Abstract
Background
The capability of OCT to examine the structure of the arterial wall before or after PCI is superior to those of other imaging modalities. Therefore the application of OCT during PCI seems logical and has the potential to enhance our performance during the PCI procedures.
Methods
OCT was performed in fifty-two patients out of which, 45 patients underwent PCI. Out of these 45 patients, in 25 patients both pre and post PCI OCT assessment was done. In 20 patients only post PCI OCT assessment was done. In seven patients PCI was not done due to nonsignificant obstruction, these seven patients were not included in final analysis.
Results
Over all OCT leads to management changes in 65% of the time it was used. Alteration of stent length was done in 56% of the cases when evaluated pre PCI. Alteration of stent diameter was done in 36% cases when evaluated pre PCI. Treatment of malapposition was done in 24% of total cases. Further balloon dilatation for vessel expansion was done in 15% of total cases. In one case left main stenting was done after proximal edge dissection.
Conclusion
OCT makes better visualization of plaque, thrombus, stent malapposition, dissection, plaque prolapse and helps in optimization of PCI results. More extensive, long-term studies will be needed to assess the prognostic implications of these findings.
Keywords: Optical coherence tomography, Percutaneous transluminal coronary angioplasty, Angiography, Coronary artery disease
1. Introduction
Atherosclerosis is a diffuse process, with uneven accumulation of plaque over the whole length of the coronary artery. The standard coronary angiogram is, in reality, a lumenogram providing useful information about lumen diameter but yielding little insight into plaque composition or lesion morphology.1 More over there is a significant inter-observer variability in the angiographic classification of lesion complexity even by skilled operators.2, 3 Angiography does not permit direct visualization of the arterial wall. In contrast, intravascular imaging (IVUS, OCT etc.) provides cross-sectional images of both the arterial lumen and wall, allowing correct assessment of true lumen dimensions and architecture as well as atherosclerotic plaque burden and vessel wall abnormalities.4 Optical coherence tomography (OCT) is an optical analog of intravascular ultrasound (IVUS) that can be used to examine the coronary arteries and has 10-fold higher resolution than IVUS.5 This technique has been found to have a high sensitivity and specificity for the classification of the different types of atherosclerotic plaques6, 7 and has also been utilized to estimate the collagen, macrophage content and to detect thin fibrous caps in atherosclerotic plaques.8, 9, 10 Furthermore, this improved resolution with OCT may help in interventional procedures and indeed OCT has been applied to assess the results of stent implantation.11, 12, 13 All of these data have been from outside India and there is no information on how OCT performs in our clinical milieu. We, therefore, present our preliminary experience of using OCT to assess and guide coronary interventions in India.
2. Methods
This study was designed as a retrospective, single center study. After baseline angiogram, consecutive patients who were posted for OCT guided PCI during Sep 2012 to Mar 2013 were studied. Patients with symptomatic ischemic heart disease, and a de novo lesion in the native coronary arteries, who gave consent for OCT were selected for the study. Exclusion criteria were ostial left main coronary artery disease, PCI to SVG graft, lesion of large vessel (reference vessel diameter > 4 mm), totally occluded lesion, lesion of severe tortuous vessel, serum creatinine > 2 mg/dL, and when use of antiplatelets and anticoagulants was contraindicated.
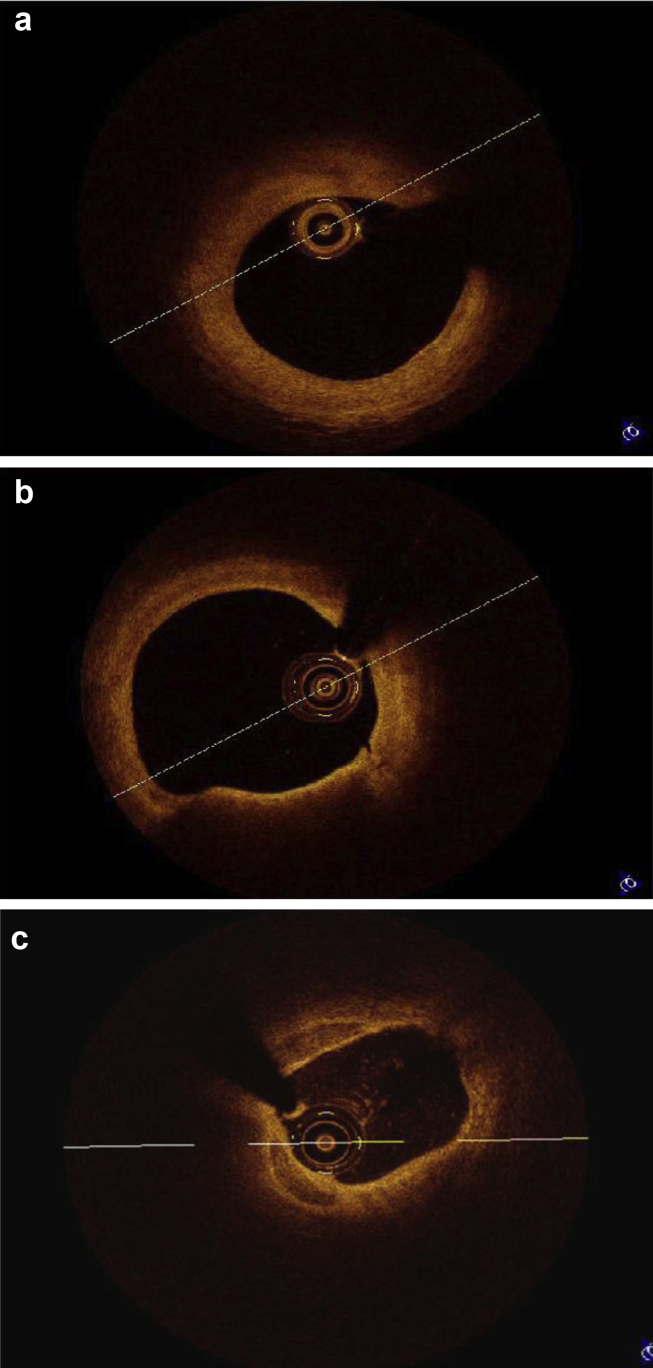
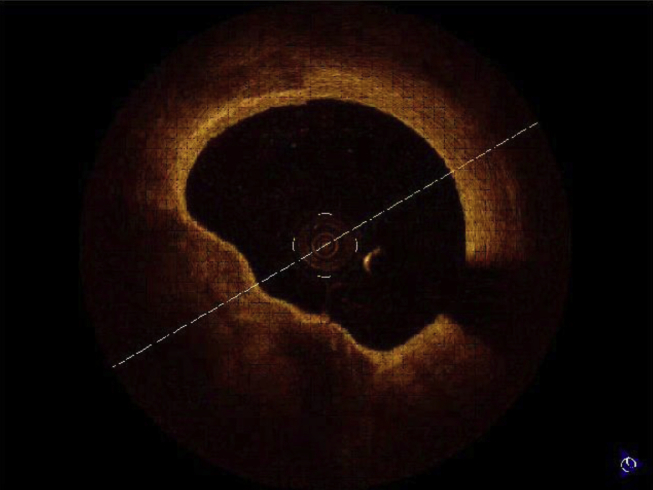
Standard protocol was used for performance & interpretation of OCT.14, 15 The use of OCT to guide PCI was left at the operator's discretion. All procedures were performed by experienced staff members with established skills. Fifty-two patients who underwent PCI or diagnostic procedures were studied by OCT. Pre PCI and post PCI assessments are done in 25 patients. In 20 patients, post PCI assessment was done. 7 patients underwent diagnostic angiogram followed by OCT. In these 7 patients PCI was not done due to nonsignificant obstruction and were excluded from final analysis. PCI was performed according to standard procedure. In patients with STEMI OCT was used after thrombus suction to see extent of thrombus and residual stenosis. OCT study was performed using Lightlab C7XR Frequency Domain OCT system. FD OCT is much superior to older TD OCT. It electronically scans the laser wavelength and gives faster images with exceptional image quality. It gives 100 frames/s, 15 mm axial resolution and 10 mm scan diameter.14, 15, 16 FD OCT systems do not require proximal occlusion. The coronary vessel is transiently rendered free of blood by a bolus injection of saline, contrast, or other solution, injected at rates of 2–4 ml/s.14, 15, 16 C7XR FD OCT system comes with a Dragonfly Catheter. It has fast flush, spiral pullback acquisition. The imaging catheter is a Rapid exchange (Rx) imaging catheter. An automated 20 mm/s pullback within the monorail rapid exchange catheter allows imaging of a 6cm long coronary segment during a 3-s injection.14, 15, 16 The acquired images are stored in the OCT console. The stored images were analyzed subsequently. Fibrous plaque produces homogenous high-intensity signal, lipid laden plaque produces low-intensity, irregular signal with indistinct and irregular borders. Calcified plaque produces low-intensity, sharp bordered, “textured” appearances (Fig. 1a–c). A thin cap fibroatheroma (TCFA) was defined as a homogenous area in the arterial wall with a low refractive index, separated from the vessel lumen by a capsule having a high refractive index and a thickness of less than 65 μm (Fig 2). Cross-sectional OCT images were analyzed at 1 mm interval.
Fig. 1.
(a) OCT image analysis – Fibrous plaque. (b) OCT image analysis – Lipid plaque. (c) OCT image analysis – Calcified plaque.
Fig. 2.
Thin cap fibrous atheroma.
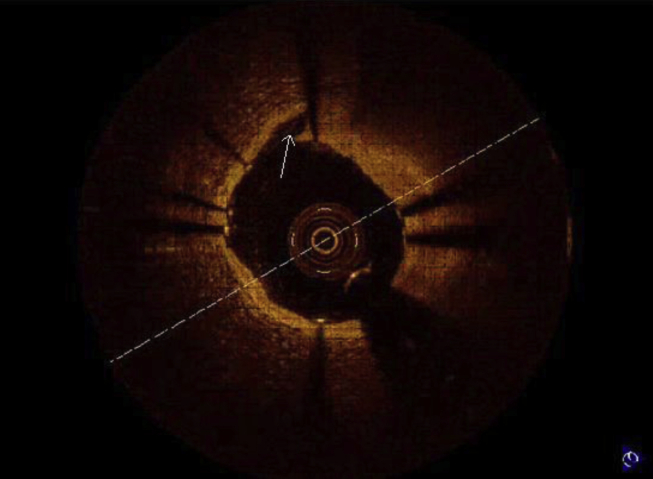
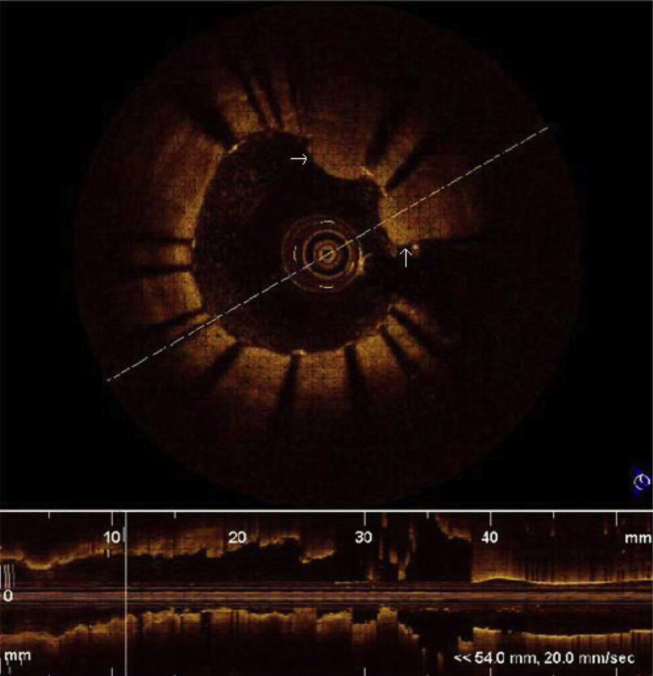
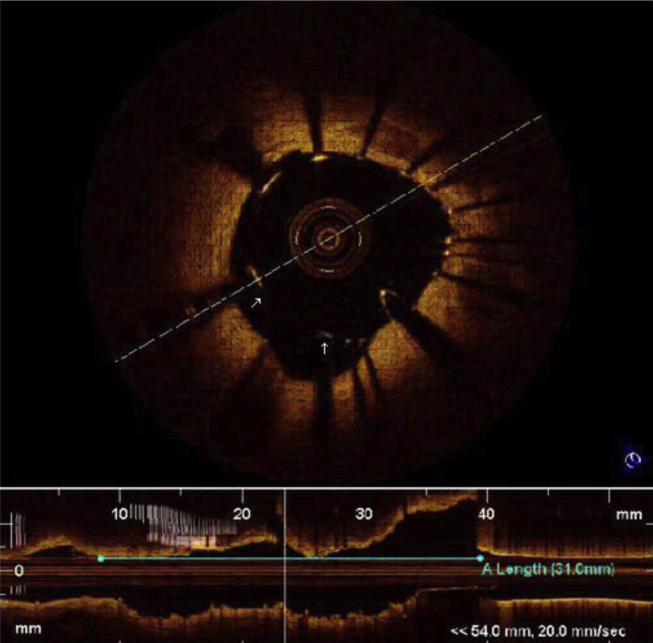
Quantitative analysis was performed to determine degree of stenosis. The presence of dissections at the stent edges, tissue prolapse through stent struts and zones of incomplete stent apposition were evaluated. A dissection flap is a linear rim of tissue with a clear separation from the vessel wall, plaque, or the stent struts (Fig 3).14, 15 Tissue prolapse was defined as protrusion of tissue through an imaginary arc connecting two adjacent struts without apparent surface disruption. It is defined as occurring if depth of protrusion is >50 μm (Fig 4).17
Fig. 3.
Edge dissection.
Fig. 4.
Tissue prolapse through stent strut.
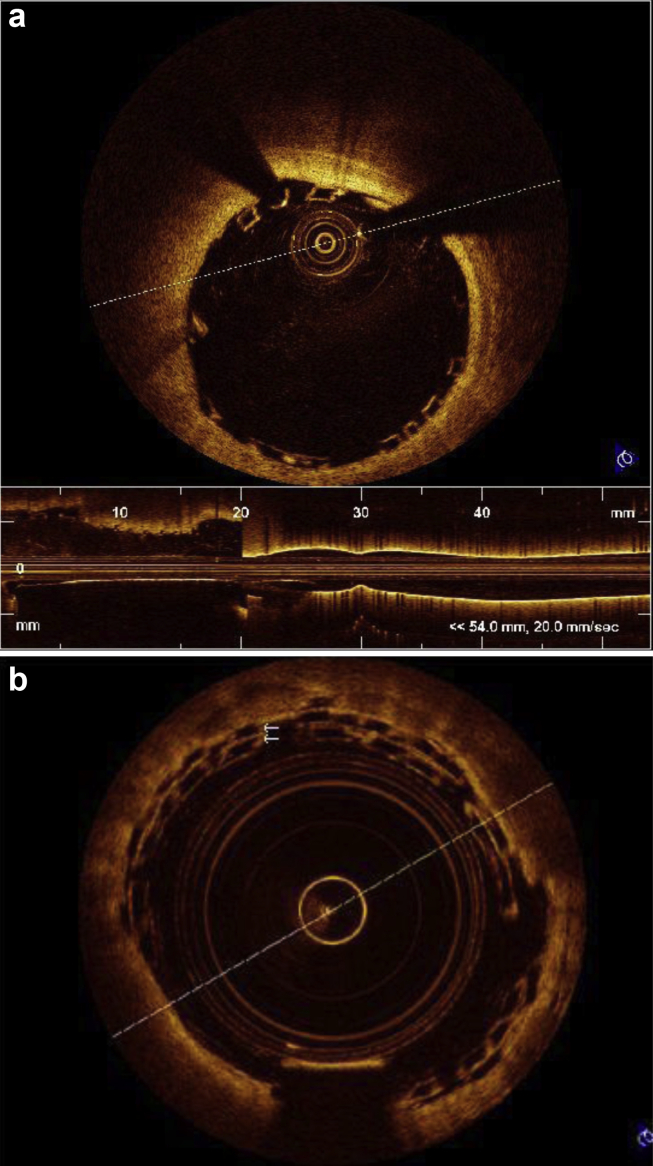
Incomplete stent apposition was defined as a measured distance greater than the strut thickness for bare metal stents or greater than the sum of the thickness of the strut plus polymer for drug eluting stents (Fig 5). OCT was also done in patients after implantation of bioabsorbable scaffold (Fig. 6a and b).
Fig. 5.
Stent malapposition.
Fig. 6.
(a) OCT picture of well apposed bioabsorbable stent. (b) Overlapping bioabsorbable stent.
3. Results
OCT was done in a total of forty-five patients who underwent successful PCI. Twenty-eight patients were male, mean age was 54 years, 24 patients were hypertensive, 27 patients were diabetics, and 13 were smokers (Table 1). PCI was performed to treat acute STEMI in 10 patients; whereas NSTEMI/UA was present in 27 patients. LAD lesion was imaged in 28 patients, LCX lesion was imaged in 9 patients, and RCA lesion was imaged in 8 patients. Baseline angiographic and procedural characteristic is given in Table 2. Stent malapposition was detected in 24% (11 patients) of intervention requiring further balloon dilatation. Thin cap fibroatheromas (TCFA) with a fibrous caps measuring between 0.02 and 0.06 mm, were detected in 3 patients (in the region of the stenosis in the 2 patients), although it was not considered as an indication of PCI. Some amount of tissue prolapse through the stent struts was observed in almost all the patients. In incomplete stent apposition, the maximum separation between the stent and the arterial wall ranged between 0.04 and 0.5 mm and it involved up to 180° of the vessel circumference. The anticoagulation used was same as that for PCI (70–100 IU of unfractionated heparin per kilogram body weight). In two patients IV Bivalirudin was used. No patient had thrombus formation around the OCT catheter. None of the patients developed angina, electrocardiographic changes, or complications during the procedure and during hospital stay. The OCT study prolonged the duration of the procedure in each patient by about 10–20 min (average of 15 min). In total, OCT was used for 70 times along with PCI. Our strategy or decision for reintervention was changed on 46 occasions out of 70 times when OCT was used (65%) (Table 3). In absolute number, strategy was changed in 28 (62%) out of 45 patients after OCT. Alteration of stent length was done in 14 patients (56% cases when evaluated pre PCI). Alteration of stent diameter was done in 9 patients (36% cases when evaluated pre PCI). Treatment of malapposition was done in 11 patients (24.4% of total cases). Further, balloon dilatation for vessel expansion in calcified lesion was done in 7 patients (15.5% of total cases). Edge dissection requiring further balloon dilatation or stenting was seen in 4 patients (8.8% of total cases).
Table 1.
Baseline clinical characteristics.
| Characteristics | Pre PCI and PCI OCT | Post PCI OCT |
|---|---|---|
| No. of patients | 25 | 20 |
| Age (years) | 54 ± 9 | 56 ± 10 |
| Men | 16 (64%) | 12 (60%) |
| Hypertension | 13 (52%) | 11 (55%) |
| DM | 15 (61%) | 12 (60%) |
| Dyslipidemia | 8 (32%) | 6 (30%) |
| Current smokers | 7 (28%) | 6 (30%) |
| Clinical Presentation | ||
| Stable angina | 14 (56%) | 5 (25%) |
| UA/NSTEMI | 7 (28%) | 4 (20%) |
| STEMI | 4 (16%) | 11 (55%) |
Table 2.
Baseline angiographic & procedural characteristics.
| Characteristics | Pre PCI and post PCI – OCT (n = 25) | Post PCI OCT (n = 20) |
|---|---|---|
| Lesion length (mm) | 23.8 ± 6.3 | 22.1 ± 6.7 |
| Stent diameter (mm) | 2.86 ± 0.28 | 2.90 ± 0.24 |
| Stent length (mm) | 25.9 ± 5.9 | 24.8 ± 6.7 |
| RVD (mm) | 2.68 ± 0.34 | 2.71 ± 0.33 |
| MLD (mm) | ||
| Pre-procedure | 0.81 ± 0.48 | 0.70 ± 0.53 |
| Post-procedure | 2.73 ± 0.39 | 2.60 ± 0.46 |
| Mean lumen CSA (mm 2) | 6.0 ± 1.5 | 6.3 ± 1.6 |
| Mean stent CSA (mm 2) | 7.4 ± 1.8 | 6.8 ± 1.5 |
| Presence of thrombi | 18 (24%) | 8 (22%) |
Table 3.
Changes in management strategy after OCT.
| Over all management changes | No. of patients |
|---|---|
| Treatment of malapposition | 11 (24.4% of total cases) |
| Further balloon dilatation for vessel expansion in calcified lesion | 7 (15.5% of total cases) |
| Further balloon dilatation/stenting for edge dissection | 4 (8.8% of total cases) |
| Alteration of stent length | 14 (56% cases when evaluated pre PCI) |
| Alteration of stent diameter | 9 (36% cases when evaluated pre PCI) |
| One case of left main stenting after detecting proximal edge dissection | |
| Total no of times OCT performed = 70 (pre & post PCI in 25pt, pre PCI only in 20pt) | |
| Total no of occasions decision was changed post OCT = 46 (65.5%) | |
One case of left main stenting was done after detecting proximal edge dissection on OCT. Small amount of tissue protrusion was not taken as criteria for reintervention.
4. Discussion
In this study we evaluated how OCT changes our strategy during stent implantation in the Indian milieu. This report shows that OCT can provide clinically useful and more importantly, clinically actionable information about sub optimal stent deployment despite satisfactory angiographic images after PCI. We found that OCT showed important residual pathology post PCI and such findings changed stent management strategies in a significant number of patients (62% of the 45 cases coming to PCI in our study). The main changes suggested after OCT were alteration of stent length (56%), stent diameter (36%) and treatment of stent malapposition (24%). OCT thus may have a useful role in PCI even in the Indian context. It should, however, be remembered that OCT use is still is in its infancy; while OCT with its superior resolution will show more abnormalities than other currently available imaging modalities, it is not certain at this time that fixing all these “OCT visualized” abnormalities will necessarily improve patient outcomes. Experience with IVUS and other imaging modalities show that the correlation between some abnormal findings seen on IVUS post PCI and subsequent clinical outcome is highly variable. While some studies have reported similar rates of adverse events in patients with and without acute incomplete stent apposition or plaque prolapse as assessed by IVUS,18, 19, 20, 21 others have demonstrated a high prevalence of abnormal findings in the IVUS study following stent implantation in patients who develop acute stent thrombosis.22, 23, 24, 25 OCT has distinct advantages in some areas. Given its high resolution, OCT is considered to be superior to IVUS for the assessment of the dissection, zones of incomplete stent apposition, tissue prolapse following PCI.11 Thrombus is poorly seen on IVUS,26 whereas it is well visualized by OCT. OCT is very helpful in assessing stent apposition with overlapping stents.27 OCT was extensively used in ABSORB trial to assess acute results of PCI as well as to determine the degree of absorption of bioabsorbable stent during follow up.28 Lesion characteristics such as lipid and calcium content can be defined prior to the intervention and these findings can be predictive of post-procedure myocardial infarction.29 OCT studies have shown the morphologic features of plaques that are potential predictors of subsequent progression and acute coronary syndromes (ACS).6, 30 Though OCT is potentially more accurate than IVUS at determining lesion severity, it still remains an anatomical imaging technique and as yet is unlikely to replace a physiological technique like FFR.31
Unlike with IVUS, there have been few prospective studies of clinical outcomes in patients undergoing OCT. In the absence of such data, there are currently no definite proven indications for the use of OCT in the clinical setting.14, 15 The potential clinical indications for OCT are similar to IVUS which include angiographically uncertain lesions, evaluation for allograft vasculopathy, lesion assessment pre PCI, and evaluation of stent deployment post PCI, evaluation of mechanism of in-stent restenosis and stent thrombosis. The results of CLI-OPCI study, which included 335 in the OCT group and 335 in the angiography group suggest that the use of OCT can improve clinical outcomes of patients undergoing PCI.32 Developed in the 1980s, intravascular ultrasound (IVUS) is currently the most commonly utilized methods for endovascular imaging. The clinical role for IVUS was established via both prospective and retrospective studies with specific guidelines for its use.33, 34 Advantages of IVUS is its penetration of 4–8 mm inside the vessel wall, unlike OCT which can only penetrate about 2–3 mm, better imaging of aorto-ostial lesions and no need of contrast. A study comparing IVUS and OCT found that, OCT guidance for stent implantation was associated with smaller stent expansion as compared to IVUS.35 On the other hand another study comparing IVUS and OCT suggests that FD OCT generates similar reference lumen dimensions, as well as better detection of malapposition, tissue prolapse and disease severity compared with IVUS.36 Ultimately, clinical outcome data will determine whether OCT will super-seed IVUS or just plain angiography and this is currently an open unresolved question. Novel hybrid IVUS and OCT imaging catheters are in the making and have synergistic advantages; preliminary ex vivo images using a hybrid IVUS-OCT catheter have demonstrated feasibility.37
5. Limitations
This study should be considered in the light of several limitations. It was a single center, case series kind of descriptive study with a limited number of patients. The study end points were subjective interpretations and given that OCT resolution is far superior to those of current methods, naturally there was no independent method to verify these findings. As there are no specific guidelines on indications of OCT, the use of OCT was left at operator's discretion and it might have been used in more challenging cases – this will limit its applicability in the more general population coming to PCI. Costs were not analyzed and it is not known if the use of OCT was cost effective. Long term follow up is lacking and it is not known if using OCT made a difference in long term outcomes. Large randomized controlled studies using OCT will be needed to prove its clinical efficacy.
6. Conclusion
OCT is changing the way we see coronary lesion pathophysiology. Our data, one of the first experiences in India, confirm that OCT allows for better visualization of lumen, plaque, thrombus, as well as stent apposition, & dissection post PCI. Presently we are at the beginning stage to establish how this imaging information might be best used to guide management and optimize the angiographic results. More extensive, long term studies will be needed to assess the clinical outcome of these interventions.
Conflicts of interest
All authors have none to declare.
References
- 1.Popma J.J., Bashore T.D. Qualitative and quantitative angiography. In: Topol E., editor. Textbook of Interventional Cardiology. WB Saunders; Philadelphia, Pa: 1994. pp. 1052–1068. [Google Scholar]
- 2.Ellis S.G., Vandormael M.G., Cowley M.J. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Circulation. 1990;82:1193–1202. doi: 10.1161/01.cir.82.4.1193. [DOI] [PubMed] [Google Scholar]
- 3.Kleiman N.S., Rodriguez A.R., Raizner A.E. Interobserver variability in grading of coronary arterial narrowings using the American College of Cardiology/American Heart Association grading criteria. Am J Cardiol. 1992;69:413–415. doi: 10.1016/0002-9149(92)90245-t. [DOI] [PubMed] [Google Scholar]
- 4.Guédès A., Tardif J.C. Intravascular ultrasound assessment of atherosclerosis. Curr Atheroscler Rep. 2004;6:219. doi: 10.1007/s11883-004-0035-4. [DOI] [PubMed] [Google Scholar]
- 5.Jang I.K., Bouma B.E., Kang D.H. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 6.Jang I.K., Tearney G.J., MacNeill B. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–1555. doi: 10.1161/01.CIR.0000159354.43778.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yabusita H., Bouma B.E., Houser S.L. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 8.Virmani R., Kolodgie F.D., Burke A.P., Farb A., Schwartz S.M. Lessons from sudden coronary death. A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 9.Giattina S.D., Courtney B.K., Herz P.R. Assessment of coronary plaque collagen with polarization sensitive optical coherence tomography (PS-OCT) Int J Cardiol. 2006;107:400–409. doi: 10.1016/j.ijcard.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Tearney G.J., Yabushita H., Houser S.L. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. doi: 10.1161/01.cir.0000044384.41037.43. [DOI] [PubMed] [Google Scholar]
- 11.Bouma B.E., Tearney G.J., Yabushita H. Evaluation of intracoronary stenting by intravascular optical coherence tomography. Heart. 2003;89:317–321. doi: 10.1136/heart.89.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Sandoval L.J., Bouma B.E., Tearney G.J., Jang I.K. Optical coherence tomography as a tool for percutaneous coronary Interventions. Catheter Cardiovasc Interv. 2005;65:492–496. doi: 10.1002/ccd.20340. [DOI] [PubMed] [Google Scholar]
- 13.Gerckens U., Lim V.Y., Grube E. Tomografía de coherencia óptica en la evaluación de las endoprótesis coronarias con capacidad de liberación de fármacos. Rev Esp Cardiol. 2005;58:1469. [PubMed] [Google Scholar]
- 14.Prati F., Regar E., Mintz G.S., Expert's OCT Review Document Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. doi: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 15.Prati F., Guagliumi G., Mintz G.S., Costa M., for the Expert's OCT Review Document Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33:2513–2522. doi: 10.1093/eurheartj/ehs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlis P., Schmitt J.M. Current and future developments in intracoronary optical coherence tomography imaging. EuroIntervention. 2009;4:529–533. doi: 10.4244/eijv4i4a89. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalo N., Serruys P.W., Okamura T. Optical coherence tomography assessment of the acute effects of stent implantation on the native vessel wall: a systematic quantitative approach. Heart. 2009;95:1913–1919. doi: 10.1136/hrt.2009.172072. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe K., Serruys P.W., Degertekin M. Incomplete stent apposition after implantation of paclitaxel-eluting stents or bare metal stents. Circulation. 2005;111:900–905. doi: 10.1161/01.CIR.0000155607.54922.16. [DOI] [PubMed] [Google Scholar]
- 19.Kimura M., Mintz G.S., Carlier S. Outcome after acute incomplete sirolimus stent apposition as assessed by serial intravascular ultrasound. Am J Cardiol. 2006;98:436–442. doi: 10.1016/j.amjcard.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 20.Hong M.K., Park S.W., Lee C.W. Long-term outcomes of minor plaque prolapsed within stents documented with intravascular ultrasound. Catheter Cardiovasc Interv. 2000;51:22–26. doi: 10.1002/1522-726x(200009)51:1<22::aid-ccd6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Futamatsu H., Sabate M., Angiolillo D.J. Characterization of plaque prolapse after drug-eluting stent implantation in diabetic patients. J Am Coll Cardiol. 2006;48:1139–1145. doi: 10.1016/j.jacc.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Uren N.G., Schwarzacher S.P., Metz J.A. Predictors and outcomes of stent thrombosis. Eur Heart J. 2002;23:124–132. doi: 10.1053/euhj.2001.2707. [DOI] [PubMed] [Google Scholar]
- 23.Alfonso F., Suárez A., Angiolillo D.J. Findings of intravascular ultrasound during acute stent thrombosis. Heart. 2004 December;90:1455–1459. doi: 10.1136/hrt.2003.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheneau E., Leborgne L., Mintz G.S. Predictors of subacute stent thrombosis: results of a systematic intravascular ultrasound study. Circulation. 2003 Jul 8;108:43–47. doi: 10.1161/01.CIR.0000078636.71728.40. [DOI] [PubMed] [Google Scholar]
- 25.Choi So-Yeon, Witzenbichler Bernhard, Maehara Akiko. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: a Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) substudy. Circ Cardiovasc Interv. 2011 Jun;4:239–247. doi: 10.1161/CIRCINTERVENTIONS.110.959791. [DOI] [PubMed] [Google Scholar]
- 26.Mintz G.S., Nissen S.E., Anderson W.D. ACC clinical expert consensus document on standards for the acquisition, measurement and reporting of intravascular ultrasound studies: a report of the American College of Cardiology Task Force on clinical expert consensus documents committee to develop a clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound. J Am Coll Cardiol. 2001 Apr;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 27.Tanigawa J., Barlis P., Dimopoulos K., Di Mario C. Optical coherence tomography to assess malapposition in overlapping drug-eluting stents. EuroIntervention. 2008;3:580–583. doi: 10.4244/eijv3i5a104. [DOI] [PubMed] [Google Scholar]
- 28.Onuma Y., Serruys P.W., Ormiston J.A. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention. 2010;6:447–453. doi: 10.4244/EIJ30V6I4A76. [DOI] [PubMed] [Google Scholar]
- 29.Yonetsu T., Kakuta T., Lee T. Impact of plaque morphology on creatine kinase-MB elevation in patients with elective stent implantation. Int J Cardiol. 2011;146:80–85. doi: 10.1016/j.ijcard.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Uemura Shiro, Ishigami Ken-ichi, Soeda Tsunenari. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J. 2012;33:78–85. doi: 10.1093/eurheartj/ehr284. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalo N., Escaned J., Alfonso F. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J Am Coll Cardiol. 2012;59:1080–1089. doi: 10.1016/j.jacc.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 32.Prati F., Di Vito L., Biondi-Zoccai G. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012 Nov 22;8:823–829. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 33.McDaniel M.C., Eshtehardi P., Sawaya F.J., Douglas J.S., Jr., Samady H. Contemporary clinical applications of coronary intravascular ultrasound. JACC Cardiovasc Interv. 2011;4:1155–1167. doi: 10.1016/j.jcin.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Levine G.N., Bates E.R., Blankenship J.C. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol. 2011 Dec 6;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Habara Maoto, Nasu Kenya, Terashima Mitsuyasu, Kaneda Hideaki, Yokota Daisuke. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv. 2012;5:193–201. doi: 10.1161/CIRCINTERVENTIONS.111.965111. [DOI] [PubMed] [Google Scholar]
- 36.Bezerra Hiram G., Attizzani Guilherme F., Sirbu Vasile. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. JACC: Cardiovasc Interv. March 2013;6:228–236. doi: 10.1016/j.jcin.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Li Brian H., Leung Annie S.O., Soong Alan. Hybrid intravascular ultrasound and optical coherence tomography catheter for imaging of coronary atherosclerosis. Catheter Cardiovasc Interv. February 2013;81:494–507. doi: 10.1002/ccd.24295. [DOI] [PubMed] [Google Scholar]