Abstract
Using a newly developed in vivo model measuring glucagon-like peptide-1 (GLP-1) in gut lymphatics in mice, we quantified GLP-1 secretion in vivo after glucose versus fat ingestion with and without concomitant DPP-4 inhibition. The mesenteric lymphatic duct was cannulated in anesthetized C57BL6/J mice and lymph was collected in 30 min intervals. Glucose or fat emulsion (IntralipidR) (0.03, 0.1 or 0.3 kcal) with or without DPP-4-inhibition (NVP DPP728; 10 μmol/kg) was administered by gastric gavage. Basal intact GLP-1 levels were 0.37 ± 0.04 pmol/l (n = 61) in lymph compared to 0.07 ± 0.03 in plasma (n = 6; P = 0.04) and basal DPP-4 activity was 4.7 ± 0.3 pmol/min/μl in lymph (n = 23) compared to 22.3 ± 0.9 pmol/min/μl in plasma (n = 8; P < 0.001). Lymph flow increased from 1.2 ± 0.1 μl/min to 2.3 ± 02 μl/min at 30 min after glucose and fat administration, with no difference between type of challenge or dose (n = 81). Lymph GLP-1 levels increased calorie-dependently after both glucose and fat but with different time courses in that glucose induced a transient increase which had returned to baseline after 90 min whereas the lipid induced a sustained increase which was still elevated above baseline after 210 min. Lymph GLP-1 appearance during 210 min was two to three-fold higher after glucose (7.4 ± 2.3 fmol at 0.3 kcal) than after isocaloric fat (2.9 ± 0.8 fmol at 0.3 kcal; P < 0.001). The slope between caloric load and lymph GLP-1 appearance was, however, identical after glucose and fat. We conclude that lymph GLP-1 is higher than plasma GLP-1 whereas lymph DPP-4 activity is lower than plasma DPP-4 activity and that both glucose and fat clearly stimulate GLP-1 secretion calorie-dependently in vivo but with different time courses.
Keywords: Lymph, Incretin secretion, DPP-4 inhibition, Glucose, Fat, GLP-1
1. Introduction
Glucagon-like peptide-1 (GLP-1) is produced in intestinal L-cells and secreted after food intake as well as after ingestion of individual macro-nutrients [1–3]. GLP-1 has attracted considerable interest as a glucose lowering strategy in the treatment of type 2 diabetes. Both GLP-1 receptor agonists and dipeptidyl peptidase (DPP)-4 inhibitors, which prevent the inactivation of endogenous GLP-1, are established as glucose-lowering therapeutics [4–6]. A future approach to harness endogenous GLP-1 in therapy would be to pharmacologically increase the circulating level of intact (active) GLP-1 through stimulating GLP-1 secretion [7,8]. In vitro studies in isolated L cells have identified several potential targets for pharmacological stimulation of GLP-1 secretion, such as G-protein coupled receptors GPR40 and GPR119 and amino acids [7]. However, to reliably develop such a strategy, models for studying endogenous GLP-1 secretion in vivo are required in animals for preclinical evaluation. Furthermore, more detailed knowledge of the in vivo regulation of GLP-1 secretion is also required, since most knowledge so far has been generated in isolated cells [9].
Under in vivo conditions, GLP-1 is rapidly inactivated by DPP-4, which cleaves the N-terminal dipeptide from intact GLP-1 making it largely inactive [10,11]. Secreted GLP-1 seems to undergo major (50%) inactivation by DPP-4 immediately after its release from the L-cells even before it reaches the local intestinal circulation [12]. Thereafter, a further inactivation (40%) occurs during a single passage through the liver [13], leaving only a small fraction of the secreted GLP-1 available for analyses in the peripheral circulation. This limits the potential for measuring GLP-1 in peripheral plasma in studies in small animals, such as in mice, and even when adding a DPP-4 inhibitor to the sample tubes, the raise of plasma intact GLP-1 after glucose administration is minimal. For example, we have found that intact GLP-1 levels are increased only during the initial 10 min in mice after oral administration of high caloric load of glucose in mice [14]. Since the mouse is an important model for pharmaceutical development of GLP-1-secretagogues as well as for physiological studies on regulation of GLP-1 secretion in wild type as well as in genetically modified animals, this limitation is a major challenge.
One method to improve the sensitivity in studies of GLP-1 secretion in vivo is the lymph fistula model. This has been described as a model to determine the secretion of incretin hormones in rats and it has been shown that GLP-1 secretion is increased by oral intake of fat and carbohydrates, but not after protein [15–18]. The rationale behind the technique is that after its secretion from the enterocytes, GLP-1 is released in the lymphatic lacteal located in the lamina propria of the microvilli [16]. It has been shown that GLP-1 is enriched in lymph compared to plasma, and, in fact, is more enriched in this compartment than other gut hormones, such as glucose-dependent insulinotropic peptide (GIP) and peptide YY (PYY) [16]. Therefore, a higher concentration of GLP-1 occurs in lymph compared to plasma, in part also because of a lower abundance of DPP-4, as found in the lymphatic fluid of rats [15–17], although whether the same scenario is true for the mice is not known.
In this study, we have developed the lymph fistula mouse model for the study of the physiological regulation of endogenous GLP-1 secretion and for pharmaceutical development of GLP-1 secretagogues. We aimed specifically to examine the relative sensitivity of L-cell release of GLP-1 after gastric gavage of glucose versus a lipid emulsion with and without concomitant DPP-4 inhibition.
2. Materials and methods
2.1. Animals and surgical preparation of lymph duct cannulation
The lymph duct cannulation technique was initially based on earlier work by Bollman, Cain and Grindlay in the 1940s [19] which was later further developed by Lu and collaborators in rats [20]. Female C57BL6/J mice (21–27 g) were injected i.p. with pain reliever (TemgesicR, RB Pharmaceuticals, Berkshire, UK, 0.03 mg/kg), anesthetized with isofluorane and placed under a microscope. After midline laparotomy, the mesenteric lymph duct was exposed. A 1.2 × 40 mm needle was pushed through the side skin from the inside on the right side of the animal, close to the liver. The needle was used to guide a PVC cannula (Dural Plastics and Engineering, Dural, Australia; ID 0.2 mm, OD 0.5 mm) to the lymph duct. Three-centimeter PVC tube was pre-mounted into a 20 cm collection PVC tube (ID 0.5 mm, OD 0.8 mm) and pre-filled with heparin (Leo Pharma AB, Malmö, Sweden, 100 IE/ml) in saline before use. A small incision was made in the lymphatic duct with a micro scissor and after leakage of the white lymph, the tube was inserted 2 mm into the duct by micro-forceps. When lymph was visualized migrating, the tube was fixed by placing a small drop of super glue (LoctiteR, Henkel Norden AB, Stockholm, Sweden) on the site of cannulation. The intestines were carefully replaced and soaked with saline. Then, to allow gastric instillation, the skin was punched with a 1.2 × 40 mm needle on the left side of the animal and a 30 cm tubing for infusion was guided through the needle and, after a triangular suture was made to incise the pyloric part of the stomach, inserted 1 cm with the tip towards the pylorus. The threads were pulled tight and secured around the tube. The abdomen was closed by continuous suture. A 20 × 1.5 cm elastic bandage was wrapped around the upper part of the torso just behind the front legs. The mouse was placed in a cage and restrained by metal bars on each side, allowing moving back- and forward. The stomach tube was connected to an infusion pump and infusion of saline was started immediately. The mouse was kept warm around 26–27 °C and let to recover 30–60 min with saline infusion before proceeding with the experiment.
After sampling of lymph for the baseline period of 30 min, saline, D-glucose (Sigma Aldrich, Stockholm, Sweden) or fat emulsion (IntralipidR, Fresenius Kabi, Uppsala, Sweden) was administered into the stomach (volume load 0.3 ml). Glucose and fat were administered at three caloric doses (0.03, 0.1 or 0.3 kcal) together with the DPP-4 inhibitor NVP DPP728 (10 μmol/kg; Tocris Bioscience, Bristol, UK); in one series of experiments, glucose and fat at 0.3 kcal were administered without the DPP-4 inhibitor, and in one series, saline (0.3 ml) was instilled. Lymph was then collected every 30 min throughout a total of 4 h study period; thereafter pentobarbital was given and the experiments terminated.
2.2. Lymph collection and analyses
Lymph was collected in 30 min portions in 0.2 ml Eppendorf tubes containing 5 μl saline with heparin for analyses of DPP-4 activity and with heparin and the DPP-4 inhibitor diprotin A (100 μM; Bachem, Bubendorf, Switzerland) for analysis of intact GLP-1. The tubes were weighed before and after lymph collection and then kept frozen at −70 °C until further analysis. Lymph flow was determined by multiplying lymph weight by density (1.02 g).
2.3. Plasma sampling
Female mice (C57BL6/J) weighing 21–27 g were kept under normal conditions and injected intraperitoneally with pain reliever (Temgesic) and placed in isoflurane anesthetics. A 25 μl blood sample was obtained from the orbital plexus; thereafter 75 mg glucose in 300 μl saline was administered by gavage. Thereafter, 25 μl blood samples were taken at regular intervals under anesthesia. Blood samples were pooled (two mice for each n) and centrifuged for analysis of GLP-1 levels and DPP-4 activity.
2.4. Analysis
DPP-4 activity was measured colorimetrically by incubating 20 μl lymph or plasma with 100 μl 1 mM Gly-Pro-p-nitroaniline in a microtiter plate for 60 min at 37 °C, as previously described [19]. Amount of p-nitroaniline was determined at 405 nm and reflects DPP-4 activity. Intact GLP-1 was determined in 25 μl lymph or plasma by sandwich immunoassay on a Meso Scale Discovery (MSD) platform (Meso Scale Discovery, Gaithersburg, MD, USA). The sensitivity of the assay is 0.036 pmol/l and the coefficient of variation is approximately 5%. Lymph content of intact GLP-1 was estimated by multiplying the lymph concentration of intact GLP-1 times flow in each 30 min collection fraction.
2.5. Statistics and ethics
Unpaired Student's t-test was used for determination of statistical significant difference between GLP-1 levels and DPP-4 activity in lymph and plasma. Lymph DPP-4 activity, lymph flow, lymph concentrations of intact GLP-1 and suprabasal lymph appearance of intact GLP-1 (lymph flow times lymph intact GLP-1) were calculated during the 30 min before gavage, during the first 30 min after gavage and during the entire 210 min study period, as pre-specified. Unpaired t-test was used for tests of significance. The experiments were undertaken according to Good Laboratory Practice and the experimental procedures were approved by the Ethics Committee of Lund University.
3. Results
3.1. Lymph flow
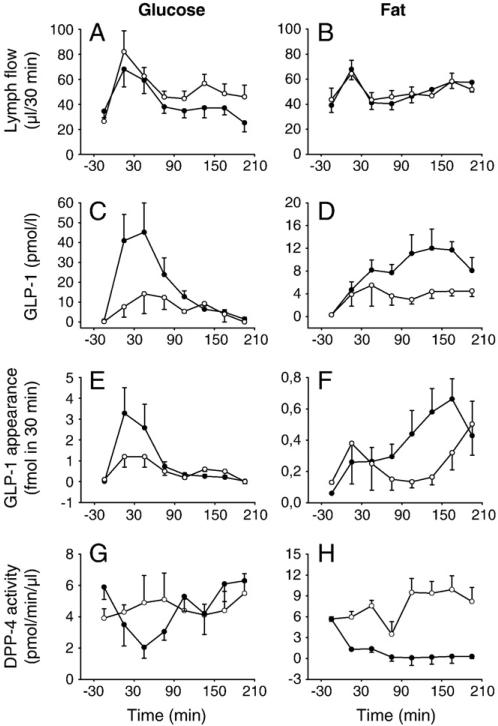
Lymph flow transiently increased after gastric instillation with an approximately doubling during the initial 30 min (Fig. 1A after 75 mg glucose and Fig. 1B after 33 mg of fat). By compiling all experiments together (n = 81), it was determined that baseline lymph flow was 37.2 ± 2.5 μl, i.e., 1.2 ± 0.1 μl/min (n = 81) which increased to 69.0 ± 5.3 μl/30 min (2.2 ± 0.1 μl/min; P < 0.001) following gastric instillation of saline, glucose or fat (0.3 ml) during the initial 30 min. There was no significant difference in changes in lymph flow when comparing instillation of saline, glucose or fat, instillation of different doses of glucose or fat, or with versus without DPP-4 inhibition (these data are not shown in detail).
Fig. 1.
Lymph flow (A, B), GLP-1 concentrations (C, D), GLP-1 appearance (E, F) and DPP-4 activity (G, H) before and after gastric instillation of 75 mg glucose (0.3 kcal) with (●-●; n = 9) or without (○-○; n = 6) DPP-4 inhibition (NVP DPP728; 10 μmol/kg) (left panels) or after gastric instillation of 33 mg fat emulsion (0.3 kcal) with (●-●; n = 7) or without (○-○; n = 7) NVP DPP728 (10 μmol/kg) (right panels) in anesthetized C57BL/6J mice (mean ± SEM).
3.2. DPP-4 activity
DPP-4 activity was 7.2 ± 0.8 pmol/min/μl (n = 9) in the lymph and 22.3 ± 0.9 pmol/min/μl in plasma (n = 8; P < 0.001). Lymph DPP-4 activity was not affected by gastric glucose or fat administration, but, as expected, reduced by the DPP-4 inhibitor NVP DPP728 (10 μmol/kg). After glucose administration, the DPP-4 inhibitor transiently reduced lymph DPP-4 activity during the initial 90 min (Fig. 1G) whereas after fat instillation, DPP-4 activity remained low after administration of NVP DPP728 throughout the 3.5 h period (Fig. 1H).
3.3. Lymph GLP-1 after glucose and fat
3.3.1. Dependency on DPP-4 inhibition
Intact GLP-1 concentration and appearance (i.e., concentration times flow) were markedly increased during the first 60 min after gastric glucose (0.3 kcal; 75 mg) in the presence but not in the absence of NVP DPP728 (Fig. 1C). Thus, in the presence of NVP DPP728, lymph GLP-1 concentrations increased from baseline of 0.5 ± 0.2 pmol/l to 41.0 ± 13.3 pmol/l during the initial 30 min (n = 9; P = 0.016) and the corresponding lymph appearance of intact GLP-1 increased from 0.02 ± 0.01 fmol to 3.23 ± 1.26 fmol (P = 0.032). After the initial 60 min after glucose, GLP-1 levels and appearance leveled off (Fig. 1C). Total GLP-1 appearance after oral glucose was 7.3 ± 2.3 fmol in the presence and 4.1 ± 0.3 fmol in the absence of the DPP-4 inhibitor. After fat administration (0.3 kcal; 33 mg), lymph GLP-1 concentration and appearance increased gradually in the presence but not in the absence of NVP DPP728 (Fig. 1E and F). Intact GLP-1 concentrations after fat instillation were at its highest after 120– 180 min after fat instillation. The total of 210 min GLP-1 appearance was 3.0 ± 0.7 fmol in the presence of NVD DPP728 (n = 6) and 1.3 ± 0.4 fmol in the absence of the DPP-4 inhibitor (n = 7; P = 0.035).
3.3.2. Calorie-dependent increase in lymph GLP-1 appearance after glucose or fat
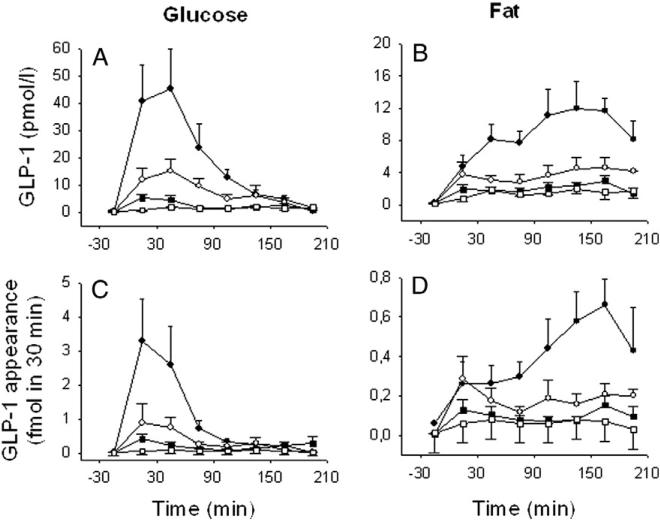
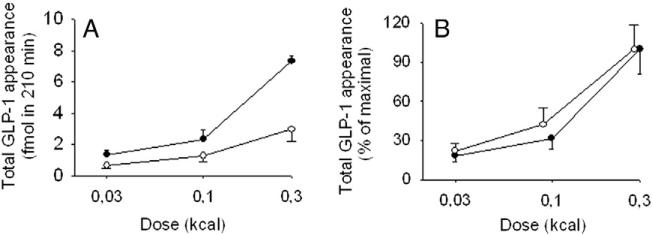
Lymph GLP-1 concentrations (Fig. 2A) and GLP-1 appearance (Fig. 2C) increased calorie dependently by gastric glucose at 8.3, 25 and 75 mg per mouse (0.03, 0.1 and 0.3 kcal). The total of 210 min increase in GLP-1 appearance was 1.4 ± 0.3 fmol (8.3 mg), 2.3 ± 0.6 fmol (25 mg) and 7.3 ± 2.3 fmol (75 mg). Lymph GLP-1 concentrations (Fig. 2B) and appearance (Fig. 2D) increased calorie-dependently also by gastric fat instillation at 3.7, 11 and 33 mg (0.03, 0.1 and 0.3 kcal). The total of 210 min increase in GLP-1 appearance was 0.7 ± 0.2 fmol (3.7 mg), 1.3 ± 0.4 fmol (11 mg) and 3.0 ± 0.8 fmol (33 mg), respectively. By comparing lymph GLP-1 appearance in relation to calorie intake after glucose versus fat, it was found to be more potently increased by glucose than by fat at the highest dose of 0.3 kcal (P = 0.041; Fig. 3A) but not at the lowest doses. When lymph GLP-1 appearance was expressed as a percent of mean of appearance at the highest dose level (0.3 kcal), the slope of the relation was almost identical between glucose and fat emulsion (Fig. 3B).
Fig. 2.
Lymph GLP-1 concentrations (A, B) and GLP-1 appearance (C, D) before and after gastric instillation of glucose (left panel) at 75 mg (0.3 kcal, n = 9), 25 mg (0.01 kcal, n = 8) or 8.3 mg (0.03 kcal, n = 6), after fat emulsion (right panel) at 33 mg (0.3 kcal, n = 7), 11 mg (0.1 kcal, n = 6) or 3.7 mg (0.03 kcal, n = 8) or after saline (0 kcal, n = 3), in all experiments with the DPP-4 inhibitor NVP DPP728 (10 μmol/kg) in anesthetized C57BL/6J mice (mean ± SEM). ●-● 0.3 kcal, ○-○ 0.1 kcal, ■-■ 0.03 kcal, □-□ 0 kcal. Points represent 30 min fractions.
Fig. 3.
Lymph GLP-1 appearance during 3.5 h after gastric administration of glucose (●-●) or fat emulsion (○-○) in anesthetized C57BL/6J mice, expressed as total appearance (A) or percent appearance of mean of 0.3 kcal for each nutrient (B). Glucose was administered at 75 mg (0.3 kcal, n = 9), 25 mg (0.1 kcal, n = 8) or 8.3 mg (0.03 kcal, n = 6) and fat emulsion was administered at 33 mg (0.3 kcal, n = 7), 11 mg (0.1 kcal, n = 6) or 3.7 mg (0.03 kcal, n = 8); all with the DPP-4 inhibitor NVP DPP728 (10 μmol/kg) (mean ± SEM).
3.4. Comparison of GLP-1 in plasma and lymph
Baseline plasma GLP-1 levels were 0.07 ± 0.03 pmol/l (n = 6) and basal lymph GLP-1 levels were 0.37 ± 0.04 pmol/l (n = 61; P = 0.04). After gastric gavage of glucose (0.3 kcal), peak plasma GLP-1 at 10 min was 0.61 ± 0.15 pmol/l and GLP-1 level in the 0–30 min lymph fraction was 41.0 ± 13.3 pmol/l (n = 9), corresponding to ≈80-fold higher levels in lymph (P < 0.001).
4. Discussion
In the present study, a novel mouse model was developed to allow in vivo studies on regulation of GLP-1 secretion by cannulation of the mesenteric lymph duct with collection of lymph. By using this novel technique we demonstrate 1) that intact GLP-1 levels are higher and DPP-4 activity lower in lymph than in plasma, 2) that both glucose and fat administration result in a marked release of intact GLP-1 into the lymph, 3) that DPP-4 inhibition further increases the lymph appearance of intact GLP-1, and 4) that glucose has a higher potency to increase GLP-1 appearance than fat during the first hours after administration, but that the calorie sensitivity of the two nutrients is similar.
In our development of this novel system, we experienced that the identification of the lymph duct is not particularly difficult in mice; instead the most challenging part of the technical procedure is achievement of a persistent lymph flow into the cannula throughout the 4-hour experiment. The success rate is approximately 80% after training and repeated experimenting of an experienced technician. With this experience, the lymph flow is ≈1–2 μl/min, which allows for collection of the lymph in 30 min fraction periods for analysis of intact GLP-1.
When we initiated the method development, it was unclear whether the lymph contains DPP-4 activity or not [16,17]. We therefore initially analyzed DPP-4 activity in the mouse lymph compared with plasma using a colorimetric assay of DPP-4 activity, which we have used before in studies in mice [21]. We found that there is indeed DPP-4 activity in the lymph, but it is only approximately 30% of that in the peripheral plasma. This confirms a previous report in rats showing DPP-4 in the mesenteric lymph duct [22]. The origin of lymph DPP-4 is probably the lymph epithelium, which previously has been shown to express DPP-4 [22]. The lymph DPP-4 activity was biologically active as evident from comparing intact GLP-1 levels in the lymph with versus without DPP-4 inhibition; it was found that DPP-4 inhibition substantially raised lymph intact GLP-1 after glucose and fat instillation. Therefore it is important to include administration of DPP-4 inhibitor in the collection protocols analyzing intact GLP-1 appearance in lymph. In this study, we used the DPP-4 inhibitor NVP DPP728, which is a cyano pyrrolidine based inhibitor that was developed in the 1990s [23]. It was used in several early experimental studies during the preclinical development of DPP-4 inhibition as glucose-lowering therapy of type 2 diabetes [24,25]. NVP DPP728 was also the DPP-4 inhibitor which we used in the very first human study on examining the proof-of-principle of the glucose-lowering treatment with DPP-4 inhibition [26].
A main outcome in the study was that lymph GLP-1 was raised by both glucose and fat administration in a clear dose-dependent manner. Both glucose and fat therefore stimulate GLP-1 secretion in vivo in the lymphatics, which corroborates previous reports of increased GLP-1 after oral glucose and fat in humans [27–29] and the previous lymph study in rats [17,30]. Since DPP-4 activity was inhibited, the increased lymph appearance of intact GLP-1 depends on stimulation of GLP-1 secretion from the enteric L-cells. Earlier in vitro studies have demonstrated different L-cell signal mechanisms between glucose and fat. Thus, whereas glucose activates L-cell secretion by being taken up by the cells through a glucose–sodium exchange mechanism governed by SGLT-1, fat works preferentially through stimulating lipid sensitivity G protein coupled receptors, mainly GPR40, GPR119 and GPR120 [31].
We also found clear differences between glucose and fat to stimulate lymph GLP-1 appearance. First, we found that glucose was more potent in increasing lymph appearance of GLP-1 than fat administration during the entire 210 min study period, whereas the calorie sensitivity in the appearance process did not seem to be different between glucose and fat, i.e., the sensitivity to increase GLP-1 appearance in relation to caloric load. The higher GLP-1 response to glucose than to intralipid seems to be at variance with previous studies in humans comparing fat versus glucose. We have thus previously compared oral glucose and oral oleic acid in equicaloric amounts (8 kcal/kg) in humans and demonstrated a higher peak level after the fat than after glucose (approximately 15 pmol/l versus approximately 11 pmol/l) [27,28]. We have also previously given intralipid (the lipid form used in the present study) to humans, but at 6 kcal/kg, and found it to have a lower effect than oleic acid, more resembling glucose (peak at 30 min at approximately 12 pmol/l; [32]). It may therefore be a difference between different lipid forms and no study has been performed comparing equimolar amounts of glucose and intralipid in humans. Second, and perhaps of importance also for the discussion on relative difference in potency between glucose and lipid, we also found that there was a difference in time-course of the effects between the nutrients. Thus, glucose instillation induced a transient, although marked, increase in intact GLP-1 appearance in lymph which was back to baseline after approximately 90 min, whereas the increase was sustained after fat where lymph GLP-1 appearance was still highly elevated at 210 min after intralipid. Also in humans, a more rapid GLP-1 release by glucose (8 kcal/kg; peak after 30 min) was evident than after oleic acid (8 kcal/kg; peak at 60 min) [27,28]. This would also be consistent with findings in the lymph fistula rat model in which dextrin provided a later peak of GLP-1 response compared to fat emulsion, which may be explained by dextrin being a more viscous solution than fat (and glucose) [17]. The difference may be due to effects of the lipid to reduce gastric emptying and prolong gut transit time and/or difference in pharmacokinetic and binding properties of DPP-4 inhibition after fat versus glucose administration. Therefore, calculating the 210 min total GLP-1 appearance might underestimate the full potency of intralipid to release GLP-1 into the lymph, which may be supported by the similarity of the two nutrients in calorie sensitivity to stimulate GLP-1 appearance. Nevertheless, during the initial 2 h after glucose and intralipid loads, there was a clear difference in that GLP-1 was released to the lymph at a much higher rate than after intralipid. The detailed mechanisms for this difference as well as its potential physiological and clinical consequences, need to be studied.
Our results thus show that collecting lymph is a model for determining GLP-1 release in mice. A reason for this is that GLP-1 concentrations are markedly higher in the lymph than in plasma and since GLP-1 is determined more closely to its origin than in peripheral plasma. The higher lymph GLP-1 levels compared to plasma were not unexpected since secreted GLP-1 is, besides the major (50%) inactivation by DPP-4 immediately after its release from the L-cells [12], also massively degraded (40%) during the first passage through the liver [13]. Hence, only a fraction of the secreted GLP-1 is available for analyses in the peripheral circulation. To this is added the lower lymph flow compared to plasma, which results in a more avid dilution of GLP-1 in the plasma pool than in the lymph. Therefore, the lymph technique is more powerful to reflect GLP-1 release than measurements of plasma GLP-1. Besides the use of the model to study GLP-1 release after different challenges, as was the aim of this study, several further studies may be proposed to further understand the kinetics and metabolism of GLP-1 and to explore the quantity and importance of GLP-1 released into the lymph. A limitation of the present study in this perspective was that we did not measure lymph and plasma GLP-1 in parallel in the same animals. The reason for this was to avoid drainage of blood during the test, but due to this limitation we cannot estimate a systemic plasma–mesenteric lymphatic comparison in this study. We did, however, measure plasma GLP-1 after glucose gavage in other animals and showed the peak lymphatic GLP-1 to be ≈80 times higher than plasma GLP-1. Further, another interesting aspect is to what extent the lymphatic GLP-1 contributes to the plasma pool of GLP-1. This would be possible to examine in future studies by measuring plasma GLP-1 after glucose gavage in mice in which lymphatics were drained versus in normal mice. Finally, a further study would be to measure the portal GLP-1 levels to examine how much of the released GLP-1 appears in the lymphatics.
We conclude that measurement of GLP-1 in mouse mesenteric lymph is suitable for quantifying GLP-1 secretion in vivo in model experiments in mice, that the concentration of intact GLP-1 is higher in lymph than in peripheral plasma, that oral DPP-4 inhibition substantially increases intact GLP-1 concentrations in the lymph, and that intake of glucose showed higher potency to increase intact GLP-1 in lymph than fat during a 210 min study period. The results will foster future studies on regulation of GLP-1 secretion in vivo to further establish its regulatory mechanisms. In future studies, also effects of administration of protein and combinations of macronutrients would be important to examine, as well as the presence of other gastrointestinal hormones than GLP-1 in lymph.
Acknowledgements
The skillful technical assistance by Kristina Andersson is gratefully acknowledged. The study was supported by grants from the Swedish Medical Research Council, Faculty of Medicine at the Lund University and Region Skåne (to Dr. Ahrén), from the Albert Påhlsson Foundation (to Dr. Ohlsson) and from the NIH DK 59630, DK 92138, and DK 76928 (to Dr. Tso), and NIH F32-091173-01 (to Dr. Kohan).
Footnotes
Author contributions
LO and BA have substantially contributed to conception and design of the study, acquisition of data and analyses and interpretation of data, drafted and critically revised the article. AK and PT have contributed to conception of the study and critically revised the article. All authors have finally approved the version to be published.
References
- 1.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696s–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 2.Ahrén B, Carr RD, Deacon CF. Incretin hormone secretion over the day. Vitam Horm. 2010;84:203–20. doi: 10.1016/B978-0-12-381517-0.00007-2. [DOI] [PubMed] [Google Scholar]
- 3.Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–13. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 4.Ahrén B. GlP-1-based therapy of type 2 diabetes: GLP-1 mimetics and DPP-IV inhibitors. Curr Diabet Rep. 2007;7:340–7. doi: 10.1007/s11892-007-0056-9. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;109:466–74. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 6.Ahrén B. Incretin therapy of type 2 diabetes. GLP-1 receptor agonists and DPP-4 inhibitors. J Eur Diabet Nurs. 2013;10:31–6. [Google Scholar]
- 7.Gribble FM. Targeting GLP-1 release as a potential strategy for the therapy of type 2 diabetes. Diabet Med. 2008;25:889–94. doi: 10.1111/j.1464-5491.2008.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahrén B. The future of incretin-based therapy. Novel avenues–novel targets. Diabetes Obes Metab. 2011;13(Suppl. 1):158–66. doi: 10.1111/j.1463-1326.2011.01457.x. [DOI] [PubMed] [Google Scholar]
- 9.Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav. 2012;6:387–93. doi: 10.1016/j.physbeh.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–31. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 11.Mentlein R. Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. doi: 10.1016/s0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 12.Hansen L, Deacon CF, Ørskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–63. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 13.Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol. 1996;271:E458–64. doi: 10.1152/ajpendo.1996.271.3.E458. [DOI] [PubMed] [Google Scholar]
- 14.Ahlkvist L, Vikman J, Pacini G, Ahrén B. Synergism by individual macronutrients explains the marked early GLP-1 and islet hormone responses to mixed meal challenge in mice. Regul Pept. 2012;178:29–35. doi: 10.1016/j.regpep.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Yoder SM, Kindel TL, Tso P. Using the lymph fistula rat model to study incretin secretion. Vitam Horm. 2010;84:221–49. doi: 10.1016/B978-0-12-381517-0.00008-4. [DOI] [PubMed] [Google Scholar]
- 16.D'Alessio D, Lu W, Sun W, Zheng S, Yang Q, Seeley R, Woods SC, Tso P. Fasting and postprandial concentrations of GLP-1 in intestinal lymph and portal plasma: evidence for selective release of GLP-1 in the lymph system. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2163–9. doi: 10.1152/ajpregu.00911.2006. [DOI] [PubMed] [Google Scholar]
- 17.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol. 2007;293:G963–71. doi: 10.1152/ajpgi.00146.2007. [DOI] [PubMed] [Google Scholar]
- 18.Arnold M, Dai Y, Tso P, Langhans W. Meal-contingent intestinal lymph sampling from awake, unrestrained rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1365–71. doi: 10.1152/ajpregu.00497.2011. [DOI] [PubMed] [Google Scholar]
- 19.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J Lab Clin Med. 1948;33:1349–52. [PubMed] [Google Scholar]
- 20.Lu WJ, Yang Q, Sun W, Woods SC, D'Alessio D, Tso P. Using the lymph fistula rat model to study the potentiation of GIP secretion by the ingestion of fat and glucose. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1130–8. doi: 10.1152/ajpgi.00400.2007. [DOI] [PubMed] [Google Scholar]
- 21.Gunnarsson PT, Winzell MS, Deacon CF, Larsen MO, Jelic K, Carr RD, Ahrén B. Glucose-induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology. 2006;147:3173–80. doi: 10.1210/en.2005-1442. [DOI] [PubMed] [Google Scholar]
- 22.Toyozaki M, Osaka M, Kondo Y, Yoshida M. High fat and high cholesterol diet induces DPP-IV activity in intestinal lymph. J Oleo Sci. 2013;62:201–5. doi: 10.5650/jos.62.201. [DOI] [PubMed] [Google Scholar]
- 23.Hughes TE, Mone MD, Russell ME, Weldon SC, Villhauer EB. NVP-DPP728 (1-[[[2-[5-cyanopyridin-2-yl)amino]ethyl]amino]acetyl]-2-cyano-(S)-pyrrolidine), a slow-binding inhibitor of dipeptidyl peptidase IV. Biochemistry. 1999;38:11597–603. doi: 10.1021/bi990852f. [DOI] [PubMed] [Google Scholar]
- 24.Balkan B, Kwasnik L, Miserendino R, Holst JJ, Li CU. Inhibition of dipeptidyl peptidase IV with NVP-DPP728 increases plasma GLP-1 (7-36 amide) concentrations and improves oral glucose tolerance in obese Zucker rats. Diabetologia. 1999;42:1324–31. doi: 10.1007/s001250051445. [DOI] [PubMed] [Google Scholar]
- 25.Reimer MK, Holst JJ, Ahrén B. Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol. 2002;146:717–27. doi: 10.1530/eje.0.1460717. [DOI] [PubMed] [Google Scholar]
- 26.Ahrén B, Simonsson E, Larsson H, Landin-Olsson M, Torgeirsson H, Jansson PA, Sandqvist M, Båvenholm P, Efendic S, Eriksson JW, Dickinson S, Holmes D. Inhibition of dipeptidyl peptidase IV improves metabolic control over a 4-week study period in type 2 diabetes. Diabetes Care. 2002;25:869–75. doi: 10.2337/diacare.25.5.869. [DOI] [PubMed] [Google Scholar]
- 27.Carr RD, Larsen MO, Winzell MS, Jelic K, Lindgren O, Deacon CF, Ahrén B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E779–84. doi: 10.1152/ajpendo.90233.2008. [DOI] [PubMed] [Google Scholar]
- 28.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahrén B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–8. doi: 10.1210/jc.2009-2054. [DOI] [PubMed] [Google Scholar]
- 29.Ohlsson L, Alsalim W, Carr RD, Tura A, Pacini A, Mari A, Ahrén B. Glucose-lowering effect of the DPP-4 inhibitor sitagliptin after glucose and non-glucose macronutrient ingestion in non-diabetic subjects. Diabetes Obes Metab. 2013;15:531–7. doi: 10.1111/dom.12062. [DOI] [PubMed] [Google Scholar]
- 30.Yoder SM, Yang Q, Kindel TL, Tso P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol. 2009;297:G299–305. doi: 10.1152/ajpgi.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Exp Rev Mol Med. 2010;5:12e1. doi: 10.1017/S146239940900132X. [DOI] [PubMed] [Google Scholar]
- 32.Lindgren O, Carr RD, Deacon CF, Holst JJ, Pacini G, Mari A, Ahrén B. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab. 2011;96:2519–24. doi: 10.1210/jc.2011-0266. [DOI] [PubMed] [Google Scholar]