Abstract
Previous studies have reported that regular practice of a device-guided slow breathing (DGB) exercise decreases resting blood pressure (BP) in hypertensive patients. The performance of DGB is associated with acute decreases in sympathetic vascular tone, and it has been suggested that the decreases in resting BP produced by regular practice of DGB over periods of weeks is due to chronic decreases in sympathetic nervous system activity. However, the kidneys respond to sympathetically-mediated changes in BP by readjusting blood volume levels within a few days. Thus, the mechanism by which DGB could produce long-term BP changes remains to be clarified. Previous research with laboratory animals and human subjects has shown that slow, shallow breathing that increases pCO2 potentiates blood pressure sensitivity to high sodium intake. These findings raise the possibility that deeper breathing during DGB that decreases BP might involve opposite changes in pCO2. The present study tested the hypothesis that performance of DGB acutely decreases a marker of pCO2, end tidal CO2 (PetCO2). Breathing rate, tidal volume, and PetCO2 were monitored before, during, and after a 15 min session of DGB by patients with elevated blood pressure. Blood pressure, heart rate, and heart rate variability (HRV) were also measured under these conditions. A control group was also studied before, during, and after a 15 min session of spontaneous breathing (SB). The DGB group, but not the SB group, showed progressive and substantial increases in tidal volume and low frequency HRV, and decreases in PetCO2 and systolic blood pressure. The PetCO2 effects persisted into the post-task, rest period. The findings are consistent with the hypothesis that habitual changes in breathing patterns of the kind observed during DGB could potentiate an antihypertensive adaptation via effects on pCO2 and its role in cardiovascular homeostasis.
Keywords: Breathing, blood pressure, end tidal CO2, respiration, tidal volume
INTRODUCTION
Numerous studies have reported that a behavioral intervention for hypertensive patients characterized by repeated practice of a device-guided slow breathing exercise (DGB) lowers resting blood pressure (BP) over periods of weeks (reviewed in Elliott & Izzo, 2006). In each of these studies, the device employed enabled subjects to synchronize their breathing pattern with a series of alternating musical tones presented over earphones during 15 min performance sessions. As well as decreasing rate progressively, DGB elongates the expiration component of the breathing cycle. Since sympathetic nervous system activity is known to increase acutely during inspiration and decrease during exhalation, it has been suggested that acute decreases in sympathetically-mediated vascular tone observed during DGB (Parati, Izzo & Gavish, 2008) participate in the long-term changes in resting BP that occur with repeated practice of DGB.
The sympathetic nervous system regulates regional distribution of blood flow at all levels of BP, but long-term control of BP is still fundamentally vested in the kidneys (Mayer, 2008; Osborn, et al 2009). For example, when resting BP level is perturbed by adrenergic stimulation, reciprocal changes in renal excretion of sodium and fluid volume gradually restore BP to preset levels. Thus, sustained impairment of the ability of the kidneys to autoregulate BP is necessary to produce chronic hypertension. This was first shown in the pioneering studies of Coleman and Guyton, (1969) who found that surgical reduction of renal mass potentiated a canine model of hypertension in response to high salt intake over periods of days. Subsequently, Anderson and colleagues found that impairment of renal function by behavioral conditioning procedures also potentiated a sodium-sensitive form of experimental hypertension (Anderson, 1994). It had been expected that the behavioral stress increased renal sympathetic activity. However, neither adrenergic antagonists nor bilateral renal denervation prevented the experimental hypertension. Instead, the behavioral stress suppressed respiration and increased pCO2, decreased plasma pH and increased renal sodium retention (Anderson, 1994; Anderson et al 1996). These findings suggested that the inhibited breathing pattern participated in the hypertensive adaptation, either directly via changes in acid-base balance on renal sodium regulation, or indirectly, via the stimulation of one or more circulating compounds that influence renal excretory activity (Bagrov et al 2009).
That paced slow breathing decreases resting BP of hypertensive patients initially seems difficult to reconcile with findings that behavioral inhibition of breathing can potentiate hypertension. However, an important difference is in the accompanying changes in tidal volume, which do not change during behavioral stress, but increase substantially during guided slow breathing. The implication of these differences is for blood gas regulation, which could affect cardiovascular regulation via a number of factors including, but not limited to, chemoreceptor reflexes.
The present study hypothesized that 15 min of DGB results in progressive decreases in a marker for pCO2, end tidal CO2 (PetCO2)in patients with elevated BP. Breathing rate, tidal volume, minute ventilation, blood pressure, and heart rate, and heart rate variability (HRV), as well as PetCO2, were recorded in a series of patients before, during, and after 15 min sessions of DGB. In addition, these measures were also recorded in a control group before, during, and after sessions of spontaneous breathing (SB). It was hypothesized that DGB would be associated with decreases in PetCO2.
METHODS
Participants
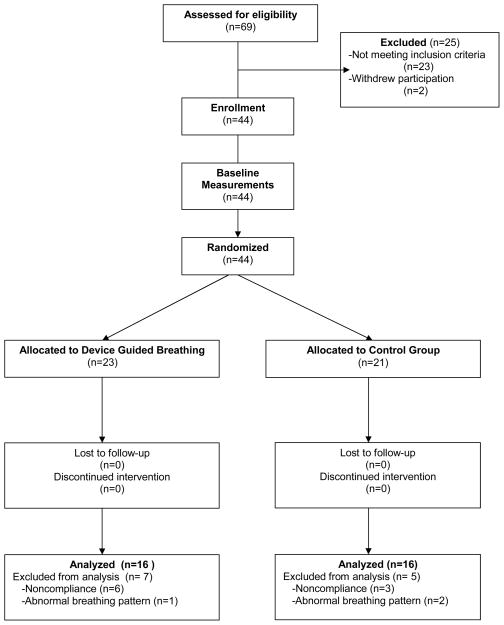
One hundred and two men and women (ages 40–70) from the surrounding community responded to local advertising, and were screened for participation via telephone. Sixty-nine potential participants visited the National Institute on Aging Clinical Research Branch at Harbor Hospital, where the purpose of the study was explained, and informed consent obtained. A physical examination was performed, and blood and urine samples collected to ensure that the participants met the study entry criteria. Exclusion criteria included cardiac, pulmonary, or kidney disease, diabetes, tobacco use, steroids, hormone replacement therapy, or antihypertensive medications with central nervous system effects. Eligibility for study inclusion was made on the basis of systolic blood pressure levels between 130 and 160 mmHg during clinic and 24-hr ambulatory monitoring. Forty-four subjects qualified and completed the study, including 18 men and 26 women, 27 European Americans and 17 African Americans. A CONSORT flow diagram showing the ultimate disposition of all subjects is provided in Figure 1.
Figure 1.
Flow diagram of disposition of all subjects who participated in the study.
Experimental design
Twenty-three subjects were randomly assigned to an experimental group that received training in DGB, and 21 subjects were assigned to the control SB condition. Twelve subjects were excluded from data analysis due to either non-compliance with the intervention or abnormal breathing pattern during baseline sessions. Table 1 describes mean and standard errors of the biometric and physiological characteristics of each group at study entry. Those assigned to the DGB group were instructed to sit comfortably with eyes closed, and arms and legs uncrossed. Individual breathing rate was initially determined from an expandable band around the torso connected to a commercially available device (RESPeRATE™, Lod, Israel) that presented distinctive tones via earphones. Each subject was instructed to inspire during ascending tones and expire during descending tones. Following two training sessions, respiration, blood pressure, and heart rate were monitored during a 10 min rest period, a 15 min guided-breathing task period, and a 10 minute recovery period during an experimental session on a subsequent day.
Table 1.
Means and standard errors of demographic characteristics and physiological measures at baseline for guided breathing and spontaneous breathing groups.
| Guided Breathing (n=16) | Spontaneous Breathing (n=16) | |
|---|---|---|
| Age (yr) | 51.9± 2.9 | 54.5± 2.0 |
| Body weight (kg) | 86.0±4.7 | 84.8± 2.8 |
| BMI (kg/m2) | 29.5±1.4 | 29.8±1.4 |
| Systolic BP (mmHg) | 144.6 ± 2.6 | 143.1 ± 2.5 |
| Diastolic BP (mmHg) | 88.9 ± 1.9 | 87.0 ± 2.0 |
| Heart Rate (bpm) | 69.5 ± 2.5 | 65.2 ± 2.6 |
| Breathing rate (breaths/min) | 14.5 ± 1.0 | 14.1 ± 1.0 |
| Tidal volume (ml) | 444.7 ± 35.2 | 409.5 ± 42.4 |
| Minute ventilation (L/min) | 6.0 ± 0.3 | 5.5 ± 0.5 |
| End tidal CO2 (mmHg) | 39.8 ± 1.1 | 37.9 ± 0.7 |
Participants in the control group were instructed to sit in the same manner, passively attend to their breathing, and silently repeat “one” during each exhalation (Benson & Klippner, 1981). If other thoughts came to mind, they were instructed to calmly refocus attention on breathing. Following two sessions of practice of SB, respiration, blood pressure, and heart rate of each subject in the control group were also monitored during a 10 min baseline, 15 min SB, and 10 min recovery.
The protocol was approved by the Institutional Review Board of the Medstar Research Institute.
Physiological recording
Breathing rate, tidal volume and, minute ventilation were monitored continuously in each participant from an elasticized vest that detected breath-to-breath expansion of the chest and abdomen via inductive plethysmography (Lifeshirt, Vivometrics, Ventura, CA)(Wilhelm et al 2005). The breathing data were recorded on a flash card in a microprocessor connected to the vest, and relayed to a computer (Dell, Model 740, Round Rock TX). Tidal volume was calibrated before each session by exhaling a fixed volume of air into an inflatable bag. Heart rate was monitored from electrocardiographic electrodes, connected to a multichannel ambulatory microcomputer which contained a flash card for data storage and transfer.
PetCO2 was monitored continuously using a respiratory gas monitor (Datex-Ohmeda, Model 5250, Fairfield, CT) via a nasal cannula. Successive 10-sec interval measurements of PetCO2 were recorded in the computer.
Blood pressure was recorded every six minutes from an inflatable arm cuff connected to an automated oscillometric device (Spacelabs, Model 90207, Redmond, WA).
Data Analysis
The significance of the mean differences in biometric and physiological measures preceding the experiment was determined by two-tailed t-tests.
Repeated measures one way analysis of variance was used to assess the significance of the differences in respiratory, blood pressure, and heart rate measures during the last five min of the baseline, DGB or SB, and post-performance periods for each group, followed by Newman Keuls multiple comparison tests.
Heart rate variability (HRV) was determined from successive interbeat intervals downloaded into Vivologic software (Vivometrics, Ventura, CA), and visually inspected for statistical outliers (i.e. two standard deviations above the session mean). The data were imported into Kubios HRV Analysis software (MATLAB, version 2 beta, Kuopio, Finland). A piecewise cubic spline interpolation method was applied before calculation of the frequency domain measures. HRV was determined for low frequency (LF range: 0.04–0.15 Hz) and high frequency (HF range: 0.15–0.40 Hz) components calculated by parametric autoregressive modeling (Oppenheim & Shafer, 1975). Analysis of variance was performed for data during the last five minutes of baseline, last five minutes of DGB or SB, and last five minutes following the performance period. Multiple comparisons were performed using Newman-Keuls methodology. All data were analyzed via GraphPad Prism, Version 5 (GraphPad Software, Inc., La Jolla, CA).
RESULTS
Subject cohort demographic data
Table 1 shows no significant differences in any pre-experimental biometric or physiological measure between the DGB and SB groups preceding the experiments.
DGB and SB effects on breathing rate and tidal volume
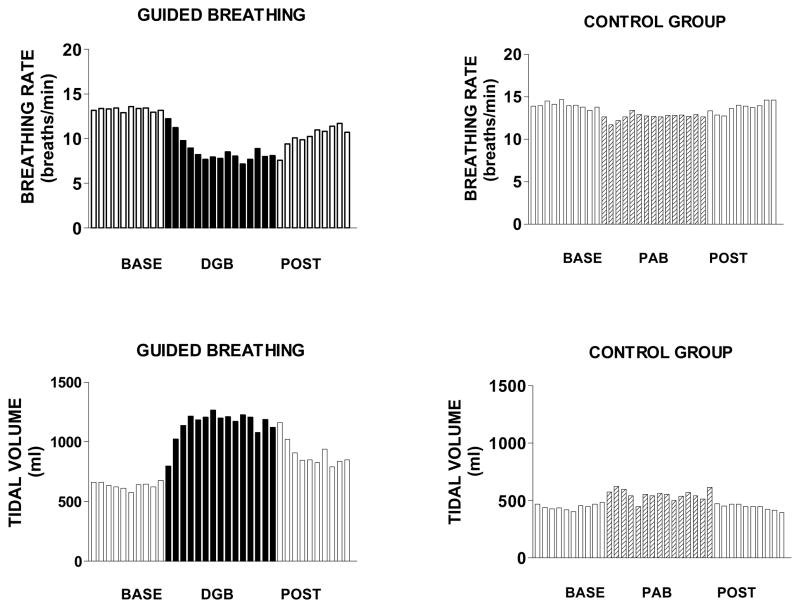
Figure 2 and Table 2 show that breathing rate of DGB subjects decreased progressively during the task (F2,51 = 7.70;p<.001), while tidal volume increased progressively (F2,51 = 5.54; p <.01). Breathing rate and tidal volume levels following DGB were intermediate between those during baseline and DGB. By contrast, Figure 1 and Table 2 show that SB by the control group had no significant effects on breathing rate (F2.51 =0.45; p <.05) or tidal volume (F2,51 = 1.49; p >.05).
Figure 2.
Mean breathing rate and tidal volume over successive one min intervals of a 10 min rest period (base), 15 min breathing exercise, and 10 minute recovery period (post) in 16 subjects in the device guided slow breathing group (DGB) and 16 subjects in the spontaneous breathing control group (SB).
Table 2.
Means and standard errors of breathing rate (breaths/min), tidal volume (L), minute ventilation (L/min), and end tidal CO2 (mmHg) over successive five minute intervals before, during, and after guided breathing (n= 16) and spontaneous breathing (n =16).
| TIME (min) | |||||||
|---|---|---|---|---|---|---|---|
| BEFORE | GUIDED BREATHING | AFTER | |||||
| Breathing rate | 13.2±4.8 | 13.3±4.8 | 10.1±3.2 | 8.0±2.6 | 8.0±3.1* | 9.4±3.6 | 11.1±4.3 |
| Tidal Volume | 0.6±0.4 | 0.6±0.3 | 1.1±0.5 | 1.2±0.5 | 1.1±0.6* | 1.0±0.4 | 0.8±0.5 |
| Minute Ventilation | 7.0±2.6 | 7.1±2.5 | 9.0±4.2 | 8.6±2.9 | 7.8±2.9 | 7.6±3.0 | 7.4±2.9 |
| End tidal CO2 | 36.2±4.2 | 36.2±3.9 | 32.1±4.4 | 29.1±5.0 | 29.3±5.6* | 30.1±5.6 | 30.8±5.8 |
| BEFORE | CONTROL GROUP | AFTER | |||||
| Breathing rate | 14.5±4.4 | 14.1±4.9 | 12.6±4.4 | 12.7±4.9 | 12.9±5.0 | 13.7±4.7 | 14.3±4.5 |
| Tidal Volume | 0.4±0.1 | 0.4±0.2 | 0.5±0.4 | 0.5±0.3 | 0.5±0.3 | 0.4±0.2 | 0.4±0.1 |
| Minute Ventilation | 5.6±1.9 | 5.6±1.8 | 5.7±2.4 | 5.7±2.5 | 5.8±2.5 | 5.6±1.8 | 5.5±1.9 |
| End tidal CO2 | 36.6±2.5 | 37.2±2.8 | 36.3±3.9 | 36.8±4.5 | 36.9±4.7 | 36.5±3.3 | 37.2±3.0 |
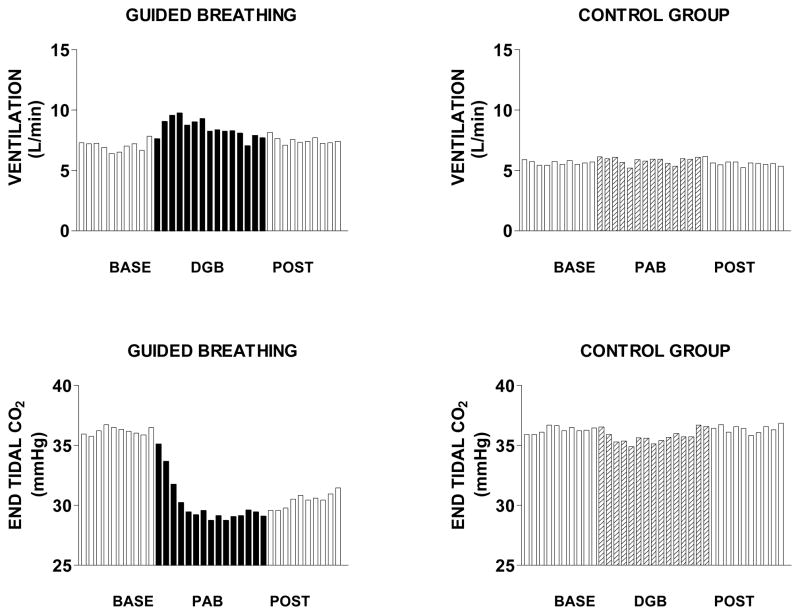
DGB and SB effects on minute ventilation and PetCO2
Figure 3 and Table 2 show that minute ventilation during the last five min of DGB was not significantly different from baseline (F2,51 = 0.35; p>.05). However, PetCO2 decreased progressively during DGB, and remained significantly below baseline levels following DGB (F2,51 =8.89; p <.001). Figure 3 and Table 2 show that SB had no significant effects on either minute ventilation (F2.51 =0.11; p <.05) or PetCO2 (F2,51 = 0.05; p >.05).
Figure 3.
Mean minute ventilation and end tidal CO2 over successive one min intervals of a 10 min rest period (base), 15 min breathing exercise, and 10 minute recovery period (post) in 16 subjects in the device guided slow breathing group (DGB) and 16 subjects in the spontaneous breathing control group (SB).
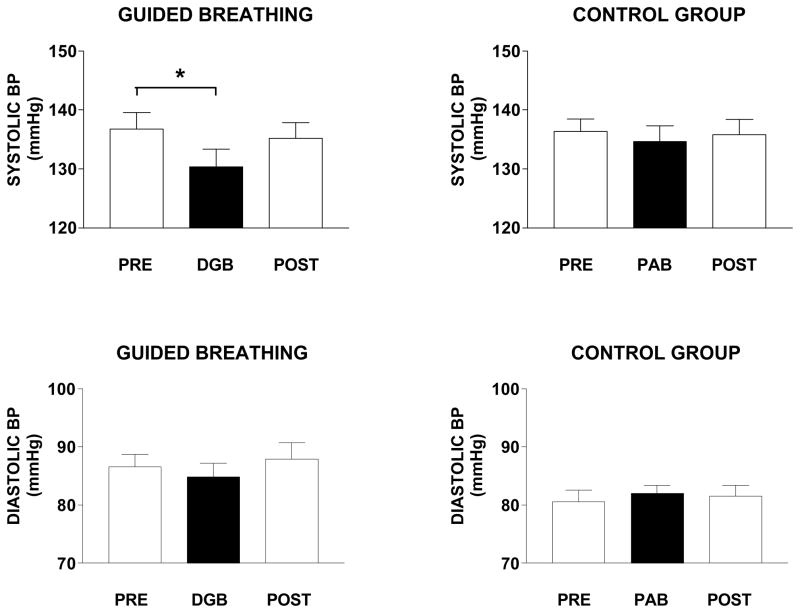
DGB and SB effects on systolic and diastolic blood pressure
Figure 4 shows that systolic BP was 6.4 ± 1.8 mmHg lower during DGB than during baseline (F2,15 =2.86; p <.05), was intermediate between baseline and DGB following DGB. No such effects were observed for systolic BP during SB by the control group (F2,15 =0.79; p >.05). Diastolic BP was not significantly different from baseline during either DGB (F2,15 =1.04; p >.05) or SB (F2,15 =0.88; p >.05) and remained unchanged following DGB or SB.
Figure 4.
Mean and standard errors of systolic and diastolic blood pressure during a 10 min rest period (base), 15 min breathing exercise, and 10 minute recovery period (post) in 16 subjects in the device guided slow breathing group (DGB) and 16 subjects in the spontaneous breathing control group (SB).
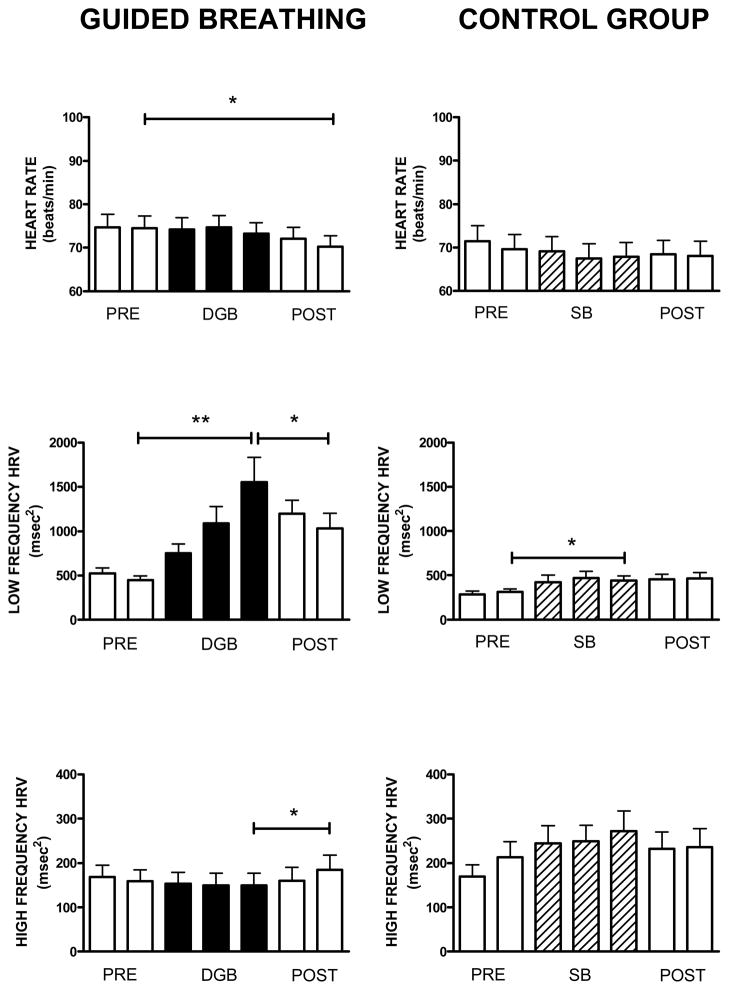
DGB and SB effects on heart rate and HRV
Figure 5 shows changes in heart rate (HR), and the LF and HF components of HRV over successive 5-min intervals of baseline, DGB and SB, and following DGB and SB. No significant changes in HR were observed during DGB compared with baseline, but HR did decrease following DGB (F6,15 = 4.56; p <.001). For the control group, HR showed no significant changes across conditions (F6,15 = 1.66; p >.201).
Figure 5.
Mean and standard errors of heart rate and low frequency and high frequency heart rate variability over successive five min intervals of a 10 min rest period (base), 15 min breathing exercise, and 10 minute recovery period (post) in 16 subjects in the device guided slow breathing group (DGB) and 16 subjects in the spontaneous breathing control group (SB).
LF HRV increased during DGB, but then decreased following DGB, though not to baseline levels (F6,15 = 9.13; p <.001). LF HRV was not significantly increased by SB, but increased slightly following SB (F6,15 = 5.82; p <.008).
HF HRV did not change significantly during DGB, but did increase following DGB (F6,15 = 4.26; p <.05). HF HRV did not change significantly during SB, and remained unchanged following SB (F6,15 = 2.97; p >.067).
DISCUSSION
The primary finding of the present study was that PetCO2 decreased progressively and substantially during a 15-min session of DGB, because concurrent increases in tidal volume more than compensated for the decreases in breathing rate. No such respiratory changes were observed during SB by the control group. Since PetCO2 co-varies with pCO2, it can be concluded that DGB also decreased pCO2. These results are in contrast to those of two previous studies of paced slow breathing that did not report any significant effects on PetCO2. In one, however, breathing was slowed for only two minutes (Joseph et al., 2005), while in the other, subjects were studied in the supine posture (Parati et al., 2002). The present findings are in accord with those in a third study in which breathing was paced at 15/min, which also found increases in tidal volume and decreases in pCO2 (Pinna, Maestri, LaRovere, Gobbi, & Fanfulla, 2005). In the present study, the instructions were to keep the lungs moving during the breathing exercise, to prevent breath pauses between inhalation and exhalation (as had been observed in some subjects during preliminary testing). The extent to which this pattern of continuous lung inflation and deflation during DGB is important for long-term BP regulation remains to be investigated.
A second finding in the present study was that systolic BP decreased acutely during DGB, but not during SB. Acute changes in BP during DGB have been examined in only a few previous studies. In one, DGB by supine normotensive subjects was accompanied by no changes in systolic or diastolic BP (Parati et al., 2002). In another, slow breathing by hypertensive subjects was associated with decreases in both systolic and diastolic BP, but paced breathing at normal rates (15/min) resulted in BP decreases of comparable magnitude (Joseph et al., 2005). The variability in results between studies may reflect differences in familiarity with the testing situation, duration of baseline observations before DGB, or other details of the experimental procedure that can affect baseline BP.
The third finding of this study, that of increased HRV during DGB, is consistent with the results of previous studies of the effects of slowed breathing (e.g. Pinheiro, Medeiros, Pinheiro & Marinho, 2007; Kulur, Haleagrahara, Adhikary, Jeganathan, 2009). Enhancement of the LF component of HRV has been observed during yoga and other forms of meditation (Peng et al., 1999; Cysarz & Bussing, 2005). Increases in LF HRV in the absence of increases in HF HRV are consistent with decreases in sympathetic influence on the heart (Valentini & Parati, 2009). The lack of changes in LF HRV during SB in the present study compared to others of meditation might reflect differences in performance between experienced and inexperienced subjects.
It has been suggested elsewhere that slow breathing may be effective as an antihypertensive intervention because of a synchronization of respiratory and cardiovascular rhythms that can occur under these conditions (Bernardi et al., 2001). Mayer waves are oscillations in BP and heart rate that have about a 10-second frequency, and are thought to be generated by a central nervous system oscillator or the effects of baroreceptor reflexes (Taylor & Eckberg, 1996). Shifts in Mayer wave frequency have been reported during the development of hypertension (Takalo, Korhonen, Majahhalme, Tuomisto, Turjanmaa,, 1999), and slow breathing procedures have been shown to change baroreceptor and chemoreceptor reflex activity (Spicuzza, Gabutti, Porta, Montano, & Bernardi, 2000; Bernardi, Porta, Spicuzza, & Sleight, 2005; Joseph et al., 2005). However, the role of such Mayer waves in the development or reversal of chronic hypertension remains to be clarified.
The interest in pCO2 that generated the present study stems from previous studies with laboratory animals which found that chronically-slowed breathing during intermittent behavioral stress elevated pCO2, and potentiated an increased sensitivity of BP to high sodium intake (Anderson, 1994). This form of experimental hypertension was accompanied by renal sodium retention that was not prevented by renal denervation. Thus, the increases in pCO2 in the animal studies were a marker for a process that affected renal sodium regulation and could have reset the set point around BP varies. That sustained hypercapnic breathing compromises renal sodium excretion while it augments renal acid excretion is a well established phenomenon that has been documented in previous studies with humans (Gennari, Goldstein, & Schwartz, 1972; Gledhill, Beirne, & Dempsey, 1975). We did not measure renal sodium excretion in the present study, in part because the 15 min DGB interval would have been too brief to observe significant effects. However, future research should be directed at possible changes in renal sodium regulation in hypertensive patients as a consequence of regular practice of DGB that decreases BP.
Effects of DGB on BP might be mediated, not only via effects on autonomic nervous system activity and renal sodium regulation, but also via effects on circulating vasoconstrictor factors, the so-called endogenous digitalis-like factors (EDLF). For example, sustained voluntary hypercapnic breathing has been shown to increased plasma concentrations of EDLF in human subjects (Bagrov, Fedorova, Austin-Lane, & Anderson, 1995). EDLF is known to increase in humans in response to a high sodium diet, but urinary excretion of EDLF was less in salt-sensitive than salt-insensitive humans (Anderson et al 2008). Additional studies are needed to determine whether DGB has effects on either plasma concentrations of or urinary excretion of EDLF.
There are a few limitations to this study. One concerns the characteristics of the subject sample, which included only individuals with resting systolic BP between 130 and 160 mmHg. The effects of DGB on PetCO2, BP, and HRV observed in this study might be different in others with lower or higher resting BP. A second limitation was the previous experience of the subjects with the breathing exercises. It remains to be determined whether subjects with extensive experience on the breathing exercises would have shown the same responses as the present group who had limited (but more than no) experience. A third concerns the fact that no measure of arterial O2 saturation was included. We did not have access to this measure in the present study, but previous research has shown that it tends to increase slightly when PetCO2 decreases (Anderson et al 1993). Finally, interpretation of the HRV data is complicated by the lack of consensus regarding the relationships of changes in LF HRV and HF HRV to changes in autonomic activity (Grossman & Taylor, 2007).
In summary, this study found that the performance of DGB decreased PetCO2, because tidal volume increased more than breathing rate slowed. The results suggest that the effects of breathing exercises on BP of hypertensive patients might be mediated by more than the effects of pulmonary reflexes on vascular tone. Specifically, it remains to future research to investigate the extent to which changes in PetCO2 during DGB are accompanied by changes in acid-base balance and renal regulation of sodium. From such studies might emerge increased understanding of the importance of respiratory behavior in the development and reversal of some forms of high blood pressure.
Acknowledgments
The authors wish to thank Deborah Grady for calling our attention to the value of the RESPeRATE in cardiovascular research, to Margaret Chesney for a careful reading of the manuscript, and to Benjamin Gavish for his advice and encouragement in all phases of the study.
Sole source of support: National Institute on Aging (NIH) Intramural Research Program.
References
- Anderson DE. Behavior analysis and the search for the origins of hypertension. Journal of the Experimental Analysis of Behavior. 1994;61:255–261. doi: 10.1901/jeab.1994.61-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DE, Austin J, Coyle K. Hemodynamic and metabolic effects of inhibitory breathing. Homeostasis. 1993;34:328–337. [Google Scholar]
- Anderson DE, Fedorova OV, French AW. Preavoidance hypercapnia and decreased hematocrit in micropigs. Physiology and Behavior. 1996;59:857–861. doi: 10.1016/0031-9384(95)02165-5. [DOI] [PubMed] [Google Scholar]
- Anderson DE, Parsons BA, McNeely JD, Miller ER. Salt sensitivity of blood pressure is accompanied by slow respiratory rate: Results of a clinical feeding study. Journal of the American Society of Hypertension. 2007;1:256–263. doi: 10.1016/j.jash.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DE, Fedorova OV, Morrell CH, Longo DL, Kashkin VA, Metzler JD, Bagrov AY, Lakatta EG. Endogenous sodium pump inhibitors and age-associated increases in salt sensitivity of blood pressure in normotensives. American Journal of Physiology: Regulative, Integrative, and Comparative Physiology. 2008;294:R1248–1254. doi: 10.1152/ajpregu.00782.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrov AY, Fedorova OV, Austin-Lane J, Anderson DE. Endogenous marinobufagenin-like immunoreactive factors and Na+, K+,- ATPase inhibition during voluntary hypoventilation. Hypertension. 1995;26:781–788. doi: 10.1161/01.hyp.26.5.781. [DOI] [PubMed] [Google Scholar]
- Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacological Reviews. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson H, Klippner MZ. The Relaxation Response. New York, New York: Wings Books; 1992. [Google Scholar]
- Bernardi L, Sleight P, Bandinelli G, Cencetti S, Fattorini L, Wdowczyc-Szulc J, Lagi A. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. British Medical Journal. 2001;323:1446–1449. doi: 10.1136/bmj.323.7327.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L, Porta C, Spicuzza L, Sleight P. Cardiorespiratory interactions to external stimuli. Archives italiennes de biologie. 2005;143:215–221. [PubMed] [Google Scholar]
- Coleman TG, Guyton AC. Hypertension caused by salt loading in the dog. III. Onset transients of cardiac output and other circulatory variables. Circulation Research. 1969;25:153–160. doi: 10.1161/01.res.25.2.153. [DOI] [PubMed] [Google Scholar]
- Cysarz D, Bussing A. Cardiorespiratory synchronization during Zen meditation. European Journal of Applied Physiology. 2005;95:88–95. doi: 10.1007/s00421-005-1379-3. [DOI] [PubMed] [Google Scholar]
- Ellliott WJ, Izzo JL. Device-guided breathing to lower blood pressure: case report and clinical overview. Medscape General Medicine. 2006;8:23. [PMC free article] [PubMed] [Google Scholar]
- Gennari FJ, Goldstein MB, Schwartz WB. The nature of the renal adaptation to chronic hypocapnia. Journal of Clinical Investigation. 1972;51:722–1730. doi: 10.1172/JCI106973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill N, Beirne GJ, Dempsey JA. Renal response to short-term hypocapnia in man. Kidney International. 1975;8:376–384. doi: 10.1038/ki.1975.130. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution, and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, Bernardi L. Slow breathing improves barorefex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- Kulur AB, Haleagrahara N, Adhikary P, Jeganathan PS. Effect of diaphragmatic breathing on heart rate variability in ischemic heart disease with diabetes. Arq Bras Cardiol. 2009;92(6):440–447. doi: 10.1590/s0066-782x2009000600008. [DOI] [PubMed] [Google Scholar]
- Mayer G. An update on the relationship between the kidney, salt, and hypertension. Wiener medizinische Wochenschrift. 2008;158:365–369. doi: 10.1007/s10354-008-0559-2. [DOI] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW. Digital Signal Processing. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1975. [Google Scholar]
- Osborn JW, Averina VA, Fink GD. Current computational models do not reveal the importance of the autonomic nervous system in long term control of arterial pressure. Experimental Physiology. 2009;94:389–396. doi: 10.1113/expphysiol.2008.043281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Fabio G, Ongaro G, Maronati A, Castiglioni P, Gavish B, Di Rienzo M, Mancia G. Acute effects of device-guided breathing on cardiovascular parameters and baroreflex sensitivity in normal subjects. American Journal of Hypertension. 2002;15:182A. [Google Scholar]
- Parati G, Izzo J, Gavish B. Respiration and Blood Pressure. In: Izzo JL, Sica D, Black HR, editors. Hypertension Primer. Dallas, Texas: American Heart Association; 2008. pp. 136–138. [Google Scholar]
- Peng CK, Mietus JE, Liy Y, Khalsa G, Douglas PS, Benson H, Goldberger AL. Exaggerated heart rate oscillations during two meditation techniques. International Journal of Cardiology. 1999;70:101–107. doi: 10.1016/s0167-5273(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Pinheiro CH, Medeiros RA, Pinheiro DG, Marinho MJ. Spontaneous respiratory modulation improves cardiovascular control in essential hypertension. Arquivos brasileiros de cardiologia. 2007;88:651–659. doi: 10.1590/s0066-782x2007000600005. [DOI] [PubMed] [Google Scholar]
- Pinna GD, Maestri R, LaRovere MT, Gobbi E, Fanfulla F. Effect of paced breathing on ventilatory and cardiovascular variability parameters during short-term investigations of autonomic function. American Journal of Physiology Heart and Circulatory Physiology. 2006;290:H424–H433. doi: 10.1152/ajpheart.00438.2005. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Gabutti A, Porta C, Montano N, Bernardi L. Yoga and chemoreflex response to hypoxia and hypercapnia. Lancet. 2000;356:1495–1496. doi: 10.1016/S0140-6736(00)02881-6. [DOI] [PubMed] [Google Scholar]
- Takalo R, Korhonen I, Majahhalme S, Tuomisto M, Turjanmaa V. Circadial profile of low-frequency oscillations in blood pressure and heart rate in hypertension. American Journal of Hypertension. 1999;12:874–881. doi: 10.1016/s0895-7061(99)00069-2. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Eckberg DL. Fundamental relations between short-term RR interval and arterial pressure oscillations in humans. Circulation. 1996;93:1527–1532. doi: 10.1161/01.cir.93.8.1527. [DOI] [PubMed] [Google Scholar]
- Valentini M, Parati G. Variables influencing heart rate. Progress in cardiovascular diseases. 2009;52:11–19. doi: 10.1016/j.pcad.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT, Sackner MA. The LifeShirt. An advanced system for ambulatory measurement of respiratory and cardiac function. Behavioral Modification. 2003;27:671–691. doi: 10.1177/0145445503256321. [DOI] [PubMed] [Google Scholar]