R., Pizzurro D., Meeker C. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985;84:384–390. doi: 10.1111/1523-1747.ep12265471. [DOI] [PubMed] [Google Scholar]
- 10.Di Cesare P., Cheung D., Perelman N., Libaw E., Peng L., Nimni M. Alteration of collagen composition and cross-linking in keloid tissues. Matrix. 1990;10:172–178. doi: 10.1016/s0934-8832(11)80166-6. [DOI] [PubMed] [Google Scholar]
- 11.Moseley R., Stewart J., Stephens P., Waddington R., Thomas D. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: lessons learned from other inflammatory diseases? Br J Dermatol. 2004;150:401–413. doi: 10.1111/j.1365-2133.2004.05845.x. [DOI] [PubMed] [Google Scholar]
- 12.Babu M., Diegelmann R., Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989;9:1642–1650. doi: 10.1128/mcb.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uitto J., Perejda A., Abergel R., Chu M., Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci U S A. 1985;82:5935–5939. doi: 10.1073/pnas.82.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang S., Pang S., Cao Y. Changes in TGF-beta 1 and type I, III procollagen gene expression in keloid and hypertrophic scar. Zhonghua Zheng Xing Shao Shang Wai Ke Za Zhi. 1999;15:283–285. [PubMed] [Google Scholar]
- 15.Sidgwick G.P., Bayat A. Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Eur Acad Dermatol Venereol. 2012;26(2):141–152. doi: 10.1111/j.1468-3083.2011.04200.x. [DOI] [PubMed] [Google Scholar]
- 16.Tredget E. The molecular biology of fibroproliferative disorders of the skin: potential cytokine therapeutics. Ann Plast Surg. 1994;33:152–154. [PubMed] [Google Scholar]
- 17.Chin G., Liu W., Peled Z. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001;108:423–429. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Duncan M., Hasan A., Berman B. Oncostatin M stimulates collagen and glycosaminoglycan production by cultured normal dermal fibroblasts: insensitivity of sclerodermal and keloidal fibroblasts. J Invest Dermatol. 1995;104:128–133. doi: 10.1111/1523-1747.ep12613623. [DOI] [PubMed] [Google Scholar]
- 19.Meyer L., Russell S., Russell J. Reduced hyaluronan in keloid tissue and cultured keloid fibroblasts. J Invest Dermatol. 2000;114:953–959. doi: 10.1046/j.1523-1747.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- 20.Kosunen A., Ropponen K., Kellokoski J. Reduced expression of hyaluronan is a strong indicator of poor survival in oral squamous cell carcinoma. Oral Oncol. 2004;40:257–263. doi: 10.1016/j.oraloncology.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Karjalainen J., Tammi R., Tammi M. Reduced level of CD44 and hyaluronan associated with unfavorable prognosis in clinical stage I cutaneous melanoma. Am J Pathol. 2000;157:957–965. doi: 10.1016/S0002-9440(10)64608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz H., Adzick N. Scarless skin wound repair in the fetus. West J Med. 1993;159:350–355. [PMC free article] [PubMed] [Google Scholar]
- 23.Adzick N., Longaker M. Scarless fetal healing. Therapeutic implications. Ann Surg. 1992;215:3–7. doi: 10.1097/00000658-199201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol. 1979;381:353–361. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 25.Sidgwick G.P., Iqbal S.A., Bayat A. Altered expression of hyaluronan synthase and hyaluronidase mRNA may affect hyaluronic acid distribution in keloid disease compared with normal skin. Exp Dermatol. 2013;22(5):377–379. doi: 10.1111/exd.12147. [DOI] [PubMed] [Google Scholar]
- 26.Taylor K., Trowbridge J., Rudisill J., Termeer C., Simon J., Gallo R. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–17084. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 27.Termeer C., Hennies J., Voith U. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 28.Termeer C., Sleeman J., Simon J. Hyaluronan – magic glue for the regulation of the immune response? Trends Immunol. 2003;24:112–114. doi: 10.1016/s1471-4906(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy C., Diegelmann R., Haynes J., Yager D. Proinflammatory cytokines differentially regulate hyaluronan synthase isoforms in fetal and adult fibroblasts. J Pediatr Surg. 2000;35:874–879. doi: 10.1053/jpsu.2000.6869. [DOI] [PubMed] [Google Scholar]
- 30.Abatangelo G. Hyaluronic acid delays or impedes reepithelialization? J Burn Care Rehabil. 1997;18:552. doi: 10.1097/00004630-199711000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Alaish S., Yager D., Diegelmann R., Cohen I. Hyaluronic acid metabolism in keloid fibroblasts. J Pediatr Surg. 1995;30:949–952. doi: 10.1016/0022-3468(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 32.Akintewe T., Alabi G. Scleroderma presenting with multiple keloids. Br Med J (Clin Res Ed) 1985;291:448–449. doi: 10.1136/bmj.291.6493.448-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1988;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.DePalma R., Krummel T., Durham L. Characterization and quantitation of wound matrix in the fetal rabbit. Matrix. 1989;9:224–231. doi: 10.1016/s0934-8832(89)80054-x. [DOI] [PubMed] [Google Scholar]
- 35.Chipev C., Simman R., Hatch G., Katz A., Siegel D., Simon M. Myofibroblast phenotype and apoptosis in keloid and palmar fibroblasts in vitro. Cell Death Differ. 2000;7:166–176. doi: 10.1038/sj.cdd.4400605. [DOI] [PubMed] [Google Scholar]
- 36.Belmont A., Kendall F., Nicolini C. Coupling of nuclear morphometry to cell geometry and growth in human fibroblasts. Cell Biophys. 1980;2:165–175. doi: 10.1007/BF02795842. [DOI] [PubMed] [Google Scholar]
- 37.Morrison H., Sherman L., Legg J. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Croce M., Boraldi F., Quaglino D., Tiozzo R., Pasquali-Ronchetti I. Hyaluronan uptake by adult human skin fibroblasts in vitro. Eur J Histochem. 2003;47:63–73. doi: 10.4081/808. [DOI] [PubMed] [Google Scholar]
- 39.Boraldi F., Croce M., Quaglino D. Cell-matrix interactions of in vitro human skin fibroblasts upon addition of hyaluronan. Tissue Cell. 2003;35:37–45. doi: 10.1016/s0040-8166(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 40.Yu Q., Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 41.Saed G., Ladin D., Olson J., Han X., Hou Z., Fivenson D. Analysis of p53 gene mutations in keloids using polymerase chain reaction-based single-strand conformational polymorphism and DNA sequencing. Arch Dermatol. 1998;134:963–967. doi: 10.1001/archderm.134.8.963. [DOI] [PubMed] [Google Scholar]
- 42.Appleton I., Brown N., Willoughby D. Apoptosis, necrosis, and proliferation: possible implications in the etiology of keloids. Am J Pathol. 1996;149:1441–1447. [PMC free article] [PubMed] [Google Scholar]
- 43.Messadi D., Le A., Berg S. Expression of apoptosis-associated genes by human dermal scar fibroblasts. Wound Repair Regen. 1999;7:511–517. doi: 10.1046/j.1524-475x.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 44.Luo Y., Prestwich G. Hyaluronic acid-N-hydroxysuccinimide: a useful intermediate for bioconjugation. Bioconjug Chem. 2001;12:1085–1088. doi: 10.1021/bc015513p. [DOI] [PubMed] [Google Scholar]
- 45.Jacob S., Berman B., Nassiri M., Vincek V. Topical application of imiquimod 5% cream to keloids alters expression genes associated with apoptosis. Br J Dermatol. 2003;149(66):62–65. doi: 10.1046/j.0366-077x.2003.05636.x. [DOI] [PubMed] [Google Scholar]
- 46.Younai S., Nichter L., Wellisz T., Reinisch J., Nimni M., Tuan T. Modulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblasts. Ann Plast Surg. 1994;33:148–151. doi: 10.1097/00000637-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Bettinger D., Mast B., Gore D. Hyaluronic acid impedes reepithelialization of skin graft donor sites. J Burn Care Rehabil. 1996;17:302–304. doi: 10.1097/00004630-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Park S., Kim J., Suh H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25:3689–3698. doi: 10.1016/j.biomaterials.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 49.Thielitz A., Vetter R.W., Schultze B. Inhibitors of dipeptidyl peptidase IV-like activity mediate antifibrotic effects in normal and keloid-derived skin fibroblasts. J Invest Dermatol. 2008;128(4):855–866. doi: 10.1038/sj.jid.5701104. [DOI] [PubMed] [Google Scholar]
- 50.Chen W., Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 51.Cho Y., Lee J., Lee J. Hyaluronic acid and silver sulfadiazine-impregnated polyurethane foams for wound dressing application. J Mater Sci Mater Med. 2002;13:861–865. doi: 10.1023/a:1016500429225. [DOI] [PubMed] [Google Scholar]
- 52.Harris P., di Francesco F., Barisoni D., Leigh I., Navsaria H. Use of hyaluronic acid and cultured autologous keratinocytes and fibroblasts in extensive burns. Lancet. 1999;353:35–36. doi: 10.1016/s0140-6736(05)74873-x. [DOI] [PubMed] [Google Scholar]
- 53.Algawi K., Agrell B., Goggin M., O'Keefe M. Randomized clinical trial of topical sodium hyaluronate after excimer laser photorefractive keratectomy. J Refract Surg. 1995;11:42–44. doi: 10.3928/1081-597X-19950101-11. [DOI] [PubMed] [Google Scholar]
- 54.Anderson I. The properties of hyaluronan and its role in wound healing. Prof Nurse. 2001;17:232–235. [PubMed] [Google Scholar]
- 55.Locci P., Marinucci L., Lilli C., Martinese D., Becchetti E. Transforming growth factor beta 1-hyaluronic acid interaction. Cell Tissue Res. 1995;281:317–324. doi: 10.1007/BF00583400. [DOI] [PubMed] [Google Scholar]
- 56.Hu M., Sabelman E., Cao Y., Chang J., Hentz V. Three-dimensional hyaluronic acid grafts promote healing and reduce scar formation in skin incision wounds. J Biomed Mater Res B Appl Biomater. 2003;67:586–592. doi: 10.1002/jbm.b.20001. [DOI] [PubMed] [Google Scholar]
- 57.Dang C., Beanes S., Soo C. Decreased expression of fibroblast and keratinocyte growth factor isoforms and receptors during scarless repair. Plast Reconstr Surg. 2003;111:1969–1979. doi: 10.1097/01.PRS.0000054837.47432.E7. [DOI] [PubMed] [Google Scholar]
- 58.Carroll L., Koch R. Heparin stimulates production of bFGF and TGF-beta 1 by human normal, keloid, and fetal dermal fibroblasts. Med Sci Monit. 2003;9:BR97–BR108. [PubMed] [Google Scholar]


