Abstract
We previously reported that the antipsychotic drug haloperidol, a multifunctional D2-like dopamine and sigma receptor subtype antagonist, has neuroprotective properties. In this study we further examined the association between neuroprotection and receptor antagonism by evaluating a panel of novel compounds with varying affinity at sigma and D2-like dopamine receptors. These compounds were evaluated using an in vitro cytotoxicity assay that utilizes a hippocampal-derived cell line, HT-22, in the presence or absence of varying concentrations (5 to 20 mM) of glutamate. While haloperidol was found to be a potent neuroprotective agent in this in vitro cell assay, the prototypic sigma 1 receptor agonist (+)-pentazocine was found not to be neuroprotective. Subsequently, the potency for the neuroprotection of HT-22 cells was evaluated for a) three SV series indoles which have nMolar affinity at D2-like receptors but varying affinity at sigma 1 receptor and b) two benzyl phenylacetamides sigma 1 receptor selective compounds which bind with low affinity at D2-like receptors but have nMolar affinity for the sigma 1 receptor. We observed that cytoprotection correlated with the affinity of the compounds for sigma 1 receptors. Based upon results from the HT-22 cell-based in vitro assay, two phenylacetamides, LS-127 and LS-137, were further evaluated in vivo using a transient middle cerebral artery occlusion (t-MCAO) model of stroke. At a dose of 100 µg/kg, both LS-127 and LS-137 attenuated infarct volume by approximately 50%. These studies provide further evidence that sigma 1 receptor selective compounds can provide neuroprotection in cytotoxic situations. These results also demonstrate that sigma 1 receptor selective benzyl phenylacetamides are candidate pharmacotherapeutic agents that could be used to minimize neuronal death after a stroke or head trauma.
Keywords: Sigma receptors, Sigma 1 receptors, Neuroprotection
1. Introduction
Sigma receptors are comprised of two pharmacologically distinct subtypes, sigma 1 and sigma 2 (also called σ-1 and σ-2). The sigma 1 receptor is an integral membrane protein which does not share amino acid homology with other known mammalian receptor proteins. However, it has homology with fungal sterol isomerase, although it lacks sterol isomerase enzymatic activity (Su and Hayashi, 2003). Hydropathy analysis of the sigma 1 receptor indicates that it is a transmembrane spanning receptor. Recently the sigma 2 receptor has been identified as being progesterone receptor membrane component 1 (PGRMC1) (Xu et al., 2011).
Both sigma receptor subtypes are expressed in the periphery and in the central nervous system (CNS). It has been suggested that sigma 1 receptors may act as intracellular modulators of signal transduction systems (Su and Hayashi, 2003). For example, stimulation of sigma receptors amplify glutamatergic, dopaminergic, IP3-related metabotropic and nerve growth-factor related signal transductions systems in vivo. Sigma receptors have also been reported to modulate calcium signaling at the endoplasmic reticulum, regulate IP3 receptors, modulate neurotransmitter release, alter psychostimulant-induced gene expression and modulate cocaine-dependent locomotor activity (Cobos et al., 2008; Guitart et al., 2004; Matsumoto et al., 2003; Monassier and Bousquet, 2002).
The sigma 1 receptor has recently been postulated to be a ligand-regulated molecular chaperone in the endoplasmic reticulum (Hayashi and Su, 2007; Maurice and Su, 2009) that regulates signal transduction, ER stress, cellular redox, cellular survival and synaptogenesis. Sigma 1 receptor selective ligands have been reported to exert antidepressant-like, anxiolytic, analgesic and robust neuroprotective actions in preclinical studies (Hayashi et al., 2011).
The endogenous ligands for sigma receptors are thought to include the neurosteroids progesterone, pregnonolone sulfate or possibly dehydroepiandrosterone (Su et al., 1988, 1990). Neurosteroids, including progesterone, testosterone and pregnenolone sulfate, have moderate affinity at sigma receptors. In addition to neurosteroids, diverse classes of pharmacologic agents, including, haloperidol, imipramine, pimozide, chlorpromazine, dextromethorphan, propranolol and phencyclidine have also been found to bind to the sigma receptor subtypes (Su, 1982).
Although haloperidol binds sigma 1 and 2 receptors with nMolar affinity, it has not been a useful ligand to study sigma receptor pharmacology and function in vivo because it also binds with high affinity to D2-like dopamine receptor subtypes. Therefore, sigma receptor selective ligands that bind with high affinity (dissociation constants in the nMolar range) and selectively (>100-fold) to the sigma 1 and sigma 2 receptor subtypes, while exhibiting low affinity at D2-like dopamine receptor subtypes, will be required to more precisely define the physiological function of sigma receptor subtypes.
There has been considerable interest in the development of novel effective and safe neuroprotective agents that might be used for the treatment of a variety of neurodegenerative disorders, including glaucoma, Alzheimer’sDisease, Parkinson’s Disease, multiple sclerosis and/or stroke (Kirk et al., 1994; Maurice and Su, 2009; Simpkins and Jankovic, 2003; Tarawneh and Galvin, 2010; Yang et al., 2005). Previous studies had shown that the putative sigma 1 agonist 4-phenyl-1-(4-phenylbutyl) piperidine can be cytoprotective for a) glucose-deprived primary mixed cortical and hippocampal neuronal cultures (Tan et al., 2010) and b) the rat caudate/putamen following transient focal ischemia (Goyagi et al., 2003). In addition, studies by Tuerxun et al. (2010) indicated that the sigma 1 receptor selective agonist SA4503 suppressed oxidative stress induced cytotoxicity of rat neurons by suppressing the mitogen-activated protein kinase/extracellular signal-regulated kinase and down regulated ionotropic glutamate receptors.
We previously reported that haloperidol potently protects against oxidative stress-related cell death in a murine hippocampal-derived cell line, HT-22, using a glutamate-based cytotoxicity assay. The protective potency of haloperidol and a number of other butyrophenones correlated with their affinity for the sigma 1 receptor. In addition, in vivo administration of haloperidol was also found to reduce lesion volume size in rats induced by a transient occlusion of the middle cerebral artery (Schetz et al., 2007).
In this communication, we further examine the role of sigma 1 selective antagonists as potential neuroprotective agents using a HT-22 cell line that is sensitive to glutamate neurotoxicity (Perez et al., 2005; Sagara and Schubert, 1998; Tan et al., 1998) to screen a panel of compounds which exhibit varying affinities at sigma 1 and D2-like dopamine receptors for cytoprotective properties (Huang et al., 1998, 2001). Based upon those results, we then investigated the ability of the most potent sigma 1 receptor selective neuroprotective compounds to prevent neurotoxicity in a transient middle cerebral artery occlusion (t-MCAO) model of stroke.
2. Results
In a previous study, we reported that the antipsychotic haloperidol has cytoprotective properties when examined using oxidative stress-induced neuronal death model using HT-22 cells. After screening a panel of clinically used antipsychotics, it appeared that the cytoprotective properties of the antipsychotics correlated with affinity at sigma 1 receptors (Schetz et al., 2007).
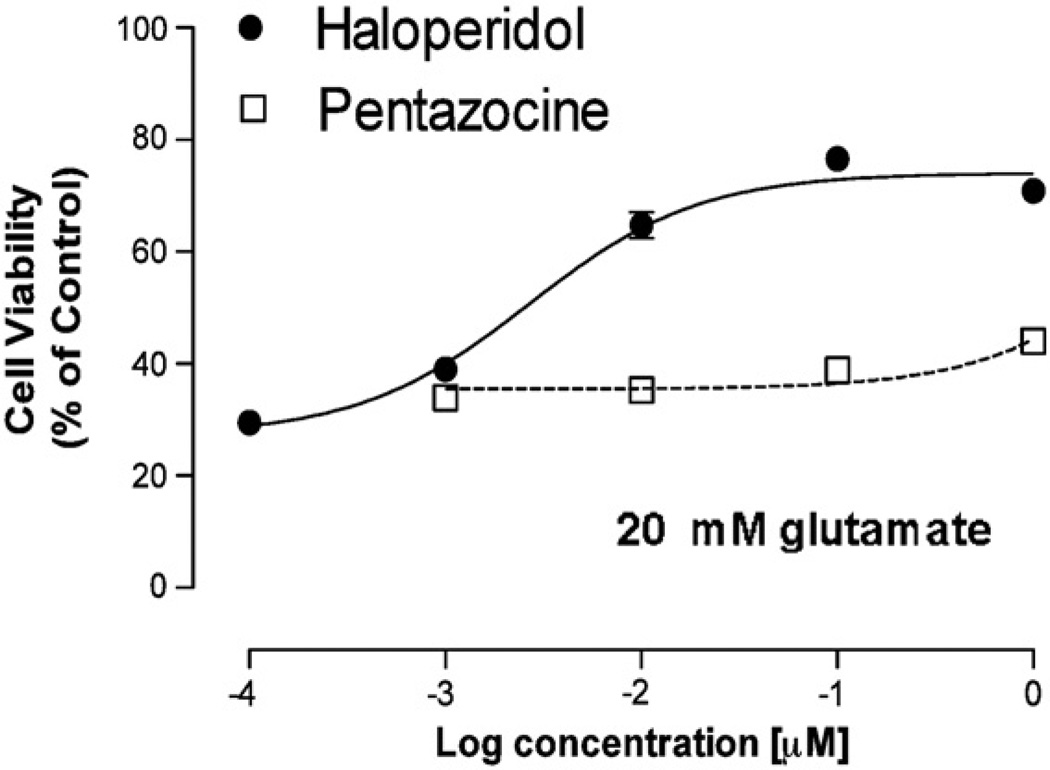
In this communication, we extend those findings by comparing the effect of the sigma 1 antagonist haloperidol with the sigma 1 selective prototypic agonist, (+)-pentazocine, using an oxidative stress-induced neuronal death model with HT-22 cells (Fig. 1). Although the affinity of haloperidol and (+)-pentazocine at sigma 1 receptors is similar (6.5 nM and 1.7 nM, respectively) (Maurice and Lockhart, 1997; Monassier and Bousquet, 2002; Vilner and Bowen, 2000), a >800-fold difference in the mean cytoprotective EC50 values (±S.E.M.) of these two compounds was observed (using 20 mM glutamate: haloperidol, EC50 = 5.9 ± 2.1 nM (n = 6); (+)-pentazocine, EC50 > 4000 nM (n = 3)) (Fig. 1). This result provides further evidence that sigma 1 receptor antagonists, but not agonists, exhibit cytoprotective properties in this assay.
Fig. 1.
Comparison of the effect of the sigma 1 antagonist haloperidol and the full agonist (+)-pentazocine in a cytotoxic assay. Dose–response curves for the effect of haloperidol (●) and (+)-pentazocine (□) on HT-22 cell viability are shown. For these studies the final concentration of glutamate used to induce oxidative stress cell death was 20 mM. Data (n = 4–8) is shown as mean values ± S.E.M. The S.E.M. is depicted in the graph but may not be visible if it is smaller than the symbol used to designate the mean value.
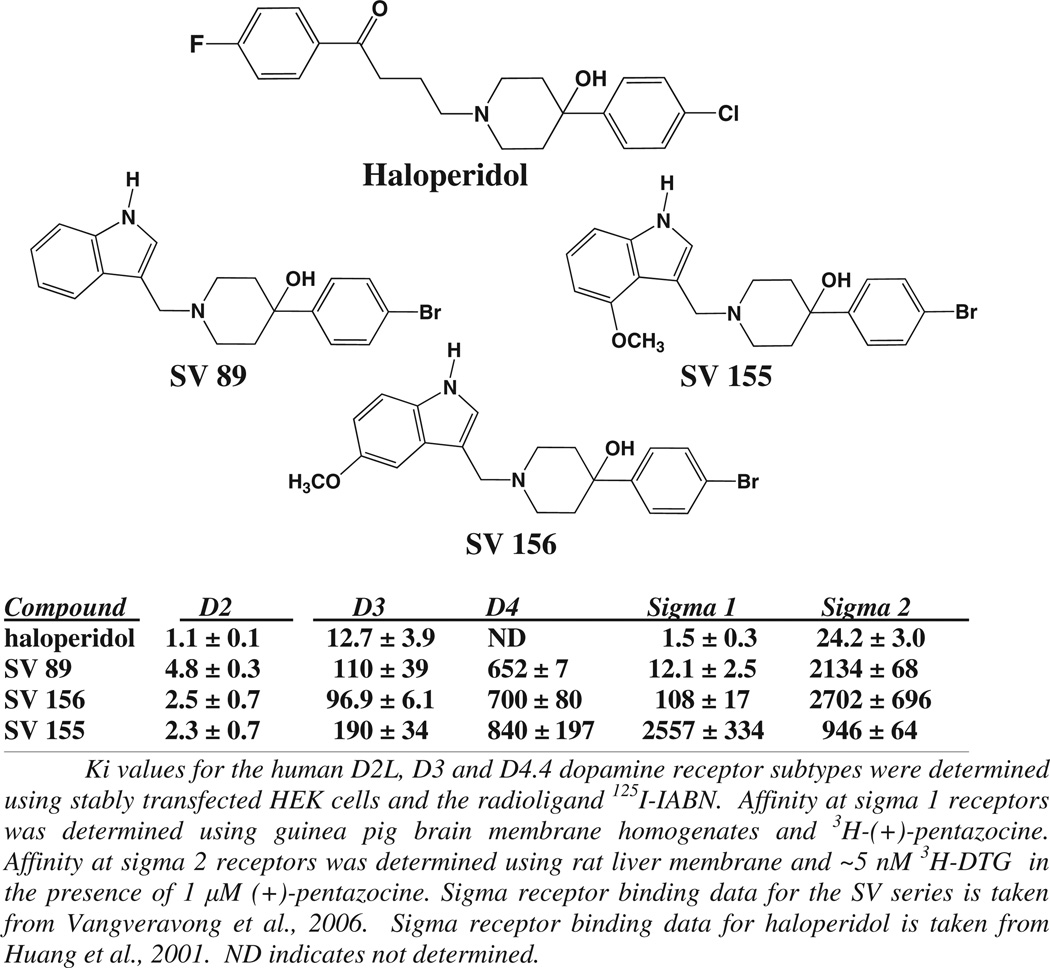
We then examined a panel of novel indoles, structurally related to haloperidol, shown to have varying affinity and selectivity for D2-like dopamine, sigma 1 and sigma 2 receptors (Vangveravong et al., 2006a). The structures and pharmacological properties of haloperidol and the three indoles examined in this study, SV-89, SV-155 and SV-156, are shown in Fig. 2. The SV series compounds had been synthesized as part of a research effort to identify D2-like dopamine receptor subtype selective compounds for the development of selective radiotracers and imaging agents for D2-like dopamine receptors. In the course of those studies the SV compounds were evaluated for binding activity at both sigma 1 and sigma 2 receptors. We identified several compounds with varying affinity and selectivity for sigma 1 receptors. All of those compounds were found to be low affinity at sigma 2 receptors (Ki values essentially ≥1000 nM). It was observed that the affinity at the D2 dopamine receptor was essentially invariant for haloperidol and the SV series compounds (1–5 nM), while the affinity of these three compounds at D3 dopamine receptors ranged from approximately 13 to 190 nM. However, the affinity at sigma 1 receptors for haloperidol and the three SV series compounds varies from approximately 2 nM to >2000 nM.
Fig. 2.
Structure and pharmacological profile of haloperidol and the SV series substituted indole compounds. The structures of haloperidol and the SV series compounds that were evaluated are shown. The affinity of these compounds at a) D2 and D3 dopamine receptors and b) sigma 1 and sigma 2 receptors is also shown. The affinity of the SV compounds was determined from competitive radioligand binding studies and the Ki values (nMolar) are presented as the mean ± S.E.M. for n > 3. Binding data is taken from Vangveravong et al., 2006a and the data for haloperidol is taken from Vangveravong et al., 2006b.
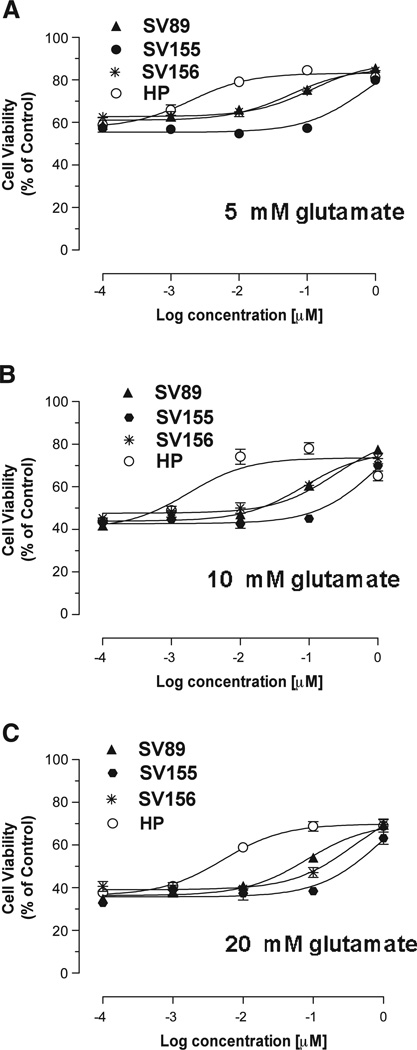
Fig. 3 shows a comparison of the cytoprotective properties of haloperidol, SV-89, SV-155 and SV-156. This analysis was performed using three different concentrations of glutamate as the insult, 5 mM, 10 mM and 20 mM. The EC50 values for in vitro cytoprotection under these three conditions indicated that the potency for HT-22 cytoprotection decreased as a function of decreasing affinity at sigma 1 receptors (Fig. 3), thus implicating the blockade of sigma 1 receptors, rather than D2-like dopamine receptors, as the primary mechanism of action.
Fig. 3.
Comparison of the effect of haloperidol and the SV series compounds in an in vitro HT-22 cytotoxicity assay. The cytoprotective properties of haloperidol and the SV series compounds (HP, haloperidol (○); SV 89 (▲); SV 155 (●); SV-156 (*)) are compared using a glutamate-dependent cytotoxic assay using HT-22 cells. Concentration-dependent analysis was performed using 5 mM (A), 10 mM (B) and 20 mM (C) glutamate. For these experiments n = 8–9. The S.E.M. is depicted in the graph but may not be visible if it is smaller than the symbol used to designate the mean value.
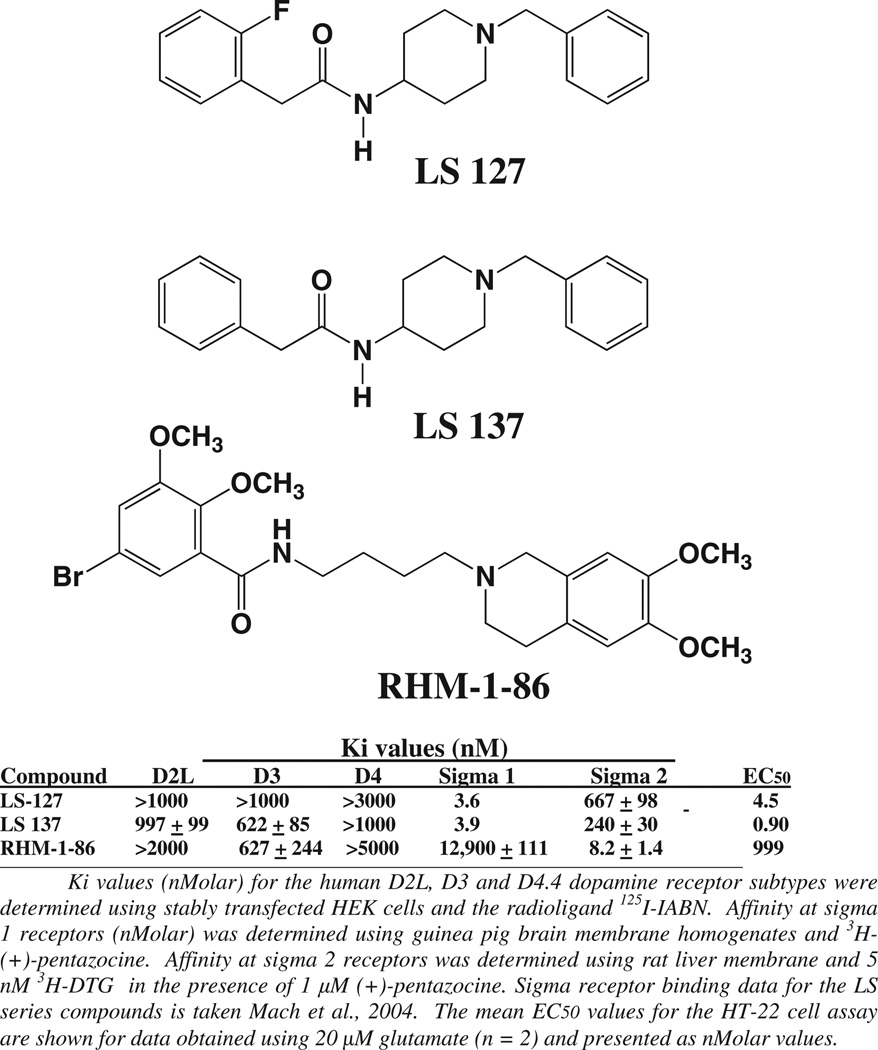
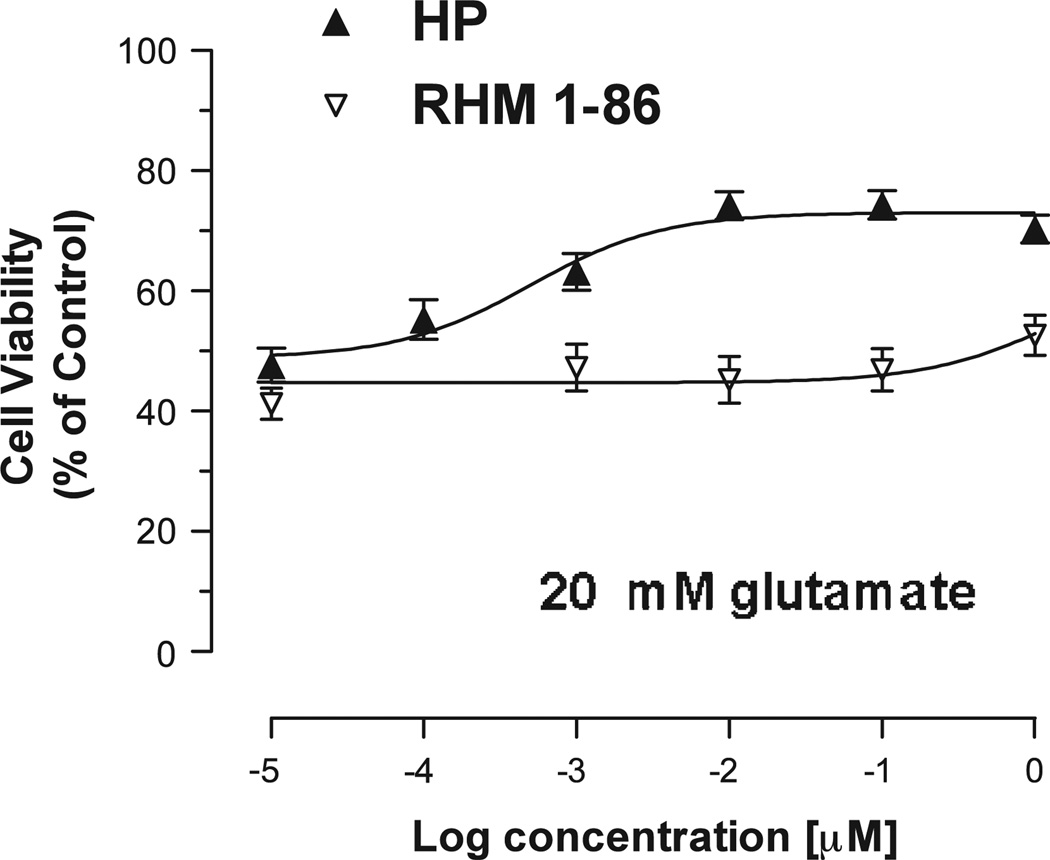
We then investigated additional compounds with selectivity at either sigma 1 or sigma 2 receptors for cytoprotective properties. During the course of our studies on the development of D2-dopamine receptor selective compounds we observed that the length of the carbon chain adjacent to the phenyl-substituted amide was an important structural component in determining D2-like and sigma receptor subtype selectivity (Mach et al., 2004). Compound RHM-1–86 is a sigma 2 selective compound (Ki value 8.2 nM), which binds with low affinity to sigma 1 receptors. It also binds to D2 and D3 receptors with low affinity (Fig. 4). Of the novel test compounds evaluated in this study, RHM-1–86 exhibited the least cytoprotective properties (mean EC50 = 999 nM for n = 2) (Fig. 5), thus further ruling out the involvement of sigma 2 receptor in HT-22 cell cytoprotection.
Fig. 4.
Structures and pharmacological profiles of the sigma 1 and sigma 2 receptor selective compounds. The structures and affinity for the benzyl-phenylacetamides LS-127, LS-137 and RHM-1–86 at D2-like (D2, D3 and D4) dopamine, sigma 1 and sigma 2 receptors are shown. The Ki values (nMolar) are presented as the mean ± S.E.M. for n ≥ 3.
Fig. 5.
Comparison of the effects of haloperidol and the sigma 2 receptor selective compound in an in vitro HT-22 glutamate cytotoxicity assay. A comparison of the cytoprotective potency of haloperidol (HP) (▲) and RHM-1–86 (▽) using an in vitro HT-22 cell-based assay is shown. The concentration of glutamate used for this experiment was 20 mM. Each point on the curve represents the mean ± S.E.M. for n = 12.
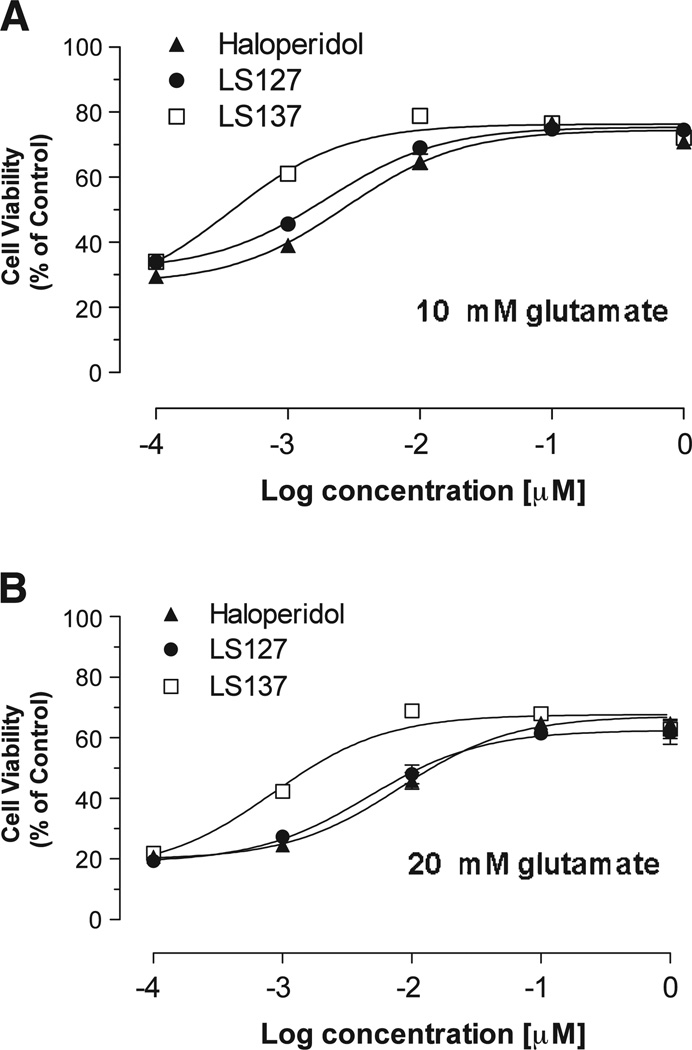
We then evaluated two of our sigma 1 selective benzyl phenylacetamide compounds, LS-127 and LS-137, for cytoprotection (Fig. 6). These two compounds bind to the sigma 1 receptor with an affinity approximately equal to the affinity of haloperidol (2–4 nM) but bind with low affinity (Ki value >500 nM) at the D2 and D3 dopamine receptor subtypes (Fig. 4). The potency of these two compounds in the HT-22 cell cytoprotective assay was found to be similar to or exceed that observed for haloperidol (Fig. 4) (using 20 mM glutamate: haloperidol, EC50 = 5.9 ± 2.1 nM (n = 6); LS-127, EC50 = 4.50 nM (n = 2); LS-137, EC50 = 0.90 nM (n = 2)), thus further ruling out D2-like dopamine receptor and sigma 2 receptor involvement in HT-22 cell cytoprotection.
Fig. 6.
Comparison of the effect of haloperidol, LS-127 and LS-137 in an in vitro cytotoxicity assay. The cytoprotective potency of haloperidol (▲), LS-127 (●) and LS-137 (□) are shown using an in vitro HT-22 cell-based assay using a concentration of glutamate at either (A) 10 mM or (B) 20mµ. Each point on the curve represents the mean ± S.E.M. The S.E.M. is depicted in the graph but may not be visible if it is smaller than the symbol used to designate the mean value.
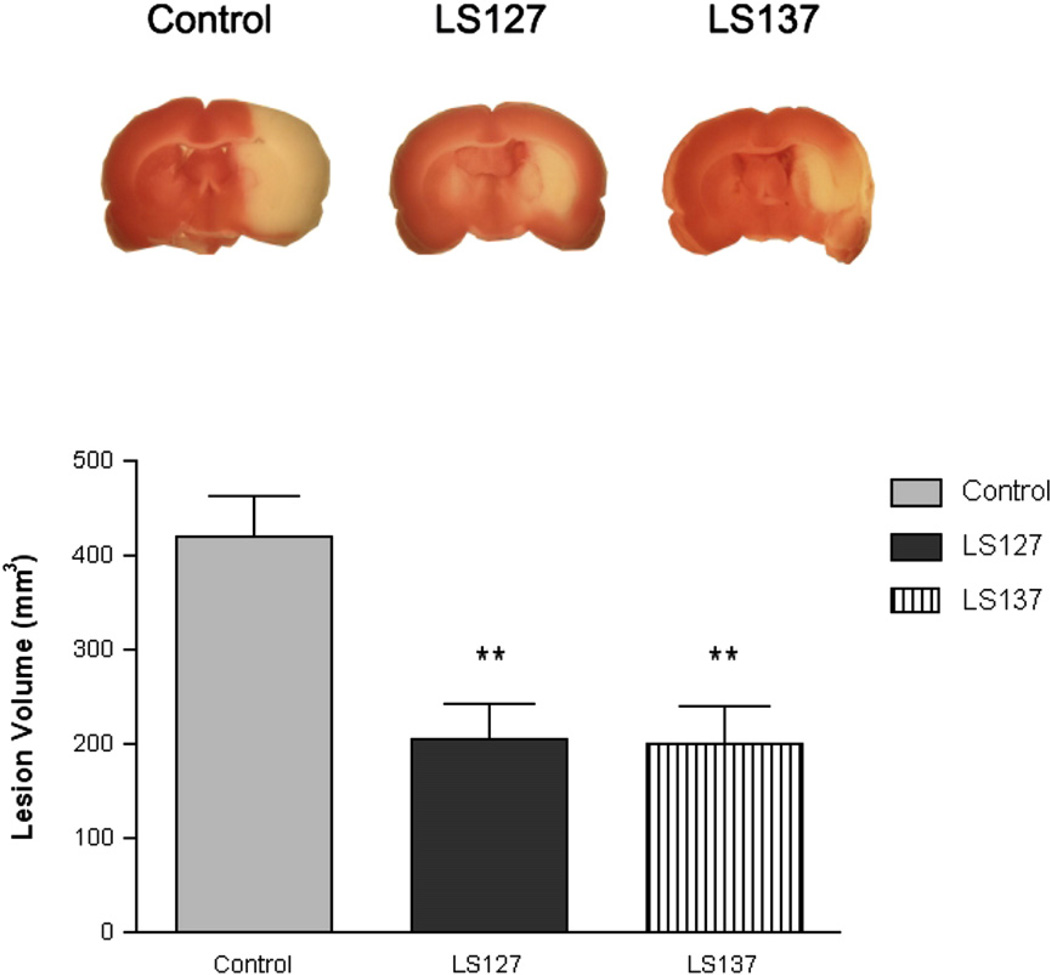
Based upon the findings from the HT-22 cell-based cytoprotection assay, LS-127 and LS-137 were selected to be evaluated for the ability to attenuate infarct volume in the t-MCAO model of stroke. At a dose of 100 µg/kg, a 50% reduction in volume of the stroke induced neuronal death was observed (Fig. 7). Based upon our previous experience with this model for stroke, the maximum achievable protection in this animal model is generally 50%, probably because of the severity of the neuronal damage that occurs in the core of the lesion during arterial occlusion (Perez et al., 2006; Yang et al., 2005). Therefore, maximal neuronal protection was achieved at the dose of LS series compounds that was used.
Fig. 7.
Sigma 1 selective compounds LS-127 and LS-137 protect against t-MCAO brain injury. (Top panel) Ischemic lesion depicted by triphenyltetrazolium chloride staining (white area) in the representative brain sections from control, LS-127, and LS-137 treated rats at 24 h after ischemic stroke. Images are representative of the widest extent of the infarct. (Bottom panel) The bar graph depicts the mean neuroprotection determined as the ischemic lesion volume ± S.E.M. The graph compares the effect on infarct volume of vehicle treated rats (solid gray bar) versus animals treated with either LS-127 (solid black bar) or LS-137 (striped bar) at a dose of 100 µg/kg. Data is presented as the mean ± S.E.M (n = 9 for vehicle treated animals and n = 9 to 10 for the animals treated with test compound). The double asterisk indicates significance compared to the vehicle control (p < 0.05).
3. Discussion
Since the pharmacological identification (Su, 1982) and determination of primary structure (Hanner et al., 1996) of the sigma 1 receptor subtype, it has been implicated in a variety of pathological disorders including psychostimulant (cocaine and methamphetamine) addiction, alcohol abuse, opioid-dependent analgesia, unipolar affective disorders, memory deficits, amnesia, neuropsychiatric disorders, neuronal destruction following stroke, retinal neuronal degradation and cancer cell proliferation (Maurice and Su, 2009). Despite the fact that the sigma 1 receptor is highly conserved and ubiquitously expressed, sigma 1 receptor gene knockout mice are viable and fertile (Langa et al., 2003), and the sigma 1 receptor knockout mice appear to exhibit normal anxiety-related behaviors (using elevated plus-maze and light/dark box tests) and normal spontaneous locomotor activity (Sabino et al., 2009) compared to wild-type mice. However, these receptor knockout mice exhibit a) reduced (formalin-induced) tonic pain (Cendán et al., 2005), b) decreased mobility in the forced swimming test (a depression-like phenotype) and c) reduced motor performance and coordination (a shorter latency to fall on the rotarod) (Mavlyutov et al., 2010).
It has been proposed that sigma 1 receptors may act as a regulator or modulator of cellular signaling rather than acting as a classical receptor which is coupled directly to a signaling pathway(s). Sigma 1 receptors have been implicated in the modulation of calcium, potassium, sodium, and N-methyl-d-aspartate channels (Maurice and Su, 2009). Sigma 1 receptors have also been implicated in regulating signaling pathways involved in cell survival and apoptosis. Ruscher and colleagues (2011) recently reported that the gene for the sigma 1 receptor was one of a panel of genes overexpressed in brain tissue from rats subjected to MCAO, that were housed in an enriched environment. Animals housed in the enriched environment appeared to have improved neurological function compared to rats housed in standard conditions. They also found that agonist activation of sigma 1 receptors in primary neuron cultures promoted neurite outgrowth and spine remodeling. Sigma receptor 1 stimulation also enhanced intracellular trafficking of components in both neurons and astrocytes involved in synaptogenesis. Studies by Antonini and coworkers (2009, 2011) indicated that agonist stimulation of sigma 1 receptors can reverse impairments in both reference and working memory caused by the administration of immunotoxin 192 IgG-saporin, which leads to the loss of cholinergic neurons in the basal forebrain. These authors suggest that agonist activation of sigma 1 receptors stabilizes inositol 1,4,5-triphosphate (IP3) receptor conformation, leading to stabilization of Ca2+ signaling from the endoplasmic reticulum into the mitochondria (Antonini et al., 2011). Allahtavkoli and Jarrott (2011) proposed that a part of the neuroprotective effects of sigma 1 receptor agonists may result because of a reduction in the levels of pro-inflammatory cytokines (including IL-1α, IL-1β, IL-2, IL-6 and TNF-α) with a concurrent enhancement of anti-inflammatory cytokine (including IL-4, IL-10 and GM-CSF) release.
Previous reports have suggested that following insult, sigma 1 receptor agonists promote cell survival, while sigma 1 receptor antagonism tends to promote cell death (Achison et al., 2007; Antonini et al., 2009; Maurice and Su, 2009; Ruscher et al., 2011; Spruce et al., 2004; Tchedre and Yorio 2008; Yang et al., 2007). The present study, which uses a novel set of D2-like dopamine and sigma receptor selective compounds, suggests that under certain conditions sigma 1 antagonists may also act as potent neuroprotectants. In this, and in our previous, study we found that sigma selective agonists (+)-pentazocine and PRE-084 were not cytoprotective in the HT-22 cell-based assay, while the prototypic sigma receptor antagonist, haloperidol, was protective. That neuroprotection obtained using HT-22 was predictive of neuroprotection using the rodent t-MCAO stroke model. These conflicting results further demonstrate the complexity of pharmacologically dissecting the functional properties of the sigma 1 receptor.
It is thought that in the HT-22 cell model (Murphy et al., 1989) extracellular glutamate leads to an inhibition of cystine uptake via its interaction with the glutamate/cystine-antiporter. Decreased intracellular cystine results in a decrease in internal glutathione, leading to the accumulation of reactive oxygen species (ROS) and activation of 12-lipoxygenase. Consequently, metabolites of arachidonic acid activate soluble guanylatecyclase, converting GTP into cyclic GMP (cGMP), which opens cGMP-dependent Ca2+-channels and leads to cell death (Breyer et al., 2007).
We previously reported the synthesis and characterization of a series of indole compounds that bound with varying selectivity to the D2 dopamine receptor compared to the D3 receptor (Vangveravong et al., 2006a). These compounds share structural elements with the classical D2-like dopamine receptor antagonist, haloperidol. Several of these were examined for affinity at sigma receptor subtypes. Both the presence and absence, as well as, the position of the methoxy group on the indole ring were pivotal for both a) D2 versus D3 receptor selectivity and b) sigma 1 receptor affinity.
We used the SV series of compounds to attempt to pharmacologically dissect the cytoprotective properties of these compounds. For haloperidol and the SV series compounds, although their affinity at D2-like receptors was similar, a decrease in affinity at sigma 1 receptors was accompanied by a decrease in the EC50 values for neuroprotection.
Furthermore, all of the SV compounds and both LS compounds bind with low affinity at sigma 2 receptors, which have recently been reported to be the progesterone receptor membrane component 1 (Xu et al., 2011). In addition, our sigma 2 receptor selective compound RHM-1–86 was not neuroprotective. These studies suggested that while sigma 1 blockade might be required for glutamate-dependent neuroprotection of HT-22 cells, sigma 2 receptor binding was not required. Furthermore, LS-127 and LS-137 both bind with low affinity at D2-like dopamine receptors, which excludes the dopaminergic activity found in SV series compounds from being responsible for the observed neuroprotection. These findings are consistent with our previous report on a series of commercially available compounds in which it was found that the cytoprotective properties of a panel of antipyschotics also correlated with affinity at sigma 1 receptors (Schetz et al., 2007).
In previous studies we found that neuroprotection in the glutamate-dependent HT-22 cell cytotoxic assay was predictive of the ability of a compound to also be neuroprotective in a rodent transient middle cerebral artery occlusion (t-MCAO) model of stroke (Perez et al., 2006; Yang et al., 2005). The latter effect is seen with a comparatively low dose (100 µg/kg) of LS-127 and LS-137. Based upon our previous studies, the level of neuroprotection observed (about 50% protection) is near the maximal achievable protection, given that the core of the infarct is not protectable (Yang et al., 2005). Although we have not performed rigorous in vivo dose response curves for our test compounds, we can say that a similar level of neuroprotection was obtained using haloperidol at a dose of 50 ug/kg (Schetz et al., 2007).
In conclusion, these results demonstrate that sigma 1 receptor selective benzyl-phenylacetamides are novel candidate pharmacotherapeutic agents that might be used to minimize neuronal death immediately after a stroke or head injury. Further studies will be required to determine if that neuroprotection can lead to protection from the cognitive deficits associated with stroke.
4. Experimental procedures
4.1. Synthesis of compounds
The methods for the synthesis for the LS series compounds have been previously described (Huang et al., 1998, 2001). The synthesis of the SV series compounds has also been previously reported (Vangveravong et al., 2006a).
4.2. Binding assays
4.2.1. Sigma receptor binding assays
LS and SV series compounds were dissolved in DMF, DMSO or ethanol, and then diluted in 50 mM Tris–HCl buffer containing 150 mM NaCl and 100 mM EDTA at pH = 7.4. The σ1 receptor binding assays were conducted in 96-well plates using guinea pig brain membrane homogenates and [3H](+)-pentazocine (Perkin Elmer, Boston, MA). The incubation time was 90 min at room temperature. Nonspecific binding was determined from samples that contained 10 µM of cold haloperidol. The reaction was terminated by the addition of ice-cold wash buffer (10 mM Tris–HCl, 150 mM NaCl, pH 7.4). The samples were harvested and filtered rapidly through a 96-well fiber glass filter plate (Millipore, Billerica, MA) that had been presoaked with 100 µL of 50 mM Tris–HCl buffer at pH 8.0 for 1 h. Each filter was washed 3 times with ice-cold wash buffer, and the filter counted in a Wallac 1450 MicroBeta liquid scintillation counter (Perkin Elmer, Boston, MA) (Tu et al., 2005, 2007, Xu et al., 2005).
The σ2 receptor binding assays were conducted using rat liver membrane homogenates and ~5 nM [3H] DTG (Perkin Elmer, Boston, MA) in the presence of 1 µM (+)-pentazocine to block σ1 sites. The incubation time was 2 h at room temperature. Nonspecific binding was defined using 10 µM of cold haloperidol. All other procedures were identical to those described for the σ1 receptor binding assay earlier (Vangveravong et al., 2006b).
Data from the competitive inhibition experiments were modeled using nonlinear regression analysis to determine IC50 values (Tu et al., 2005; Tu et al., 2007, Xu et al., 2005). Competitive curves were best fit to a one-site fit. Ki values were calculated using the method of Cheng and Prusoff (1973). Mean Ki values ± S.E.M. are reported for at least three independent experiments (Xu et al., 2005).
4.2.2. D2-like dopamine receptor binding assays
The binding properties of membrane-associated receptors were characterized by a filtration binding assay. For human D2long, D3, and D4.4 dopamine receptors expressed in HEK 293 cells, 50 µL of membrane homogenates was suspended in 50 mM Tris–HCl/150 mM NaCl/10 mM EDTA buffer, pH = 7.5 and incubated with 50 µL of 125I-IABN (Luedtke et al., 2000) at 37 °C for 60 min, using 20 uM (+)-butaclamol to define the non-specific binding. The radioligand concentration was equal to approximately 0.5 times the Kd value and the concentration of the competitive inhibitor (50 µL) ranged over 5 orders of magnitude for competition experiments. For each competition curve, two concentrations of inhibitor per decade were used and triplicates were performed. Binding was terminated by the addition of the cold wash buffer (10 mM Tris–HCl/150 mM NaCl, pH = 7.5) and filtration over a glass-fiber filter (Schleicher and Schuell No. 32). A Packard Cobra gamma counter was used to measure the radioactivity. The concentration of inhibitor that inhibits 50% of the specific binding of the radioligand (IC50 value) was determined by using nonlinear regression analysis to analyze the data of competitive inhibition experiments. Competition curves were modeled for a single site and the IC50 values were converted to equilibrium dissociation constants (Ki values) using the Cheng and Prusoff (1973) correction. Mean Ki values ± S.E.M. are reported for at least three independent experiments.
4.3. HT-22 cell-based cytoprotection assay
4.3.1. Cell culture
HT-22 is a murine hippocampal cell line that is maintained in Dulbecco’s modified Eagle’s (DMEM) media (GIBCO, Gaithersburg, PA) supplemented with 10% charcoal-stripped fetal bovine serum (HyClone, Logan, UT) and 20 µg/mL gentamycin (Sigma, St. Louis, MO) (5% CO2, 95% air, 37° C) (Perez et al., 2006). HT-22 cells (passages 18–25) were seeded into Costar 96-well plates (Corning, NY) at a density of 5000 cells per well. Compounds were administered simultaneously with glutamate insult. HT-22 cells were incubated with glutamate for 16 h then assessed for viability. Glutamate cell death in HT-22 cells is through an inhibition of the glutamate/cystine antiporter, leading to the depletion of glutathione and oxidative stress (Perez et al., 2006).
4.3.2. Cell viability
Cell viability was determined by calcein acetoxymethyl (calcein AM) assay (Molecular Probes, Eugene, OR). The calcein AM assay measures cellular esterase activity and plasma membrane integrity. To determine complete (100%) cell death, several wells received either 1% sodium dodecyl sulfate or methanol for 15 min before the calcein AM assay. Media was removed and replaced with a 1 µM solution of calcein AM in PBS. After incubation at room temperature for 15 min, fluorescence was determined (excitation 485 nm, emission 530 nm) using a fluorescence FL600 microplate reader (Biotek, Winooski, VT). Sigmoidal standard curves were created using a 4-parameter log curve with constant top and used to determine EC50 values (GraphPad Prism, version 3.02 for Windows, GraphPad Software, San Diego, CA).
4.4. Rodent middle cerebral artery occlusion model of stroke
4.4.1. Animals
Female Charles River Sprague–Dawley rats (250 g, Wilmington, MA) were acclimatized to animal facilities three days prior to surgery. Bilateral ovariectomy was performed 2 weeks prior to our studies with test drug to avoid any possible confound of the neuroprotective properties of endogenous estrogen. Drug administration and t-MCAO were performed under anesthesia with intraperitoneal (i.p.) injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). All animal procedures were approved by the University of North Texas Health Science Center Animal Care and Use Committee.
4.4.2. Preparation of drug
To achieve a formulation that is aqueous soluble and therefore suitable for intravenous administration, LS-127 and LS-137 were dissolved in aqueous 30% 2-hydroxypropyl-β-cyclodextrin (HPβCD) solution at a concentration of 100 µg/mL to yield the 1 mL/kg injection volume. Compounds (100 µg/kg) in HPβCD were administered through the jugular vein immediately after the onset of the MCAO. For control, ovariectomized females (OVX) were treated with equivalent volumes of 30% HPβCD. The number of animals per group was as follows: a) vehicle, n = 9, b) LS-127, n = 10 and c) LS-137, n = 9.
4.4.3. Cerebral ischemia reperfusion injury and lesion volume determination
Two weeks after ovariectomy, animals were anesthetized by intraperitoneal (i.p.) injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). During the procedures, rectal temperature was monitored and maintained between 36.5 °C and 37 °C with heating lamps and pads. The left middle cerebral artery was transiently occluded using previously described methods (Perez et al., 2006). Briefly, with the aid of an operating microscope (Zeiss, Jena, Germany), the left common carotid artery and internal carotid artery were exposed through a midline cervical skin incision. A 3.0-cm length of 3–0 monofilament suture with a rounded-tip was introduced into the internal carotid artery via the external carotid artery lumen and advanced until resistance was encountered. The distance between the common carotid artery bifurcation and the resistant point was 2.0 cm. The MCA was occluded for 1 h and then the suture was withdrawn for reperfusion. Animals were decapitated 24 h after reperfusion. Brains were harvested and placed in a brain matrix for slicing (Harvard Apparatus, Holliston, MA). Seven slices were made at 3, 5, 7, 9, 11, 13 and 15 mm posterior to the olfactory bulb. Slices were incubated for 30 min in 2% solution of 2,3,5-triphenyltetrazolium chloride at 37 °C, and then fixed in 10% formalin. The stained slices were photographed and subsequently measured for the ischemic lesion volume (Image-Pro Plus 4.1, Media Cybernetics, Silver Spring, MD).
4.5. Data analysis and statistics
Statistical analysis was performed using Graphpad Prism software (GraphPad Software, Inc, San Diego, CA, U.S.A.). All data are presented as means ± S.E.M. Ischemic lesion volumes were compared by one-way ANOVA followed by Tukey tests. A probability of <0.05 was considered significant.
Acknowledgments
The authors would like to thank Dr. David Schubert of the Salk Institute, San Diego, CA for providing us with the HT-22 cells line. This research was supported by grants from NS050658-01A1 (RHM), AG22550 (JWS), NS054687 (SY) and NS054651 (SY). The authors would like to thank Suzy Griffin and Michelle Taylor for their assistance in the preparation of this manuscript.
REFERENCES
- Achison M, Boylan MT, Hupp TR, Spruce BA. HIF-1alpha contributes to tumour-selective killing by the sigma receptor antagonist rimcazole. Oncogene. 2007;26:1137–1146. doi: 10.1038/sj.onc.1209890. [DOI] [PubMed] [Google Scholar]
- Allahtavkoli M, Jarrott B. Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, proinflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain Res. Bull. 2011;85:219–224. doi: 10.1016/j.brainresbull.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Antonini V, Prezzavento O, Coradazzi M, Marrazzo A, Ronsisvalle S, Arena E, Leanza G. Anti-amnesic properties of (+/−)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats. J. Neurochem. 2009;109:744–754. doi: 10.1111/j.1471-4159.2009.06000.x. [DOI] [PubMed] [Google Scholar]
- Antonini V, Marrazzo A, Kleiner G, Coradazzi M, Ronsisvalle S, Prezzavento O, Ronsisvalle G, Leanza G. Anti-amnesic and neuroprotective actions of the sigma-1 receptor agonist (−)-MR22 in rats with selective cholinergic lesion and amyloid infusion. J. Alzheimers Dis. 2011;24:569–586. doi: 10.3233/JAD-2011-101794. [DOI] [PubMed] [Google Scholar]
- Breyer A, Elstner M, Gillessen T, Weiser D, Elstner E. Glutamate-induced cell death in neuronal HT22 cells is attenuated by extracts from St. John’s wort. (Hypericum perforatum L.) Phytomedicine. 2007;14:250–255. doi: 10.1016/j.phymed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Cendán CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur. J. Pharmacol. 2005;21:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Entrena JM, Nieto FR, Cendán CM, Del Pozo E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr. Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyagi T, Bhardwaj A, Koehler RC, Traystman RJ, Hurn PD, Kirsch JR. Potent sigma 1-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine provides ischemic neuroprotection without altering dopamine accumulation in vivo in rats. Anesth. Analg. 2003;96:532–538. doi: 10.1097/00000539-200302000-00043. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacol. 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. (Berl.). [DOI] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl Acad. Sci. USA. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tsai SY, Mori T, Fujimoto M, Su TP. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin. Ther. Targets. 2011;15:557–577. doi: 10.1517/14728222.2011.560837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Hammond PS, Whirrett BR, Kuhner RJ, Wu L, Childers SR, Mach RH. Synthesis and quantitative structure-activity relationships of N-(1-benzylpiperidin-4-yl) phenylacetamides and related analogues as potent and selective sigma 1 receptor ligands. J. Med. Chem. 1998;44:4404–4415. doi: 10.1021/jm980032l. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hammond PS, Wu L, Mach RH. Synthesis and structure-activity relationships of N-(1-benzylpiperidin-4-yl) arylacetamide analogues as potent sigma1 receptor ligands. J. Med. Chem. 2001;44:4404–4415. doi: 10.1021/jm010384j. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Reddy NL, Fischer JB, Wolcott TC, Knapp AG, McBurney RN. In vitro neuroprotection by substituted guanidines with varying affinities for the N-methyl-D-aspartate receptor ionophore and for sigma sites. J. Pharmacol. Exp. Ther. 1994;271:1080–1085. [PubMed] [Google Scholar]
- Langa F, Codony X, Tovar V, Lavado A, Giménez E, Cozar P, Cantero M, Dordal A, Hernández E, Pérez R, Monroy X, Zamanillo D, Guitart X, Montoliu L. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur. J. Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Freeman RA, Boundy VA, Martin MW, Mach RH. Characterization of 125I-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38:438–449. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Mach RH, Huang Y, Freeman RA, Wu L, Vangveravong S, Luedtke RR. Conformationally-flexible benzamide analogues as dopamine D(3) and sigma(2) receptor ligands. Bioorg. Med. Chem. Lett. 2004;14:195–202. doi: 10.1016/j.bmcl.2003.09.083. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. σ Receptors: potential medications development target for anti-cocaine agents. Eur. J. Pharmacol. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Maurice T, Lockhart BP. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog Neuropsychopharmacol Biol. Psychiatry. 1997;21:69–102. doi: 10.1016/s0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavlyutov TA, Epstein ML, Andersen KA, Ziskind-Conhaim L, Ruoho AE. The sigma-1 receptor is enriched in postsynaptic sites of C-terminals in mouse motoneurons. An anatomical and behavioral study. Neurosci. 2010;167:247–255. doi: 10.1016/j.neuroscience.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monassier L, Bousquet P. Sigma receptors: from discovery to highlights of their implications in the cardiovascular system. Fundam. Clin. Pharmacol. 2002;16:1–8. doi: 10.1046/j.1472-8206.2002.00063.x. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron. 1989;2:1547–1558. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res. 2005;1038:216–222. doi: 10.1016/j.brainres.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Perez E, Cai ZY, Covey DF, Simpkins JW. Neuroprotective effects of estratriene analogs: structure-activity relationships and molecular optimization. Drug Dev. Res. 2006;68:1–15. [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, Toresson H, Ruslim-Litrus L, Oksenberg D, Urfer R, Johansson BB, Nikolich K, Wieloch T. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134:732–746. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y, Schubert D. The activation of metabotropic glutamate receptors protects nerve cells from oxidative stress. J. Neurosci. 1998;18:6662–6671. doi: 10.1523/JNEUROSCI.18-17-06662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetz JA, Perez E, Liu R, Chen S, Lee I, Simpkins JW. A prototypical sigma-1 receptor antagonist protects against brain ischemia. Brain Res. 2007;1181:1–9. doi: 10.1016/j.brainres.2007.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins N, Jankovic J. Neuroprotection in Parkinson disease. Arch. Intern. Med. 2003;163:1650–1654. doi: 10.1001/archinte.163.14.1650. [DOI] [PubMed] [Google Scholar]
- Spruce BA, Campbell LA, McTavish N, Cooper MA, Appleyard MV, O’Neill M, Howie J, Samson J, Watt S, Murray K, McLean D, Leslie NR, Safrany ST, Ferguson MJ, Peters JA, Prescott AR, Box G, Hayes A, Nutley B, Raynaud F, Downes CP, Lambert JJ, Thompson AM, Eccles S. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004;64:875–886. doi: 10.1158/0008-5472.CAN-03-3180. [DOI] [PubMed] [Google Scholar]
- Su TP. Evidence for sigma opioid receptor: binding of [3H] SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982;223:284–290. [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr. Med. Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Su TP, Schell SE, Ford-Rice FY, London ED. Correlation of inhibitory potencies of putative antagonists for sigma receptors in brain and spleen. Eur. J. Pharmacol. 1988;148:467–470. doi: 10.1016/0014-2999(88)90130-6. [DOI] [PubMed] [Google Scholar]
- Su TP, Shukla K, Gund T. Steroid binding at sigma receptors: CNS and immunological implications. CIBA Found. Symp. 1990;153:107–113. doi: 10.1002/9780470513989.ch6. [DOI] [PubMed] [Google Scholar]
- Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J. Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- Tan F, Guio-Aguilar PL, Downes C, Zhang M, O’Donovan L, Callaway JK, Crack PJ. The σ 1 receptor agonist 4-PPBP elicits ERK1/2 phosphorylation in primary neurons: a possible mechanism of neuroprotective action. Neuropharmacol. 2010;59:416–424. doi: 10.1016/j.neuropharm.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Tarawneh R, Galvin JE. Potential future neuroprotective therapies for neurodegenerative disorders and stroke. Clin. Geriatr. Med. 2010;26:125–147. doi: 10.1016/j.cger.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchedre KT, Yorio T. Sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Investig. Ophthalmol. Vis. Sci. 2008;49:2577–2588. doi: 10.1167/iovs.07-1101. [DOI] [PubMed] [Google Scholar]
- Tu Z, Dence CS, Ponde DE, Jones L, Wheeler KT, Welch MJ, Mach RH. Carbon-11 labeled sigma2 receptor ligands for imaging breast cancer. Nucl. Med. Biol. 2005;32:423–430. doi: 10.1016/j.nucmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Tu Z, Xu J, Jones LA, Li S, Dumstorff C, Vangveravong S, Chen DL, Wheeler KT, Welch MJ, Mach RH. Fluorine-18-labeled benzamide analogues for imaging the sigma2 receptor status of solid tumors with positron emission tomography. J. Med. Chem. 2007;50:3194–3204. doi: 10.1021/jm0614883. [DOI] [PubMed] [Google Scholar]
- Tuerxun T, Numakawa T, Adachi N, Kumamaru E, Kitazawa H, Kudo M, Kunugi H. SA4503, a sigma-1 receptor agonist, prevents cultured cortical neurons from oxidative stress-induced cell death via suppression of MAPK pathway activation and glutamate receptor expression. Neurosci. Lett. 2010;469:303–308. doi: 10.1016/j.neulet.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Vangveravong S, McElveen E, Taylor M, Griffin SA, Luedtke RR, Mach RH. Identification and pharmacologic characterization of D2 dopamine receptor selective antagonists. Bioorg. Med. Chem. 2006a;14:815–825. doi: 10.1016/j.bmc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Vangveravong S, Xu J, Zeng C, Mach RH. Synthesis of N-substituted 9-azabicyclo[3.3.1]nonan-3alpha-yl carbamate analogs as sigma2 receptor ligands. Bioorg. Med. Chem. 2006b;14:6988–6997. doi: 10.1016/j.bmc.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. J. Pharmacol. Exp. Ther. 2000;292:900–911. [PubMed] [Google Scholar]
- Xu J, Tu Z, Jones LA, Vangveravong S, Wheeler KT, Mach RH. [3H]N-[4-(3,4-dihydro-6,7-dimethoxyisoquinolin- 2(1H)-yl)butyl]-2-methoxy-5-methylbenzamide: a novel sigma-2 receptor probe. Eur. J. Pharmacol. 2005;525:8–17. doi: 10.1016/j.ejphar.2005.09.063. [DOI] [PubMed] [Google Scholar]
- Xu J, Zeng C, Chu W, Pan F, Rothfuss JM, Zhang F, Tu Z, Zhou D, Zeng D, Vangveravong S, Johnston F, Spitzer D, Chang KC, Hotchkiss RS, Hawkins WG, Wheeler KT, Mach RH. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011;5:380–386. doi: 10.1038/ncomms1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wang X, Simpkins JW. Estrogens as protectants of the neurovascular unit against ischemic stroke. Curr. Drug Targets CNS Neurol. Disord. 2005;4:169–177. doi: 10.2174/1568007053544174. [DOI] [PubMed] [Google Scholar]
- Yang S, Bhardwaj A, Cheng J, Alkayed NJ, Hurn PD, Kirsch JR. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth. Analg. 2007;104:1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]