Abstract
Development of effective treatments for Alz-heimer’s disease is complicated by the poor understanding of its pathophysiology. Recent work suggests mitochondria may play a primary role in neurodegeneration, due to alterations in mitochondria turnover and that the brain is specifically susceptible, due to high energy demand. Mitochondria are the major source of cellular energy through oxidative phosphorylation and regulate intracellular calcium levels and survival pathways. Hypoxia has been implicated in several neurodegenerative diseases including Alzheimer’s disease. During hypoxic events, mitochondrial complex III produces high levels of reactive oxygen species (ROS). These ROS seem to have a primary role in the regulation of the transcription factor hypoxia inducible factor 1α that triggers death effectors. Here we discuss the role of mitochondria in AD putting focus on the activation of hypoxia-mediated mitochondrial pathways, which could eventually lead to cell degeneration and death.
Keywords: Mitochondria, Hypoxia, ROS
Introduction
Alzheimer’s disease (AD) affected upwards of 26.6 million people worldwide in 2006, a number that could quadruple by 2050 (Brookmeyer et al. 2007). The sporadic nature of more than 95% of AD cases, the differential susceptibility to and the course of the illness, as well as the late age onset of the disease, suggest that epigenetic and environmental components play a role in its etiology (Zawia et al. 2009). AD is a chronic disorder that slowly destroys neurons and causes severe cognitive disability. The neuropathological hallmarks of AD include a progressive loss of neurons, synaptic degeneration, intracellular deposits of hyperphosphorylated tau, neurofibrillary tangles and extracellular deposits of amyloid β (Aβ), senile plaques (Bojarski et al. 2008; Moreira et al. 2009). The aggregates of Aβ may occur in brain parenchyma and in the walls of small brain arteries, leading to cerebral amyloid angiopathy (CAA). Such alterations will disturb neuronal and synaptic function and even at its earliest stage, Aβ deposits around brain vessels could certainly interfere with the dynamic adaptation of cerebral blood flow (CBF) to changing brain function.
Data from clinical imaging, epidemiological, and phar-macotherapy studies have indicated that vascular changes play an important role in AD pathogenesis (de la Torre 2004; Bell and Zlokovic 2009). Many vascular risk factors for AD, such as atherosclerosis, stroke and cardiac disease in the aging individual, could result in cerebrovascular dysfunction and trigger AD pathology (Rocchi et al. 2009). Magnetic resonance imaging (MRI), transcranial doppler measurements, and single photon excitation computed tomography (SPECT) in humans have established that the resting CBF is significantly reduced in AD patients, and this may be an early event in AD pathogenesis. Arterial spin-labeling MRI has demonstrated cerebral hypoperfusion in AD patients (Johnson et al. 2005). Functional MRI (fMRI) studies using blood oxygenation level dependent (BOLD) measure increases in CBF during tasks that assess episodic memory have established that there is a delay in CBF response in patients with mild cognitive impairment (MCI), and that this delay in fMRI-BOLD signal becomes more pronounced in AD patients (Rombouts et al. 2005). This suggests that CBF reductions are present in the early stages of AD pathogenesis.
A reduction in blood flow leads to a decrease in oxygen delivery (hypoxia) in brain tissue (Roy and Rauk 2005) suggesting hypoxia may alter the synaptic plasticity and promote mitochondrial dysfunction, oxidative stress, and apoptosis in the cerebral cortex, hippocampus, and striatum (Askew 2002; Maiti et al. 2006, 2007, 2008a, b). Also, disruption of calcium homeostasis, following hypoxia, may contribute to the neurotoxicity of Aβ and subsequent development of AD (Kawahara and Kuroda 2000). Sun and colleagues (Sun et al. 2006) defined the molecular mechanism of hypoxia leading to dementia and showed that hypoxia leads to increased β-secretase activity and production of Aβ. Guglielmotto and collaborators (Guglielmotto et al. 2009) also demonstrated that hypoxia up-regulates β-secretase potentiating the production of Aβ. Furthermore, the authors reported that this effect is mediated by mitochondrial reactive oxygen species (ROS) (Guglielmotto et al. 2009). In this chapter we give an overview of the role of mitochondria in physiologic and neuropathologic conditions, particularly in AD and discuss the mitochondrial pathways mediated by hypoxia putting focus on the role of hypoxia inducible factor 1α (HIF-1α).
Mitochondria and the brain
The brain integrates diverse central and peripheral signals to maintain homeostasis (Ronnett et al. 2009) and has evolved for tight regulation of oxygen and glucose homeostasis in order to avoid or, at least, minimize brain damage (Acker and Acker 2005).
This is driven by the extraordinary energy requirement needed to maintain ion gradients across the neuronal plasma membrane that are critical for the generation of action potentials. This intense energy requirement is continuous and even brief periods of oxygen or glucose deprivation can result in neuronal dysfunction or death (Simpkins and Dykens 2007). Despite its high energy demand to maintain normal functions, the brain stores little energy. Cerebral energy only sustains brain function for a few minutes before irreversible injury, which results from metabolic failure (Issam 1993). Mitochondria are intracellular organelles that play an essential role in cellular energy production (Dykens 1997; Green and Kroemer 2004). Mitochondrial oxidative phosphorylation (OXPHOS) is the primary source of high-energy compounds in the cell (Beal 2005; Niizuma et al. 2009). The metabolism of glucose through the tricarboxylic acid (TCA) cycle generates the electron donors NADH and succinate that donate electrons to complex I and complex II, respectively. Electrons from these complexes are transferred to coenzyme Q, complex III, cytochrome c, complex IV and finally to molecular oxygen which is reduced to water. The electron transport system is organized in this way in order to regulate ATP production (Fig. 1). Part of the energy of those electrons is used to pump protons into the mitochondrial matrix creating a voltage gradient which is used in ATP synthase to generate ATP from ADP (Fig. 1). The ATP generated during this cycle is utilized to provide the energy necessary to carry out active cellular processes.
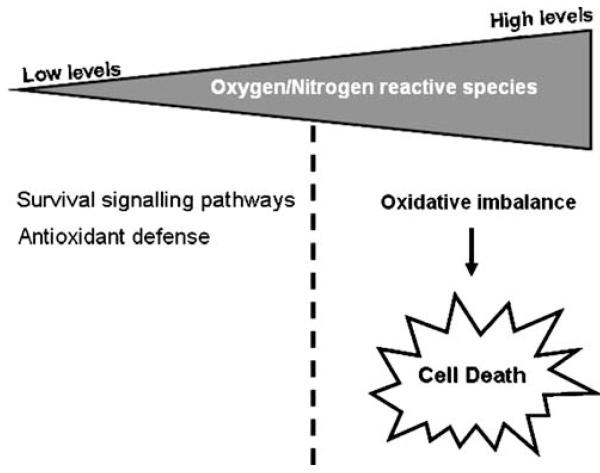
Fig. 1.
Dual role of mitochondrial reactive oxygen species. The continuous electron leak from the respiratory chain leads to the generation of damaging reactive oxygen species (ROS) that play a dual role. Low levels of ROS induce the expression of several genes involved in antioxidant defense. However, high levels of ROS promote oxidative stress and activate anomalous signalling mechanisms that will ultimately lead to cell death
These intracellular organelles are also an important source of reactive oxygen species (ROS) due to the unavoidable OXPHOS chemistry undergoing regulated proton leak (Shigenaga et al. 1994; Kroemer et al. 1995; Benzi and Moretti 1995; Ozawa 1997). The continuous leak from the respiratory chain leads to the generation of ROS and possibly thermogenic processes (Richter and Kass 1991; Beal 1996; Cay and Jones 1999; Ricquier and Bouillard 2000). Both ROS and reactive nitrogen species (RNS) are products of normal cellular metabolism (Valko et al. 2007). The most abundant reactive species, superoxide anion radical (O ·−2), hydroxyl radical (HO−), nitric oxide (NO·), peroxynitrite (ONOO−) and hydrogen peroxide (H2O2) (Goetz and Luch 2008) may play a dual role, deleterious or beneficial depending on their levels (Valko et al. 2006).
Low/moderate levels of ROS and RNS are involved in physiological roles such as cell survival signalling pathways and defense against infectious agents. Low levels of reactive species induce the expression of several genes involved in antioxidant defense including manganese superoxide dismutase (MnSOD), catalase, glutathione reductase (Gred), and intracellular signalling and regulation (Dröge 2002). Most cell types have been shown to elicit a small oxidative burst generating low concentrations of ROS when they are stimulated by cytokines, growth factors and hormones, e.g. interleukin-1α (IL-1α), interleukin 6 (IL-6), interleukin 3 (IL-3), tumor necrosis factor-α (TNF-α) and angiotensin II (ANGII), among others (Thannickal and Fanburg 2000). This led to the assumption that the initiation and/or proper functioning of several signal transduction pathways rely on the action of ROS as signalling molecules.
However, high levels of ROS promote oxidative stress and activate anomalous signaling mechanisms related to various disease states (Brown and Borutaite 2001; Sheu et al. 2006). In the physiological process of aging or in the pathological processes, the production of reactive species exceeds the scavenging capacity of endogenous systems, resulting in the damage of cellular components such as proteins, lipids, and nucleic acids. Besides being one major source of ROS, mitochondria are also one of the preferential targets of reactive species. This susceptibility of mtDNA is probably due to its lack of protective histones, limited repair capabilities, and proximity to the electron transport chain (Wallace 1992; Linnane et al. 1989; Miquel 1991; Moreira et al. 2008a). Oxidative stress could impair mitochondrial function potentiating the activation of the intrinsic death pathway (Hoye et al. 2008).
Is mitochondrial dysfunction a necessary step in neurodegeneration?
Subtle functional alterations in essential cellular energy can lead to insidious pathological changes in neurons (Swerdlow et al. 1996, 1997, 1998). Reduced glucose utilization and energy metabolism and oxidative stress are key players involved in the onset and progression of AD (Moreira et al. 2006, 2007a, b, 2009; Zhu et al. 2006). A growing body of evidence suggests that mitochondrial abnormalities and ROS generation are involved in AD pathogenesis (Moreira et al. 2008a, b; Zhu et al. 2006), with oxidative stress occurring prior to cytopathology (Nunomura et al. 2001; Hirai et al. 2001). Evidence for mitochondrial dysfunction in AD pathogenesis comes from impaired activity of three tricarboxylic acid cycle (TCA) complexes, pyruvate dehydrogenase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase, observed in postmortem AD brain and fibroblasts (Huang et al. 2003; Bubber et al. 2005). Reduced respiratory chain complexes I, III and IV activities have also been found in platelets and lymphocytes from AD patients and postmortem brain tissue (Kish et al. 1992; Parker et al. 1994; Bosetti et al. 2002; Valla et al. 2006).
Evidence from studies in experimental models and in AD brain tissue, demonstrate that the underlying neurodegeneration is associated with morphological and biochemical features of apoptosis. An imbalanced level of some molecular apoptotic markers such as pro-apoptotic (Bax, Bak and Bad) and anti-apoptotic (Bcl-2 and Bcl-xL)proteins, members of Bcl-2 protein family [83,84], and the initiator caspases 8 and 9 and the effector caspases 3 and 6 have been reported in post-mortem brains of AD patients (Stadelmann et al. 1999; Behl 2000; Albrecht et al. 2007; Calissano et al. 2009). Expression profiling analysis of gene expression in brain tissue samples from AD and age-matched control patients has revealed a marked decrease in expression of some anti-apoptotic gene such as NCKAP1 (Suzuki et al. 2000). In addition, immunocytochemical and biochemical studies report the presence of active caspase(s) and caspase-cleaved substrates in neurons, around senile plaques and neurofibrillary tangles (Kitamura et al. 1996; Gastard et al. 2003; Cribbs et al. 2004) and also in postsynaptic densities (Louneva et al. 2008). Both caspase-cleaved AβPP and activated caspase 3 have been shown to be present and associated to granulovacuolar degeneration, a diagnostic AD neuropathological sign in brains of affected patients (Su et al. 2002). Finally, a marked co-localization of pathological hyperphosphorylated tau, cleaved caspase-3 and caspase-6 have been recently reported in TUNEL-positive neurons in the brainstem of AD patients (Wai et al. 2009).
Altogether, these studies indicate that mitochondrial dysfunction, oxidative stress, and apoptotic cell death are intimately involved with AD pathophysiology.
The hypoxia-induced mitochondrial dysfunction: a cause of AD?
Although the diverse triggers of neurodegeneration and their interactions are still the topic of extensive debate, the possible contribution of cerebrovascular deficiencies has been vigorously promoted in recent years (Farkas and Luiten 2001). Various forms of cerebrovascular insufficiency such as reduced blood supply to the brain or disrupted microvascular integrity in cortical regions may occupy an initiating or intermediate position in the chain of events ending with cognitive failure. The capillary endothelial cells are further characterized by a relatively high number of mitochondria, which can provide the energy needed for the working of the specific blood brain barrier (BBB) transport proteins (e.g. glucose and amino acid transporters) (Farkas and Luiten 2001). A decreased CBF, lower metabolic rates of glucose and oxygen and a compromised structural integrity of the cerebral vasculature with special attention to micro-vessels are representative degenerative features of the vascular system of the aging and degenerative brain (Fig. 2). Indeed, recent evidence suggests endothelial dysfunction in AD-related cerebral hypoperfusion and AD pathophysiology (Lange-Asschenfeldt and Kojda 2008). Also Aβ deposition in brain vessels occurs in many AD patients and results in CAA and decreased oxygen delivery to the brain (Fig. 2) (Moreira et al. 2009; Xiong et al. 2009). Application of exogenous Aβ to normal blood vessels ex vivo causes endothelium-dependent vasoconstriction with decrease in blood flow and, consequently, oxygen levels (Thomas et al. 1996; Townsend et al. 2002).
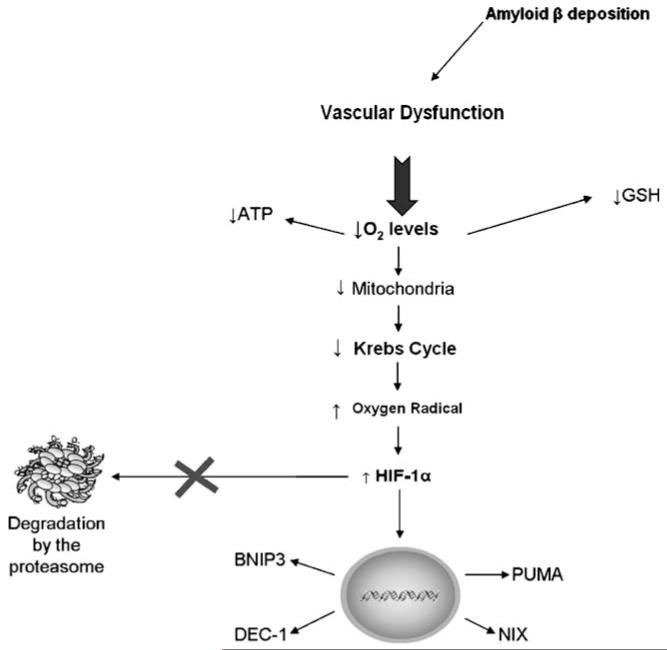
Fig. 2.
Hypoxia-mediated cell death in neurodegeneration. Hypoxia has been implicated in several pathologies of the central nervous system. In Alzheimer’s disease the deposition of the amyloid β (Aβ) protein in brain vessels potentiate the occurrence of hypoxic phenomena. A drop in tissue oxygen levels to the point where oxygen demand exceeds supply rapidly leads to a metabolic crisis putting in danger the ongoing physiological functions. This metabolic crisis comprises a severe energy (ATP) drop that results from an impairment of mitochondria function, including the inhibition of the tricarboxylic acid (TCA) cycle. These alterations are intimately associated with an increase in the production of reactive oxygen species (ROS) as well as reactive nitrogen species, namely nitric oxide (NO) and a concomitant decrease in antioxidants namely reduced glutathione (GSH), the first line of defense against oxidative stress. Hypoxia-inducible factor-1 (HIF-1 α) is a transcription factor that is oxygen sensitive. In physiological conditions HIF-1α is continuously degraded by the proteasome. However, in the presence of low levels of oxygen (O2) and increased levels of ROS, HIF-1α is activated and translocated to the nucleus where it will bind to hypoxia response elements (HREs) increasing the expression of pro-apoptotic proteins, namely the defective chorion-1 (DEC-1), Bcl2/adenovirus E1B 19kD-interacting protein-3 (BNIP3), its orthologue Nip3-like protein X (NIX), PUMA and cyclin G2 leading to cell death. See text for more complete information
A drop in tissue oxygen levels to the point where oxygen demand exceeds supply (termed hypoxia) leads rapidly to metabolic crisis and represents a severe threat to ongoing physiological function and, ultimately, viability (Taylor 2008). Hypoxia has been implicated in CNS pathology in a number of disorders including stroke, head trauma, neoplasia and neurodegenerative disease (Acker and Acker 2005; Bharke and Hale 1993; Hainsworth et al. 2007).
Cellular and molecular pathways underlying hypoxic neurotoxicity and cell death are multifaceted and complex involving several cellular responses, including oxidative stress, altered ionic homeostasis, mitochondrial dysfunction, and activation of apoptotic cascades (Maiti et al. 2008a; Brookmeyer et al. 2007; Zawia et al. 2009; Hota et al. 2007). Also, hypoxia triggers free radical generation and depletion of antioxidant status, thus leading to oxidative damage of vital cellular components (Adams 1975; Chandel et al. 1998). Changes in synaptic efficacy occur very early during hypoxia and may, indeed, be the first response of the neuron to ischemic insult (Aoyagi et al. 1998; Fleidervish et al. 2001).
Studies have suggested that hypoxia can induce apoptosis dependent on transcriptional activation of apoptotic factors (Harris 2002). Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator involved in adaptation to hypoxic stress. HIF-α subunits are oxygen sensitive due to the fact that they are substrates of a family of proline and asparagine hydroxylases which utilize dioxygen, ferrous iron (Fe2+) and 2-oxoglutarate to catalyse the hydroxylation of specific residues on HIF-αsubunits. In hypoxia, this repression is removed and the HIF pathway becomes rapidly activated (Taylor 2008) (Fig. 2). HIF works as a master transcriptional regulator of hypoxia-dependent gene expression and its transcriptional activation is a stress response developed through evolution to allow cells to avoid a bioenergetic crisis in low oxygen levels. Whether and to what extent the HIF-system may participate in the disease process remains to be elucidated.Indeed, current data would support a dual role of the HIF-system, depending on whether it is the cause or the consequence (Acker and Acker 2005).
Initial studies in exploring the mechanism by which mitochondria regulate the stability of HIF-1α demonstrated that ROS levels, produced at complex III, increase in hypoxic conditions (Bell and Chandel 2007). This increase in ROS production seems required and sufficient to stabilize HIF-1α protein avoiding its degradation by the proteasome (Fig. 2) (Bell and Chandel 2007; Chandel et al. 2000; Hirota and Semenza 2001). However this topic remains controversial in view of the fact that other reports have shown a general ROS decrease in hypoxia (Görlach et al. 2003) and further documented that a functioning mitochondrial respiratory chain may not be necessary for HIF-α regulation (Acker and Acker 2005; Srinivas et al. 2001; Vaux et al. 2001).
Although the mechanism is not fully understood, HIF system activates a physiological pathway that covers a wide array of physiological responses to hypoxia, ranging from mechanisms that increase cell survival to those inducing cell cycle arrest or even apoptosis (Acker and Acker 2005). These mechanisms combine cooperatively to activate HIF to maximal levels under decreasing oxygen concentrations (Acker and Acker 2005). Previous studies reported that initially HIF activates a survival pathway that involves the expression of angiogenic and vasodilators genes such as vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS) anderythropoietin(EPO) (Bernaudin et al. 1999; Brines et al. 2000; Jin et al. 2000; Jin et al. 2002; Aminova et al. 2005). Long-lasting HIF activation includes responses with adverse effects on cell function by inducing cell-cycle-arrest-specific and pro-apoptotic proteins such as defective chorion-1 (DEC-1), Bcl2/adenovirus E1B 19kD-interacting protein-3 (BNIP3), its orthologue Nip3-like protein X (NIX), PUMA and cyclin G2 expression. In addition, direct stabilization through the pro-apoptotic protein p53 has been suggested by studies demonstrating physical and functional interactions between HIF-1α and p53 (Acker and Plate 2002). The protein p53 is a master regulator of cell death by inducing apoptosis through the control of apoptosis-related gene expression (Schmitt et al. 2002). In response to certain death stimuli, a fraction of stabilized p53 rapidly translocates to mitochondria, launching a rapid pro-apoptotic response in a transcription-independent manner that jump-starts and amplifies the slower transcription dependent response (Marchenko et al. 2000; Mihara et al. 2003; Erster et al. 2004).
Several studies have been conducted to establish the role of hypoxia in neurodegeneration and, specifically, in AD. It has been previously shown that cerebral hypoxia results in increased activity of caspase-9 and caspase-3 in the cerebral cortex of newborn piglets (Khurana et al. 2002; Mishra and Delivoria-Papadopoulos 2006). The mechanism of activation of caspase-9 during hypoxia that leads to initiation of programmed cell death in mammalian brain tissue is not known, but data indicate that the decrease in ATP levels and cytochrome c release had primary roles in this process (Fig. 2) (Delivoria-Papadopoulos et al. 2008). Also the increase in NO· levels induced by hypoxia has been shown to activate caspase-9 through a transcription-dependent mechanism. Indeed, there is a NO-mediated increase in pro-apoptotic proteins such as Bax and Bad during hypoxia that may lead to APAF-1 activation resulting in the conversion of procaspase-9 into active caspase-9 and subsequent activation of caspase-3 while down-regulating anti-apoptotic proteins of the Bcl-2 (Barhwal et al. 2007). Additionally, expression of HIF-1-regulated “pro-death” BH3-only family members, such as BNIP3, has also been shown to increase following cerebral ischemia (Chen et al. 2001; Yin et al. 2000; Shibata et al. 2002; Schmidt-Kastner et al. 2004).
The inhibition of TCA cycle and depletion in reduced glutathione (GSH) levels as a result of its greater use for quenching the accelerated free radical generation accompanied by a concomitant increase in GSSG was also observed in hippocampal cells under hypoxia (Fig. 2) (Taylor 2008; Barhwal et al. 2008). Recently, Sarada et al. (Sarada et al. 2008) reported that neuroblastoma cells exposed to hypoxia present increased free radical production and apoptosis and decreased GSH content and Gred, glutathione peroxidase (GPx) and SOD activities.
Wang and collaborators (Wang et al. 2006) demonstrated that the expression of APH-1A, a component of the γ-secretase complex, and the γ-secretase mediated Aβ and Notch intracellular domain generation are regulated by HIF-1. Another study showed a functional hypoxia-responsive element in the β-site AβPP cleavage enzyme 1 (BACE1) gene promoter (Sun et al. 2006). The authors report that hypoxia up-regulated γ-secretase cleavage of AβPP and Aβ production by increasing BACE1 gene transcription and expression both in vitro and in vivo. Hypoxia treatment markedly increased Aβ deposition and neuritic plaque formation and potentiated the memory deficit in Swedish mutant AβPP transgenic mice (Sun et al. 2006). These results clearly demonstrate that hypoxia can facilitate AD pathogenesis, and they provide a molecular mechanism linking vascular factors to AD. Zhang and collaborators (Zhang et al. 2007) also showed that acute hypoxia increases the expression and the enzymatic activity of BACE1 by up-regulating the level of BACE1 mRNA, resulting in increases in the AβPP C-terminal fragment-β and Aβ. Recently, Guglielmotto and collaborators (Guglielmotto et al. 2009) observed that hypoxia significantly increased BACE1 gene transcription through an early up-regulation dependent on the release of mitochondrial ROS and a late up-regulation due to the overexpression and activation of the HIF-1α, resulting in increased BACE1 activity and Aβ production. Further-more, the authors reported that the oxidative stress-mediated up-regulation of BACE1 is mediated by c-jun N terminal kinase pathway (Guglielmotto et al. 2009). This study strengthens the hypothesis that oxidative stress is a basic common mechanism of Aβ accumulation.
Concluding remarks
ROS production contributes to mitochondrial damage in a range of pathologies and is also important in redox signalling from the organelle to the rest of the cell. In response to decreased oxygen levels, cells activate HIF, which leads to metabolic adaptation to hypoxia including activation of HIF. Although the mechanism is not fully understood, HIF system activates several pathways that cover a wide array of responses to hypoxia, ranging from mechanisms that increase cell survival to those inducing cell cycle arrest or even apoptosis. Recent evidence shows that hypoxia favours Aβ production. Although AD is intimately associated with mitochondrial dysfunction, oxidative stress and hypoxic episodes, the role of HIF is only emerging. Major new insights into the molecular mechanisms that regulate HIF are needed to evaluate the value of this transcription factor as a therapeutic target in neurodegenerative diseases associated to hypoxia.
Contributor Information
Cristina Carvalho, Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal; Department of Zoology – Faculty of Sciences and Technology, University of Coimbra, Coimbra, Portugal.
Sónia C. Correia, Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal; Department of Zoology – Faculty of Sciences and Technology, University of Coimbra, Coimbra, Portugal
Renato X. Santos, Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal; Department of Zoology – Faculty of Sciences and Technology, University of Coimbra, Coimbra, Portugal
Susana Cardoso, Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal; Department of Zoology – Faculty of Sciences and Technology, University of Coimbra, Coimbra, Portugal.
Paula I. Moreira, Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal; Institute of Physiology – Faculty of Medicine, University of Coimbra, Coimbra, Portugal
Timothy A. Clark, UTSA Institute for Neuroscience and Department of Biology, College of Sciences, University of Texas, San Antonio, TX 78249, USA
Xiongwei Zhu, Department of Pathology, Case Western Reserve University, Cleveland, OH, USA.
Mark A. Smith, Department of Pathology, Case Western Reserve University, Cleveland, OH, USA
George Perry, UTSA Institute for Neuroscience and Department of Biology, College of Sciences, University of Texas, San Antonio, TX 78249, USA.
References
- Acker T, Acker H. J Exp Biol. 2005;207:3171. doi: 10.1242/jeb.01075. [DOI] [PubMed] [Google Scholar]
- Acker T, Plate KH. J Mol Med. 2002;80:562. doi: 10.1007/s00109-002-0355-1. [DOI] [PubMed] [Google Scholar]
- Adams JA. Br J Anaesth. 1975;47:1221. [Google Scholar]
- Albrecht S, Bourdeau M, Bennett D, Mufson EJ, Bhattacharjee M, LeBlanc AC. Amer J Pathol. 2007;170:1200. doi: 10.2353/ajpath.2007.060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminova LR, Chavez JC, Lee J, Ryu H, Kung A, LaManna JC, Ratan RR. J Biol Chem. 2005;280:3996. doi: 10.1074/jbc.M409223200. [DOI] [PubMed] [Google Scholar]
- Aoyagi A, Saito H, Abe K, Nishiyama N. Brain Res. 1998;799:130. doi: 10.1016/s0006-8993(98)00465-x. [DOI] [PubMed] [Google Scholar]
- Askew EW. Toxicology. 2002;180:107. doi: 10.1016/s0300-483x(02)00385-2. [DOI] [PubMed] [Google Scholar]
- Barhwal K, Singh SB, Hota SK, Jayalakshmi K, Ilavazhagan G. Eur J Pharmacol. 2007;570:97. doi: 10.1016/j.ejphar.2007.05.063. [DOI] [PubMed] [Google Scholar]
- Barhwal K, Hota SK, Prasad D, Singh SB, Ilavazhagan G. Hypoxia-induced Deactivation of NGF-mediated ERK1/2 Signaling in Hippocampal Cells: Neuroprotection by Acetyl-L-Carnitine. J Neurosci Res. 2008;86:2705. doi: 10.1002/jnr.21722. [DOI] [PubMed] [Google Scholar]
- Beal MF. Curr Opin Neurobiol. 1996;6:661. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- Beal MF. Annu Neurol. 2005;58:495. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Behl C. J Neural Transm. 2000;107:1325. doi: 10.1007/s007020070021. [DOI] [PubMed] [Google Scholar]
- Bell EL, Chandel NS. Essays Biochem. 2007;43:17. doi: 10.1042/BSE0430017. [DOI] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Acta Neuropathol. 2009;118:103. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi G, Moretti A. Neurobiol Aging. 1995;16:661. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. J Cereb Blood Flow Metab. 1999;19:643. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Bharke M, Hale SB. Sports Med. 1993;16:97. doi: 10.2165/00007256-199316020-00003. [DOI] [PubMed] [Google Scholar]
- Bojarski L, Herms J, Kuznicki J. J Neurochem Int. 2008;52:621. doi: 10.1016/j.neuint.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Neurobiol Aging. 2002;23:371. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Proc Natl Acad Sci USA. 2000;97:10526. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi MH. Alzheimers Dement. 2007;3:186. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. IUBMB Life. 2001;52:189. doi: 10.1080/15216540152845993. [DOI] [PubMed] [Google Scholar]
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Ann Neurol. 2005;57:695. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- Calissano P, Matrone C, Amadoro G. Commun Integrative Biol. 2009;2:163. doi: 10.4161/cib.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cay J, Jones DP. J Bioenerg Biomembr. 1999;31:327. doi: 10.1023/a:1005423818280. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Proc Natl Acad Sci USA. 1998;95:11715. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano SE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. J Biol Chem. 2000;275:25130. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. J Biol Chem. 2001;276:30724. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Poon WW, Rissman RA, Blurton-Jones M. Amer J Pathol. 2004;165:353. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre JC. Lancet Neurol. 2004;3:184. doi: 10.1016/S1474-4422(04)00683-0. [DOI] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M, Ashraf QM, Mishra OP. Neurosci Lett. 2008;438:38. doi: 10.1016/j.neulet.2008.03.078. [DOI] [PubMed] [Google Scholar]
- Dröge W. Physiol Rev. 2002;82:47. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dykens JA. In: In Neurodegenerative Diseases: Mitochondria and Free Radicals in Pathogenesis. Beal MF, Bodis-Wollner I, Howell N, editors. John Wiley & Sons; 1997. p. 29. [Google Scholar]
- Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. Mol Cell Biol. 2004;24:6728. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E, Luiten PM. Prog Neurobiol. 2001;64:575. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fleidervish I, Gebhardt C, Astman N, Gutnick MJ, Heinemann U. J Neurosci. 2001;21:4600. doi: 10.1523/JNEUROSCI.21-13-04600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastard MC, Troncoso JC, Koliatsos VE. Ann Neurol. 2003;54:393. doi: 10.1002/ana.10680. [DOI] [PubMed] [Google Scholar]
- Goetz M, Luch A. Cancer Lett. 2008;266:73. doi: 10.1016/j.canlet.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Görlach A, Berchner-Pfannschmidt U, Wotzlaw C, Cool RH, Fandrey J, Acker H, Jungermann K, Kietzmann T. Thromb Diath Haemorrh. 2003;89:926. [PubMed] [Google Scholar]
- Green DR, Kroemer G. Science. 2004;305:626. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. J Neurochem. 2009;108:1045. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- Hainsworth R, Drinkhil MJ, Rivera-Chira M. Clin Auton Res. 2007;17:13. doi: 10.1007/s10286-006-0395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Nat. Rev. Cancer. 2002;2:38. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. J Neurosci. 2001;21:3017. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Semenza GL. J Biol Chem. 2001;276:21166. doi: 10.1074/jbc.M100677200. [DOI] [PubMed] [Google Scholar]
- Hota SK, Barhwal K, Singh SB, Ilavazhagan G. Neurochem Int. 2007;51:384. doi: 10.1016/j.neuint.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Hoye A, Davoren J, Wipf P. Acc Chem Res. 2008;41:87. doi: 10.1021/ar700135m. [DOI] [PubMed] [Google Scholar]
- Huang HM, Ou HC, Xu H, Chen HL, Fowler C, Gibson GE. J Neurosci Res. 2003;74:309. doi: 10.1002/jnr.10756. [DOI] [PubMed] [Google Scholar]
- Issam A. In: AANS publications committee neurosurgical topics. Awad MD, editor. American Association of Neurological Surgeons; 1993. p. 327. [Google Scholar]
- Jin KL, Mao XO, Greenberg DA. Proc Natl Acad Sci USA. 2000;97:10242. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Proc Natl Acad Sci USA. 2002;99:11946. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Radiology. 2005;234:851. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara M, Kuroda Y. Brain Res. Bull. 2000;53:389. doi: 10.1016/s0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- Khurana P, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Neurochem Res. 2002;27:931. doi: 10.1023/a:1020347732741. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN. J Neurochem. 1992;59:776. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Kamoshima W, Ota T, Matsuoka Y, Nomura Y, Smith MA, Perry G, Whitehouse PJ, Taniguchi T. Brain Res. 1996;780:260. doi: 10.1016/s0006-8993(97)01202-x. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. FASEB J. 1995;9:1277. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Kojda G. Exp Gerontol. 2008;43:499. doi: 10.1016/j.exger.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Marzuki S, Ozawa T, Tanaka M. Lancet. 1989;1:642. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, Bennett DA, Trojanowski JQ, Arnold SE. Amer J Pathol. 2008;173:1488. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti P, Singh SB, Sharma AK, Muthuraju S, Banerjee PK, Ilavazhagan G. Neurochem Int. 2006;49:709. doi: 10.1016/j.neuint.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Maiti P, Singh SB, Muthuraju S, Veleri S, Ilavazhagan G. G Brain Res. 2007;1175C:1. doi: 10.1016/j.brainres.2007.06.106. [DOI] [PubMed] [Google Scholar]
- Maiti P, Muthuraju S, Ilavazhagan G, Singh SB. Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav Res. 2008a;189:233. doi: 10.1016/j.bbr.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Maiti P, Singh SB, Mallick BN, Muthuraju S, Ilavazhagan G. J Chem Neuroanat. 2008b;36:227. doi: 10.1016/j.jchemneu.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Marchenko ND, Zaika A, Moll UM. J Biol Chem. 2000;275:16202. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. Molecular Cell. 2003;11:577. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Miquel J. Arch Gerontol Geriatr. 1991;12:99. doi: 10.1016/0167-4943(91)90022-i. [DOI] [PubMed] [Google Scholar]
- Mishra OP, Delivoria-Papadopoulos M. Neurosci Lett. 2006;401:81. doi: 10.1016/j.neulet.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Cardoso SM, Santos MS, Oliveira CR. J Alzheimer’s Dis. 2006;9:101. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Oliveira CR. Antioxid. Redox Signal. 2007a;9:1621. doi: 10.1089/ars.2007.1703. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Seiça R, Oliveira CR. J Neurolog Sci. 2007b;257:206. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Free Rad Biol Med. 2008a;44:1493. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, Perry G. CNS & Neurolog Disord Drug Targets. 2008b;7:3. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. J Alzheimer’s Dis. 2009;16:741. doi: 10.3233/JAD-2009-0972. [DOI] [PubMed] [Google Scholar]
- Niizuma K, Endo H, Pak H. J Neurochem. 2009;109:133. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. J Neuropathol Exp Neurol. 2001;60:759. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Ozawa T. Physiol Rev. 1997;77:425. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK. Neurology. 1994;44:1090. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- Richter C, Kass GE. Chemico-biol Interact. 1991;77:1. doi: 10.1016/0009-2797(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Ricquier D, Bouillard F. J Physiol. 2000;529:3. doi: 10.1111/j.1469-7793.2000.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi A, Orsucci D, Tognoni G, Ceravolo R, Siciliano G. Curr Alzheimer Res. 2009;6:224. doi: 10.2174/156720509788486644. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. J Neurochem. 2009;109:17. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Neuroimage. 2005;26:1078. doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Roy S, Rauk A. Med Hypotheses. 2005;65:123. doi: 10.1016/j.mehy.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Sarada SK, Himadri P, Ruma D, Sharma SK, Pauline T, Mrinalini Brain Res. 2008;1209:29. doi: 10.1016/j.brainres.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Aguirre-Chen C, Kietzmann T, Saul I, Busto R, Ginsberg MD. Brain Res. 2004;1001:133. doi: 10.1016/j.brainres.2003.11.065. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Cancer Cell. 2002;1:289. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Sheu S, Nauduri D, Anders M. Biochim et Biophysica Acta. 2006;1762:256. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y. J Cereb Blood Flow Metab. 2002;22:810. doi: 10.1097/00004647-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Proc Natl Acad Sci USA. 1994;91:10771. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Dykens JA. Brain Res Rev. 2007;57:421. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Srinivas V, Leshchinsky I, Sang N, King MP, Minchenko A, Caro J. J Biol Chem. 2001;276:21995. doi: 10.1074/jbc.C100177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K, Lassmann H. Amer J Pathol. 1999;155:1459. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JH, Kesslak JP, Head E, Cotman CW. Acta Neuropathol. 2002;104:1. doi: 10.1007/s00401-002-0548-2. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Proc Natl Acad Sci USA. 2006;103:18727. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nishiyama K, Yamamoto A, Inazawa J, Iwaki T, Yamada T, Kanazawa I, Sakaki Y. Genomics. 2000;63:246. doi: 10.1006/geno.1999.6053. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Ann Neurol. 1996;40:663. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr, Davis RE, Parker WD., Jr Neurology. 1997;49:918. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Trimmer PA, Miller SW, Maguire DJ, Sheehan JP, Maguire RS, Pattee G, Juel V, Phillips LH, Tuttle JB, Bennett JP, Jr, Davis RE, Parker WD., Jr Exp Neurol. 1998;153:135. doi: 10.1006/exnr.1998.6866. [DOI] [PubMed] [Google Scholar]
- Taylor CT. Biochem J. 2008;409:19. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Amer J Physiol Lung Cell Mol Physiol. 2000;279:L1005. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. Nature. 1996;380:168. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- Townsend KP, Obregon D, Quadros A, Patel N, Volmar C, Paris D, Mullan M. Ann New York Acad Sci. 2002;977:65. doi: 10.1111/j.1749-6632.2002.tb04799.x. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Chemico-biol Interact. 2006;160:1. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. J. Int J Biochem Cell Biol. 2007;39:44. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Mitochondrion. 2006;6:323. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux C, Metzen E, Yeates KM, Ratcliffe PJ. Blood. 2001;98:296. doi: 10.1182/blood.v98.2.296. [DOI] [PubMed] [Google Scholar]
- Wai MS, Liang Y, Shi C, Cho EY, Kung HF, Yew DT. Biogerontology. 2009;10:457. doi: 10.1007/s10522-008-9189-8. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Science. 1992;256:628. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhang YW, Zhang X, Liu R, Zhang X, Hong S, Xia K, Xia J, Zhang Z, Xu H. FASEB J. 2006;20:1275. doi: 10.1096/fj.06-5839fje. [DOI] [PubMed] [Google Scholar]
- Xiong H, Callaghan D, Jones A, Bai J, Rasquinha I, Smith C, Pei K, Walker D, Lue LF, Stanimirovic D, Zhang W. J Neurosci. 2009;29:5463. doi: 10.1523/JNEUROSCI.5103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Luo Y, Cao G, Bai L, Pei W, Kuharsky DK, Chen J. J Biol Chem. 2000;277:42074. doi: 10.1074/jbc.M204991200. [DOI] [PubMed] [Google Scholar]
- Zawia NH, Lahiri DK, Cardozo-Pelaez F. Free Radic Biol Med. 2009;46:1241. doi: 10.1016/j.freeradbiomed.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhou K, Wang R, Cui J, Lipton SA, Liao FF, Xu H, Zhang YW. J Biol Chem. 2007;282:10873. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, Hirai K, Smith MA. J Alzheimer’s Dis. 2006;9:147. doi: 10.3233/jad-2006-9207. [DOI] [PubMed] [Google Scholar]