Abstract
This work reviews results of in vivo dosimetry (IVD) for total skin electron beam (TSEB) therapy, focusing on new methods, data emerged within 2012. All quoted data are based on a careful review of the literature reporting IVD results for patients treated by means of TSEB therapy. Many of the reviewed papers refer mainly to now old studies and/or old guidelines and recommendations (by IAEA, AAPM and EORTC), because (due to intrinsic rareness of TSEB-treated pathologies) only a limited number of works and reports with a large set of numerical data and proper statistical analysis is up-to-day available in scientific literature. Nonetheless, a general summary of the results obtained by the now numerous IVD techniques available is reported; innovative devices and methods, together with areas of possible further and possibly multicenter investigations for TSEB therapies are highlighted.
Keywords: In vivo dosimetry, Total skin electron beam therapy, Systematic review
1. Background
In last decades, TSEB has become an important medical service for the treatment of rare skin malignancies (see references in5,10 for historical background). On the basis of epidemiological data, only a low percentage of patients need this kind of treatments; anyway, various rare skin diseases such as mycosis fungoides, T-Cell lymphoma, Sèzary syndrome, Kaposis sarcoma and inflammatory breast cancer are usually treated with TSEB.20 Related uncommon setup42 evaluation and special tools requirements, which need to be implemented for successful treatments were already described by major reports and available guidelines.1 At present, in-depth explanation of the process of commissioning and evaluation is already available, with due mathematical formalism for proper dose calculations2,3; moreover, preliminarily needed measurements have been extensively described, and related in vivo dosimetry problems discussed in detail.3,4 Many approximations and relatively large tolerance levels concerning the results obtained by in vivo measurements are to be ordinarily expected and commonly accepted in the course of TSEB therapy by involved care center and professionals. At any rate, suitable in vivo dosimetry measures must be provided for safety treatment procedure and the bulk of data here reviewed confirm the need of these procedures for such complex techniques as the ones involved in TSEB treatments. Specifically, many works and authors (see e.g.4,26,28 and references therein) report in vivo dosimetry as a must for TSEB therapy for:
-
•
providing objective data for the radiation oncologist;
-
•
prescribing supplemental fields of treatment;
-
•
avoiding possible toxicity areas and infections that could have severe implications for patients Quality of Life (QoL) and patients follow-up.
2. Systematic review of literature criteria
Our method of bibliographic search and systematic review made use of Medline, Cochrane Library and Science Direct search engines for the major clinical relevant articles and Medical Physics and JACMP for specific physics articles. Using proper key words (see above Abstract), the temporal range 2000–2012 was imposed as a constraint. We also extended our search to include the main guidelines and reports available from IAEA, ASTRO, AAPM and ESTRO. Additional studies were included after the bibliographic search, using both our professional knowledge and advice by other experts of TSEB sector to provide more exhaustive data and/or mention of innovative approaches in TSEB fields. General search map and results are detailed in Graph 1. Basically, only papers dealing with results of patient treatments were considered. Many articles discussing only phantom simulation and/or techniques of implementation were not considered as a main topic of the present review. In the absence of comparative data of in vivo dosimetry, measurement techniques with innovative approach was considered as an ‘extra inclusion’ criterion. In all, we tried to report mainly studies with patient data, but without advancing any interpretation about clinical relevance and without favoring any personal judgment about measurement techniques. Due to the limited number of publications, during our systematic review we included some other papers, reports and guidelines with evaluation of the articles reporting data of in vivo dosimetry applied to the TSEB, not reachable by the above-mentioned libraries, but currently available by the Google Scholar engine.
Graph 1.
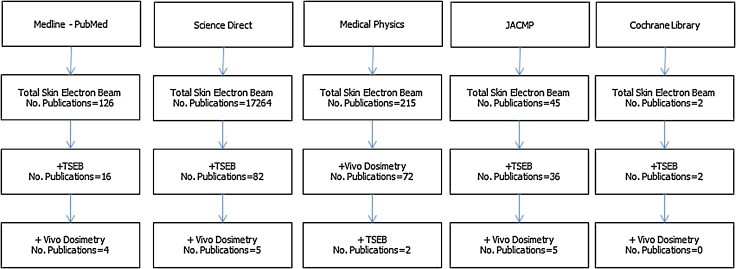
Systematic review of the literature and adopted keywords.
Finally, data for each study were reported in separate evidence in a synoptic table (Table 1). Following above-specified rationale, our in vivo results summarizing table include: authors; number of treated patients with in vivo data (whenever reported); number of measure points over the patients skin; the kind of IVD detectors or devices used; the irradiation technique adopted in treatments; and an indication of the results obtained by the studies as their conclusions.
Table 1.
Review of the results of in vivo dosimetry for patients treated with TSEB therapy.
| Authors (years) | No. patients | Proposed No. of readings | In vivo dosimetry device(s) | Irradiation technique | Main results |
|---|---|---|---|---|---|
| Antolak et al. (1998) | 72 patients | 22 points | TLD | 6 static dual fields + scatter plate | Tables 3 and 4 in Ref.5 |
| Yaparpalvi et al. (2000) | 360 patients | 809 in vivo dose measurements | Diode detectors | n.a. | 11.5% of the measurements outside the threshold of ±6% |
| Anacak (2003) | 67 patients | 10 points | TLD | 6 static dual fields | ±15.4%a |
| Piotrowski et al. (2003) | 3 patients | 736 points (34 points/patient) | TLD | Rotational | ±10% |
| Marre and Marinello (2004) | n.a. | 20 fractions | p-Type diode | n.a. | ±1% |
| Gamble et al. (2005) | 2 patients | n.a. | Film Gaf-chromic | 6 static dual fields | ±10% of the prescription |
| Piotrowski et al. (2006) | 3 patients | 22 points (proposed by Antolak) 12 points (proposed by other authors) |
TLD | Rotational dual fields + scatter plate | See Table 3 in Ref.15 |
| Bufacchi et al. (2007) | 4 patients | 20 points (TLD) 6 points (Gaf-chromic vs. TLD) |
TLD film Gaf-chromic | 6 static dual fields | TLD: ±1.8% Film: ±3.7% |
| Xu et al. (2008)17 | 1 patient | n.a. | MOSFET | Rotational | ±5% |
| Poli et al. (2010) | n.a. | n.a. | TLD | ±6.5 to ± 50% | |
| Al-Mohammed et al. (2011) | 6 patients | 10 | MOSFET | Stanford twelve-fields | ±5.5% |
| Bao et al. (2012) | 2 infants | 12 points | OSL | Rotational | ±10% of the prescription |
n.a., not available.
Mean deviation of all measurements.
3. Materials and methods
Different approaches and strategies to deliver TSEB therapy are described in literature (for a review, see45 and references therein): rotational TSEB, multiple field techniques (Stanford Technique)7, single scatterer horizontal beam, translational techniques, patient lying on technique and possible combination of these techniques.11,19,20 Each modality of delivery requires accurate calibration and evaluation of the IVD data, as this kind of dosimetry has become a routine procedure for many centers providing TSEB therapy. Anyway, due to large fields of irradiation used in therapy and the multiplicity of involved and/or emerging techniques, the determination of the absolute dose delivered for specific anatomical site may often require specific evaluation and proper rationale concerning the obtained results.
Best results for IVD were obtained in past years by thermo-luminescent dosimetry (TLD) and film dosimetry.5,6,11,15,21 Recently, new devices and related dosimetric techniques have appeared (radiochromic films,14 MOSFET detectors24 diodes and optically stimulated luminescent (OSL) devices18,22) highlighting some important advantages (such as two-dimensional mapping apart from other opportunities) of new technologies over more traditional ones, mostly in association with new IVD techniques. But some cautions are to be taken into considerations in order for the new IVD techniques and new devices to actually overcome and substitute the more traditional ones. For example, different and sometimes even conflicting conclusions from many authors are reported about the use of the radiochromic film. Some groups report evidence of good agreement between measure with TLD and Gaf-chromic film,14,16,19,44 but some groups call for more caution and conclude with the need of requirements for further or deeper investigation in particular for large variation due to low dose exposures.16,23 Few of the scientific papers available describe Monte Carlo calculations (see e.g.43) as an alternative or to confirm TSEB in vivo film dosimetry; but Monte Carlo simulations appear to be still far from taking into affordable account complex details, like room geometry and different off-axis distances.13,22 Moreover, both simulations and observed measures may have inaccuracies, due to intricate details (patient skin distance variations, patient motion and inappropriate setup, which could actually account for the discrepancies between simulation and IVD results. Major faults of the IVD devices traditionally used for TSEB therapy are related to their lack of real-time measurements. Mostly because of the delay between exposure and reading time, usual IVD devices and techniques are highly time consuming in terms of calibration, pre-irradiation or preparation. The evolution of new medical devices will allegedly open new areas of investigation, providing actual real-time IVD for TSEB (and in total body irradiation as well). For example, some institutions have explored IVD using OSL or MOSFET as a pioneering approach to patient safety protection instead of reserving them only for radiation protection.24,34–36 The integration of related data with routine tools and other medical devices could undeniably open new frontiers for in vivo dosimetry. We actually report here some experiences of IVD for TSEB using semiconductor diode technology,1,8,9 although some authors conclude that these devices, contrary to some suggestions, could be actually convenient for real-time dose delivery verification, but with some anomalies.8 Anyway, it is evident that extensive reference to measurements using TLD remains necessary to reliably evaluate dose distribution, to discover the overlapping of dose and to demonstrate the effectiveness of delivered dose. Excluding the IVD studies using TLD, many of the alternative papers about new in vivo dosimetry tools still concern a too restricted number of patients and measures to ensure robust effectiveness and efficiency of new IVD methods. Finally, still very few experiences are reported, concerning TSEB therapy for infants (anyway, see21 and references therein).
4. Results
During the last decade, because of the patients safety approach of their centers, many authors have shown a great interest in in vivo dosimetry procedures and problems in TSEB treatments. Replacement of complex and long management required by the traditional use of TLD still remains the aim of many studies in order to replace old rigid approaches with more flexible and less time-consuming solutions. Some authors conclude that innovative device and new methods of IVD could actually be integrated with other systems and software already available, such as e-chart and specific software for IVD database.18 In our opinion, comparisons of new techniques for IVD still assess TLD measures as a gold standard. When new methods (OSL, MOSFET, Gaf-Chromic) are uncritically used, possible bias of statistical analysis could be introduced, primarily because of the low level of statistical data available so far. An alternative could be the introduction of Monte Carlo cross-reference calculations but (as above mentioned) it is reported that many factors can influence dose calculations, thereby inducing almost unavoidable discrepancies with in vivo measures.13
On the other hand, there is a tendency to cite only advantages of new techniques, thereby partially hiding problems which are still relevant and connected to them. It should be underlined that data coming from new techniques could be misinterpreted and could underestimate the criticality of the in vivo dosimetry and the possible effects of irradiation on patients. In addition to this, it is not often easily identifiable, if the improvements of the IVD techniques using new devices are actually correlated with the new practice, or if they could be simply considered as statistical events related to the small number of patients analyzed. Nonetheless, these new options can be considered, with a proper degree of caution, for commissioning purposes and cross-reference techniques validation.
To minimize, to prevent and possibly to avoid toxicity and to help proper evaluation of undesirable effects, each IVD procedure should be accurate and as reproducible as possible during the entire course of treatments; especially in infants, and wherever clinical need demands for additional irradiation above all, e.g. using local fields. New IVD techniques and devices have not yet been extensively used to monitor (late) toxicity effects, and it is not possible to identify on the basis of published studies if identical anatomical regions of analysis have actually been considered. Most of the recent studies make use of new in vivo devices in the region of uniformity or in position of excellent geometry with respect to the electron beams. But it is known that some of the criticality for the electron dose calculation and/or dose deposition is induced by the presence of cavities and/or by irregular body shape. Mapping on of phantom-surface dose distributions is actually available both for rotational partial skin electron irradiation39 and for general TSEB,40,41 whereas in vivo dose variations with depth and body locations during TSEB are almost systematically reported in cited papers, but usually only for measurements with traditional TLD dosimeters. At any rate, dose uniformity remains difficult to reach, because of the varying curvature of body surface over the stray field14,25,27 and some parts of the patient body can of course shield other parts. The main solution to assure, even in those locations, the prescribed dose homogeneity within proper limits (say, around reference limits of ±10%) is to carry out proper (and possibly real-time) in vivo measurements during patient irradiation.
A great part of recent IVD studies displays a major bias due to unavoidable reduced statistical numerousness of data and measures on patients, because of the intrinsic rareness of TBET cared pathologies.14,16,19 As reported in Table 1, only few studies report a large number of cases, specifying number of patients and number and location of points of measure over patient skin. Moreover, only a few authors have given details about IVD accuracy, correlation with specific anatomy position and error associated with specific points of measure. In this way, a further small bias into the statistical results is probably introduced, due to the indetermination of the points of measure. For example, TLD techniques have already reported many discrepancies between the dose planned and the dose delivered for specific anatomical areas,15 but analogous data are not yet available for newer in vivo devices and techniques.
When measurements are performed, problems of over/under dosage can obviously appear due to inhomogeneities in air cavities (e.g. ears) or behind bone structures (e.g. mouth). In these cases in vivo dosimeters should be used with extreme care. Detailed investigations reveal that many dose discrepancies are due to inaccurate IVD device orientation and positioning, especially in the regions with rapidly changing contours and/or sloping surfaces. Relying on the solid base of TLD measures of the last decades, it is now possible to identify the body part with maximal criticalities. Some authors, using TLD in vivo dosimetry, actually report critical dose distribution related to the position of the TLD: particularly for the scalp, hands, biceps, shoulder, perineum, feet and finger; the same complications during IVD procedures using new devices are only partly described. Thus, hypotheses about new IVD protocols and related devices should take into consideration these previous experiences and reproduce analogous and expectedly better results before being routinely implemented12,23,25 (and references therein).
5. Discussion
In vivo dosimetry has as its justification principles both the safety of patients and the reduction of possible errors. One major aim of the in vivo dosimetry process is to provide the correct information of the cumulated dose during radiation treatment and, if possible, to monitor in real-time the dose delivered. Safety procedures and commissioning are detailed in depth in many reports and guidelines for the use of external radiation therapy with electrons (e.g. AAPM, ESTRO, IAEA).1–4 The health risk management has developed statistical analysis to evaluate the procedure performance and safety (e.g. FMEA/FMECA and RCA; see28–31) to prevent incorrect administration of complex procedures to patients resulting from direct/indirect improper interventions and/or errors. This could reasonably be prevented not only by the use of proper in vivo techniques, but even with the build up of adequate staffs with adequate training and/or license to perform these complex procedures. Obviously, all of these should be associated with adequate calibration, careful commissioning, adequate QA programs, specific training and in-depth knowledge of the adopted TSEB therapy technique(s). Adverse risks related to wrong or improper TSEB delivery can always appear, due to improper use or absence of professionals for in vivo dosimetry or to underestimation of the importance of these tasks during the treatment sessions.
The review of the TSEB/IVD results should also be discussed in depth and proved by mutual (possibly, by multi-center) studies, to determine actual accuracy and to shape standards of in vivo doses for TSEB treatments. Some groups have already tried to interrelate and inter-evaluate performances for TSEB therapy and IVD techniques, but they have concluded that occurring ascertained variations in dose distribution among different TSEB treatment techniques require further investigation, which should include clinical evidence and practical considerations.23
Most of the authors quoted in Table 1, according to TLD in vivo measurement, agree to the tolerance range of ±10% of the TSEB therapy prescribed dose. The new above-mentioned techniques of IVD seem (see Table 1) apt to promise a much greater accuracy (±1% for p-type diode12 up to ±5.5% for MOSFET24). Anyway, to obtain new measure (gold) standards and to avoid possible unexpected results or toxicity, new methods and devices need to be further investigated and validated, with more statistical data relying on precise indication of the anatomical skin points/regions of measure.
As widely recommended in guidelines and reports, the use of boost fields requires careful clinical judgment based on in vivo dosimetry15 (and references therein), because wrong or inappropriate information could originate misjudgments and causeless skin lesions. Skin dose associated with radiation therapy is of course of primary interest in clinical evaluation and for investigation about possible risks of late effects. But it should be always taken into considerations that skin dose is in no way an intuitive matter and it is difficult to measure. Even here, literature is supported by some Monte Carlo simulations13; nonetheless, at least the above-mentioned native problems of the TSEB techniques require to be properly solved, before thinking about greatly reducing the need of in vivo measurements. At present, using a set of general rules, equations and models, skin dose can be estimated with reasonable accuracy for patients receiving in-depth radiotherapy; of course, related safety standards and follow up protocols must be extended to TSEB techniques. It should be underlined that in vivo dosimetry cannot by itself alone guarantee the TSEB optimality: patient safety and consent should be attested by both mandatory dosimetry protocols and medical physics consultations. Of course, appropriate safety checks and real-time acknowledgment could minimize some of the risks of inappropriate patient TSEB treatments. Thus, therapists must always supervise patients during treatments, both visually – by carefully oriented closed circuit and television camera(s) and by proper speak/audio communication system with the patient inside the bunker. Finally, patients condition must be assessed by radiation oncologist during the entire course of treatment to manage and possibly to prevent any acute toxicity with the aid of IVD data evaluation, to provide care support, and to take shared decisions about patch treatments and/or any other TSEB-feasible technical option.
6. Measurement tools for in vivo TSEB conditions: an overview
During many years (starting from the eighties), in vivo TSEB dosimetry has concentrated upon clinical use of thermoluminescent dosimeters and – somewhat later – diode dosimeters (see Van Dam and Marinello,4 chapters III and IV for an extended review; Furetta and Weng32 for a comprehensive description of the dosimetric technique; and Furetta33 for a general physical treatise about this mostly consolidated technique). More recent dosimetric alternatives (and more expensive too, but usually much less cumbersome and much less time-consuming, particularly in comparison with thermoluminescent dosimeters) are given by MOSFET Dosimeters (see Bloemen-van Gurp – particularly Chapter 3 – for an extended Review about the use of MOSFET devices34), by optically stimulated dosimeters (OSL) (see Esquivel et al.35 for general IVD using these devices; and Viamonte et al.36 for an example of a commercially available OSL system, used for general external beam radiotherapy) and by electron paramagnetic resonance (EPR) dosimeters (see Shultka el al. for the comparison between l-alanine EPR dosimetry measurements and data from a clinical TPS, reporting discrepancies within a range of about 0.6%37; and Schauer et al. for a general review of this technique in medical dosimetry38).
7. Conclusions
Our review of the results for in vivo dosimetry in TSEB therapy has shown that many topics still remain under investigation. The advent of methods for real-time dosimetry and the availability of more user-friendly and/or simpler devices have opened new perspectives in related medical physics area. As a further perspective, technical problems could be solved by networks of hospitals over regional area, possibly providing remote reading of instruments and tools with direct network data access by professionals, thereby greatly helping future shaping of newly coded procedures. These advantages could spread and expand new techniques – and possibly a renewed use of the old ones – to more centers, as the demand for TSEB techniques, due to remembered rareness of related pathologies, remains very limited. To possibly overcome problems of limited number of cases and related poor statistical significance (particularly for more recent and new techniques IVD results), both national and international collaboration should be claimed and arranged.
Most of the TSEB irradiation centers have locally solved some problems of in vivo dosimetry and many authors have explored new approaches and methods in order to be both flexible and accurate, to provide the radiation oncologist with the correct information for adequate dose assessments and care planning, and in order to standardize the techniques and methods for in vivo dosimetry. It must be remarked that the introduction of the MOSFET, semiconductor diodes and OSL devices (with their small dimensions and possible isotropicity with respect to the direction of incident beam) actually allows the beginnings of real-time IVD dosimetry, otherwise hardly possible (or impossible) to obtain with the TLD and FILM techniques. These new IVD systems can be quickly read, their data can be promptly integrated with chart, database and spreadsheets for calculation, and their use can be furthermore optimized by personnel and staff organization, using coded procedures and adequate training for the operators. On the same ground, these tools could actually even open new landscapes for in vivo dosimetry, as they could be applied in network of hospitals sharing devices and professionals, thereby collecting data from multiple sites. Consequently, these new options could also open opportunities for expanding the TSEB irradiation therapies to a greater number of sites and to standardize the procedures over wider regions (thereby furthermore overcoming above-mentioned statistical numeracy problems). But before all of this is possible, the efficacy and accuracy of the new procedures, both in general and special conditions must be robustly proved, so as to adequately increase the reference data bases, hopefully becoming, as soon as possible, part of nationally and internationally available guidelines.
In complex treatments, such as the ones involved in TSEB, data from clinical evaluation about treated patients are self-evidently of crucial importance, especially in the implementation of new methods and devices, with the constant aim of avoiding possible and/or unexpected late effects, due to inappropriate use of standardized techniques. In this respect, it is noticeable that wherever a TSEB therapy medical service is provided, most of the procedures are already supported with extensive commissioning, QA programs and in vivo measurements for safety purpose and accurate dose delivery. Moreover, currently, many organizations already include international-protocols-for-electron-beams based IVD, as a must for their TSEB therapy treatments. To avoid critical events, inefficacy and/or inefficiency of the TSEB techniques, all needed and/or useful information must be collected in teams of dedicated professionals, thereby ensuring real usefulness of related in vivo dosimetry techniques. Facing specific IVD technical problems, wherever no guidelines exist or no other reliable sources are available, systematic reviews of published clinical trials data and/or experts consensus, advice and review should be taken into consideration. Critical use of medical textbooks, journals, articles and government publications can be an acceptable and feasible solution as well.
Conflict of interest
None declared.
Financial disclosure
None declared.
Contributor Information
Gabriele Guidi, Email: Guidi.Gabriele@policlinico.mo.it, gabrieleguidi@yahoo.com.
Giovanni Gottardi, Email: Gottardi.Giovanni@policlinico.mo.it.
Paola Ceroni, Email: Ceroni.Paola@policlinico.mo.it.
Tiziana Costi, Email: Costi.Tiziana@policlinico.mo.it.
References
- 1.1987. Total skin electron therapy: technique and dosimetry. AAPM Report No. 23. [Google Scholar]
- 2.2005. Diode in-vivo dosimetry for patients receiving external beam radiation therapy. AAPM Report No. 87. [Google Scholar]
- 3.IAEA—Absorbed dose determination in external beam radiotherapy: an international code of practice for dosimetry based on standards of absorbed dose to water. Technical Reports Series No. 398
- 4.Van Dam J., Marinello G. 2006. Methods for in vivo dosimetry in external radiotherapy. ESTRO Booklet No. 1. [Google Scholar]
- 5.Antolak J.A., Cundiff H., Ha C.S. Utilization of thermoluminescent dosimetry in total skin electron beam radiotherapy of mycosis fungoides. Int J Radiat Oncol Biol Phys. 1998;40:101–108. doi: 10.1016/s0360-3016(97)00585-3. [DOI] [PubMed] [Google Scholar]
- 6.Van Der Merwe D.G. Dosimetry in total skin electron beam radiotherapy of mycosis fungoides—a technique which can be implemented on a conventional electron linear accelerator. Int J Radiat Oncol Biol Phys. 1993;27(2):391–396. doi: 10.1016/0360-3016(93)90252-q. [DOI] [PubMed] [Google Scholar]
- 7.El-Khatib E., Hussein S., Nikolic O., Voss J.S., Parsons C. Variation of electron beam uniformity with beam angulation and scatterer position for total skin irradiation with the Stanford technique. Int J Radiat Oncol Biol Phys. 1995;33(2):469–474. doi: 10.1016/0360-3016(95)00112-C. [DOI] [PubMed] [Google Scholar]
- 8.Yaparpalvi R., Fontenla D.P., Vikram B. Clinical experience with routine diode dosimetry for electron beam radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(4):1259–1265. doi: 10.1016/s0360-3016(00)00763-x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu R. Entrance dose measurements for in-vivo diode dosimetry: comparison of correction factors for two types of commercial silicon diode detectors. J Appl Clin Med Phys. 2000;1:100–107. doi: 10.1120/jacmp.v1i3.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anacak Y., Arican Z., Bar-Deroma R., Tamir A., Kuten A. Total skin electron irradiation: evaluation of dose uniformity throughout the skin surface. Med Dosim. 2003;28(1 (Spring)):31–34. doi: 10.1016/S0958-3947(02)00235-2. [DOI] [PubMed] [Google Scholar]
- 11.Piotrowski T., Fundowicz D., Pawlaczyk M., Malicki J. Thermoluminescent dosimetry in rotary-dual technique of the total skin electron irradiation. Neoplasma. 2003;50:2. [PubMed] [Google Scholar]
- 12.Marre D., Marinello G. Comparison of p-type commercial electron diodes for in vivo dosimetry. Med Phys. 2004;31:50. doi: 10.1118/1.1630492. [DOI] [PubMed] [Google Scholar]
- 13.Ye S.J., Pareek P.N., Spencer S., Duan J., Brezovich I.A. Monte Carlo techniques for scattering foil design and dosimetry in total skin electron irradiations. Med Phys. 2005;32(June (6)):1460–1468. doi: 10.1118/1.1924368. [DOI] [PubMed] [Google Scholar]
- 14.Gamble L.M., Farrell T.J., Jones G.W., Hayward J.E. Two-dimensional mapping of underdosed areas using radiochromic film for patients undergoing total skin electron beam radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62(3):920–924. doi: 10.1016/j.ijrobp.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 15.Piotrowski T., Malicki J. The rotary dual technique for total skin irradiation in the treatment of mycosis fungoides—a description of the applied method. Rep Pract Oncol Radiother. 2006;11(1):29–37. [Google Scholar]
- 16.Bufacchi A., Carosi A., Adorante N. In vivo EBT radiochromic film dosimetry of electron beam for total skin electron therapy TSET. Phys Med. 2007;23:67–72. doi: 10.1016/j.ejmp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Xu M., Sethi A., Glasgow G.P. TU-FF-A2-02: dual-fields rotational total skin electron irradiation/therapy. Med Phys. 2008;35:2920. [Google Scholar]
- 18.Esquivel C., Smith M., Stathakis S., Gutierrez A., Shi C., Papanikolaou N. SU-FF-T-267: total skin electron therapy skin: dose validation using optically stimulated luminescent dosimeters. Med Phys. 2009;36:2582. [Google Scholar]
- 19.Schiapparelli P., Zefiro D., Massone F., Taccini G. Total skin electron therapy: a reimplementation using radiochromic films and IAEA TRS-398 code of practice. Med Phys. 2010;37(July (7)):3510–3517. doi: 10.1118/1.3442301. [DOI] [PubMed] [Google Scholar]
- 20.Diamantopoulos S., Platoni K., Dilvoi M. Clinical implementation of total skin electron beam (TSEB) therapy: a review of the relevant literature. Phys Med. 2011;27(April (2)):62–68. doi: 10.1016/j.ejmp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Bao Q., Hrycushko B., Dugas J., Hager F., Solberg T. A technique for pediatric total skin electron irradiation. Br J Radiol. 2011;84:1125–1130. doi: 10.1186/1748-717X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Khatib ZT. Total skin electron therapy using beam modifiers. Post Graduate Diploma in Medical Physics 2006 (Al-Yarmouk University)/School of Applied Sciences, Royal Melbourne Institute of Technology (Jordanian University) (http://researchbank.rmit.edu.au/eserv/rmit:9498/Al_Khatib.pdf).
- 23.Baugh G., Al-Alawi, Fletcher L., Mills J.A., Grieve J. A preliminary comparison of total skin electron treatment techniques to demonstrate the application of a mid-torso phantom for measurement of dose penetration. Br J Radiol. 2011;84(December (1008)):1125–1130. doi: 10.1259/bjr/52924135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Mohammed H.I. Measuring patient's skin dose during total skin electron therapy using MOSFET. Biomed Res. 2011;22(1):9–14. [Google Scholar]
- 25.Kry S.F., Smith S.A., Weathers R., Stovall M. Skin dose during radiotherapy: a summary and general estimation technique. J Appl Clin Med Phys. 2012;13(3):373. doi: 10.1120/jacmp.v13i3.3734. May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones G.W., Kacinski B.M., Wilson L.D. Total skin electron radiation in the management of mycosis fungoides: consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group; EORTC Cutaneous Lymphoma Project Group. J Am Acad Dermatol. 2002;47(3):364–370. doi: 10.1067/mjd.2002.123482. [DOI] [PubMed] [Google Scholar]
- 27.Poli M.E.R., Todo A.S., Campos L.L. 2010. Dose Measurements in the treatment of mycosis fungoides with total skin irradiation using a 4 MeV electron beam.http://www.irpa.net/irpa10/cdrom/00534.pdf [Google Scholar]
- 28.Total skin electron beam therapy (TSEBT): a guide for patients and care. The Clatterbridge Cancer Centre NHS Foundation Trust. (www.clatterbridgecc.nhs.uk). 2012.
- 29.World Health Organization; 2008. Radiotherapy risk profile. Technical manual.http://www.who.int/patientsafety/activities/technical/radiotherapy_risk_profile.pdf [Google Scholar]
- 30.The Pennsylvania patient safety authority: errors in radiation therapy, vol. 6, No. 3; September 2009.
- 31.IAEA—radiation protection of patients. <https://rpop.iaea.org/RPOP/RPoP/Content/index.htm>.
- 32.Furetta C., Weng P.S. World Scientific Publishing Co; 1998. Operational thermoluminescence dosimetry. [Google Scholar]
- 33.Furetta C. World Scientific Publishing Co; 2003. Handbook of thermoluminescence. [Google Scholar]
- 34.Bloemen-Van Gurp E.J. Proefschrift Pers Universitair Maastricht; 2009. In vivo dosimetry using MOSFET detectors in radiotherapy.http://arno.unimaas.nl/show.cgi?fid=16798 [Google Scholar]
- 35.Esquivel C., Smith M., Stathakis S., Gutiérrez A., Shi C., Papanikolaou N. SU-FF-T-260. In vivo dose measurements for total body irradiation using optically stimulated luminescent dosimeters. Med Phys. 2009;36(6):2580. [Google Scholar]
- 36.Viamonte A., De La Rosa L.A.R., Bukley L.A., Cherpak A., Cygler J.E. Radiotherapy dosimetry using a commercial OSL system. Med Phys. 2008;35(4):1261–1266. doi: 10.1118/1.2841940. [DOI] [PubMed] [Google Scholar]
- 37.Schultka K., Ciesielski B., Wysocka B. In vivo dosimetry in electron beam teletherapy using electron paramagnetic resonance in l-alanine. Nowotwory J Oncol. 2005;55(6):448–451. [Google Scholar]
- 38.Schauer D.A., Iwasaki A., Romanyukha A.A., Swartz H.M., Onori S. Electron paramagnetic resonance (EPR) in medical dosimetry. Radiat Meas. 2007;21:S117–S123. [Google Scholar]
- 39.Müller-Sievers K., Ertan E., Kober B. Dosimetry of rotational partial-skin electron irradiation. Radiother Oncol. 2001;58:187–192. doi: 10.1016/s0167-8140(00)00329-7. [DOI] [PubMed] [Google Scholar]
- 40.Weaver R.D., Bruce B.S., Gerbi J., Dusenbery K.E. Evaluation of dose variation during total skin electron irradiation using thermoluminescent dosimeters. Int J Radiat Oncol Biol Phys. 1995;33(2):475–478. doi: 10.1016/0360-3016(95)00161-Q. [DOI] [PubMed] [Google Scholar]
- 41.Reynard E.P., Evans M.D.C., Devic S. Rotational total skin electron irradiation with a linear accelerator. J Appl Clin Med Phys. 2008;9(4):2793–2805. doi: 10.1120/jacmp.v9i4.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platoni K., Diamantopoulos S., Panayiotakis G. First application of total skin electron beam irradiation in Greece: setup, measurements and dosimetry. Phys Med. 2012;28:174–182. doi: 10.1016/j.ejmp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 43.O'Shea T.P., Foley M.J., Rajasekar D. Electron beam therapy at extended source-to-surface distance: a Monte Carlo investigation. J Appl Clin Med Phys. 2008;9(4):57–67. doi: 10.1120/jacmp.v9i4.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiapparelli P., Zefiro D., Massone F., Taccini G. Total skin electron therapy (TSET). A reimplementation using radiochromic films and IAEA TRS-398 code of practice. Med Phys. 2010;37(7):3510–3517. doi: 10.1118/1.3442301. [DOI] [PubMed] [Google Scholar]
- 45.Piotrowski T., Milecki P., Skórska M., Fundowicz D. Total skin electron irradiation techniques: a review. Postep Derm Alergol. 2013;1:50–55. doi: 10.5114/pdia.2013.33379. [DOI] [PMC free article] [PubMed] [Google Scholar]


