Abstract
Peripheral T-cell non-Hodgkin lymphomas (T-NHL) are rare diseases, with a worse prognosis compared to their B-cell counterparts. Allogeneic hematopoietic stem cell transplantation may have a role in the treatment of relapsed/refractory disease or high-risk histologies in the upfront setting. However, there is limited information on the efficacy of allogeneic transplantation for these diseases, as well as what factors may predict outcomes. We therefore performed a retrospective study of 34 patients who received an allogeneic transplant for the treatment of TNHL at a single center between January 1, 1992 and December 31, 2009. The median follow-up for survivors was 45 months (range 9-160 months). The two-year overall survival (OS) was 0.61 (95% CI: 0.43-0.75) with a plateau at twenty-eight months. Ki-67 expression ≤ 25% was predictive of improved OS (p < 0.01), and transplant in complete remission was predictive of a decreased cumulative incidence of events (p= 0.04). Three patients received DLI; and two patients demonstrated a response, supporting a graft-versus-lymphoma effect. These data demonstrate that allogeneic transplantation is a viable option for the treatment of T-NHL and merits prospective evaluation.
Keywords: Allogeneic HSCT, graft-versus-lymphoma effect, T-cell Non Hodgkin’s Lymphoma
INTRODUCTION
T-cell non-Hodgkin’s lymphomas (T-NHL) represent a heterogeneous array of non-Hodgkin’s lymphoma (NHL). They typically account for less than 10% of all newly diagnosed NHL in the US [1]. The most common subtypes of peripheral T/NK cell neoplasms in North America are peripheral T cell lymphoma not otherwise specified (PTCL), anaplastic large cell lymphoma (ALCL), and angioimmunoblastic T cell lymphoma (AITL). With the exception of anaplastic lymphoma kinase-positive {alk(+)} ALCL, the T cell phenotype is an independent poor risk factor. In a large prospective study of 1883 patients with NHL, the 288 patients with T-NHL had significantly lower complete remission (CR), overall survival (OS) and event free survival (EFS) relative to the patients with B-cell lymphoma [2]. Five year OS and EFS were 41% and 33% respectively for the T-NHL group. Excluding ALCL from the analysis, OS was 31%, likely reflecting the superior prognosis of alk (+) ALCL.
While there is currently no standard treatment for T-NHL, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) comprise the most commonly used regimen. However, assessing optimal therapy is complicated by the heterogeneous nature of this disease group. For example, even among the poorer prognosis subtypes, there is considerable heterogeneity in response to anthracycline-containing regimens with alk(-) ALCL patients achieving a median survival of nearly 5 years as opposed to only 9 months for aggressive HTLV-1 associated lymphoma [3,4]. Phase II studies support high dose therapy followed by autologous stem cell transplant (HD-ASCT) in first remission for T-NHL [5]. Results have been less promising for HD-ASCT in the relapsed or refractory setting [6].
While data have emerged supporting allogeneic hematopoietic stem cell transplantation (allo-HSCT) for T-NHL, the published experience remains limited [7-10]. These studies have demonstrated favorable overall survival rates and support the presence of a graft-versus-lymphoma (GVL) effect. The heterogeneity of patient populations, sample size, different stages at HSCT and the potential role of additional prognostic factors account in part for the variation in results between different studies. To expand the published experience and improve characterization of prognostic predictors to enhance patient selection, we analyzed the Memorial Sloan-Kettering Cancer Center (MSKCC) experience with allo-HSCT for T-NHL.
MATERIALS AND METHODS
Study Design
This is a retrospective study of all thirty-four patients who received an allo-HSCT for the treatment of peripheral T-NHL at MSKCC between January 1992 and December 2009. Written informed consent for treatment was obtained from all patients. A waiver of authorization for this retrospective review was obtained from the Institutional Review and Privacy Board. An MSKCC pathologist confirmed the pathologic diagnosis for each patient. Eligibility criteria for transplant included a diagnosis of relapsed, refractory, or high risk T-NHL per the judgment of the treating/attending; availability of an HLA-identical or single-antigen mismatched donor or double umbilical cord units; absence of active infection; and lack of coexisting cardiac, pulmonary, hepatic, or renal dysfunction that would preclude administration of the cytoreductive regimen.
Patients who were in complete remission at the time of transplant were offered allogeneic transplantation over autologous transplantation at the transplant attending’s discretion. For patients in CR1 (n=5) allogeneic transplantation was offered because the risk of relapse was felt to be unacceptably high with autologous transplant due to specific histology (n=3), persistent minimal residual disease noted in the peripheral blood (n=1), and a concomitant diagnosis of chronic myelomonocytic leukemia (n=1). For patients in CR2 (n=10), allogeneic transplantation was offered because of previous autologous transplant (n=1), short remission duration (n=4), significant bony and presumed marrow involvement at diagnosis (n=1), a specific histology believed to be at high risk for relapse (n=2), previous organ toxicity eliminating the possibility of high dose therapy (n=1) and for an unknown reason (n=1). The single patient in CR3 underwent allogeneic transplantation because of short remission durations. Patients who were not in complete remission at the time of transplantation were preferentially considered for allogeneic transplantation if their clinical status and donor availability permitted.
Pre-transplant variables were assessed for their effects on overall survival. Patients were classified as either being in CR or having another disease status at the time of transplant. CR was defined as the absence of any morphologic evidence of disease. The number of previous courses of chemotherapy was distinguished between ≤ 2 or > 2. Second-line international prognostic factor index (IPI) [11,12] and prognostic index for peripheral T cell lymphoma (PIT) score [13] were determined at admission for transplant and were characterized as ≤1 or >1 because we have previously shown that the second line age-adjusted IPI (sAAIPI) determined at time of allo-HSCT is predictive of clinical outcomes [12]. Ki-67 analysis, determined by immunohistochemistry (IHC) pre-salvage therapy, was demarcated as ≤25% or >25%. Preparative regimens were either ablative or reduced intensity [14]. Acute graft-versus-host disease (GVHD) was graded according to CIBMTR criteria [15]. Chronic GVHD was characterized as either limited or extensive. The primary transplant outcomes for 16 of the patients who underwent T-cell depleted (TCD) allo-HSCT have been previously reported [12].
Statistical analysis
Analyses were performed as of 6/30/2010. OS was calculated from date of transplant to the date of death or loss to follow-up. Progression free survival (PFS) was calculated from the date of transplant date to the date of progression/relapse or death from any cause. The Kaplan-Meier method was used to estimate survival functions. Further, the cumulative incidence of relapse was calculated considering an event as disease relapse or progression. Death before the event (n=6) was considered a competing event. The cumulative incidence function was therefore used to estimate the probabilities for the time to event. Log-rank test and Gray’s test were used respectively to determine whether survival and cumulative incidence functions differed by covariates of interests. The following variables were tested for an association with OS and cumulative incidence of events: prior HD-ASCT, IPI, PIT, Ki-67, prior radiotherapy, number of previous courses of therapy, degree of marrow ablation, T cell depletion (TCD), and disease status at transplant. Fisher exact test was used to compare the frequency distribution of acute GVHD between conventional and TCD allografts.
RESULTS
Patient characteristics
Table I details the patient characteristics. The patient population was heavily pretreated. The median number of prior chemotherapeutic regimens was 3 (range 1-8) with 10 patients (29.4%) also receiving prior involved field radiotherapy. The median time from diagnosis to transplant was 339 days (range 29 days to 1739 days). Eighteen patients received unmodified grafts and 16 patients received ex vivo TCD transplant [12,16,17]. Ten patients received grafts whose T cells were removed from bone marrow grafts by sequential soybean lectin agglutination and sheep red blood cell (sRBC)-rosette depletion [18]. Six patients received granulocyte colony stimulating factor-mobilized PBSCs that was depleted of T cells by positive selection of CD34+ stem cells using the ISOLEX 300i Magnetic Cell Separator and subsequent sRBC-rosette depletion.
Table I.
Patient characteristics
| Age | |
| Median | 37 |
| Range | 5-68 |
|
| |
| Sex | |
| Female | 9 (26%) |
| Male | 25 (74%) |
|
| |
| Diagnosis | |
| Anaplastic large cell lymphoma | 8 (24%) |
| Alk(+) | 4(12%) |
| Alk(−) | 4(12%) |
| Peripheral T cell lymphoma NOS | 7 (21%) |
| Hepatosplenic Gamma Delta T cell lymphoma | 5 (15%) |
| Angioimmunoblastic T cell lymphoma | 4 (12%) |
| Panniculitic T cell lymphoma | 3 (9%) |
| HTLV-1 associated T cell lymphoma | 2 (6%) |
| Mycoses Fungoides | 2 (6%) |
| Cutaneous gamma-delta T cell lymphoma | 1 (3%) |
| Enteropathy-type T cell lymphoma | 1 (3%) |
| NK- cell lymphoma | 1 (3%) |
|
| |
| Number of lines of chemotherapy | |
| ≤2 | 14 (41%) |
| >2 | 20 (59%) |
|
| |
| Prior radiotherapy | |
| No | 24 (71%) |
| Yes | 10 (29%) |
|
| |
| Prior autologous transplant | |
| No | 30 (88%) |
| Yes | 4 (12%) |
|
| |
| Pre-salvage Ki-67 | |
| ≤ 25% | 11 (32%) |
| >25% | 12 (35%) |
| Not available | 11 (32%) |
|
| |
| IPI at transplant | |
| ≤1 | 22 (65%) |
| >1 | 12 (35%) |
|
| |
| PIT at transplant | |
| ≤1 | 28 (82%) |
| >1 | 6 (18%) |
|
| |
| Disease status at transplant | |
| CR1 | 5 (15%) |
| CR2 | 10 (29%) |
| CR3 | 1 (3%) |
| PR | 13 (38%) |
| SD | 2 (6%) |
| Refractory | 1 (3%) |
| Unknown/other | 2 (6%) |
|
| |
| Preparative Regimens | |
| Myeloablative: | |
| TBI-baseda | 16 (47%) |
| Chemotherapy-basedb | 5 (15%) |
| Reduced Intensity/Non-myeloablative: | |
| Melphalan-basedc | 4 (12%) |
| Non-melphalan basedd | 9 (26%) |
|
| |
| Graft source | |
| Peripheral blood | 24 (71%) |
| Bone Marrow | 8 (24%) |
| Double unit umbilical cord | 2 (6%) |
|
| |
| Degree of matching | |
| Matched related | 18 (53%) |
| Matched unrelated | 10 (29%) |
| Mismatched related | 1 (3%) |
| Mismatched unrelated | 5 (15%) |
|
| |
| GVHD prophylaxis | |
| Ex-vivo T cell depletion | 16 (47%) |
| Cyclosporin/mycophenolate mofetil | 5 (15%) |
| Tacrolimus/sirolimus/methotrexate | 4 (12%) |
| Alemtuzumab/cyclosporin | 3 (9%) |
| Tacrolimus/methotrexate | 3 (9%) |
| Othere | 3 (9%) |
TBI(1375cGy-1550cGy)/Thiotepa/Cyclophosphamide (120 mg/kg): 11 patients; TBI (1375cGy-1550cGy)/Thiotepa/Fludarabine: 5 patients
Cyclophosphamide (120 mg/kg)/Fludarabine/TBI (1375cGy-1550cGy): 2 patients; Busulfan/Melphalan/Fludarabine: 1 patient; Busulfan/Melphalan/Thiotepa; 1 patient; Thiotepa/Melphalan/Fludarabine: 1 patient
Melphalan/Fludarabine/Campath: 3 patients; Melphalan/Fludarabine: 1 patient
Cyclophosphamide (50 mg/kg)/Fludarabine/TBI (200cGy): 7 patients; Fludarabine/TBI (200cGy): 1 patient; Cyclophosphamide (50 mg/kg)/Fludarabine/Rituximab (concurrent B-cell malignancy): 1 patient
Cyclosporin/mycophenolate mofetil/methotrexate: 1 patient; Mycophenolate mofetil /sirolimus: 1 patient; tacrolimus/sirolimus: 1 patient
Transplant related toxicity
Six patients died from transplant-related mortality (TRM, 18%). Three patients died from GVHD, one from non-engraftment, one from organ failure, and one from infection. For patients in CR, two patients died from TRM (both with GVHD, 13%). This risk compares favorably to the risk of relapse with autologous transplantation in CR [5]. The latest TRM (non-engraftment) occurred on day +122 following allo-HSCT. Fourteen patients (41%) developed acute GVHD, including seven patients (21%) with grade III-IV aGVHD. Seven patients (25% of patients alive at d+100) developed chronic GVHD, including one patient with extensive chronic GVHD. One additional patient developed grade II acute GVHD of the skin that responded to topical therapy following donor leukocyte infusion (DLI). As expected, the rate of acute GVHD was significantly greater following unmodified grafts compared with TCD (66.7% vs 18.8%, p = 0.01). The three patients who received Campath as part of their preparative regimen were excluded from this analysis. One of these three patients was diagnosed with grade II acute GVHD.
Two patients had a documented diagnosis of Epstein-Barr virus lymphoproliferative disorder (EBV-LPD) after allo-HSCT. One patient developed a donor-derived EBV associated Hodgkin lymphoma following a TCD allograft and is without evidence of disease following therapy with doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD). A second patient developed an EBV-LPD after a double umbilical cord blood allograft. This patient is currently in remission after therapy with third-party EBV-specific cytotoxic T-lymphocytes [19].
Overall survival , progression free survival and cumulative incidence of events
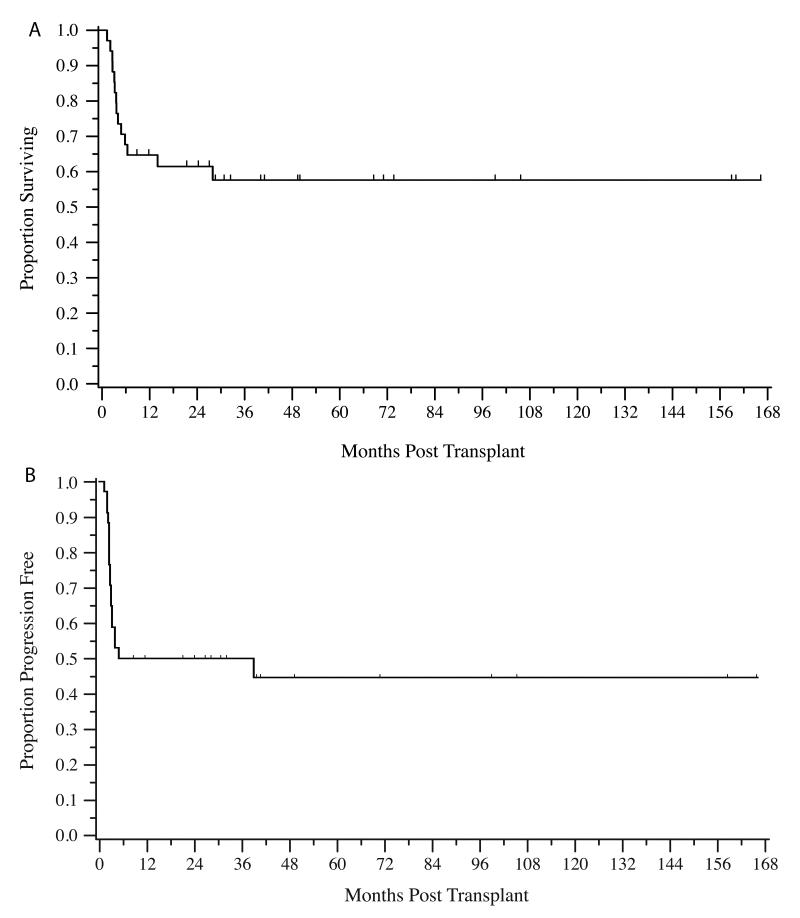
The median follow-up for survivors was 45 months (range 9-160 months). Fourteen patients (41%) had expired at the conclusion of the follow-up period. The two-year OS was 0.61 (95% CI: 0.43-0.75) (Figure 1A). Median survival has not been reached. Notably, there is a plateau on the OS curve with no events noted after 28 months post transplant. The two-year PFS was 0.50 (95% CI: 0.32-0.65) with a similar plateau noted (figure 1B).
Figure 1.
Kaplan Meier estimate of overall survival (A) and progression-free survival (B)
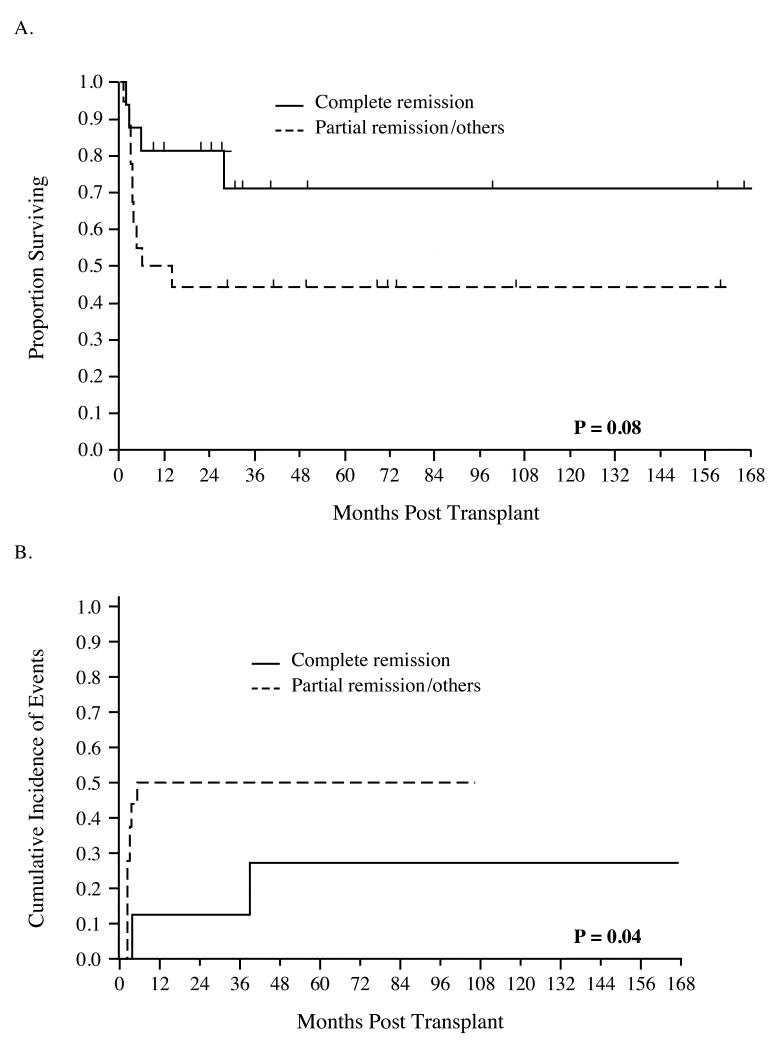
The univariate log-rank test was used to evaluate associations between clinical factors and survival. In this small series of patients, there was a trend toward improved OS based on lower IPI (p=0.07) and disease status in remission at transplant (Figure 2A, p=0.08). In 23 evaluable patients, Ki-67 ≤ 25% at last biopsy pre-salvage therapy predicted enhanced OS (p < 0.01). Although eight patients (24%) died following relapse or progression of disease, only two of 16 patients who underwent allo-HSCT in CR died as a result of relapse or disease progression. In contrast, prior HD-ASCT (p= 0.50), PIT (≤1 vs > 1, p=0.12), TCD (p=0.31), prior radiotherapy (p=0.16), number of previous chemotherapy regimens (≤2 vs > 2, p=0.94), and conditioning intensity (myeloablative vs. reduced intensity, p=0.31) were not associated with OS. Due to a small cohort, and only one clinical factor reaching the threshold for significance, multivariate analysis was not performed.
Figure 2.
Remission status at transplant predicts OS (A) and cumulative incidence of events (B)
At the conclusion of our follow-up period, 12 events (either relapse or progression) were noted. The cumulative incidence of events at 6 months was 33%. Univariate Gray’s test indicated that the cumulative incidence of events was decreased in patients in remission at the time of transplant (p=0.04, Figure 2B), and there was a trend favoring improved cumulative incidence of events in patients who had received 2 or fewer prior chemotherapy regimens (p = 0.07). There were no associations between cumulative incidence of events and IPI (p=0.87), TCD (p=0.35), degree of myeloablation (p=0.64), prior radiotherapy (p =0.23), KPS (p=1.0), and Ki-67 score (p=0.48). Test of association was not performed between prior HD-ASCT, PIT and incidence of events due to few or no events in strata.
Donor leukocyte infusions
Three patients underwent DLI to treat disease recurrences, and two responded. The first patient was given DLI for relapsed mycoses fungoides (MF) 8 months following TCD allo-HSCT. This patient developed overall grade II acute GVHD limited to skin after DLI, which responded to topical therapy only. He achieved a CR, which was maintained for 5 years prior to being discharged from BMT clinic. Another patient with hepatosplenic gamma-delta T cell lymphoma (HSL) received DLI nine months after unmodified alemtuzumab containing allo-HSCT for molecular evidence of disease in the bone marrow. Molecular disease then resolved, and his CR continued for 2.5 years when clinical relapse was noted. A second DLI did not achieve a response, although the viability of this cryopreserved cell product may have been compromised. One further patient with HTLV-1 associated lymphoma who received a TCD allo-HSCT received DLI for significant, diffuse disease and expired 13 days following infusion.
DISCUSSION
While information supporting allogeneic HSCT for T-NHL is limited, preliminary data suggest a benefit [7-10]. Initial case reports described a GVL effect following non-myeloablative allo-HSCT, both in the context of DLI and withdrawal of immunosuppression in patients with cutaneous T-cell lymphomas [20,21]. Corradini et al [7] conducted a phase II trial evaluating the efficacy of a non-myeloablative allo-HSCT for PTCL. With a median follow-up of 28 months, 12/17 (70%) patients remained in complete remission. Notably, DLI induced a response in two patients who progressed after transplant. The largest reported experience to date describes 77 patients who received an allo-HSCT for aggressive T-NHL [8]. In this heterogeneous group, the five-year OS and EFS were 57% and 53%. Predictors for poor OS included chemoresistant disease and grade III or IV acute GVHD. Disease status at transplantation was predictive of event free survival.
In the current study, we were able to demonstrate favorable OS, PFS and cumulative incidence of events following allo-HSCT for T-NHL despite significant prior therapy. Our OS of 61% and PFS of 50% are consistent with previous reports. Notably, a plateau was reached approximately 2 years after allo-HSCT with a median follow-up of almost 3 years among survivors, implying this treatment may be curative for T-NHL, even in heavily treated patients. It is especially provocative that only two patients who underwent allo-HSCT in CR died from relapsed disease, supporting evaluation of allo-HSCT as a consolidation strategy earlier in the disease course. However, in patients who did not receive functional imaging the distinction between CR and PR may be difficult. Our experience also provides further evidence for a GVL effect in these diseases because 2 of 3 patients responded to DLI.
In this study, we evaluated the predictive value of prognostic markers prior to transplantation. The IPI has been validated in the upfront treatment of T-NHL [11], and we have previously shown that the sAAIPI determined at the time of allo-HSCT is predictive of outcomes in patients with NHL undergoing ablative TCD allo-HSCT [12]. In addition, other groups have proposed prognostic models specific for T-NHL including the prognostic index for peripheral T cell lymphoma (PIT) [13]. Including age, performance status, LDH and bone marrow involvement, the PIT had superior predictive capacity compared with IPI in T-NHL. In our cohort, there is a suggestion that both IPI and PIT measured at the time of transplantation may predict survival, although the small number of patients in this cohort limited our ability to reach significance. It would be interesting to test prospectively if second line IPI or PIT should influence the timing of allogeneic transplantation. The proliferation rate measured by Ki-67 staining has previously been shown to predict response to standard therapy for extranodal NK/T cell lymphoma [22] and for PTCL [23]. Our study is the first to evaluate pre-salvage Ki-67 as a prognostic measurement for survival following allo-HSCT. Despite the relatively small numbers, there was a significant detrimental effect on OS for patients whose pre-salvage Ki-67 was > 25%. Therefore, we now show that Ki-67 expression also predicts response to allo-HSCT, a finding consistent with the fact that tumors with a lower proliferative rate may be more likely to benefit from GVL. We describe that 2of 3 patients had a response to DLI, with one durable response noted. This is a small cohort of patients and, thus, no definitive conclusions can be drawn. However, it is interesting to note that the patient with a durable response had a diagnosis of MF. This response is consistent with previous reports of response to DLI or withdrawal of immune suppression for this disease [20,21]. It is likely that the degree of graft-versus-lymphoma effect seen following allo-HSCT for T-NHL differs between histologies consistent with what is seen in B-cell histologies.
While these results, including the prognostic value of Ki-67, are of great interest, they are based on retrospective data in a patient population that is heterogeneous with regard to histology and transplantation approach. The study also has a small number of patients with a prior autologous transplant and our findings may, therefore, not apply to this group of patients. Finally, our study spans a prolonged period of time. However, given the rarity of these diseases, it is difficult to gather a large uniform series of patients, evidenced by the very limited literature on allo-HSCT for the treatment of these diseases.
Despite these potential limitations, our experience with allo-HSCT for T-NHL further supports the feasibility, safety, and efficacy of this treatment. Prognostic markers, such as Ki-67 may improve patient selection and outcomes. Thus far, there is only one published, small, prospective trial evaluating allo-HSCT for the treatment of T-NHL [7]. The completion of larger prospective studies could answer whether allo-HSCT is a curative therapy for T-NHL and which patients would most benefit from this treatment.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert care provided to these patients by the fellows, housestaff, and nurses of Memorial Sloan-Kettering Cancer Center.
Grant Support: Supported in part by P01 CA23766 (NIH).
Footnotes
POTENTIAL CONFLICTS OF INTEREST: There are no potential conflicts of interest to declare.
There are no financial disclosures for all authors.
REFERENCES
- 1.Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 2002;13:140–149. doi: 10.1093/annonc/mdf033. [DOI] [PubMed] [Google Scholar]
- 2.Gisselbrecht C, Gaulard P, Lepage E, et al. Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA) Blood. 1998;92:76–82. [PubMed] [Google Scholar]
- 3.Savage KJ, Harris NL, Vose JM, et al. ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- 4.Suzumiya J, Ohshima K, Tamura K, et al. The International Prognostic Index predicts outcome in aggressive adult T-cell leukemia/lymphoma: analysis of 126 patients from the International Peripheral T-Cell Lymphoma Project. Ann Oncol. 2009;20:715–721. doi: 10.1093/annonc/mdn696. [DOI] [PubMed] [Google Scholar]
- 5.Reimer P, Rudiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27:106–113. doi: 10.1200/JCO.2008.17.4870. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez J, Munsell M, Yazji S, et al. Impact of high-dose chemotherapy on peripheral T-cell lymphomas. J Clin Oncol. 2001;19:3766–3770. doi: 10.1200/JCO.2001.19.17.3766. [DOI] [PubMed] [Google Scholar]
- 7.Corradini P, Dodero A, Zallio F, et al. Graft-versus-lymphoma effect in relapsed peripheral T-cell non-Hodgkin’s lymphomas after reduced-intensity conditioning followed by allogeneic transplantation of hematopoietic cells. J Clin Oncol. 2004;22:2172–2176. doi: 10.1200/JCO.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 8.Le Gouill S, Milpied N, Buzyn A, et al. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J Clin Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 9.Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2010;116:1369–1376. doi: 10.1182/blood-2009-10-247510. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen ED, Kim HT, Ho VT, et al. A large single-center experience with allogeneic stem-cell transplantation for peripheral T-cell non-Hodgkin lymphoma and advanced mycosis fungoides/Sezary syndrome. Ann Oncol. 2011 doi: 10.1093/annonc/mdq698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansell SM, Habermann TM, Kurtin PJ, et al. Predictive capacity of the International Prognostic Factor Index in patients with peripheral T-cell lymphoma. J Clin Oncol. 1997;15:2296–2301. doi: 10.1200/JCO.1997.15.6.2296. [DOI] [PubMed] [Google Scholar]
- 12.Perales MA, Jenq R, Goldberg JD, et al. Second-line age-adjusted International Prognostic Index in patients with advanced non-Hodgkin lymphoma after T-cell depleted allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45:1408–1416. doi: 10.1038/bmt.2009.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 14.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 17.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kernan NA, Flomenberg N, Collins NH, O’Reilly RJ, Dupont B. Quantitation of T lymphocytes in human bone marrow by a limiting dilution assay. Transplantation. 1985;40:317–322. doi: 10.1097/00007890-198509000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116:5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burt RK, Guitart J, Traynor A, et al. Allogeneic hematopoietic stem cell transplantation for advanced mycosis fungoides: evidence of a graft-versus-tumor effect. Bone Marrow Transplant. 2000;25:111–113. doi: 10.1038/sj.bmt.1702099. [DOI] [PubMed] [Google Scholar]
- 21.Herbert KE, Spencer A, Grigg A, Ryan G, McCormack C, Prince HM. Graft-versus-lymphoma effect in refractory cutaneous T-cell lymphoma after reduced-intensity HLA-matched sibling allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:521–525. doi: 10.1038/sj.bmt.1704641. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Kim BS, Choi CW, et al. Ki-67 expression is predictive of prognosis in patients with stage I/II extranodal NK/T-cell lymphoma, nasal type. Ann Oncol. 2007;18:1382–1387. doi: 10.1093/annonc/mdm183. [DOI] [PubMed] [Google Scholar]
- 23.Went P, Agostinelli C, Gallamini A, et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24:2472–2479. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]