Abstract
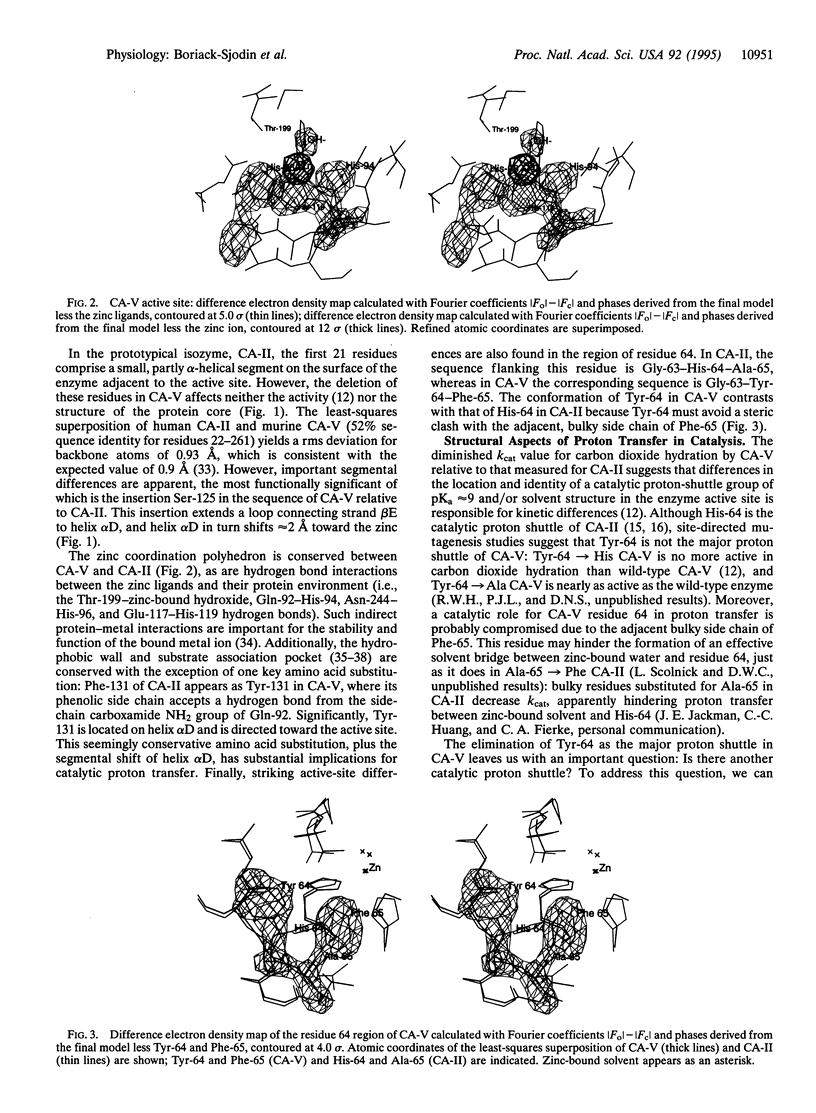
The three-dimensional structure of murine mitochondrial carbonic anhydrase V has been determined and refined at 2.45-A resolution (crystallographic R factor = 0.187). Significant structural differences unique to the active site of carbonic anhydrase V are responsible for differences in the mechanism of catalytic proton transfer as compared with other carbonic anhydrase isozymes. In the prototypical isozyme, carbonic anhydrase II, catalytic proton transfer occurs via the shuttle group His-64; carbonic anhydrase V has Tyr-64, which is not an efficient proton shuttle due in part to the bulky adjacent side chain of Phe-65. Based on analysis of the structure of carbonic anhydrase V, we speculate that Tyr-131 may participate in proton transfer due to its proximity to zinc-bound solvent, its solvent accessibility, and its electrostatic environment in the protein structure. Finally, the design of isozyme-specific inhibitors is discussed in view of the complex between carbonic anhydrase V and acetazolamide, a transition-state analogue. Such inhibitors may be physiologically important in the regulation of blood glucose levels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amor-Gueret M., Levi-Strauss M. Nucleotide and derived amino-acid sequence of a cDNA encoding a new mouse carbonic anhydrase. Nucleic Acids Res. 1990 Mar 25;18(6):1646–1646. doi: 10.1093/nar/18.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balboni E., Lehninger A. L. Entry and exit pathways of CO2 in rat liver mitochondria respiring in a bicarbonate buffer system. J Biol Chem. 1986 Mar 15;261(8):3563–3570. [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brünger A. T. Free R value: a novel statistical quantity for assessing the accuracy of crystal structures. Nature. 1992 Jan 30;355(6359):472–475. doi: 10.1038/355472a0. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Burbaum J. J., Ohlmeyer M. H., Reader J. C., Henderson I., Dillard L. W., Li G., Randle T. L., Sigal N. H., Chelsky D., Baldwin J. J. A paradigm for drug discovery employing encoded combinatorial libraries. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6027–6031. doi: 10.1073/pnas.92.13.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 1986 Apr;5(4):823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994 Sep 1;50(Pt 5):760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- DATTA P. K., SHEPARD T. H., 2nd Intracellular localization of carbonic anhydrase in rat liver and kidney tissues. Arch Biochem Biophys. 1959 Mar;81(1):124–129. doi: 10.1016/0003-9861(59)90182-1. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Cherian K. Mitochondrial carbonic anhydrase is involved in rat renal glucose synthesis. Am J Physiol. 1989 Dec;257(6 Pt 1):E791–E796. doi: 10.1152/ajpendo.1989.257.6.E791. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd Carbonic anhydrase: inhibition results in decreased urea production by hepatocytes. J Appl Physiol (1985) 1986 Feb;60(2):646–652. doi: 10.1152/jappl.1986.60.2.646. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd Inhibition of CA V decreases glucose synthesis from pyruvate. Arch Biochem Biophys. 1986 Nov 15;251(1):198–204. doi: 10.1016/0003-9861(86)90066-4. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson A. E., Jones T. A., Liljas A. Refined structure of human carbonic anhydrase II at 2.0 A resolution. Proteins. 1988;4(4):274–282. doi: 10.1002/prot.340040406. [DOI] [PubMed] [Google Scholar]
- Heck R. W., Tanhauser S. M., Manda R., Tu C., Laipis P. J., Silverman D. N. Catalytic properties of mouse carbonic anhydrase V. J Biol Chem. 1994 Oct 7;269(40):24742–24746. [PubMed] [Google Scholar]
- Håkansson K., Carlsson M., Svensson L. A., Liljas A. Structure of native and apo carbonic anhydrase II and structure of some of its anion-ligand complexes. J Mol Biol. 1992 Oct 20;227(4):1192–1204. doi: 10.1016/0022-2836(92)90531-n. [DOI] [PubMed] [Google Scholar]
- Jewell D. A., Tu C. K., Paranawithana S. R., Tanhauser S. M., LoGrasso P. V., Laipis P. J., Silverman D. N. Enhancement of the catalytic properties of human carbonic anhydrase III by site-directed mutagenesis. Biochemistry. 1991 Feb 12;30(6):1484–1490. doi: 10.1021/bi00220a006. [DOI] [PubMed] [Google Scholar]
- Khalifah R. G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem. 1971 Apr 25;246(8):2561–2573. [PubMed] [Google Scholar]
- Krebs J. F., Rana F., Dluhy R. A., Fierke C. A. Kinetic and spectroscopic studies of hydrophilic amino acid substitutions in the hydrophobic pocket of human carbonic anhydrase II. Biochemistry. 1993 May 4;32(17):4496–4505. doi: 10.1021/bi00068a004. [DOI] [PubMed] [Google Scholar]
- Lindskog S., Engberg P., Forsman C., Ibrahim S. A., Jonsson B. H., Simonsson I., Tibell L. Kinetics and mechanism of carbonic anhydrase isoenzymes. Ann N Y Acad Sci. 1984;429:61–75. doi: 10.1111/j.1749-6632.1984.tb12315.x. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Ellison A. C. A study of renal carbonic anhydrase. Mol Pharmacol. 1967 Nov;3(6):503–508. [PubMed] [Google Scholar]
- Nagao Y., Platero J. S., Waheed A., Sly W. S. Human mitochondrial carbonic anhydrase: cDNA cloning, expression, subcellular localization, and mapping to chromosome 16. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7623–7627. doi: 10.1073/pnas.90.16.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao Y., Srinivasan M., Platero J. S., Svendrowski M., Waheed A., Sly W. S. Mitochondrial carbonic anhydrase (isozyme V) in mouse and rat: cDNA cloning, expression, subcellular localization, processing, and tissue distribution. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10330–10334. doi: 10.1073/pnas.91.22.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama T., Sato S., Zhu X. L., Waheed A., Sly W. S. Human carbonic anhydrase IV: cDNA cloning, sequence comparison, and expression in COS cell membranes. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1315–1319. doi: 10.1073/pnas.89.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. N., Tu C., Chen X., Tanhauser S. M., Kresge A. J., Laipis P. J. Rate-equilibria relationships in intramolecular proton transfer in human carbonic anhydrase III. Biochemistry. 1993 Oct 12;32(40):10757–10762. doi: 10.1021/bi00091a029. [DOI] [PubMed] [Google Scholar]
- Steiner H., Jonsson B. H., Lindskog S. The catalytic mechanism of carbonic anhydrase. Hydrogen-isotope effects on the kinetic parameters of the human C isoenzyme. Eur J Biochem. 1975 Nov 1;59(1):253–259. doi: 10.1111/j.1432-1033.1975.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Ross B. D. The impact of acetazolamide on renal ammoniagenesis and gluconeogenesis. J Lab Clin Med. 1983 Oct;102(4):536–542. [PubMed] [Google Scholar]
- Tu C. K., Silverman D. N., Forsman C., Jonsson B. H., Lindskog S. Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochemistry. 1989 Sep 19;28(19):7913–7918. doi: 10.1021/bi00445a054. [DOI] [PubMed] [Google Scholar]
- Vidgren J., Liljas A., Walker N. P. Refined structure of the acetazolamide complex of human carbonic anhydrase II at 1.9 A. Int J Biol Macromol. 1990 Dec;12(6):342–344. doi: 10.1016/0141-8130(90)90040-h. [DOI] [PubMed] [Google Scholar]
- Whitney P. L., Briggle T. V. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem. 1982 Oct 25;257(20):12056–12059. [PubMed] [Google Scholar]