Abstract
Rodent apoA-IV is expressed predominantly in small intestine and also expressed to a small extent in liver and hypothalamus. ApoA-IV has been shown to inhibit food intake in rats when injected centrally. In the current study, we hypothesize that a specific sequence within rat apoA-IV is responsible for mediating the anorectic effect. We use a bacterial expression system to generate truncation mutants (Δ249–371, Δ117–371 and Δ1–61) of rat apoA-IV and assess the ability of various regions of the molecule to inhibit food intake. The results indicate that a responsible sequence exists within the N-terminal 61 amino acids of rat apoA-IV. Synthetic peptides (1–30 EVTSDQVANVMWDYFTQLSNNAKEAVEQLQ, 1–15 EVTSDQVANVMWDYF and 17–30 QLSNNAKEAVEQLQ) were used to specify the region in between residues 1 and 30. A 14-mer peptide (17–30) encompassing this sequence was capable of reducing food intake in a dose-dependent manner whereas a peptide designed on a more C-terminal region (211–232) of apoA-IV (QEKLNHQMEGLAFQMKKNAEEL) failed to exhibit the dose-dependent anorectic effect. The isolation of this sequence provides a valuable tool for future work directed at identifying apoA-IV binding proteins and is a key step for exploring the potential of therapeutic manipulation of food intake via this pathway.
Keywords: Apolipoprotein A-IV, Food intake, Truncation mutation
1. Introduction
Apolipoprotein A-IV is a lipid-binding protein mainly synthesized by the small intestine and secreted into intestinal lymphatics associated with chylomicrons [1,2]. Examination of the amino acid sequence indicates the presence of amphipathic helical repeat structures that likely mediates its interactions with lipids [3] which is a function shared by other exchangeable apolipoproteins such as apoA-I. ApoA-IV has been suggested to play several similar roles as apoA-I [4]. For example, apoA-IV has been shown to activate lecithin: cholesterol acyl transferase [5], cholesterol ester transfer protein [6] and to promote cholesterol efflux from cells [7]. ApoA-IV clearly plays an important role in the clearance of dietary fat as it is a key component of chylomicrons and variants of the protein can affect postprandial responses in triglyceride rich lipoproteins [8]. Mice made transgenic with either human [9] or mouse [10] apoA-IV are significantly protected from atherosclerosis. However, in terms of lipoprotein metabolism, there is not yet a clear function for apoA-IV that is not shared by other apolipoproteins.
Interestingly, unlike other exchangeable apolipoproteins, apoA-IV mRNA levels and protein secretion are markedly increased during lipid absorption [11]. Under conditions of high lipid load, apoA-IV accounts for up to 3% of total gut secreted protein [11,12]. Several studies have suggested that levels of apoA-IV may also be an important feedback for the regulation of food intake. Fujimoto [13] reported that mesenteric lymph from lipid infused donor rats exerted an anorectic effect when intravenously infused into fasted rat recipients. When this lymph was immunodepleted of apoA-IV, the anorectic effect was abolished. Importantly, the effect was not mediated by infused apoA-I. Furthermore, it was demonstrated that apoA-IV had a direct effect on food intake when injected into the third ventricle of the rat brain [14]. By contrast, antibodies to apoA-IV injected at the same location increased food intake.
More experiments have investigated the connection between apoA-IV levels and effects on appetite. Fukagawa et al. [15] have shown a relationship between the circadian rhythm of apoA-IV in circulation and periods of active feeding in rats. In addition, apoA-IV has been shown to inhibit the rate of gastric emptying and gastric acid secretion by acting through the central nervous system [16,17]. Both effects may offer potential mechanisms for the regulation of appetite by apoA-IV. Intriguingly, Liu et al. have reported that the hypothalamus can produce apoA-IV de novo [18]. During periods of fasting, hypothalamic apoA-IV mRNA levels decrease and then rise upon lipid feeding [19]. However, this responsiveness of brain apoA-IV levels to the diet tends to degrade over prolonged periods of fat absorption [20], implying that a chronic high fat diet may reduce the responsiveness of the satiety feedback pathway resulting in long-term over eating. Indeed, the potential role of apoA-IV in the long-term effects of obesity is illustrated by the fact that certain polymorphisms of apoA-IV are associated with a higher body mass index than others [21].
In terms of structure and function relationship, it has been shown that the region of apoA-IV from 117–160 is required for LCAT activation [5]. For lipid binding, apoA-IV has an ‘inhibitory’ region located at the C-terminus between residues 333 and 343 [22]. However, it is unknown in regard to the structural basis for the impact of apoA-IV on food intake. In the current study, we hypothesize that apoA-IV mediates its anorectic effects through a molecular interaction that requires a specific sequence within the protein. To test this, we performed deletion mutagenesis on rat apoA-IV and produced synthetic peptides for various regions of the sequence. The results indicate that a domain in the N-terminus of apoA-IV is responsible for its anorectic effects centrally.
2. Experimental procedures
2.1. Materials
Primer synthesis and DNA sequencing were performed by IDT (Coralville, IA) or the University of Cincinnati DNA Core (Cincinnati, OH). Restriction enzymes were purchased from New England Biolabs (Beverly, MA). IgA protease (Igase) was purchased from MoBiTec (Germany). BL-21 (DE3) Escherichia coli, pET30 vector and His-bind resin were from Novagen (Madison, WI). Isopropyl-β-Dthiogalactoside (IPTG) was purchased from Fisher Scientific (Pittsburg, PA). His-bind resin was purchased from Novagen (Madison, WI). Restriction enzymes were purchased from New England Biolabs (Beverly, MA). SDS-PAGE gels were obtained from Bio-Rad (Hercules, CA). Centriprep centrifugal concentrators were purchased from Millipore Amicon Bioseparations (Bedford, MA). All chemical reagents are of the highest quality available.
2.2. Mutagenesis of rat ApoA-IV
The development of the rat apoA-IV expression system has been described previously [23]. The wild type (WT) apoA-IV construct in the pET-30 vector was used as the template for PCR-based mutagenesis. The C-terminal mutations were created by performing PCR-based site-directed mutagenesis in order to insert stop codons at positions 249 or117, respectively (Fig. 1). The Δ1–61 mutant was generated by a ‘flap’ PCR approach in which a forward primer was designed with a clamp region that matched nucleotides beginning at amino acid 62 and a 5′ flap region that contained the His-tag and an NcoI site for ligation back into the pET30 vector [24]. The sequence of each mutant construct was verified on an Applied Biotechnology System DNA sequencer at the University of Cincinnati DNA Core.
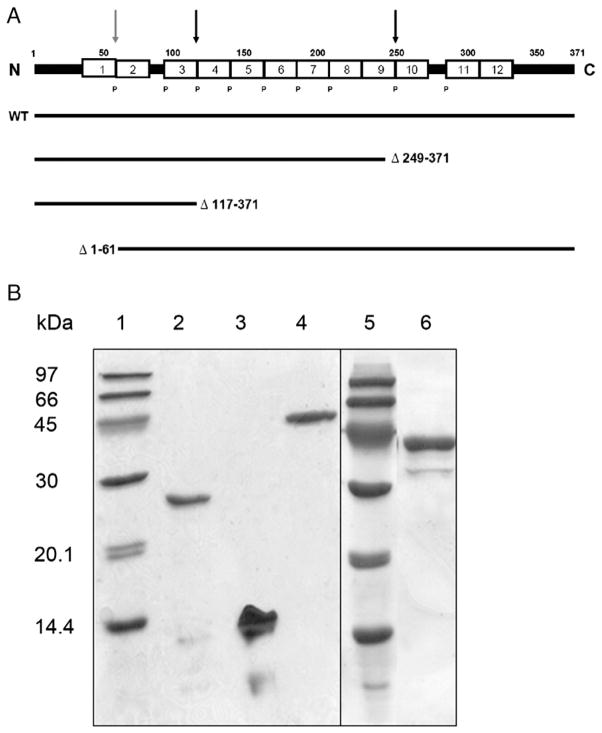
Fig. 1.
Linear diagram and SDS-PAGE analysis of mature rat apoA-IV and recombinant deletion mutants. A: The sequence is numbered according to the mature apoA-IV sequence. The white boxes represent putative 22 amino acid amphipathic alpha helices. Most of the repeats are punctuated by proline residues (P). The arrows are placed at sites where deletions were made either in the C- or N-terminus. Each deletion mutant used in this study is represented by a solid black line showing the sequence that was left intact. B: After expression and purification as described in Experimental Procedures, 4 μg samples of apolipoprotein were electrophoresed on an 18% denaturing polyacrylamide gel and stained with Coomassie blue. Lane 1: Amersham low molecular weight protein standards; Lane 2: apoA-IV Δ249–371; Lane3: apoA-IV Δ117–371; Lane 4: WT rat apoA-IV; Lane 5: standards; Lane 6: Δ1–61.
2.3. Protein expression and purification
The expression constructs were transfected into E. Coli BL-21 cells and grown on Luria-Bertani (LB) agar plates containing 30 μg/ml kana-mycin (Calbiochem, San Diego, CA) for 16 h at 37 °C. Cell colonies were picked and grown in LB culture media with 30 μg/ml kanamycin overnight in 10 ml culture tubes at 37 °C. These were then used to inoculate 100 ml LB cultures that were grown to an A600 of between 0.6 and 0.7. IPTG was added for 2 h to induce over-expression of the protein. Cell pellets were collected and brought up in His-bind buffer (5 mM Imidazole, 0.5 M NaCl, 20 mM Tris–HCl, pH 7.9). Cells were sonicated, centrifuged, and the supernatant was filtered and added to His-bind columns according to the manufacturer’s instruction. The protein was eluted, concentrated and cut using Igase. Next, the sample was passed through the His-bind column a second time to remove the His-tag. The samples were then concentrated and dialyzed against saline for storage at 1 mg/ml.
2.4. Removal of lipopolysaccharide (LPS)
Bacterial LPS was removed from the fully purified apoA-IV samples by passing it through an AffinityPak Detoxi-Gel endotoxin removing gel (Pierce, Rockford, IL). The protein was diluted to a final concentration of 1.5 mg/ml in STB. The column was equilibrated in STB buffer and then 1 ml of sample was loaded to each column and incubated for 1 h at R.T. Each column was then eluted with 10 ml of STB and concentrated. Post column samples were sent to Cambrex-BioWhittaker for a KineticQCL colorimetric assay of endotoxin levels.
2.5. Native rat apoA-IV purification
Rat plasma was obtained from abdominal aorta blood. Potassium bromide was added to adjust the density to 1.21 g/ml and lipoproteins of d < 1.21 g/ml were isolated by flotation ultracentrifugation. The lipoproteins were then lyophilized and delipidated with a series of incubation in ethanol-ether and the protein pellet was dissolved in 0.1 M Tris, pH 8.2, containing 1% sodium decyl sulfate (SDS). Protein samples were subjected to 8% polyacrylamide gel electrophoresis (PAGE) containing 0.2% SDS. After electrophoresis for 6 h at 10 mA, individual apolipoproteins were located by staining. Area of the gels corresponding to apoA-IV was eluted with 150 ml of 0.05 M NH4HCO3, containing 0.1% SDS, 0.1 M sodium azide and 0.1 mM PMSF. apoA-IV solutions were then dialyzed against 10 mM Tris–HC1, 2 mM SDS, 1 mM EDTA, and 0.01% sodium azide, at 4 °C [11].
2.6. Peptide synthesis
Synthetic peptides were generated by automated solid phase peptide synthesis on a Milligen 9050 Peptide Synthesizer at the Louisiana State University core facility. They were washed in diethyl ether and freeze-dried for use. Electrospray mass spectrometry was performed on each peptide to determine purity and correct molecular weight. In some cases, the peptides were sequenced directly using an Applied Biosystems 477A Protein Sequencer. The sequences of the peptides used in this study were as follows:
ApoA-IV (1–30) EVTSDQVANVMWDYFTQLSNNAKEAVEQLQ, apoA-IV (1–15) EVTSDQVANVMWDYF, apoA-IV (17–30) QLSNNAKEAVEQLQ, and ApoA-IV (211–232) QEKLNHQMEGLAFQMKKNAEEL.
2.7. Food intake studies
Male Sprague–Dawley rats (275–300 g, Harlan, Indianapolis, IN) were individually housed under stable environmental conditions with a 12-h dark/12-h light cycle with free access to pelleted food and water. For 3rd ventricular cannulation, rats were anesthetized with 1 ml/kg i.p. injections of a mixture of 70 mg/kg ketamine and 2 mg/kg xylazine, thereafter were placed in a stereotaxic instrument with the skull held horizontally and exposed. A 21-gauge stainless-steel guide cannula was lowered directly on the midline, 2.2-mm posterior to bregma and 7.5-mm ventral to the dura, and fixed to the skull with anchor screws and dental acrylic. Obturators that extended 0.5 mm beyond the cannula tips were inserted. All procedures were performed in accordance with the guidelines of the University Institutional Animal Care and Use Committee at the University of Cincinnati. Cannula placement was verified one week after surgery by i3vt injection of angiotensin II (10 ng). Only those animals consuming more than 5 ml of water in 60 min post-injection were included in the study. Two weeks after cannula implantation, rats were fasted for 24 h and assigned to weight-matched groups. At the onset of dark, rats received i3vt injections (4 μl) containing saline, or the indicated apoA-IV mutant or peptide in saline. The rats were visually observed, and food and water consumption was measured at 0.5, 1, 2, 4 h post-injection.
2.8. Statistical analysis
Results are presented as mean ± S.E.M. Data were analyzed by using Student’s-test or one-way analysis of variance (ANOVA) followed by Tukey’s test. For all analyses, a P < 0.05 was considered to be statistically significant.
3. Results
3.1. Compare the effect of native and recombinant rat apoA-IV on food intake
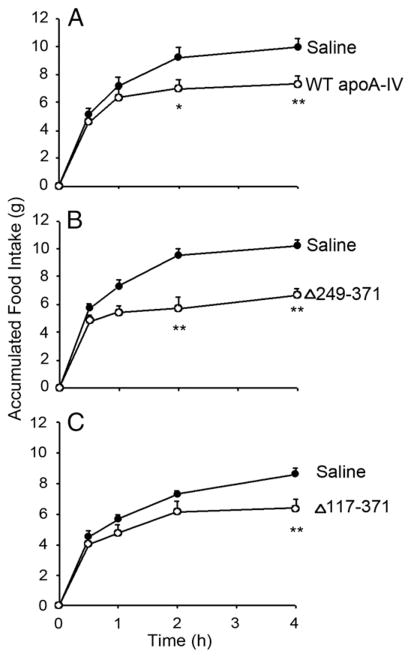
To test the hypothesis that apoA-IV contains a specific sequence that is responsible for its anorectic effect, we used deletion mutagenesis to divide the molecule into approximate thirds and evaluate the ability of the remaining protein to mediate the anorectic effect (Fig. 1). We previously demonstrated that the recombinant protein produced in our expression system is structurally and functionally similar to native rat apoA-IV in terms of its ability to bind lipid and inhibit food intake in rats [23]. An example of this is shown in Fig. 2A. When injected into the 3rd ventricle of the rat brain prior to the onset of dark, recombinant apoA-IV significantly reduced 4 h food intake in a dose-dependent manner. A 4 μg dose of native rat apoA-IV also significantly suppressed food intake over 4 h with a magnitude similar to the recombinant protein. This observation indicates that the recombinant form of apoA-IV recapitulates the effects of the native protein. However, before initiating studies with a series of mutants, we addressed a potential complication of injecting bacterial expressed protein into a living brain. It is well known that bacterial cells contain significant levels of lipopolysaccharide (LPS), also known as endotoxin, in the cell wall. LPS is a known neurotoxin that elicits inflammatory responses in many tissues. If present in our purified protein preparations, it is possible that LPS may cause the reduction of food intake that is independent of the apoA-IV effect. To assure that the LPS did not have an effect on the rat food intake studies, we removed it by column chromatography on an endotoxin affinity column. LPS analysis showed that this treatment significantly reduced the LPS in the apoA-IV preparation. The untreated sample [LPS (+)] contained 31 endotoxin units per μg of apoA-IV while the column-treated sample [LPS (−)] contained approximately 1 endotoxin unit per μg of apoA-IV. We then compared the two preparations of apoA-IV in a rat food intake experiment. Fig. 2B shows a time course of food intake over 4 h post infusion of these two samples vs. saline alone. It is clear that both recombinant apoA-IV samples significantly reduced food intake at 2 and 4 h vs. the saline control. Furthermore, the presence of the miniscule amount of LPS in the untreated sample had no significant impact on the anorectic effect. Because of pragmatic considerations, we chose to perform the remainder of the studies without further removal of the LPS from our samples.
Fig. 2.
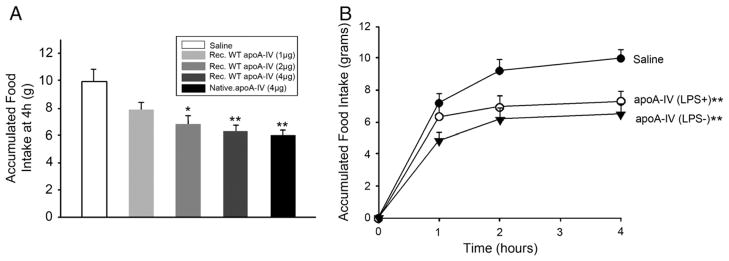
Food intake measurements in i3vt cannulated rats. A: 24 h fasted rats were injected with either saline alone or the indicated amount of apoA-IV (recombinant or native rat apoA-IV) immediately before dark-onset and accumulated food intake was measured up to 4 h. *P < 0.05, **P < 0.01 vs. Saline, n = 5. B: Accumulated food intake was plotted as a function of time post injection of 4 μg recombinant apoA-IV. LPS (+) refers to a preparation of recombinant apoA-IV as derived from the His-binding column described in Experimental Procedures prior to injection into the 3rd ventricle of the rat brain. LPS (−) refers to a similar sample that had been passed down an endotoxin affinity column, **P < 0.01 vs. Saline, n = 5.
3.2. Food intake studies with truncation mutations of rat apoA-IV
Fig. 1B shows an SDS-PAGE analysis of the purified C-terminal mutants. WT rat apoA-IV (lane 4) migrated to an apparent MW of about 43 kDa in agreement with its predicted mass of 42,635 Da (including the Thr-Pro- on the N-terminus of the protein). ApoA-IV Δ249–371 and apoA-IV Δ117–371 migrated to apparent masses of 28 kDa (predicted 28.6 kDa) and 14 kDa (predicted 13.4 kDa), respectively. It is clear that the recombinant proteins were highly homogeneous. The ability of the C-terminal truncation mutants of rat apo A-IV to inhibit food intake in fasted rats was then assessed. Fig. 3A confirms that the WT recombinant apoA-IV reduced food intake at both 2 and 4 h compared to the saline control. Similarly, apoA-IV Δ249–371 inhibited food intake at the same time points (Fig. 3B). Apo A-IV Δ117–371 also exerted an anorectic effect at 4 h, and a trend toward inhibited food intake at 2 h (Fig. 3C). It follows from the results in Fig. 3 that a sequence located in the N-terminal 116 amino acids contains a site that is responsible for food intake inhibition, as this sequence was intact in all the mutants. To probe further, we generated an N-terminal mutant that lacked the first 61 amino acids of the protein. This site was chosen because it divided the N-terminal 117 residues roughly in half and is located at the helical junction between repeats 1 and 2 of apoA-IV. Fig. 1B shows the SDS-PAGE analysis of this mutant. A minor contaminant band is apparent at about 32 kDa that is likely a proteolytic cleavage product of the truncation mutant. Interestingly, as shown in Fig. 4, Δ1–61 mutant did not significantly inhibit food intake compared to saline control. This strongly suggests that the active sequence locates in the N-terminal 116 amino acids, in which 1–61 amino acids is the critical region.
Fig. 3.
Effect of C-terminal truncation mutants of apoA-IV on rat food intake when injected centrally. Accumulated food intake is displayed according to time post injection of 4 μg recombinant apoA-IV. *P < 0.05, **P < 0.01 vs. Saline, n = 5.
Fig. 4.
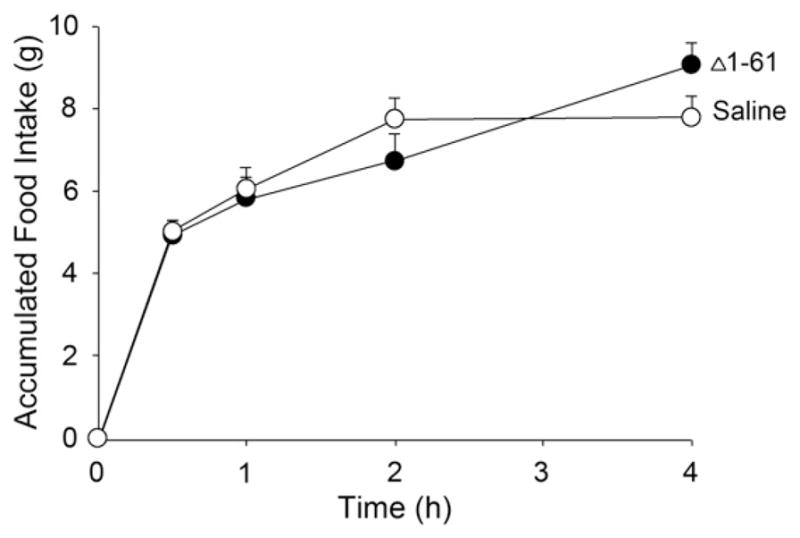
Effect of an N-terminal deletion of apoA-IV on rat food intake when injected centrally. Accumulated food is displayed according to time post injection of 4 μg recombinant apoA-IV, n = 5.
3.3. Food intake studies with synthetic peptides of rat apoA-IV
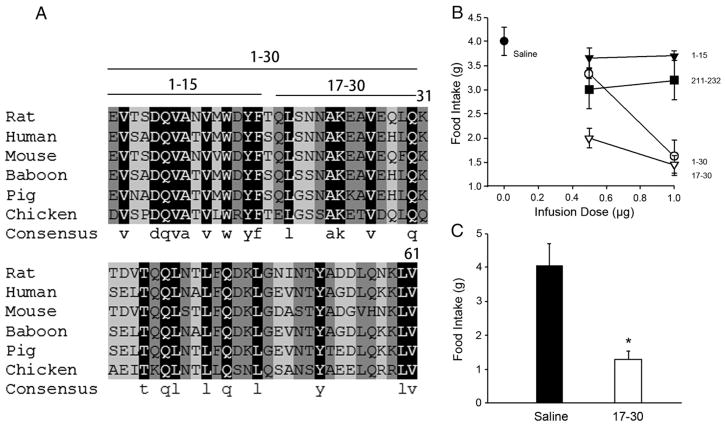
Furthermore, we generated synthetic peptides corresponding to the rat apoA-IV sequence. Fig. 5A shows an overlay of the N-terminal 61 amino acids of rat apoA-IV compared to the sequences of human, mouse, baboon, pig and chicken. It is clear that this domain contains significantly conserved regions, especially within the first 30 amino acids. We generated a series of three peptides encompassing the first 30 amino acids of the protein (1–30, 1–15, and 17–30). An additional peptide that contained an amphipathic helical sequence from the C-terminal region of the protein, apoA-IV (211–232), was also included as a control. These were injected into the 3rd ventricle of rats to assess the impact on food intake. Fig. 5B showed the effects of this series of peptides at two doses on rat food intake 30 min after injection. Compared to saline, the apoA-IV (1–15) peptide preparations were similar to the saline control at both doses tested. Similarly, the C-terminal peptide apoA-IV (211–232), although it exhibited a slight anorectic effect at the low dose, failed to mediate a further effect at the higher dose. By contrast, apoA-IV (1–30) exhibited a small decrease in food intake at the low dose and a significant effect at the high dose. ApoA-IV (17–30) exhibited the most potent anorectic effect at 0.5 μg which became more pronounced at the higher dose. The effect of apoA-IV (17–30) is further demonstrated in Fig. 5C with larger groups of rats at the 1.0 μg dose. Since both active peptides contain the sequence 17–30, the most likely explanation for the data is that this region contains a sequence capable of mediating the anorectic effect.
Fig. 5.
Effect of N-terminal and C-terminal synthetic peptides of rat apoA-IV on food intake when injected centrally. A: multi-species sequence alignment of the N-terminal 61 amino acids of apoA-IV. Amino acids highlighted in black are completely conserved across all species. Those highlighted in dark gray are conserved across all but one species and those in light gray are conserved in at least 3 of 6 species. The solid lines above the sequences indicate the N-terminal peptides used in this study. B: Accumulated food intake for 30 min is displayed at infusion dose of the relevant peptide in saline. n = 4. C: Direct comparison of food intake at 30 min after i3vt injection of either saline or 1 μg of apoA-IV peptide (17–30), *P < 0.05 vs. Saline, n = 7.
4. Discussion
In the present report, we provide evidence from recombinant deletion mutants and synthetic peptides that a region near the N-terminus between residues 17 and 30 of apoA-IV is capable of mediating an anorectic effect when administered to the 3rd ventricle of the rat brain. The fact that only a single 14-mer peptide can, at least partially, recapitulates the effects of the full-length protein indicates that the effect probably depends on the amino acid sequence and not on the functionality of the fully folded protein The significance of these findings with respect to the mechanism of the anorectic effect of apoA-IV and its therapeutic potential are discussed.
It is clear that apoA-IV suppressed food intake when administered centrally into the rat. This effect is not shared by apoA-I. Conversely, when endogenous levels of apoA-IV in the brain are immuno-depleted, food intake increases. The initial signaling event could occur via two general mechanisms. The first could be a direct interaction, such as through a specific protein–protein interaction (i.e. a receptor). There have been several reports of specific binding of apoA-IV to various cell types [25–27] although a specific binding protein has not been identified. Another possibility is that apoA-IV might be able to work through an indirect pathway such as manipulating the lipid content of the neuronal membrane to exert its activity. However, we suggest that the indirect mechanism is less likely for several reasons. First, apoA-I is known to be a more effective lipid binding molecule [28,29] and is expected to be a more readily manipulated membrane lipid content than apoA-IV [24], yet it does not exhibit the anorectic effect [13]. Second, the 14-mer peptide that we identified in this study is in a domain that does not appear to contain significant amphipathic helical character, a characteristic that is likely critical for lipid interactions. We suggest that the ability of the 14-mer peptide to promote the effect in the absence of the rest of the apoA-IV molecule argues for the possibility of a direct protein–protein interaction. Confirmation of this hypothesis must await further studies directed at isolating the putative binding protein. The active 14-mer peptide will likely be a useful tool for such pursuits.
There are two main ways that anorectic peptides can signal the brain, either through peripheral nerves or through receptors in the brain itself [30]. The de novo production of apoA-IV in hypothalamus could have a direct effect on inhibiting food intake. On the other hand, circulating apoA-IV, or a peptide derived from it, may cross the blood–brain barrier (BBB) and exert its anorectic effects centrally. However, our previous data have shown that the full-length of apoA-IV protein does not cross the BBB [31]. Our observations in this study that a small peptide can be efficacious raises the possibility that apoA-IV can undergo proteolytic cleavage in the circulation resulting in a small biologically active peptide that would be more likely to diffuse across the blood–brain barrier. Further studies will be required to determine if apoA-IV is susceptible to such a cleavage both in vitro and in vivo. Again, if no form of apoA-IV is crossing the BBB, it will be very important to look at whether vagus nerve stimulation has any effect on food intake. The afferent fibers of the vagus nerve have been shown to transmit signals for several meal-related metabolites as well as various mechanical and chemical stimuli [32,33]. Previous studies have demonstrated that CCK binds with CCK1 receptors on local vagal afferent nerve terminals and activates ascending vagal fibers for relaying information to the brain [32,34]. Lo et al. [35] reported that intraperitoneal injection of apoA-IV exerted anorectic effect as fast as in 15 min which is in accordance with quick signal transduction through nerve system. It is also suggested that apoA-IV interacts with CCK to inhibit food intake. But the effect of apoA-IV plus CCK was only partially mediated by CCK1 receptor. It is possible that apoA-IV acts locally by activating the vagus nerve through a receptor other than CCK1 receptor, and it may also interact with other peripheral anorectic signals in addition to CCK. It is still unknown whether the N-terminal fragment is required for apoA-IV anorectic effect when administered peripherally. Therefore, it will be informative to perform food intake studies in normal rats injected peripherally with mutant apoA-IV and also in rats that have had a vagotomy.
Regarding the structure of the anorectic domain itself, the sequence is relatively well-conserved among the species with known apoA-IV sequences. An exception to this is the chicken sequence, which contains only 5 identical residues among the 14 (Fig. 5A). Chicken apoA-IV is a more hydrophobic protein than that from mammals and it lacks a unique glutamine-rich domain present at the C-terminus [36]. It has been proposed that the addition of the C-terminal domain evolved in mammals to assist in chylomicron formation with the advent of apoB mRNA editing [37]. Based on structural arguments, we have suggested that this C-terminal domain may have imparted dramatically different lipid binding characteristics on mammalian apoA-IV vs. the chicken, perhaps facilitating a change in apoA-IV function from a lipid binding structural protein to a signaling protein with a role in food intake [38]. Following this logic, it may be possible that the anorectic sequence in the N-terminus also evolved as a binding site for this signaling function in mammals. In summary, we have shown in this study several implications for the treatment of obesity. The identification of the anorectic domain within apoA-IV raises the possibility that stable derivatives of the peptide could be used for the pharmaceutical manipulation of appetite. In addition, this work strongly suggests that there is a specific binding protein for apoA-IV that mediates its effects on food intake in the nervous system. Identification of such mediators will be important for further understanding the complex and redundant set of pathways which regulate food intake.
HIGHLIGHTS.
We determined the specific sequence within rat apoA-IV responsible for its anorectic effect.
We used a bacterial expression system to generate truncation mutants of rat apoA-IV.
We found that a sequence within the N-terminal 61 amino acids of rat apoA-IV was responsible for its anorectic action.
We used synthetic peptides to specify the functionality of the N-terminal region.
We found that a 14-mer peptide (residue 17 to 30) was capable of reducing food intake.
Acknowledgments
This work was supported by grants from the National Institute of Health: DK076928, DK092138, DK059630 (to P.T.), and HL67093, HL82734 (to W. S. D.)
References
- 1.Swaney JB, Reese H, Eder HA. Polypeptide composition of rat high density lipoprotein: characterization by SDS-gel electrophoresis. Biochem Biophys Res Commun. 1974;59:513–9. doi: 10.1016/s0006-291x(74)80010-0. [DOI] [PubMed] [Google Scholar]
- 2.Elshourbagy NA, Boguski MS, Liao WS, Jefferson LS, Gordon JI, Taylor JM. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc Natl Acad Sci U S A. 1985;82:8242–6. doi: 10.1073/pnas.82.23.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boguski MS, Elshourbagy N, Taylor JM, Gordon JI. Comparative analysis of repeated sequences in rat apolipoproteins A-I, A-IV, and E. Proc Natl Acad Sci U S A. 1985;82:992–6. doi: 10.1073/pnas.82.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karathanasis SK, Yunis I, Zannis VI. Structure, evolution, and tissue-specific synthesis of human apolipoprotein AIV. Biochemistry. 1986;25:3962–70. doi: 10.1021/bi00361a034. [DOI] [PubMed] [Google Scholar]
- 5.Emmanuel F, Steinmetz A, Rosseneu M, Brasseur R, Gosselet N, Attenot F, et al. Identification of specific amphipathic alpha-helical sequence of human apolipoprotein A-IV involved in lecithin:cholesterol acyltransferase activation. J Biol Chem. 1994;269:29883–90. [PubMed] [Google Scholar]
- 6.Main LA, Ohnishi T, Yokoyama S. Activation of human plasma cholesteryl ester transfer protein by human apolipoprotein A-IV. Biochim Biophys Acta. 1996;1300:17–24. doi: 10.1016/0005-2760(95)00228-6. [DOI] [PubMed] [Google Scholar]
- 7.Remaley AT, Stonik JA, Demosky SJ, Neufeld EB, Bocharov AV, Vishnyakova TG, et al. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280:818–23. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 8.Ostos MA, Lopez-Miranda J, Ordovas JM, Marin C, Blanco A, Castro P, et al. Dietary fat clearance is modulated by genetic variation in apolipoprotein A-IV gene locus. J Lipid Res. 1998;39:2493–500. [PubMed] [Google Scholar]
- 9.Duverger N, Tremp G, Caillaud JM, Emmanuel F, Castro G, Fruchart JC, et al. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 1996;273:966–8. doi: 10.1126/science.273.5277.966. [DOI] [PubMed] [Google Scholar]
- 10.Cohen RD, Castellani LW, Qiao JH, Van Lenten BJ, Lusis AJ, Reue K. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J Clin Invest. 1997;99:1906–16. doi: 10.1172/JCI119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi H, Nutting DF, Fujimoto K, Cardelli JA, Black D, Tso P. Transport of lipid and apolipoproteins A-I and A-IV in intestinal lymph of the rat. J Lipid Res. 1990;31:1613–25. [PubMed] [Google Scholar]
- 12.Tso P, Liu M, Kalogeris TJ, Thomson AB. The role of apolipoprotein A-IV in the regulation of food intake. Annu Rev Nutr. 2001;21:231–54. doi: 10.1146/annurev.nutr.21.1.231. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–6. doi: 10.1152/ajpgi.1992.262.6.G1002. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto K, Fukagawa K, Sakata T, Tso P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J Clin Invest. 1993;91:1830–3. doi: 10.1172/JCI116395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukagawa K, Gou HM, Wolf R, Tso P. Circadian rhythm of serum and lymph apolipoprotein AIV in ad libitum-fed and fasted rats. Am J Physiol. 1994;267:R1385–90. doi: 10.1152/ajpregu.1994.267.5.R1385. [DOI] [PubMed] [Google Scholar]
- 16.Okumura T, Fukagawa K, Tso P, Taylor IL, Pappas TN. Apolipoprotein A-IV acts in the brain to inhibit gastric emptying in the rat. Am J Physiol. 1996;270:G49–53. doi: 10.1152/ajpgi.1996.270.1.G49. [DOI] [PubMed] [Google Scholar]
- 17.Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol. 1998;274:R1834–8. doi: 10.1152/ajpregu.1998.274.6.R1834. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Doi T, Shen L, Woods SC, Seeley RJ, Zheng S, et al. Intestinal satiety protein apolipoprotein AIV is synthesized and regulated in rat hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1382–7. doi: 10.1152/ajpregu.2001.280.5.R1382. [DOI] [PubMed] [Google Scholar]
- 19.Liu M, Shen L, Liu Y, Tajima D, Sakai R, Woods SC, et al. Diurnal rhythm of apolipo-protein A-IV in rat hypothalamus and its relation to food intake and corticosterone. Endocrinology. 2004;145:3232–8. doi: 10.1210/en.2003-1554. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Shen L, Liu Y, Woods SC, Seeley RJ, D’Alessio D, et al. Obesity induced by a high-fat diet downregulates apolipoprotein A-IV gene expression in rat hypothalamus. Am J Physiol Endocrinol Metab. 2004;287:E366–70. doi: 10.1152/ajpendo.00448.2003. [DOI] [PubMed] [Google Scholar]
- 21.Lefevre M, Lovejoy JC, DeFelice SM, Keener JW, Bray GA, Ryan DH, et al. Common apolipoprotein A-IV variants are associated with differences in body mass index levels and percentage body fat. Int J Obes Relat Metab Disord. 2000;24:945–53. doi: 10.1038/sj.ijo.0801260. [DOI] [PubMed] [Google Scholar]
- 22.Pearson K, Tubb MR, Tanaka M, Zhang XQ, Tso P, Weinberg RB, et al. Specific sequences in the N and C termini of apolipoprotein A-IV modulate its conformation and lipid association. J Biol Chem. 2005;280:38576–82. doi: 10.1074/jbc.M506802200. [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Maiorano N, Shen L, Pearson K, Tajima D, Zhang DM, et al. Expression of biologically active rat apolipoprotein AIV in Escherichia coli. Physiol Behav. 2003;78:149–55. doi: 10.1016/s0031-9384(02)00959-9. [DOI] [PubMed] [Google Scholar]
- 24.Pearson K, Saito H, Woods SC, Lund-Katz S, Tso P, Phillips MC, et al. Structure of human apolipoprotein A-IV: a distinct domain architecture among exchangeable apolipoproteins with potential functional implications. Biochemistry. 2004;43:10719–29. doi: 10.1021/bi048978m. [DOI] [PubMed] [Google Scholar]
- 25.Dvorin E, Gorder NL, Benson DM, Gotto AM, Jr, Apolipoprotein A-IV. A determinant for binding and uptake of high density lipoproteins by rat hepatocytes. J Biol Chem. 1986;261:15714–8. [PubMed] [Google Scholar]
- 26.Savion N, Gamliel A. Binding of apolipoprotein A-I and apolipoprotein A-IV to cultured bovine aortic endothelial cells. Arteriosclerosis. 1988;8:178–86. doi: 10.1161/01.atv.8.2.178. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg RB, Patton CS. Binding of human apolipoprotein A-IV to human hepatocellular plasma membranes. Biochim Biophys Acta. 1990;1044:255–61. doi: 10.1016/0005-2760(90)90311-k. [DOI] [PubMed] [Google Scholar]
- 28.Rifici VA, Eder HA, Swaney JB. Isolation and lipid-binding properties of rat apolipoprotein A-IV. Biochim Biophys Acta. 1985;834:205–14. doi: 10.1016/0005-2760(85)90157-2. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg RB, Ibdah JA, Phillips MC. Adsorption of apolipoprotein A-IV to phospholipid monolayers spread at the air/water interface. A model for its labile binding to high density lipoproteins. J Biol Chem. 1992;267:8977–83. [PubMed] [Google Scholar]
- 30.Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–83. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 31.Shen L, Pearson KJ, Xiong Y, Lo CM, Tso P, Woods SC, et al. Characterization of apo-lipoprotein A-IV in brain areas involved in energy homeostasis. Physiol Behav. 2008;95:161–7. doi: 10.1016/j.physbeh.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol. 1997;272:R1245–51. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 33.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short-and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–7. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 34.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–41. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 35.Lo CM, Zhang DM, Pearson K, Ma L, Sun W, Sakai RR, et al. Interaction of apolipoprotein AIV with cholecystokinin on the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1490–4. doi: 10.1152/ajpregu.00329.2007. [DOI] [PubMed] [Google Scholar]
- 36.Steinmetz A, Hermann M, Nimpf J, Aebersold R, Ducret A, Weinberg RB, et al. Expression and conservation of apolipoprotein AIV in an avian species. J Biol Chem. 1998;273:10543–9. doi: 10.1074/jbc.273.17.10543. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg RB, Anderson RA, Cook VR, Emmanuel F, Denefle P, Hermann M, et al. Structure and interfacial properties of chicken apolipoprotein A-IV. J Lipid Res. 2000;41:1410–8. [PubMed] [Google Scholar]
- 38.Tubb MR, Silva RA, Pearson KJ, Tso P, Liu M, Davidson WS. Modulation of apolipoprotein A-IV lipid binding by an interaction between the N and C termini. J Biol Chem. 2007;282:28385–94. doi: 10.1074/jbc.M704070200. [DOI] [PubMed] [Google Scholar]