Abstract
BACKGROUND
Although a large body of literature has been devoted to examining the relationship between eicosapentaenoic and docosahexaenoic acids (EPA+DHA) and blood pressure, past systematic reviews have been hampered by narrow inclusion criteria and a limited scope of analytical subgroups. In addition, no meta-analysis to date has captured the substantial volume of randomized controlled trials (RCTs) published in the past 2 years. The objective of this meta-analysis was to examine the effect of EPA+DHA, without upper dose limits and including food sources, on blood pressure in RCTs.
METHODS
Random-effects meta-analyses were used to generate weighted group mean differences and 95% confidence intervals (CIs) between the EPA+DHA group and the placebo group. Analyses were conducted for subgroups defined by key subject or study characteristics.
RESULTS
Seventy RCTs were included. Compared with placebo, EPA+DHA provision reduced systolic blood pressure (−1.52mm Hg; 95% confidence interval (CI) = −2.25 to −0.79) and diastolic blood pressure (−0.99mm Hg; 95% CI = −1.54 to −0.44) in the meta-analyses of all studies combined. The strongest effects of EPA+DHA were observed among untreated hypertensive subjects (systolic blood pressure = −4.51mm Hg, 95% CI = −6.12 to −2.83; diastolic blood pressure = −3.05mm Hg, 95% CI = −4.35 to −1.74), although blood pressure also was lowered among normotensive subjects (systolic blood pressure = −1.25mm Hg, 95% CI = −2.05 to −0.46; diastolic blood pressure = −0.62mm Hg, 95% CI = −1.22 to −0.02).
CONCLUSIONS
Overall, available evidence from RCTs indicates that provision of EPA+DHA reduces systolic blood pressure, while provision of ≥2 grams reduces diastolic blood pressure.
Keywords: blood pressure, docosahexaenoic acid, eicosapentaenoic acid, fish oil, hypertension, meta-analysis, omega-3, randomized controlled trials, systematic review
Thirty-one percent of Americans are hypertensive, 30% are prehypertensive, and approximately 20% are hypertensive yet unaware of their status.1,2 Only 47% of those with hypertension are adequately controlled.1 Prior research shows that diet and lifestyle modifications, including physical activity, sodium reduction, and fish oil supplementation, can reduce blood pressure (BP), enhance antihypertensive drug efficacy, and decrease cardiovascular disease (CVD) risk.3
The active ingredients in fish oil considered responsible for its antihypertensive effect are the long-chain omega-3 fatty acids eicosapentaenoic acid (EPA; 20:5 n-3) and docosahexaenoic acid (DHA; 22:6 n-3). Although previous meta-analyses of fish oil supplementation and BP have been published,4–7 none have been designed with inclusion criteria sufficient to examine the extensive scope of literature available in this active area of investigation. For example, the most recently published meta-analysis excluded trials that examined food sources of EPA and DHA (herein referred to as EPA+DHA) and those that were less than 8 weeks in duration.7 Therefore, our main objective was to update the state of the science by conducting the most comprehensive meta-analysis of its kind of randomized controlled trials (RCTs) that examined EPA+DHA in relation to BP.
METHODS
Literature review
A comprehensive literature search was conducted by the University of Colorado Denver Health Science Library using Ovid/Medline, Embase, and the Cochrane Library. A PubMed search was performed in February 2013 to identify any publications not yet indexed by Ovid/Medline. Literature searches covered studies published from 1946 through February 2013 and published in all languages. Level 1 screening included review of all titles and/or abstracts. Full-text publications of any studies not eliminated at level 1 were retrieved for complete review at level 2 screening. Supplementary literature searches included examining the reference lists of all relevant studies, pertinent review articles, and meta-analyses.
Eligibility criteria for study selection
Included studies were RCTs that examined the effect of EPA+DHA on BP in nonhospitalized adults (aged ≥18 years). Eligible outcomes were systolic and diastolic BP values (SBP and DBP, respectively). The exclusion criteria in this review were as follows:
Hypertensive subjects treated with BP-lowering medications;
Less than 3-week treatment duration;
Crossover RCTs with less than a 4-week washout period between treatments;
Studies that did not specify the amount of EPA+DHA provided or without required data to be used meta-analytically (all authors of otherwise eligible studies were contacted for missing data); and
Studies conducted in populations not representative of the general adult population, including pregnant and nursing women and individuals with preexisting CVD or significant disease process (e.g., renal disease or cancer) or secondary hypertension.
Data extraction and quality assessment
The following qualitative and quantitative information was extracted from all RCTs: publication year, population demographic characteristics, geographic location, baseline hypertensive status, other relevant baseline health characteristics, medication use, sample size, the specific dose of EPA+DHA, the type of food or supplement, outcome assessment method, and means and SDs for BP outcomes.
Data synthesis
Random-effects meta-analysis models were used to calculate weighted group mean differences (postintervention minus preintervention), 95% confidence intervals (CIs), and corresponding P values for heterogeneity between the EPA+DHA group and the placebo group. The weight of each study was based on the inverse of the variance, which is a measure that accounts for the sample size in each group. The macro-level models included data on all subjects at all dose levels. Subgroup analyses were conducted to identify potential sources of heterogeneity or between-study variability and to estimate the effect of EPA+DHA according to key study characteristics. Categorical dose–response analyses were performed to discern potential patterns or thresholds of effect. Sensitivity and influence analyses were conducted by evaluating the impact of adding or removing studies based on important study characteristics and outlier status. The relative weight of each study was appreciated for each meta-analysis model to determine the influence that each study had on the overall summary effect. The presence of publication bias was assessed visually by examining a funnel plot measuring the SE as a function of effect size, as well as performing Egger’s regression method and the Duval and Tweedie imputation method.8 All analyses were performed using Comprehensive Meta-Analysis (version 2.2.046; Biostat, Englewood, NJ).
RESULTS
Study Characteristics
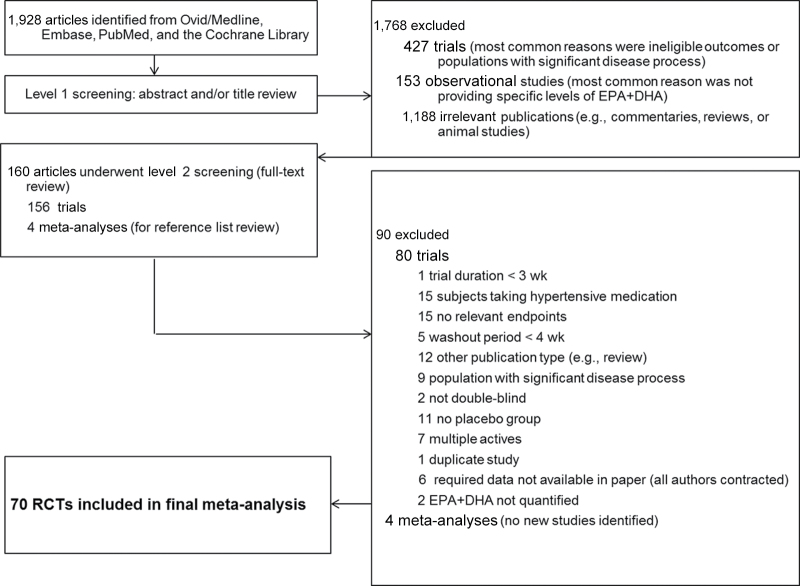
A flow diagram of the search strategy, including reasons for exclusion, is shown in Figure 1. A total of 70 RCTs9–78 met all eligibility criteria and were included in the meta-analysis. The main study characteristics are shown in Table 1 (hypertensive populations) and Table 2 (normotensive populations, with 1 prehypertensive population).17 Ramel et al.63 examined hypertensive and normotensive subjects combined; these data are included in Table 1 but were not meta-analyzed in the subgroup analyses by hypertension status. Approximately 40% of the included RCTs were conducted in North America, with the remaining distributed primarily between Nordic countries (20%), European countries other than the Nordic countries (27%), and Australia (13%). The mean study duration was 69 days, the mean EPA+DHA dose was 3.8g/day, and sources of EPA+DHA included different types of seafood, EPA+DHA–fortified foods, fish oil, algal oil, and purified ethyl esters. Olive oil was the most commonly used placebo, with the remainder consisting predominately of other vegetable oils (e.g., safflower, corn, and sunflower oils).
Figure 1.
Flow diagram of literature search and selection of randomized controlled trials (RCTs) for meta-analysis of eicosapentaenoic and docosahexaenoic acids (EPA+DHA) and blood pressure.
Table 1.
Characteristics of the randomized controlled trials in hypertensive study populationsa
| First author | Year | Country | Age, yb | Sex, M/Fc | Duration, d | Intervention regimen | Control | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention type | Dose, g/dd | DHA, g/d | EPA, g/d | DHA+ EPA, g/de | |||||||
| Bonaa 14 | 1990 | Norway | 20–61 | 156 (M+F) | 70 | Fish oil (EE) | 6 | 1.8 | 3.2 | 5.1 | Corn oil |
| Hill 38 | 2007 | Australia | 25–65 | 28/53 | 84 | Fish oil | 6 | 1.6 | 0.4 | 1.9 | Sunflower oil |
| Hughes 39 | 1990 | United States | NR | 26/0 | 30 | Fish oil | 10 | 1.5 | 3.5 | 5.0 | Wheat germ oil |
| Knapp 41 | 1989 | United States | 30–71 | 36/0 | 28 | Fish oil | 50 | 6.0 | 9.0 | 15.0 | Safflower oil |
| 10 | 1.2 | 1.8 | 3.0 | Mixed vegetable oils | |||||||
| Landmark 42 | 1993 | Norway | 33–64 | 18/0 | 28 | Fish oil (EE) | 10 | 2.8 | 1.8 | 4.8 | Olive oil |
| Levinson 43 | 1990 | United States | 18–75 | 17 (M+F) | 42 | Fish oil | 50 | 6.0 | 9.0 | 15.0 | Vegetable oil |
| Meland 48 | 1989 | Norway | 26–66 | 40/0 | 42 | Fish oil | 20 | 2.4 | 3.6 | 6.4 | Olive + corn oil |
| Mundal 54 | 1993 | Norway | 33–64 | 18/0 | 28 | Fish oil | ― | 2.8 | 1.8 | 4.6 | Olive oil |
| Passfall 60 | 1993 | Germany | 40–61 | 4/6 | 42 | Fish oil | 9 | 0.9 | 1.3 | 2.2 | Olive oil |
| Prisco 61 | 1998 | Italy | 33–57 | 32/0 | 120 | Fish oil (EE) | 4 | 1.4 | 2.0 | 3.6 | Olive oil |
| Radack 62 | 1991 | United States | ≥ 18 | 19/14 | 84 | Fish oil | 6 | 0.8 | 1.2 | 2.0 | Safflower oil |
| Ramel63,f | 2010 | Iceland, Spain, Ireland | 20–40 | 138/186 | 56 | Fish oil | 6 | ― | ― | 2.1 | Sunflower oil |
| Cod | 64 | ― | ― | 1.3 | |||||||
| Salmon | 64 | ― | ― | 0.3 | |||||||
| Sagara 65 | 2011 | United Kingdom | 45–59 | 38/0 | 35 | DHA-enriched bread | 2 | 2.0 | 0.0 | 2.0 | Olive oil–enriched bread |
| Steiner 69 | 1989 | Switzerland | 44 (13) | 17/11 | 28 | Fish oil | 4 | 0.5 | 1.1 | 2.0 | Salad oil |
| Toft 71 | 1995 | Norway | 20–61 | 50/28 | 112 | Fish oil (EE) | 4 | ― | ― | 3.4 | Corn oil |
| Wang 77 | 2008 | China | 42 (3) | 14/7 | 56 | Fish oil | 3 | 0.4 | 0.5 | 0.9 | Vegetable oil |
Abbreviations: DHA, docosahexaenoic acid; EE, ethyl esters; EPA, eicosapentaenoic acid; F, female; M, male; NR, not reported.
a Two study populations (Hughes et al. 1990 39 and Steiner et al. 1989 69 ) were stratified by hypertensive status; therefore, only study
characteristics for hypertensives are shown here.
b Mean (SD) is shown when range was not provided.
c The total sample size is shown plus M+F to indicate both men and women were included when the distribution by sex was not provided.
d Dose of entire fish oil supplement or food.
e May include small amounts of docosapentaenoic acid.
Table 2.
Characteristics of the randomized controlled trials in normotensive study populationsaf Not included in hypertensive-only meta-analysis because only a portion of the population (32%) was hypertensive
| First author | Year | Country | Age, yb | Sex, M / Fc | Duration, d | Intervention regimen | Control | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention type | Dose, g/dd | DHA, g/d | EPA, g/d | DHA+ EPA, g/de | |||||||
| Armstong 10 | 2012 | United States | 20–59 | 35/81 | 42 | Fish oil (EE) | 5 | 1.0 | 2.0 | 3.0 | Corn + soy oil |
| Atar 11 | 2012 | Iran | 45–65 | 0/78 | 56 | Fish oil | 2 | 0.5 | 0.7 | 1.2 | Cornstarch |
| Bach 12 | 1989 | United States | 31 (9) | 16/14 | 35 | Fish oil | 6 | 1.4 | 1.1 | 2.5 | Fractionated coconut oil |
| Barcelo-Coblijn 13 | 2008 | Canada | 36–43 | 50/3 | 84 | Fish oil | 0.6 | 0.1 | 0.3 | 0.4 | Sunflower oil |
| 1.2 | 0.3 | 0.5 | 0.8 | ||||||||
| Browning 15 | 2007 | United Kingdom | < 50 | 0/33 | 84 | Fish oil | ― | 2.9 | 1.3 | 4.2 | Oleic + linoleic acid oil |
| Buckley 16 | 2009 | Australia | 22 (1) | 25/0 | 35 | Fish oil | 6 | 1.6 | 0.4 | 1.9 | Sunflower oil |
| Carter17,f | 2012 | United States | 24 (2) | 18/20 | 56 | Fish oil | 9 | 1.1 | 1.6 | 2.7 | Olive oil |
| Cazzola 18 | 2007 | Italy | 18–42 | 100/0 | 84 | Fish oil | 3 | 0.3 | 1.4 | 1.6 | Corn oil |
| 6 | 0.5 | 2.7 | 3.2 | ||||||||
| 9 | 0.8 | 4.1 | 4.9 | ||||||||
| Chin 19 | 1993 | Australia | 18–32 | 29/0 | 28 | Fish oil | 5 | 0.6 | 0.9 | 1.5 | Palm + safflower + olive oil |
| 10 | 1.2 | 1.8 | 2.9 | ||||||||
| 20 | 2.3 | 3.6 | 5.9 | ||||||||
| Cobiac 20 | 1991 | Australia | 30–60 | 31/0 | 35 | Salmon + sardines | 164 | 3.0 | 1.5 | 4.5 | Mixed vegetable oil |
| Fish oil | 15 | 1.6 | 3.0 | 4.6 | |||||||
| Conquer 21 | 1999 | Canada | 30 (2) | 19/0 | 42 | Seal oil | 20 | 1.7 | 1.3 | 3.8 | Vegetable oil |
| Croset 22 | 1990 | France | 86 (4) | NR | 60 | Fish oil (EE) | 0.1 | 0.0 | 0.1 | 0.1 | Placebo oil (NFS) |
| Demke 23 | 1988 | United States | 18–60 | 8/23 | 28 | Fish oil | 5 | ― | ― | 1.7 | Safflower oil |
| Derosa 25 | 2009 | Italy | ≥18 | 164/169 | 180 | Fish oil (EE) | 3 | 1.5 | 0.9 | 2.4 | Sucrose, mannitol and mineral salts |
| Derosa 24 | 2012 | Italy | 18–75 | 82/85 | 180 | Fish oil (EE) | 3 | 1.4 | 1.2 | 2.6 | Sucrose, mannitol and mineral salts |
| Deslypere 26 | 1992 | Belgium | 21–90 | 58/0 | 365 | Fish oil | 3 | 0.2 | 0.8 | 1.1 | Olive oil |
| 6 | 0.3 | 1.6 | 2.1 | ||||||||
| 9 | 0.5 | 2.4 | 3.2 | ||||||||
| Dewell 27 | 2011 | United States | 50 (10) | 64/36 | 60 | Fish oil | 2 | 0.5 | 0.7 | 1.2 | Soybean oil |
| 6 | 1.5 | 2.1 | 3.6 | ||||||||
| Dyerberg 28 | 2004 | Denmark | 20–60 | 87/0 | 56 | Fish oil | 12 | 1.3 | 2.0 | 3.3 | Palm oil |
| Finnegan 29 | 2003 | United Kingdom | 25–72 | 87/63 | 120 | EPA+DHA-enriched margarine | 25 | 0.3 | 0.2 | 0.5 | Margarine (sunflower + safflower oil-based) |
| EPA+DHA–enriched margarine + fish oil capsules | 28 | ― | ― | 1.3 | Margarine + capsules (both sunflower + safflower oil-based) | ||||||
| Flaten 30 | 1990 | Norway | 35–45 | 56/0 | 42 | Fish oil | 14 | 2.9 | 3.6 | 6.8 | Olive Oil |
| Geelen 31 | 2003 | Nether-lands | 50–70 | 36/38 | 84 | Fish oil | 3.5 | 0.6 | 0.7 | 1.3 | Sunflower oil |
| Ginty 32 | 2012 | United States | NR | 8/26 | 21 | Fish oil | ― | 0.4 | 1.0 | 1.4 | Corn oil |
| Grimsgaard 33 | 1998 | Norway | 36–56 | 224/0 | 49 | Fish oil (EE) | 4 | 0.0 | 4.0 | 4.0 | Corn oil |
| 4 | 4.0 | 0.0 | 4.0 | ||||||||
| Gustafsson 34 | 1996 | Sweden | 48 (9) | 24 (M+F) | 21 | EPA+DHA-enriched food products | 57 | ― | ― | 3.2 | Sunflower-enriched food products |
| Hallund 35 | 2010 | Denmark | 40–70 | 45/0 | 56 | Marine trout | 150 | 2.0 | 0.9 | 3.2 | Chicken |
| Harris 36 | 2008 | United States | 21–70 | 14 /19 | 112 | Fish oil (EE) | 23 | 0.0 | 1.0 | 1.0 | Soybean oil |
| Hellsten 37 | 1993 | Sweden | 30–60 | 40 (M+F) | 150 | Cod liver oil | 6 | ― | ― | 2.0 | Corn oil |
| Hughes 39 | 1990 | United States | NR | 26/0 | 30 | Fish oil | 10 | 1.5 | 3.5 | 5.0 | Wheat germ oil |
| Kelley 40 | 2007 | United States | 54 (2) | 34/0 | 90 | Fish oil | 7.5 | 3.0 | ― | 3.0 | Olive oil |
| Lindqvist 78 | 2009 | Sweden | 35–60 | 35/0 | 42 | Baked herring | 150 | ― | ― | 1.2 | Baked lean pork + chicken |
| Lofgren 44 | 1993 | United States | 40–60 | 23/0 | 84 | Fish oil | 20 | 2.4 | 3.6 | 6.0 | Safflower oil |
| Mackness 45 | 1994 | 7 European countries | 30–71 | 55/24 | 98 | Fish oil | 4 | ― | ― | 3.4 | Corn oil |
| Maki 46 | 2009 | United States | 35–64 | 8/42 | 28 | Krill oil | 2 | 0.1 | 0.2 | 0.3 | Olive oil |
| Fish oil | 2 | 0.2 | 0.2 | 0.4 | |||||||
| McVeigh 47 | 1994 | Ireland | 45–61 | 16/4 | 42 | Fish oil | 10 | 1.2 | 1.8 | 3.0 | Olive oil |
| Mills 50 | 1989 | Canada | 22–34 | 20/0 | 28 | Fish oil | 9 | 1.0 | 1.6 | 2.6 | Olive oil |
| Mills 49 | 1990 | Canada | 19–31 | 29/0 | 28 | Fish oil | 4.5 | 0.5 | 0.8 | 1.3 | Safflower Oil |
| Monahan 51 | 2004 | United States | 18–35 | 10/8 | 30 | Fish oil | 10 | 2.0 | 3.0 | 5.0 | Olive Oil |
| Mori 52 | 1999 | Australia | 20–65 | 56/0 | 42 | Fish oil (EE) | 4 | 0.0 | 3.8 | 3.8 | Olive oil |
| 4 | 3.7 | 0.0 | 3.7 | ||||||||
| Mortensen 53 | 1983 | Denmark | 25–40 | 20/0 | 28 | Fish oil | 10 | 0.5 | 0.8 | 1.4 | Vegetable oil |
| Murphy 55 | 2007 | Australia | 20–65 | 35/39 | 190 | EPA+DHA–enriched foods | ― | ― | ― | 1.0 | Same foods, without DHA+EPA enrichment |
| Neff 56 | 2010 | United States | 18–65 | 15/21 | 112 | Algal oil | 5 | 2.0 | 0.0 | 2.0 | Corn + soybean oil |
| Nestel 57 | 2002 | United States | 40–69 | 21/17 | 49 | Fish oil (EE) | 4 | 0.1 | 3.0 | 3.1 | Olive oil |
| 4 | 2.8 | 0.2 | 3.5 | ||||||||
| Nordoy 58 | 2001 | Norway | 28–61 | 32/10 | 35 | Fish oil (EE) | 2 | 0.8 | 0.9 | 1.7 | Corn oil |
| Noreen 59 | 2012 | United States | 19–55 | 14/26 | 42 | Fish oil | 4 | 1.0 | 1.8 | 3.1 | Safflower oil |
| Ryu 64 | 1990 | United States | 20–39 | 20/0 | 28 | Fish oil | 6 | 0.9 | 2.1 | 3.0 | Wheat germ oil |
| Sanders 66 | 2006 | United Kingdom | 33 (13) | 39/40 | 28 | Fish oil | 4 | 1.5 | 0.0 | 1.5 | Olive oil |
| Sjoberg 67 | 2010 | Australia | 53 (17) | 17/16 | 84 | Fish oil | 2 | 0.5 | 0.1 | 0.7 | Sunola oil |
| 4 | 1.0 | 0.2 | 1.3 | ||||||||
| 6 | 1.6 | 0.3 | 2.0 | ||||||||
| Stark 68 | 2004 | Canada | 45–70 | 0/32 | 28 | Fish oil | 6 | 2.8 | 0.0 | 2.8 | Corn + soy oil |
| Steiner 69 | 1989 | Switzer-land | 44 (13) | 17/11 | 28 | Fish oil | 4 | 0.5 | 1.1 | 2.0 | Salad oil |
| Theobald 70 | 2007 | United Kingdom | 45–65 | 20/19 | 90 | Fish oil | 1.5 | 0.7 | 0.0 | 0.7 | Olive oil |
| THPCRG 9 | 1992 | United States | 30–54 | 245/105 | 180 | Fish oil | 6 | 1.0 | 1.4 | 3.6 | Olive oil or other placebo |
| Vakhapova 72 | 2011 | Israel | 50–90 | 67/63 | 105 | Fish oil | 3 | 0.0 | 0.0 | 0.0 | Cellulose |
| Vandongen 73 | 1993 | Australia | 30–60 | 51/0 | 84 | Fish oil | 6 | 0.9 | 1.3 | 2.3 | Olive + palm + safflower oils |
| 12 | 1.7 | 2.6 | 4.7 | ||||||||
| Vericel 74 | 1999 | France | 70–83 | 6/14 | 42 | Fish oil | 0.6 | 0.2 | 0.0 | 0.2 | Sunflower oil |
| von Houwelingen 75 | 1987 | Nether-lands | 20–45 | 82/0 | 42 | Mackarel paste | 135 | 3.0 | 1.7 | 4.7 | Meat paste |
| Walser 76 | 2008 | United States | 20–51 | 14/7 | 42 | Fish oil | 10 | 2.0 | 3.0 | 5.0 | Safflower oil |
Abbreviations: DHA, docosahexaenoic acid; EE, ethyl esters; EPA, eicosapentaenoic acid; NFS, not further specified; NR, not reported; THPCRG, Trials of Hypertension Prevention Collaborative Research Group.
a Two study populations (Hughes et al. 39 and Steiner et al. 69 ) were stratified by hypertensive status; therefore, only study
characteristics for normotensives are shown here.
b Mean (SD) is shown when range was not provided.
c The total sample size is shown plus M+F to indicate both men and women were included when the distribution by sex was not provided.
d Dose of entire fish oil supplement or food.
e May include small amounts of docosapentaenoic acid.
f Population includes normotensive and prehypertensive subjects.
Results from meta-analysis
Meta-analysis results for all analyses are reported in Table 3, and results for selected analyses are illustrated in Figures 2 and 3 and Supplementary Figures S1 and S2.
Table 3.
Summary of meta-analysis results
| Model | No. of data points | WGMD | Lower 95% CI | Upper 95% CI | P value for heterogeneity |
|---|---|---|---|---|---|
| Systolic blood pressure | |||||
| All studiesa | 93 | −1.52 | −2.25 | −0.79 | 0.001 |
| Supplement only | 82 | −1.75 | −2.55 | −0.94 | 0.001 |
| Food only | 11 | 0.10 | −1.31 | 1.50 | 0.50 |
| US studies | 25 | −1.78 | −3.33 | −0.23 | 0.03 |
| Non-US studies | 68 | −1.33 | −2.16 | −0.50 | 0.007 |
| Duration ≥60 days | 41 | −1.63 | −2.67 | −0.59 | 0.08 |
| Dose 0 to <1 g | 12 | −2.38 | −5.14 | 0.38 | 0.009 |
| Dose 1 to <2 g | 19 | −1.81 | −3.59 | −0.03 | 0.47 |
| Dose 2 to <3 g | 18 | −0.21 | −1.85 | 1.43 | 0.007 |
| Dose 3 to <4 g | 22 | −3.85 | −5.55 | −2.15 | 0.05 |
| Dose 4 to <5 g | 11 | −0.86 | −1.84 | 0.13 | 0.97 |
| Dose ≥5 g | 10 | −0.36 | −2.95 | 2.23 | 0.17 |
| Hypertensive subjects | 15 | −4.51 | −6.12 | −2.83 | 0.72 |
| Normotensive subjects | 73 | −1.25 | −2.05 | −0.46 | 0.01 |
| EPA only | 7 | −4.61 | −8.35 | −0.86 | 0.01 |
| DHA only | 8 | −1.27 | −3.37 | 0.84 | 0.28 |
| Ethyl ester | 15 | −2.24 | −3.72 | −0.76 | 0.002 |
| Other marine oils | 67 | −1.45 | −2.39 | −0.50 | 0.007 |
| Diastolic blood pressure | |||||
| All studiesa | 92 | −0.99 | −1.54 | −0.44 | 0.00 |
| Supplement only | 81 | −1.11 | −1.72 | −0.50 | 0.00 |
| Food only | 11 | −0.38 | −1.46 | 0.70 | 0.75 |
| US studies | 24 | −1.35 | −2.48 | −0.21 | 0.02 |
| Non-US studies | 68 | −0.88 | −1.52 | −0.25 | 0.00 |
| Duration ≥60 days | 41 | −0.95 | −1.56 | −0.34 | 0.31 |
| Dose 0 to <1 g | 10 | 0.04 | −1.48 | 1.56 | 0.78 |
| Dose 1 to <2 g | 21 | 0.40 | −1.10 | 1.91 | 0.001 |
| Dose 2 to <3 g | 18 | −1.09 | −2.08 | −0.11 | 0.16 |
| Dose 3 to <4 g | 22 | −1.86 | −2.67 | −1.06 | 0.36 |
| Dose 4 to <5 g | 11 | −0.59 | −1.37 | 0.19 | 0.94 |
| Dose ≥5 g | 10 | −1.97 | −3.96 | 0.02 | 0.06 |
| Hypertensive subjects | 15 | −3.05 | −4.35 | −1.74 | 0.17 |
| Normotensive subjects | 72 | −0.62 | −1.22 | −0.02 | 0.002 |
| EPA only | 5 | −0.81 | −1.99 | −0.37 | 0.55 |
| DHA only | 8 | −0.84 | −2.29 | 0.62 | 0.32 |
| Ethyl ester | 16 | −0.80 | −1.49 | −0.11 | 0.28 |
| Other marine oils | 64 | −1.20 | −2.02 | −0.37 | 0.00 |
Abbreviations: CI, confidence interval; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; WGMD, weighted group mean difference.
a Includes all studies, regardless of dose, duration, region, hypertensive status, and source of EPA+DHA (supplement or food).
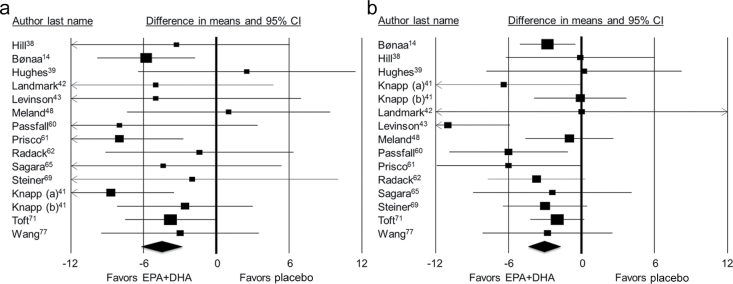
Figure 2.
Results from meta-analyses of randomized controlled trials examining eicosapentaenoic and docosahexaenoic acids (EPA+DHA) provision and (a) systolic blood pressure and (b) diastolic blood pressure among hypertensive subjects. The squares represent average change in blood pressure in individual randomized controlled trials, or individual trial strata, with 95% confidence intervals (CIs). The diamond represents the pooled summary estimate. Knapp (a) is a higher-dose subgroup, and Knapp (b) is a lower-dose subgroup.
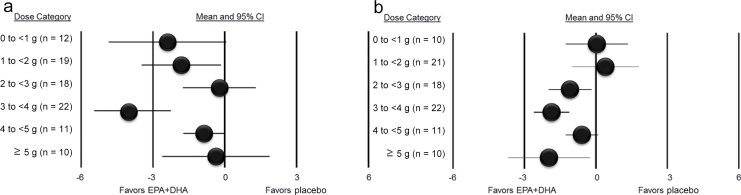
Figure 3.
Results from meta-analyses of randomized controlled trials examining eicosapentaenoic and docosahexaenoic acids (EPA+DHA) and (a) systolic blood pressure and (b) diastolic blood pressure by EPA+DHA dose category. The circle represents the pooled summary estimate across all studies within each dose category, with 95% confidence intervals (CIs). n indicates the number of data points in each dose category, which may be greater than the number of individual studies.
In the overall meta-analysis model of 93 data points from 70 RCTs, SBP decreased by 1.52mm Hg (95% CI = −2.25 to −0.79) and DBP by 0.99mm Hg (95% CI = −1.54 to −0.44), compared with placebo, after EPA+DHA provision (Table 3; Supplementary Figures S1 and S2). Studies with multiple entries in the meta-analysis models reflect results presented separately by the authors for different subgroups (e.g., low-dose and high-dose EPA+DHA). In the meta-analysis of hypertensive subjects, significant reductions in SBP (−4.51mm Hg; 95% CI = −6.12 to −2.83) and DBP (−3.05mm Hg; 95% CI = −4.35 to −1.74) were observed (Figure 2). The meta-analysis of studies among normotensive subjects also found a significant reduction of SBP (−1.25mm Hg; 95% CI = −2.05 to −0.46) and DBP (−0.62mm Hg; 95% CI = −1.22 to −0.02) (Table 3). The summary estimates were modified by source of EPA+ DHA and by study region (Table 3). In the meta-analysis of supplement-only studies, SBP decreased by 1.75mm Hg (95% CI = −2.55 to −0.94) and DBP by 1.11 (95% CI = −1.72 to −0.50) after EPA+DHA provision, compared with placebo. Among US-only studies, reductions of 1.78mm Hg (95% CI = −3.33 to −0.23) in SBP and 1.35mm Hg (95% CI = −2.48 to −0.21) in DBP were observed. Because relatively few studies evaluated EPA+DHA as individual fatty acids, there was insufficient statistical power to detect a meaningful difference between EPA and DHA separately on lowering either SBP or DBP.
The subgroup analyses by dose are depicted in Figure 3. There was no clear pattern of dose–response between EPA+DHA and SBP. Significant reductions were observed with doses of 1 to <2g/d (−1.81; 95% CI = −3.59 to −0.03) and 3 to <4g/d (−3.85; 95% CI = −5.55 to −2.15). No apparent effect on DBP was observed for dose levels <2g/day, whereas significant reductions were observed for 2 to <3g/day (−1.09; 95% CI = −2.08 to −0.11) and 3 to <4g/day (−1.86; 95% CI = −2.67 to −1.06).
An examination of potential publication bias indicated a modest proclivity for published studies that found a significant SBP reduction with EPA+DHA provision (Supplementary Figure S3). There was a slight indication of publication bias with a proclivity for publication of results that showed a significant DBP reduction with EPA+DHA provision, which was modified by study region. Non-US studies were more likely to publish findings showing DBP reduction with EPA+DHA provision, whereas US studies were more likely to publish null findings or an increase in DBP with EPA+DHA provision.
DISCUSSION
This meta-analysis of RCTs that examined EPA+DHA provision and BP provides the most comprehensive quantitative summary of the evidence to date. Before this meta-analysis, the most recent published meta-analysis7 excluded studies <8 weeks in duration and those that examined food sources of EPA+DHA. By liberalizing the duration restriction to 3 weeks, including RCTs conducted with EPA+DHA–rich and –fortified foods, and capturing recent RCTs published in the past 2 years, our meta-analysis evaluated an additional 53 studies not included by Campbell et al.7 The considerably larger volume of studies enhanced the statistical power to perform important subgroup analyses by factors such as dose, geographic region, hypertensive status, and source of EPA+DHA.
The results from our analysis demonstrate that EPA+DHA are as effective, and in some cases more effective, than other lifestyle-related interventions, including increasing physical activity and restricting alcohol and sodium,79 for lowering BP among hypertensive populations not taking antihypertensive medication. These results are consistent with findings from Campbell et al.7 as well as other earlier meta-analyses.80,81 Lowered systemic vascular resistance through changes in endothelial function is considered a primary mechanism by which EPA+DHA may lower BP.82 Recent systematic reviews and a meta-analysis of RCTs found improved endothelial function in response to EPA+DHA, particularly among patients with risk factors for CVD, including hypertension, but not consistently among healthy young and middle-aged subjects.83,84 This observation may explain the greater response of unmedicated hypertensive subjects when compared with normotensive subjects in our meta-analysis.
The reductions in BP observed in this analysis are not only statistically significant but also are clinically meaningful. Among adults, SBP rises by approximately 0.6mm Hg per year; among those aged ≥50 years, the lifetime risk of hypertension is 90%.85 Furthermore, only 1mm Hg SBP separates each stage of hypertension. The statistically significant reduction in SBP of 1.25mm Hg noted among normotensive individuals in our analysis would represent a delay of age-related SBP increase by 2 years and progression from prehypertensive to hypertensive status. The 4.51mm Hg decrease observed among hypertensive populations not taking antihypertensive medication could prevent an individual from requiring medication to control their hypertension or could help maintain an individual in a lower stage of progressive hypertension. Lowered systemic vascular resistance and BP can reduce risk of coronary plaque rupture, stroke, and complications of stroke, including related cognitive decline, thus improving clinical outcomes for higher-risk populations.82
Overall, there was no clear discernible pattern of a dose–response effect for EPA+DHA on BP, which is similar to findings from past meta-analyses.7,81,86 Although less data are available that examine EPA+DHA–rich or –fortified food and BP outcomes (n = 8 studies in this meta-analysis), EPA+DHA–rich or –fortified foods were less effective than supplements with regards to lowering BP. It is important to note, however, that there are barriers to frequent fish consumption, which may explain in part the discrepant findings between food and supplement studies. Among the general population, barriers to frequent fish consumption include dislike of taste, unpleasant smell, and “concerns about bones.”87,88 Five of the 8 food studies in the current meta-analysis required daily consumption of oily fish, including sardines, mackerel, and salmon. Although compliance was not routinely reported among all food studies, von Houwelingen et al.,75 who provided subjects with mackerel, reported compliance of only 78%. In addition, the researchers noted that a tendency for compliance decreased over the course of the study, as assessed by urinary lithium excretion. Only 3 studies of EPA+DHA–fortified foods (e.g., margarine and bread)29,55,65 were identified for inclusion in our meta-analysis, which challenges efforts to fully investigate the role of these EPA+DHA sources as part of an overall healthy dietary pattern.
Collectively, the evidence from RCTs indicates that provision of ≥2g/d EPA+DHA may reduce both SBP and DBP, with the strongest benefits observed among hypertensive individuals who are not on antihypertensive medication. In addition, a lower dose (between 1 and 2g/d) may reduce SBP but not DBP. From a clinical and public health perspective, provision of EPA+DHA may lower BP and ultimately reduce the incidence of associated chronic diseases.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
This work was supported by the Global Organization for EPA and DHA Omega-3s (GOED). GOED had no role in the study design or conduct; the acquisition, extraction, management, or analysis of data; the interpretation of research findings; or the writing of the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge GOED for their partial support of this research.
REFERENCES
- 1. Centers for Disease Control and Prevention. Vital signs: prevalence, treatment, and control of hypertension—United States, 1999–2002 and 2005–2008. MMWR Morbid Mortal Wkly Rep 2011; 60:103–108. [PubMed] [Google Scholar]
- 2. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 2012; 125:e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. Cabo J, Alonso R, Mata P. Omega-3 fatty acids and blood pressure. Br J Nutr 2012; 107:S195–S200. [DOI] [PubMed] [Google Scholar]
- 5. Hartweg J, Farmer AJ, Holman RR, Neil HAW. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on haematological and thrombogenic factors in type 2 diabetes. Diabetologia 2007; 50:250–258. [DOI] [PubMed] [Google Scholar]
- 6. Xin W, Wei W, Li X. Effect of fish oil supplementation on fasting vascular endothelial function in humans: a meta-analysis of randomized controlled trials. PLoS One 2012; 7:e46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell F, Dickinson HO, Critchley JA, Ford GA, Bradburn M. A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur J Prev Cardiol 2013; 20:107–120. [DOI] [PubMed] [Google Scholar]
- 8. Rothstein H, Sutton A, Borenstein M, eds. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. John Wiley and Sons: Chichester, England, 2005. [Google Scholar]
- 9. Trials of Hypertension Prevention Collaborative Research Group (THPCRG). The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA 1992; 267:1213–1220. [DOI] [PubMed] [Google Scholar]
- 10. Armstrong P, Kelley DS, Newman JW, Staggers FE, Sr, Hartiala J, Allayee H, Stephensen CB. Arachidonate 5-lipoxygenase gene variants affect response to fish oil supplementation by healthy African Americans. J Nutr 2012; 142:1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Atar MJH, Hajianfar H, Bahonar A. The effects of omega-3 on blood pressure and the relationship between serum visfatin level and blood pressure in patients with type II diabetes. ARYA Atheroscler 2012; 8:27–31. [PMC free article] [PubMed] [Google Scholar]
- 12. Bach R, Schmidt U, Jung F, Kiesewetter H, Hennen B, Wenzel E, Schieffer H, Bette L, Heyden S. Effects of fish oil capsules in two dosages on blood pressure, platelet functions, haemorheological and clinical chemistry parameters in apparently healthy subjects. Ann Nutr Metab 1989; 33:359–367. [DOI] [PubMed] [Google Scholar]
- 13. Barcelo-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: A multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr 2008; 88:801–809. [DOI] [PubMed] [Google Scholar]
- 14. Bønaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromsø study. N Engl J Med 1990; 795–801. [DOI] [PubMed] [Google Scholar]
- 15. Browning LM, Krebs JD, Moore CS, Mishra GD, O’Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab 2007; 9:70–80. [DOI] [PubMed] [Google Scholar]
- 16. Buckley JD, Burgess S, Murphy KJ, Howe PRC. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J Sci Med Sport 2009; 12:503–507. [DOI] [PubMed] [Google Scholar]
- 17. Carter JR, Schwartz CE, Yang H, Joyner MJ. Fish oil and neurovascular control in humans. Am J Physiol Heart Circ Physiol 2012; 303:H450–H456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cazzola R, Russo-Volpe S, Miles EA, Rees D, Banerjee T, Roynette CE, Wells SJ, Goua M, Wahle KWJ, Calder PC, Cestaro B. Age- and dose-dependent effects of an eicosapentaenoic acid-rich oil on cardiovascular risk factors in healthy male subjects. Atherosclerosis 2007; 193:159–167. [DOI] [PubMed] [Google Scholar]
- 19. Chin JP, Gust AP, Nestel PJ, Dart AM. Marine oils dose-dependently inhibit vasoconstriction of forearm resistance vessels in humans. Hypertension 1993; 21:22–28. [DOI] [PubMed] [Google Scholar]
- 20. Cobiac L, Clifton PM, Abbey M, Belling GB, Nestel PJ. Lipid, lipoprotein, and hemostatic effects of fish vs fish-oil n-3 fatty acids in mildly hyperlipidemic males. Am J Clin Nutr 1991; 53:1210–1216. [DOI] [PubMed] [Google Scholar]
- 21. Conquer JA, Cheryk LA, Chan E, Gentry PA, Holub BJ. Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thromb Res 1999; 96:239–250. [DOI] [PubMed] [Google Scholar]
- 22. Croset M, Vericel E, Rigaud M, Hanss M, Courpron P, Dechavanne M, Lagarde M. Functions and tocopherol content of blood platelets from elderly people after low intake of purified eicosapentaenoic acid. Thromb Res 1990; 57:1–12. [DOI] [PubMed] [Google Scholar]
- 23. Demke DM, Peters GR, Linet OI, Metzler CM, Klott KA. Effects of a fish oil concentrate in patients with hypercholesterolemia. Atherosclerosis 1988; 70:73–80. [DOI] [PubMed] [Google Scholar]
- 24. Derosa G, Cicero AFG, Fogari E, D’Angelo A, Bonaventura A, Romano D, Maffioli P. Effects of n-3 PUFAs on postprandial variation of metalloproteinases, and inflammatory and insulin resistance parameters in dyslipidemic patients: evaluation with euglycemic clamp and oral fat load. J Clin Lipid 2012; 6:553–564. [DOI] [PubMed] [Google Scholar]
- 25. Derosa G, Maffioli P, D’Angelo A, Salvadeo SAT, Ferrari I, Fogari E, Gravina A, Mereu R, Randazzo S, Cicero AFG. Effects of long chain omega-3 fatty acids on metalloproteinases and their inhibitors in combined dyslipidemia patients. Expert Opin Pharmacother 2009; 10:1239–1247. [DOI] [PubMed] [Google Scholar]
- 26. Deslypere JP. Influence of supplementation with N-3 fatty acids on different coronary risk factors in men—a placebo controlled study. Verh K Acad Geneeskd Belg 1992; 54:189–216. [PubMed] [Google Scholar]
- 27. Dewell A, Marvasti FF, Harris WS, Tsao P, Gardner CD. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J Nutr 2011; 141:2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dyerberg J, Eskesen DC, Andersen PW, Astrup A, Buemann B, Christensen JH, Clausen P, Rasmussen BF, Schmidt EB, Tholstrup T, Toft E, Toubro S, Stender S. Effects of trans- and n-3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr 2004; 58:1062–1070. [DOI] [PubMed] [Google Scholar]
- 29. Finnegan YE, Minihane AM, Leigh-Firbank EC, Kew S, Meijer GW, Muggli R, Calder PC, Williams CM. Plant- and marine-derived n-3 polyunsaturated fatty acids have differential effects on fasting and postprandial blood lipid concentrations and on the susceptibility of LDL to oxidative modification in moderately hyperlipidemic subjects. Am J Clin Nutr 2003; 77:783–795. [DOI] [PubMed] [Google Scholar]
- 30. Flaten H, Hostmark AT, Kierulf P, Lystad E, Trygg K, Bjerkedal T, Osland A. Fish-oil concentrate: effects on variables related to cardiovascular disease. Am J Clin Nutr 1990; 52:300–306. [DOI] [PubMed] [Google Scholar]
- 31. Geelen A, Zock PL, Swenne CA, Brouwer IA, Schouten EG, Katan MB. Effect of n-3 fatty acids on heart rate variability and baroreflex sensitivity in middle-aged subjects. Am Heart J 2003; 146:E4. [DOI] [PubMed] [Google Scholar]
- 32. Ginty AT, Conklin SM. Preliminary evidence that acute long-chain omega-3 supplementation reduces cardiovascular reactivity to mental stress: a randomized and placebo controlled trial. Biol Psychol 2012; 89:269–272. [DOI] [PubMed] [Google Scholar]
- 33. Grimsgaard S, Bonaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr 1998; 68:52–59. [DOI] [PubMed] [Google Scholar]
- 34. Gustafsson IB, Ohrvall M, Ekstrand B, Vessby B. Moderate amounts of n-3 fatty acid enriched seafood products are effective in lowering serum triglycerides and blood pressure in healthy subjects. J Hum Nutr Diet 1996; 9:135–145. [Google Scholar]
- 35. Hallund J, Madsen BO, Bugel SH, Jacobsen C, Jakobsen J, Krarup H, Holm J, Nielsen HH, Lauritzen L. The effect of farmed trout on cardiovascular risk markers in healthy men. Br J Nutr 2010; 104:1528–1536. [DOI] [PubMed] [Google Scholar]
- 36. Harris WS, Lemke SL, Hansen SN, Goldstein DA, DiRienzo MA, Su H, Nemeth MA, Taylor ML, Ahmed G, George C. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids 2008; 43:805–811. [DOI] [PubMed] [Google Scholar]
- 37. Hellsten G, Boman K, Saarem K, Hallmans G, Nilsson TK. Effects on fibrinolytic activity of corn oil and a fish oil preparation enriched with omega-3-polyunsaturated fatty acids in a long-term study. Curr Med Res Opin 1993; 13:133–139. [DOI] [PubMed] [Google Scholar]
- 38. Hill AM, Buckley JD, Murphy KJ, Howe PRC. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr 2007; 85:1267–1274. [DOI] [PubMed] [Google Scholar]
- 39. Hughes GS, Ringer TV, Watts KC, DeLoof MJ, Francom SF, Spillers CR. Fish oil produces an atherogenic lipid profile in hypertensive men. Atherosclerosis 1990; 84:229–237. [DOI] [PubMed] [Google Scholar]
- 40. Kelley DS, Siegel D, Vemuri M, Mackey BE. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am J Clin Nutr 2007; 86:324–333. [DOI] [PubMed] [Google Scholar]
- 41. Knapp HR, FitzGerald GA. The antihypertensive effects of fish oil. A controlled study of polyunsaturated fatty acid supplements in essential hypertension. N Engl J Med 1989; 320:1037–1043. [DOI] [PubMed] [Google Scholar]
- 42. Landmark K, Thaulow E, Hysing J, Mundal HH, Eritsland J, Hjermann I. Effects of fish oil, nifedipine and their combination on blood pressure and lipids in primary hypertension. J Hum Hypertens 1993; 7:25–32. [PubMed] [Google Scholar]
- 43. Levinson PD, Iosiphidis AH, Saritelli AL, Herbert PN, Steiner M. Effects of n-3 fatty acids in essential hypertension. Am J Hypertens 1990; 3:754–760. [DOI] [PubMed] [Google Scholar]
- 44. Lofgren RP, Wilt TJ, Nichol KL, Crespin L, Pluhar R, Eckfeldt J. The effect of fish oil supplements on blood pressure. Am J Pub Health 1993; 83:267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mackness MI, Bhatnagar D, Durrington PN, Prais H, Haynes B, Morgan J, Borthwick L. Effects of a new fish oil concentrate on plasma lipids and lipoproteins in patients with hypertriglyceridaemia. Eur J Clin Nutr 1994; 48:859–865. [PubMed] [Google Scholar]
- 46. Maki KC, Reeves MS, Farmer M, Griinari M, Berge K, Vik H, Hubacher R, Rains TM. Krill oil supplementation increases plasma concentrations of eicosapentaenoic and docosahexaenoic acids in overweight and obese men and women. Nutr Res 2009; 29:609–615. [DOI] [PubMed] [Google Scholar]
- 47. McVeigh GE, Brennan GM, Cohn JN, Finkelstein SM, Hayes RJ, Johnston GD. Fish oil improves arterial compliance in non-insulin-dependent diabetes mellitus. Arterioscler Thromb 1994; 14:1425–1429. [DOI] [PubMed] [Google Scholar]
- 48. Meland E, Fugelli P, Laerum E, Ronneberg R, Sandvik L. Effect of fish oil on blood pressure and blood lipids in men with mild to moderate hypertension. Scand J Prim Health Care 1989; 7:131–135. [DOI] [PubMed] [Google Scholar]
- 49. Mills DE, Mah M, Ward RP, Morris BL, Floras JS. Alteration of baroreflex control of forearm vascular resistance by dietary fatty acids. Amer J Physiol 1990; 259:R1164–R1171. [DOI] [PubMed] [Google Scholar]
- 50. Mills DE, Prkachin KM, Harvey KA, Ward RP. Dietary fatty acid supplementation alters stress reactivity and performance in man. J Hum Hypertens 1989; 3:111–116. [PubMed] [Google Scholar]
- 51. Monahan KD, Wilson TE, Ray CA. Omega-3 fatty acid supplementation augments sympathetic nerve activity responses to physiological stressors in humans. Hypertension 2004; 44:732–738. [DOI] [PubMed] [Google Scholar]
- 52. Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 1999; 34:253–260. [DOI] [PubMed] [Google Scholar]
- 53. Mortensen JZ, Schmidt EB, Nielsen AH, Dyerberg J. The effect of N-6 and N-3 polyunsaturated fatty acids on hemostasis, blood lipids and blood pressure. Thromb Haemost 1983; 50:543–546. [PubMed] [Google Scholar]
- 54. Mundal HH, Gjesdal K, Landmark K. The effect of N-3 fatty acids and nifedipine on platelet function in hypertensive males. Thromb Res 1993; 72:257–262. [DOI] [PubMed] [Google Scholar]
- 55. Murphy KJ, Meyer BJ, Mori TA, Burke V, Mansour J, Patch CS, Tapsell LC, Noakes M, Clifton PA, Barden A, Puddey IB, Beilin LJ, Howe PRC. Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr 2007; 97:749–757. [DOI] [PubMed] [Google Scholar]
- 56. Neff LM, Culiner J, Cunningham-Rundles S, Seidman C, Meehan D, Maturi J, Wittkowski KM, Levine B, Breslow JL. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J Nutr 2011; 141:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr 2002; 76:326–330. [DOI] [PubMed] [Google Scholar]
- 58. Nordoy A, Hansen JB, Brox J, Svensson B. Effects of atorvastatin and omega-3 fatty acids on LDL subfractions and postprandial hyperlipemia in patients with combined hyperlipemia. Nutr Metab Cardiovas 2001; 11:7–16. [PubMed] [Google Scholar]
- 59. Noreen EE, Brandauer J. The effects of supplemental fish oil on blood pressure and morning cortisol in normotensive adults: a pilot study. J Compl Integr Med 2012; 9:1–16. [DOI] [PubMed] [Google Scholar]
- 60. Passfall J, Philipp T, Woermann F, Quass P, Thiede M, Haller H. Different effects of eicosapentaenoic acid and olive oil on blood pressure, intracellular free platelet calcium, and plasma lipids in patients with essential hypertension. Clin Investigator 1993; 71:628–633. [DOI] [PubMed] [Google Scholar]
- 61. Prisco D, Paniccia R, Bandinelli B, Filippini M, Francalanci I, Giusti B, Giurlani L, Gensini GF, Abbate R, Neri Serneri GG. Effect of medium-term supplementation with a moderate dose of n-3 polyunsaturated fatty acids on blood pressure in mild hypertensive patients. Thromb Res 1998; 91:105–112. [DOI] [PubMed] [Google Scholar]
- 62. Radack K, Deck C, Huster G. The effects of low doses of n-3 fatty acid supplementation on blood pressure in hypertensive subjects. A randomized controlled trial. Arch Intern Med 1991; 151:1173–1180. [PubMed] [Google Scholar]
- 63. Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I. Moderate consumption of fatty fish reduces diastolic blood pressure in overweight and obese European young adults during energy restriction. Nutrition 2010; 26:168–174. [DOI] [PubMed] [Google Scholar]
- 64. Ryu J, Lerner J, Sullivan JM. Unresponsiveness of forearm hemodynamics to omega-3 polyunsaturated fatty acids and aspirin. Prostaglandins 1990; 39:339–347. [DOI] [PubMed] [Google Scholar]
- 65. Sagara M, Njelekela M, Teramoto T, Taguchi T, Mori M, Armitage L, Birt N, Birt C, Yamori Y. Effects of docosahexaenoic acid supplementation on blood pressure, heart rate, and serum lipids in scottish men with hypertension and hypercholesterolemia. Int J Hypertens 2011; 2011:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sanders TA, Gleason K, Griffin B, Miller GJ. Influence of an algal triacylglycerol containing docosahexaenoic acid (22:6n-3) and docosapentaenoic acid (22:5n-6) on cardiovascular risk factors in healthy men and women. Br J Nutr 2006; 95:525–531. [DOI] [PubMed] [Google Scholar]
- 67. Sjoberg NJ, Milte CM, Buckley JD, Howe PRC, Coates AM, Saint DA. Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br J Nutr 2010; 103:243–248. [DOI] [PubMed] [Google Scholar]
- 68. Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women recieving and not receiving hormon replacement therapy. Am J Clin Nutr 2004; 79:765–773. [DOI] [PubMed] [Google Scholar]
- 69. Steiner A, Oertel R, Battig B, Pletscher W, Weiss B, Germinger P, Vetter W. Effect of fish oil on blood pressure and serum lipids in hypertension and hyperlipidaemia. J Hypertens 1989; 7:S73–S76. [PubMed] [Google Scholar]
- 70. Theobald HE, Goodall AH, Sattar N, Talbot DCS, Chowienczyk PJ, Sanders TAB. Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J Nutr 2007; 137:973–978. [DOI] [PubMed] [Google Scholar]
- 71. Toft I, Bonaa KH, Ingebretsen OC, Nordoy A, Jenssen T. Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med 1995; 123:911–918. [DOI] [PubMed] [Google Scholar]
- 72. Vakhapova V, Richter Y, Cohen T, Herzog Y, Korczyn AD. Safety of phosphatidylserine containing omega-3 fatty acids in non-demented elderly: a double-blind placebo-controlled trial followed by an open-label extension. BMC Neurol 2011; 11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vandongen R, Mori TA, Burke V, Beilin LJ, Morris J, Ritchie J. Effects on blood pressure of omega 3 fats in subjects at increased risk of cardiovascular disease. Hypertension 1993; 22:371–379. [DOI] [PubMed] [Google Scholar]
- 74. Vericel E, Calzada C, Chapuy P, Lagarde M. The influence of low intake of n-3 fatty acids on platelets in elderly people. Atherosclerosis 1999; 147:187–192. [DOI] [PubMed] [Google Scholar]
- 75. von Houwelingen R, Nordoy A, van der Beek E, Houtsmuller U, de Metz M, Hornstra G. Effect of a moderate fish intake on blood pressure, bleeding time, hematology, and clincical chemistry in healthy males. Am J Clin Nutr 1987; 46:424–436. [DOI] [PubMed] [Google Scholar]
- 76. Walser B, Stebbins CL. Omega-3 fatty acid supplementation enhances stroke volume and cardiac output during dynamic exercise. Eur J Appl Physiol 2008; 104:455–461. [DOI] [PubMed] [Google Scholar]
- 77. Wang S, Ma AQ, Song SW, Quan QH, Zhao XF, Zheng XH. Fish oil supplementation improves large arterial elasticity in overweight hypertensive patients. Eur J Clin Nutr 2008; 62:1426–1431. [DOI] [PubMed] [Google Scholar]
- 78. Lindqvist HM, Langkilde AM, Undeland I, Sandberg AS. Herring (Clupea harengus) intake influences lipoproteins but not inflammatory and oxidation markers in overweight men. Br J Nutr 2009; 101:383–390. [DOI] [PubMed] [Google Scholar]
- 79. Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, Cook JV, Williams B, Ford GA. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens 2006; 24:215–233. [DOI] [PubMed] [Google Scholar]
- 80. Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993; 88:523–533. [DOI] [PubMed] [Google Scholar]
- 81. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 2002; 20:1493–1499. [DOI] [PubMed] [Google Scholar]
- 82. Mozaffarian D. Fish, n-3 fatty acids, and cardiovascular haemodynamics. J Cardiovasc Med (Hagerstown) 2007; 8:S23––S26. [DOI] [PubMed] [Google Scholar]
- 83. Wang Q, Liang X, Wang L, Lu X, Huang J, Cao J, Li H, Gu D. Effect of omega-3 fatty acids supplementation on endothelial function: a meta-analysis of randomized controlled trials. Atherosclerosis 2012; 221:536–543. [DOI] [PubMed] [Google Scholar]
- 84. Egert S, Stehle P. Impact of n-3 fatty acids on endothelial function: results from human interventions studies. Curr Opin Clin Nutr Metab Care 2011; 14:121–131. [DOI] [PubMed] [Google Scholar]
- 85. Appel LJ, American Society of Hypertension Writing Group, Giles TD, Black HR, Izzo JL, Jr, Materson BJ, Oparil S, Weber MA. ASH position paper: dietary approaches to lower blood pressure. J Clin Hypertens (Greenwich) 2009; 11:358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Appel LJ, Miller ER, 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with “fish oil” reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med 1993; 153:1429–1438. [PubMed] [Google Scholar]
- 87. Ruxton CHS. The benefits of fish consumption. Nutr Bull 2011; 36:6–19. [Google Scholar]
- 88. Adophus K, Biac S. Oily fish consumption in young adults: current intakes, knowledge, barriers and motivations. J Hum Nutr Diet 2011; 24:375. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.