Abstract
Aim
To evaluate the current treatment of mycosis fungoides (MF) and Sézary syndrome (SS) focusing on the role of radiotherapy (RT), its principles and indications, and the perspectives of the novel irradiation technologies.
Background
MF and SS are rare lymphoproliferative diseases whose incidence is increasing. For a long time RT has been used as a single modality or in integrated treatment programs for these diseases.
Materials and methods
The latest systematic reviews, primary studies and new diagnostic and treatment guidelines on MF and SS were analyzed. Clinical outcomes together with the technical aspects and the role of RT were also evaluated.
Results
New data are available on pathogenesis, diagnostic criteria, classification and staging procedures for MF and SS and several local and systemic therapies are proposed. Localized RT can cure “minimal stage” MF while total skin electron beam irradiation (TSEI) may cure initial-stage disease and may offer important symptom relief (itch, erythroderma) in a more advanced setting. Despite its efficacy, RT is not largely used, mainly because of some technical difficulties but new RT technologies may be proposed to treat large skin surfaces.
Conclusions
New treatment programs offer good results, with median survival of more than 12 years in early-stage MF, but the median survival of 2.5 years or less in advanced stages is still a challenge. RT remains an option for all stages with a good cost/effectiveness ratio in a curative or palliative setting. New RT technologies can overcome some technical problems of treating large skin surfaces.
Keywords: Radiotherapy, Mycosis fungoides, Sézary syndrome, Total skin electron irradiation
1. Background
Cutaneous T-cell lymphomas (CTCLs) are a group of rare non-Hodgkin's lymphomas (NHLs) that primarily develop in the skin (primary cutaneous lymphomas) with clonal accumulation of neoplastic T lymphocytes, and at times progress to involve lymph nodes, blood and visceral organs.1 The classification of primary cutaneous lymphoma (PCL) has evolved over the past 50 years. In 2005, WHO and EORTC classifications were combined to obtain the current PCL classification.2,3 The WHO-EORTC classification for cutaneous lymphomas distinguishes between cutaneous T-cell lymphomas (CTCL), cutaneous B-cell lymphomas (CBCL), lymphomas from Natural Killer cells (NK) and a group of temporarily unclassifiable lymphomas with undefined phenotypic characteristics.4,5 CTCLs account for 71% of cases of primary cutaneous lymphomas compared with 29% for cutaneous B-cell lymphomas.6 The annual incidence of CTCLs in USA is 0.3–1 per 100,000 people. Incidence continuously rises for unknown causes,6,7 and it is correlated with high physician density, high family income and higher social setting.7 mycosis fungoides (MF) is the most common type of CTCLs, and accounts for about 50–70% of CTCL cases, while Sézary Syndrome (SS), a leukemic variant of CTCLs, accounts for 1–3% of cases.4,6,8
The present paper aims at reviewing the role of radiotherapy within the framework of the current management of MF and SS and is based on the analysis of data regarding diagnostic and treatment programs derived from the latest systematic reviews, primary studies and new guideline publications; radiotherapy role is discussed in terms of expected outcomes, toxicity and technical issues.
2. Diagnosis
MF is an extranodal NHL of mature T-cells with primary cutaneous involvement, defined as a chronic, indolent disease with slow progression. It is characterized by the development of patches, plaques or tumors in the skin. SS is an erythrodermic, leukemic variant of CTCL, and it is characterized by circulating, atypical, malignant T lymphocytes with cerebriform nuclei (Sézary cells), erythroderma and lymphadenopathy. Patients with CTCL may also develop visceral organ involvement. One unique feature of CTCL is that the malignant T cell clone expands at the expense of normal T cells, creating an immunodeficiency, so patients with CTCL have an increased risk of second malignancies, and can die due to bacterial infections and septicemia or viral infections.9–12
Malignant T lymphocytes in MF and SS are characterized by the following immunophenotype: CD2+, CD3+, CD4+, CD5+, CCR4+, CD45RO+, rarely CD8+, and they lack certain T-cell markers, CD7 and CD26. T cells also express cutaneous lymphocyte antigen (CLA) and TH2 cytokines.13
In a subgroup of patients with MF it has been a large cell transformation (LCT) documented that is diagnosed when large cells are present in more than 25% of lymphoid/tumor cell infiltrates in a skin lesion biopsy.14,15 Diagnosis is based on the integration of clinical, histopathologic, immunopathologic and molecular biological characteristics.16 Complete physical examination (examination of entire skin, palpation of peripheral lymph nodes, palpation for organomegaly), biopsy of suspicious skin sites and immunohistochemical studies of skin biopsy are essential to confirm the diagnosis. Biopsy of suspicious lymph nodes and assessment of peripheral blood for Sézary cells are recommended in the absence of a definitive skin diagnosis. Lymph node biopsy is also recommended for clinically abnormal nodes (≥1.5 cm in diameter).17 Visceral disease with the involvement of an organ (e.g., spleen, liver) other than the skin, nodes or blood should be documented using imaging studies. PET-CT scan of the body is recommended in patients with unfavorable features (T2 or higher, folliculotropic MF or large cell transformation, palpable adenopathy or abnormal blood test results).18 Bone marrow biopsy may be helpful only in the case of suspected marrow involvement or evident unexplained blood count abnormalities.17
3. Staging and prognosis
MF/SS are classified into 4 clinical stages based on the TNMB (tumor–node–metastasis–blood), the classification which takes into account the extent of skin involvement based on the percentage of body surface area (BSA), the presence of lymph node or visceral disease and the detection of Sézary cells in the peripheral blood (Table 1).17,19 MF/SS are generally considered incurable conditions, but it is important to recognize that the majority of patients have an indolent form of the disease with 65–85% of patients with MF having stage IA or IB disease20,21 and living for many years. The most significant prognostic factors for survival in patients with MF include age at presentation, extent and type of skin involvement (T classification), overall stage, presence of extracutaneous disease and peripheral blood involvement.22,23 Long-term follow-up data from a retrospective study on 525 patients with MF and SS showed an independent prognostic value of these factors in a multivariate analysis.23 The risk of disease progression, development of extracutaneous disease and death due to MF was correlated with initial T classification. The extent of blood involvement was significantly correlated with survival outcomes. In a study based on data from a large number of patients with MF/SS (N = 1502) registered in a large cutaneous lymphoma database, multivariate analysis showed that advanced skin (T) stage, peripheral blood involvement, elevated LDH, and folliculotropic MF were independent factors predictive of an increased risk of disease progression and death.22 The majority of patients with early-stage disease (stages IA, IB, and IIA) do not progress to more advanced-stage disease, and patients presenting with isolated patch or plaque disease (T1–T2) may have a median survival of more than 12 years.23,24
Table 1.
Tumor–node–metastasis–blood (TNMB) classification in mycosis fungoides and Sézary syndrome (ISCL/EORTC revision).17,19
| T (skin) | ||
| T1 | Limited patches, papules, and/or plaques covering <10% of the skin surface. May further stratify into T1a (patch only) vs. T1b (plaque ± patch) | |
| T2 | Patches, papules or plaques covering ≥10% of the skin surface. May further stratify into T2a (patch only) vs. T2b (plaque ± patch) | |
| T3 | One or more tumors (diameter ≥1 cm) | |
| T4 | Confluence of erythroderma covering >80% of BSA | |
| N (nodes) | ||
| N0 | No clinically abnormal peripheral lymph nodes, no biopsy required | |
| N1 | Clinically abnormal peripheral lymph nodes, histopathology: Dutch grade 1 or NCI LN0–2 | |
| 1a | Clone negative | |
| 1b | Clone positive | |
| N2 | Clinically abnormal peripheral lymph nodes, histopathology: Dutch grade 2 or NCI LN3 | |
| 2a | Clone negative | |
| 2b | Clone positive | |
| N3 | Clinically abnormal peripheral lymph nodes, histopathology: Dutch grade 3–4 or NCI LN4, clone negative or positive | |
| Nx | Clinically abnormal peripheral lymph nodes, not confirmed by histopathology | |
| M (viscera) | ||
| M0 | No visceral organ involvement | |
| M1 | Visceral organ involvement (organ specified and confirmed by histopathology) | |
| B (blood) | ||
| B0 | Absence of significant blood involvement: ≤5% of peripheral blood lymphocytes are atypical (Sézary) cell | |
| B0a | Clone negative | |
| B0b | Clone positive | |
| B1 | Low blood tumor burden: >5% of peripheral blood lymphocytes are atypical (Sézary) cells but does not meet the criteria of B2 | |
| B1a | Clone negative | |
| B1b | Clone positive | |
| B2 | High blood tumor burden: ≥1000/mcL Sézary cells with positive clone; one of the following can be substituted for Sézary cells: CD4/CD8 ≥ 10, CD4 + CD7-cells ≥ 40% or CD4 + CD26-cells ≥ 30% | |
| Classification of stages in mycosis fungoides and Sézary syndrome | ||||
|---|---|---|---|---|
| Stage | Tumor | Node | Metastasis | Blood |
| IA | 1 | 0 | 0 | 0–1 |
| IB | 2 | 0 | 0 | 0–1 |
| IIA | 1–2 | 1–2 | 0 | 0–1 |
| IIB | 3 | 0–2 | 0 | 0–1 |
| IIIA | 4 | 0–2 | 0 | 0 |
| IIIB | 4 | 0–2 | 0 | 1 |
| IVA1 | 1–4 | 0–2 | 0 | 0–2 |
| IVA2 | 1–4 | 3 | 0 | 0–2 |
| IVB | 1–4 | 0–3 | 0–2 | |
BSA, body surface area; NCI, National Cancer Institute (NCI-VA classification); Dutch, Dutch system. Sézary syndrome is defined as meeting T4 plus B2 criteria.
Moreover, patients with stage IA disease do not appear to have a decreased survival compared with an age-, sex-, and race-matched population.25 Patients with advanced-stage disease (stages IIB, III, and IVA) with tumors, erythroderma, and lymph node or blood involvement, but no visceral involvement, have a median survival of 5 years from time of presentation. It has to be noted that patients with tumors (T3) have an inferior outcome compared to those with erythroderma (T4). Patients with visceral involvement (stage IVB) are rare and have a median survival of only 2.5 years or less.20,23,25–27
4. Current treatment options other than radiotherapy
Several treatment options do exist for the management of mycosis fungoides or Sézary syndrome, and they are all summarized in Table 2. The current management is based on the stage of disease, and it includes skin-directed therapies and localized radiation treatment for limited disease as well as systemic therapy supplemented by or integrated with total skin electron irradiation for more advanced stages.
Table 2.
Stage-based treatment options and results for mycosis fungoides or Sézary syndrome.
| Stage | Results (5 years DSS23) | Treatment options | |
|---|---|---|---|
| IA | 100% | Local RT | |
| Topical corticosteroids | |||
| Topical chemotherapy (i.e., nitrogen mustard or carmustine) | |||
| Topical retinoids (i.e., bexarotene or tazarotene) | |||
| Topical imiquimod | |||
| Phototherapy (UVB for patch or thin plaques; PUVA for thicker plaques) | |||
| IB–IIA | IB (95%) IIA (84%) |
TSEI in particular for patients with severe skin symptoms or generalized thick plaque or tumor disease (TSEI followed by systemic therapies such as interferon or bexarotene to maintain response) | |
| IA–IIA disease with B1 blood involvement | Not available | More intensive treatments as described for stage III with B1 blood involvement | |
| Patients with histological evidence of folliculotropic or large cell transformation (LCT) are usually managed as described for treatment of stage IIB disease | |||
| IIB | 56% | Limited extent tumor disease with or without patch/plaque disease | Local RT and an adjuvant systemic therapy (retinoids, IFN, HDAC inhibitors, ECP, denileukin diftitox, methotrexate) |
| Systemic therapy with or without RT and with or without skin-directed therapy | |||
| Generalized tumor disease | TSEI or systemic therapy (when use TSEI, adjuvant therapy with systemic therapies can be considered to improve response duration) | ||
| Patient with LCT | Systemic therapy | ||
| III | 65% | Patients with no significant blood involvement | Generalized skin-directed therapies |
| ECP | |||
| Biologic systemic therapies with or without skin-directed therapy | |||
| IV | 30% | Sézary syndrome | Single agent biologic systemic therapy or combination therapies |
| Non-Sézary or solid organ disease | Systemic chemotherapy with or without RT for local control (adjuvant biologic therapy may be considered following chemotherapy to improve response duration) | ||
Palliative treatment (see text). DSS, disease specific survival.
The management of MF/SS is centered on a “stage-based” approach and an evaluation by a multidisciplinary team is preferred. Initial treatment in patients with patch/plaque disease includes skin-directed therapies (localized or generalized), with the addition of systemic biologic therapy for refractory, or progressive disease. In patients with unfavorable prognostic features (e.g., folliculotropic or large-cell transformed MF, or B1 involvement) systemic biologic therapies may be introduced earlier in the treatment algorithm. Patients who do not respond to biologic therapy or those with very aggressive or extracutaneous disease may be treated with chemotherapy.28,29
4.1. Localized skin-directed therapies other than radiotherapy
Corticosteroids: frequently prescribed in patient with limited patch-stage MF (IA), producing response rates of over 90%.30,31 Class I (potent) topical corticosteroids, such as betamethasone dipropionate 0.05% or mometasone furoate 0.1%, are the most effective, but their long-term of use may lead to skin atrophy or striae formation, and their use on large skin surfaces may lead to systemic absorption. Patients with T1 disease have an approximately 60–65% complete response (CR) rate and a 30% partial response (PR) rate with topical steroids. Patients with T2 disease have a 25% CR rate and a 57% PR rate.31
Topical chemotherapy (mechlorethamine and carmustine): used for the management of MF for many decades.32,33 The efficacy of topical nitrogen mustard at concentrations of 0.1–0.2% in an aqueous or ointment base is demonstrated, and a complete response rate of up to 72% in early stage MF with long-term remissions (>8 years) is reported. However, long-term use is associated with the risk of secondary epidermal cancer and a high rate of hypersensitivity.34
Synthetic retinoids (bexarotene and tazarotene) and imiquimod: bexarotene gel is the only FDA approved synthetic retinoid for topical therapy in patients with MF and SS. A phase I–II trial involving 67 patients with early stage MF demonstrated a 63% ORR (CR in 21%) with a median response duration of 99 weeks.35 In a small number of series Imiquimod has been proven to be active in patients with early stage MF refractory to other therapies.36–38 As skin irritation is frequently observed, these agents may be used for localized treatment on limited areas.
4.2. Generalized skin-directed therapies other than radiotherapy
These treatments are indicated in patients with widespread skin involvement.
Phototherapy [PUVA (psoralen and UVA) and UVB]: Psoralen plus ultraviolet (UV)-A light therapy (PUVA) with orally administered 8-methoxypsoralen (8-MOP), which sensitizes the skin to UVA irradiation, is an important treatment for early-stage disease.39,40 The 8-MOP may also be added to either a medical bath (bath-PUVA) or a topical ointment (cream-PUVA). The efficacy of broadband UVB tends to be limited to the patch stage, whereas PUVA is also effective in clearing plaques.
In a retrospective study on patients with early-stage MF (stages IA–IIA; N = 114), treatment with narrowband UVB (N = 19) and PUVA (N = 95) also resulted in similar CR rates (68% vs. 62%) and median time to relapse (11.5 months vs.14 months).41 The 15-year survival rates were 82% (IA) and 58% (IB/IIA).40 The most common acute adverse effects are: erythema, pruritus, and nausea, which are usually mild at presentation and generally manageable with dose adjustments of UVA, 8-MOP, or dose interruptions. Gastrointestinal adverse effects are associated to orally given 8-MOP.40 Patients treated with PUVA have an increased risk of chronic photodamage and secondary cutaneous malignancies.42 It should be noted that cumulative doses of UV are associated with an increased risk of UV-associated skin malignancies. Thus, phototherapy may not be appropriate for patients with a history of squamous or basal cell carcinoma or melanoma.
4.3. Systemic therapies
Systemic therapies with extracorporeal photopheresis (ECP), interferons, systemic retinoids, or histone deacetylase (HDAC) inhibitors are preferred to traditional chemotherapy for patients that are not responding to initial skin-directed therapies. Multiagent chemotherapy is applied only to patients who do not respond to multiple prior therapies (including single-agent chemotherapy and combination regimens), or having bulky lymph node or parenchimal disease.
Interferon: Interferon-α (INF-α) is one of the most widely used first-line treatments in CTCL. It has a wide range of biologic effects, including antiviral, antiproliferative, and immunomodulatory actions, and the overall response rate (ORR) is greater than 70% with CR rates greater than 20%.43 IFN gamma has been shown to be effective in the treatment of patients with various stages of CTCL that is refractory to IFN alpha and other topical or systemic therapies.44 IFN-α is proposed as a first-line therapy in stage IIB–III disease and as a second-line treatment in early stage disease. It is generally given as a long-term therapy. Combination therapy with IFN-α and PUVA has very high response rates (ORR 92%, complete response 62–70%) and is superior to other combinations with retinoids or extracorporeal photopheresis.45,46 Almost all patients initially develop temporary flu-like symptoms. Chronic side effects can include anorexia, fatigue, depression, alopecia, cytopenia, and impaired liver function.45
Retinoids: Retinoids are vitamin A derivatives that have important effects on cell growth, terminal differentiation, and apoptosis. They are strongly teratogenic, so conception control before, during and after treatment is necessary in all female patients of child-bearing age.
ORR to retinoids (all-trans retinoic acid, 13-cis-retinoic acid, and the synthetic analogs isotretinoin, etretinate, and acitretin) ranged from 44% to 67% and complete response rates from 21% to 35%, with a median response duration of about eight months. Common side effects include skin and mucous membrane dryness.47 Oral bexarotene, a new synthetic retinoid, has been evaluated for the treatment of refractory or persistent early- and advanced-stage CTCL in two multicenter clinical trials48,49 that demonstrated in early stage disease (stages IA–IIA) refractory to prior treatment an ORR of 54% and a disease progression rate of 21%.49 In patients with advanced CTCL (stages IIB–IVB) refractory to prior treatments, clinical CR and PR were observed in 45% of patients with ORR of 55%, including 13% clinical CR.48
HDAC inhibitors: New class of drugs, potent inducers of histone acetylation, cell cycle arrest and apoptosis. Vorinostat was the first HDAC inhibitor approved by FDA for the treatment of patients with progressive, persistent, or recurrent CTCL, or following two systemic therapies. The activity and safety of the HDAC inhibitors vorinostat and romidepsin has been evaluated in patients with refractory CTCL.50,51 In a phase II study involving 74 patients (median 3 prior therapies) with persistent, progressive or refractory stage IB-IVA MF/SS, vorinostat resulted in an ORR of 30% with median time to progression of 5 months.51 The response rates and median response durations were comparable to those obtained with bexarotene capsules and denileukin diftitox.
Romidepsin is approved by the FDA for the treatment of CTCL in patients who failed after at least one prior systemic therapy.
Denileukin diftitox: is an Immunotoxin, a recombinant fusion protein that contains the portion of IL-2 that interacts with the IL-2 receptor (IL-2R) coupled with a truncated portion of diphtheria toxin (DT). Denileukin diftitox was FDA approved for the treatment of patients with persistent or recurrent CTCL based on phase III studies.52,53
Conventional cytotoxic systemic chemotherapy: Primary treatment only for patients with advanced disease or large cell transformation and for second-line therapy for early-stage disease refractory to skin-directed therapies and systemic biologic therapies. It consists of single-agent and combination chemotherapy that have been associated with high response rates, but short lived durations. Options involve single-agent or multi-agent chemotherapy including methotrexate, chlorambucil, vincristine, doxorubicin, cyclophosphamide, etoposide, gemcitabine, nucleoside analogs. Combination regimens include CHOP, CVP. Low-dose methotrexate has been used to treat early-stage MF and SS for many years but only limited data are available.54,55 Low-dose MTX has been successfully combined with IFN-α.56
Gemcitabine as a single agent has been evaluated in patients with advanced, heavily pretreated CTCL and as front-line therapy in untreated patients.57–59 Pentostatin has shown activity as a single agent or in combination with IFN alpha in patients with advanced MF or SS.
Limited data also suggest some activity for the oral temozolomide and the proteasome inhibitor bortezomib in patients with previously treated MF.60,61
Pegylated liposomal doxorubicin has shown substantial single-agent activity in patients with pretreated, advanced or refractory CTCL.62
Pralatrexate: is a folate analog indicated for patients with relapsed/refractory peripheral T-cell lymphoma (PTCL), and has also demonstrated activity in patients with CTCL.63
Extracorporeal photopheresis (ECP): is an immunomodulatory therapy using psoralen and UVA radiation extracorporeally. It consists in the removal of leukocytes by leukapheresis, which are then treated with 8-methoxypsoralen, exposed to UVA and returned to the patient. ECP is particularly indicated in patients with or at risk of blood involvement (erythrodermic stage III disease or IVA with SS).64,65 A suggested mechanism of action is the induction of apoptosis in circulating malignant T lymphocytes and the subsequent release of tumor antigens that lead to a systemic antitumor response against the malignant T cell clone. This treatment is of particular benefit in both erythrodermic MF and SS with circulating neoplastic T cells. A meta-analysis of 19 studies (5 studies using ECP as monotherapy and 14 studies as combination therapy) involving more than 400 patients with CTCL showed a combined ORR for all stages of CTCL of 56% (18% CR). The response rates were 58% (15% CR) for erythrodermic disease (T4) and 43% (9.5% CR) for SS.
5. Localized radiation treatments
Radiotherapy (RT) is an important treatment option in the management of these patients either for those with limited stage or those with advanced stage disease. Due to the rarity of this disease, there are no randomized trials evaluating indications for RT in comparison to the other aforementioned treatment approaches. RT can be delivered to a variable extension of the skin surface and with palliative or curative intent, it can also be delivered together with other treatment options, more often not concomitantly, but in a sequential schedule. Mycosis fungoides, like other hematological diseases, is extremely radiosensitive, hence low dose treatments can achieve a high level of response rate.66,67 The cell survival curves indicate a D0 of 0.9 Gy and a N of less than 2. In linear quadratic model terms, this means that there is a large component of alpha-associated cell kill with limited repair of initial damage and the alpha beta ratio is greater than 10.68 Impressive responses to radiations had been already documented at the beginning of the use of radiotherapy, leading to an increasing use of this technique among dermatologists at that time.69–72 Dose–response relationships were widely examined in several studies showing a direct correlation between disease free interval and radiation dose.68,73 Partial regression of disease was observed with single doses as low as 1.0 Gy but complete response required a dose of 7.0 Gy or higher. In a study using fractionated treatment and doses ranging from less than 10 Gy to 40 Gy for individual plaques and tumors, Cotter et al.74 demonstrated a similar response rate, i.e. 89–96%, across the entire range of dose. However, the likelihood of local recurrence after treatment was inversely related to dose, and was 42% for doses less than 10 Gy, 32% for doses of 10–20 Gy, 21% for doses of 20–30 Gy, and no recurrences after doses higher than 30 Gy.
5.1. RT in unilesional disease
Several studies reported the efficacy of RT in this setting of patients. A recent review evaluated the results of localized RT for the treatment of 10 patients affected by MF with unilesional or oligolesional disease (localized set of lesion, up to three or four covering <5% of body surface area) treated from 1997 to 2010 at Cleveland University.75 In general, each patient's lesion was treated with an en-face field using 6 MeV or 9 MeV electrons prescribed to the 80–90% isodose line depending on the thickness of the lesion. A 0.5–1-cm tissue equivalent bolus was used daily. The treated margin was at least 2 cm. A dose of 30.6–36 Gy was delivered at 1.8 Gy per fraction. The radiation was delivered daily, 5 days each week. In this series all patients treated with radiation therapy achieved a complete response verified by clinical examination within 2 months of treatment. After diagnosis, none of the patients progressed to a more advanced stage of disease, within a follow-up period ranging from 15 to 182.5 months after diagnosis (mean follow-up of 63.9 months). The mean overall duration of response to radiation therapy was over 40.1 months. Thirty per cent of patients reported a relapse, with two-thirds occurring in the previously irradiated area. Among the three patients who relapsed the mean time to relapse was 42.3 months. Acute erythema was the most common adverse event that was recorded within 2 months after the completion of radiation therapy. Other adverse events included acute fatigue, acute desquamation, chronic ulceration, chronic erythema, and acute and chronic dyspigmentation. In this experience, RT was well tolerated, with few side-effects, such as moderate erythema at the site of radiation for a few weeks following treatment. Previous studies76–85 with a low number of patients regarding RT for unilesional MF were also analyzed in the same review performing a pooled data analysis showing that the 1- and 5-year disease-free survival rates after radiation therapy were 92.7% and 83.4%, respectively. Doses delivered in these studies ranged from 20 Gy to 40 Gy and NCCN guidelines also recommend a dose of 12–36 Gy.86 A good toxicity profile emerged from these studies. However, an increased risk of development of squamous cell and basal cell carcinomas within the radiation field has been observed.87 Thus, the risks of radiation therapy must be weighed against the potential for cure of early-stage CTCL, a disease in which approximately 10–20% of cases can progress to advanced disease if left untreated.88
5.2. Local palliative RT
A retrospective study of palliative superficial radiotherapy showed a CR rate of 95% for plaques and small tumors (<3 cm) and a CR rate of 93% for large tumors (>3 cm), irrespective of dose. Use of low-dose electron beam (4 Gy in 2 Gy fractions) allows overlapping fields to treat lesion at any site.74,89 We have treated some cases of refractory widespread skin involvement disease in Modena with low dose fraction treatments (2 fraction of 2 Gy/die up to 4 Gy) using electron beam directed to the symptomatic lesions with a very good response rate without side effects. These patients could also be retreated to the same lesions or to other lesions if necessary without any problem (Fig. 1a).
Fig. 1.
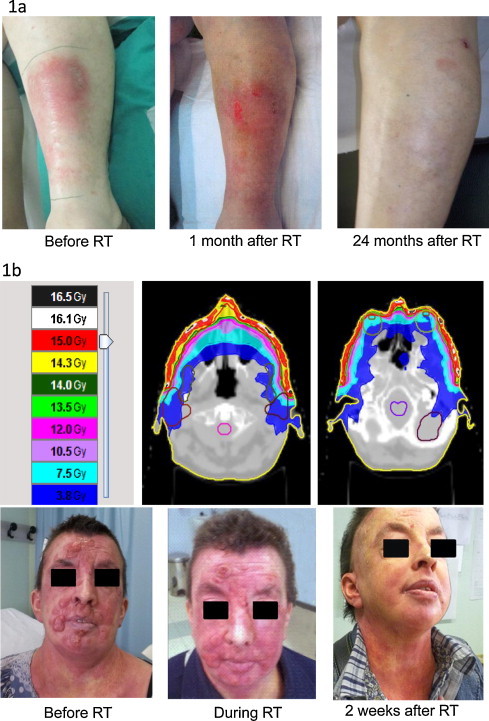
(a) Regional palliative treatment of a lesion of MF (2 Gy/fraction up to 4 Gy; 8 MeV electron beam with 0.5 cm bolus): clinical response. (b) Tomotherapy treatment of MF face lesions (3 Gy/fraction up to 15 Gy): dose distribution and clinical response.
6. Total skin electron beam irradiation (TSEI)
Modern TSEI has an overall response rate approaching 100% and it remains a very important treatment for mycosis fungoides, with no other treatment having shown such a high response rate. However, it is a very complicated treatment requiring a skilled multidisciplinary team, highly experienced in the management of cutaneous lymphoma.90 Although preliminary experiences of total-skin irradiation using X-rays were attempted by several dermatologists during the first decades of the twentieth century,91,92 TSEI could be developed only after the implementation of linear accelerators (second part of the twentieth century) due to the availability of electron beam therapy. In fact, the use of X-rays was burdened of systemic effects, like acute dermatitis, agranulocytosis and death, while the depth dose characteristics of the electron beam made it possible to treat large surfaces of the skin in a single field, concentrating the dose of irradiation in the superficial tissue including epidermis and upper dermis, while limiting the radiation dose that reaches the deep dermis and subcutaneous tissue.68
6.1. Treatment techniques
A conventional linear accelerator for total skin treatment with electrons was first utilized at Stanford,93 but the use of electron beams to treat the entire skin was already described in 1952. Trump and coworkers used a well-collimated electron beam (“cathode ray ribbon beam”) generated by a Van de Graff accelerator aimed downward at a recumbent patient on a motorized table to irradiate the entire skin.94 IAEA's classification of TSEI techniques includes three different modalities.95
With translational techniques, the patient is translated on a stretcher through an electron beam of sufficient width to cover the patient's transverse dimensions; with large electron field techniques, a standing stationary patient is treated at a large SSD with a single large electron beam or a combination of large electron beams; finally, with rotational techniques the patient is standing on a rotating platform in a large electron field. Dosimetric, geometric and patient positioning details are reported in AAPM Report No. 23.96
A recent review of Diamantopoulos et al. compared different techniques describing their advantages and disadvantages as well as the dosimetric problems.97 Single scattered horizontal beam needs large treatment rooms in order to obtain a correct distance of the patient from the accelerator (SSD 7 meters) determining homogenous electron field and is burdened of X-ray contribution to total absorbed dose.96 Multiple dual field technique combines 4–8 pairs of angled electron beams. Each pair creates a composite large field that corresponds to a different orientation of the body. At Stanford, a four-field technique was utilized at first, and later, a six-field technique of treatment was introduced.93,98 In general, the dosimetry of total skin electron irradiation improves as the number of treatment fields increases.99 With a four-field technique, there is significant overlap of adjacent fields, creating “hot spots” which may result in long-term effects such as telangiectasia, subcutaneous fibrosis and even necrosis.68 In a typical set up, patients are treated in the standing position at a distance of 3.5 m from the isocenter. A Lucite plate is placed as close as possible to the patient surface in order to degrade and further scatter the electrons. During treatment, the machine is angled upwards or downwards at an angle of 18°. The combination of these two fields for treating each body surface results in an adequate homogeneous dose distribution at the patient's surface and minimizes photon contamination, which is greatest in the central axis of the beam. The most widely used variant of this technique is the six-pair dual field technique also known as “Stanford technique”100 (Fig. 2). The patient is placed in all six positions (anterior, posterior and four opposed oblique fields), with a dual-field irradiation at each position, the six patient orientations being spaced at 60° intervals. Treatment is administered over a 2-day period. On day 1, the anterior and two posterior oblique fields are treated at each of the two accelerator angles. On day 2, the posterior and two anterior oblique fields are treated at each of the two accelerator angles. The major advantage of this modality is its ease of application even in small treatment rooms. Furthermore, as the two central axes of each beam point outwards patient's body, X-ray contribution to total absorbed dose becomes less significant as its main concentration is close to the central axis of the beam. The proper angle of the component beams ranges from ±10° to ±25° and depends on each beam characteristics and the source-to-skin distance used in every center.97 Rotational TSEI reduces dramatically total treatment time, a horizontal beam101 can be used but a combination of two large electron fields can also be used.102,103 The first translational technique was developed at the Christie Hospital in England104; a modification of this initial technique was applied at the Northern Israel Oncology Center105 and it demands a stationary reclined patient at the same SSD while the large electron field is created by 4–5 pairs of transversally angled beams. This technique and its modifications overcome the difficulties related to available treatment space and patient comfort, but they add to the dosimetric uncertainty about field junctions.106 A comparison between the Modified Christie Hospital Translational Technique and the Stanford Technique revealed no statistical difference in response rate, disease-free survival and overall survival between the two irradiation techniques, but it also showed that the Stanford technique was significantly less toxic.107 Combined techniques, combining Stanford-like fields with different patient set-ups in total skin treatment are very common and some clinics developed their own variants. A very usual modification is the use of Stanford fields on a rotating patient. This mode enables treatment to be carried out at a short period of time and at relatively short source skin distances.97 “Six dual field technique” or “Stanford technique” and its variants108 is used in more than 80% of radiotherapy centers carrying out TSEI during two or three-day treatment. Rotational techniques either with one field at a large SSD or Stanford-like dual field directed toward a rotating patient, are employed in 12% of cases.97 Techniques that use a reclined patient position are the minority in TSEI treatment. Although they are not widespread, these techniques have the advantage of turning TSEI irradiation into a rather comfortable procedure for the patient. The widespread use of the six dual field technique can be related to its dosimetric results that can be achieved with single field techniques109 even in small rooms (SSD = 2.85 m110 to 4.25 m111). The modified Stanford technique using modern linear accelerators represents, therefore, a standard technique in current clinical trial protocols. The Stanford protocol involves treating three positions each day so that a full cycle of treatment to the six standing positions is delivered over 2 days. The full-dose Stanford schedule prescribed is 2 Gy per cycle to a total dose of 30–36 Gy over 9–10 weeks. A week gap in treatment can be added to provide relief from the skin radiation reaction, and to provide patients who travel a rest. Treatment typically takes 30–40 min each day.90
Fig. 2.
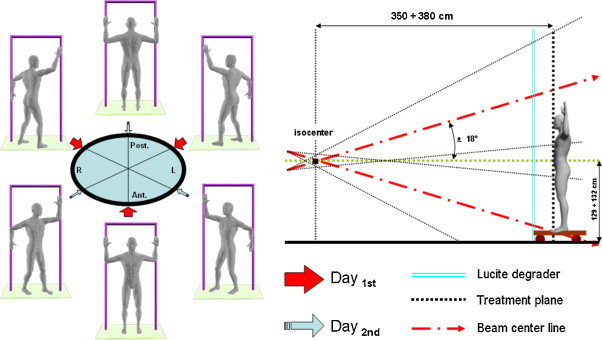
“Stanford technique”: six set-up positions of patient (anterior, posterior and four opposed oblique fields), with a dual-field irradiation at each position; all orientations are spaced at 60° intervals. (modified from: Smith124 and AAPM report 2396).
6.2. Dose prescription
The Stanford data112 showed an increased rate of complete response for increasing dose with complete response rate of 18% for doses less than 10 Gy, 55% for 10–20 Gy, 66% for 20–25 Gy, 75% for 25–30 Gy, and 94% for doses of 30 Gy or greater. Maintenance of complete response with time was greater among patients treated with high dose (25 Gy or higher). The impact was most dramatic among patients with generalized plaque disease, where 50% maintained a complete response after high-dose therapy, but only about 10% remained disease-free after low-dose therapy. Split-dose experiments showed no difference in tumor regrowth at treatment intervals of one or seven days, suggesting little recovery between treatment fractions; this allows the use of protracted fractionation schemes, limiting the effects on normal tissues and also increasing the “therapeutic ratio”.68
According to the EORTC recommendations,113 objectives of any total skin electron radiation technique should be: (1) to align the dose distribution to the target volume; (2) to be practical, comfortable, and efficient for the patient; (3) to provide sufficient dose within the target volume; (4) to reliably attain cutaneous remission; (5) to minimize toxicity; (6) to produce beneficial long-term clinical results; (7) to accommodate repeated administration as required.
The primary target volume includes the epidermis, adnexal structures, and the dermis114,115 which may receive the majority of the deposited ionizing energy. The 80% isodose should be at a depth of at least 4 mm under the skin surface, and the dose at a depth of 20 mm should be less than 20% of the maximum dose at the skin surface. The photon contamination at the level of the bone marrow must be less than 0.7 Gy for a full course of TSEI to avoid hematological sequelae.
The primary goal is to administer at least 26 Gy at a depth of 4 mm in truncal skin along the primary axes.116 For an effective electron energy of about 4 to 5.5 MeV, the truncal surface must receive about 31–36 Gy. The human body has a complex surface that presents the greatest technologic challenge for any technique of TSEI. A homogeneous dose-distribution should be gained increasing the number of fields, it is also necessary to position the patient to maximize unfolding of skin in order to reduce as much as possible the underdosed areas and to top-up regions of lesser dose (i.e. setting the joints of the limbs at appropriately large angles, slightly bending the torso and hips to expose folds in the lower trunk). Use of the Stanford University technique requires patients to stand during treatment, in contrast to a prone-supine method. When standing, some patients may benefit from support provided by a posterior frame against which they may lean or by thin encircling straps that help hold them up and orient them to their positions. At least 6 beams must be administered, unless a method of dynamic rotation is used.117,118 The globe of the eye must not receive more than 15% of the prescribed skin surface dose. A combination of internal and external eye shields will ensure that 26 Gy is administered to the eyelids while protecting the globes. Optional shields (e.g., to the scalp or testes) are not recommended. It may be necessary to place small shields over pacemakers and ostomy sites, but this may compromise efficacy.
6.3. Regional patch and boost treatments
Usually, some parts of the body surface are “shadowed” and receive relatively lower total doses of irradiation.119,120 These areas include the top of the scalp, the perineum and the soles of the feet. Other areas may be problematic in individual patients because of body habitus, such as underneath the breasts of some women and under the panniculus of obese individuals. Separate treatments can be administered to these areas using a direct electron field. Without a scalp reflector, the vertex can be patch-treated. A comparison of published 10-year data from Hamilton, Yale, and Stanford Universities and several other series suggest that progression-free survival might be improved by 10–20% by applying patch treatments.113 The dose prescribed at peak (at the skin surface of the soles) is 26–28 Gy. Results vary among patients, and measurements may influence the prescription of patch treatments. Measurements with thermoluminescent or film dosimeters are typically conducted during the first 2 weeks of TSEI and over one cycle of all fields or beams.
Some patients with a discrete number of tumor lesions will receive boost treatment to these tumors at the outset of electron beam therapy in order to reduce their thickness and permit better penetration by the TSEI electrons. Usually, doses of 15 Gy in 1.5–3.0-Gy fractions using 6–9-MeV electrons are adequate for this purpose.68 Boost treatments may reduce symptoms on the hands, feet, or face and may begin the healing of any skin ulcers. When the dose is administered 1–2 weeks before TSEI, a prompt response facilitates a course of TSEI reducing immediate adverse effects. Custom lead made goggles are proposed to shield the eyes, and the eyelids can be patch treated with radiotherapy following TSEI. Perspex hand blocks or lead-lined gloves can be used for the hands, shielding can be added to the TSEI frame to shield the lower legs and the head and neck area if needed. Lead finger nail shields can be used if there is no involvement of these areas. The testicles can be shielded by using a lead-lined cricket box.
6.4. TSEI toxicity
TSEI is generally not burdened by heavy acute and late toxicity.121 Usually, patients who are fit can continue normal activities of daily life. With the dose of 30–36 Gy, patients may experience some temporary short-term side-effects: namely, fatigue is common but generally not severe or debilitating, MF skin lesions become more erythematous and the skin develops a mild to moderate radiation dermatitis. Complete, temporary scalp alopecia (usually after 2 weeks of treatment) and temporary nail stasis appear in 100% of the patients. Hair typically returns, but the color and texture of the hair when it regrows may be different. Nails may become brittle, and half of the patients can develop some edema of hands and feet. Less than 10% of patients can develop minor nosebleeds. Other uncommon side effects are: blisters on the fingers and feet (<5%, soft comfortable footwear is advised to prevent it), self-limiting anhidrosis, minor parotiditis, and gynecomastia in males (<3% each), corneal tears from internal eye shields (<1%), chronic nail dystrophy, chronic xerosis, partial but permanent alopecia of the scalp, and fingertip dysesthesias that persist for more than a year (<1% each).113 Infertility is possible in men but it is not common in women.
Acute or late mortality related to the procedure has never been described. Patients often find the lead goggles used to shield the eyes uncomfortable. It is important to monitor for infection during treatment. In patients with early disease, the reported incidence of infection is 1%,121 but in patients with more advanced disease the incidence of skin infection is higher. The lesions of mycosis fungoides continue to respond for up to 3 months, and the assessment of response to TSEI should not be carried out too early. Yale published on patients’ perspective of TSEI and found out that TSEI is perceived to be a successful treatment but also difficult to perform and to recover from in comparison with other treatments.122
6.5. TSEI clinical indications
According to EORTC guidelines,113 TSEI can be used for patients at all stages of mycosis fungoides, and remains a very important treatment for these patients, even for those with SS. The response rates and duration of response are higher in earlier stage disease. The aim of treatment (curative or palliative) varies depending on the stage. In a retrospective series of 141 patients with initial-stage disease IA (T1 N0 M0) and IB (T2 N0 M0) of the University Hospital of Dijon123 treated with a 6-MeV linear accelerator to a mean total dose of 30 Gy, 2 Gy/day, 4 days/week, for 4 weeks, the overall response rate was 94.7%. A complete response was achieved in 87.5% of T1 and 84.8% of T2 patients. After a median follow-up of 114 months for the whole group, 5-year DFS rate was 50% and OS rate was 65%. Electron-beam irradiation is more penetrating than other skin-directed treatments (e.g., topical nitrogen mustard [HN2] or phototherapy) and is generally considered a rational first line therapy for patients with T2-classified (i.e. generalized patch or plaque) and T3-classified (i.e. tumors) disease.68,124 The efficacy of TSEI in erythrodermic MF (i.e. T4 disease) is more controversial.125 The Stanford University126 recently reported its experience with TSEI in the treatment of patients with mycosis fungoides at stage IB (generalized patch or plaque T2N0M0) and stage IIB (tumor T3N0M0). They analyzed retrospectively data of 180 patients treated at their institution from 1970 through 2007 with TSEI as monotherapy comparing this group with subgroups receiving adjuvant nitrogen mustard (HN2). The TSEI was administered using the six dual field technique, with a dose of 1.5–2 Gy per 2-day cycle.100 Most patients received a total dose of 36 Gy (range, 30–40 Gy), and local boost treatments (10–15 Gy) were often delivered to tumor lesions or thick plaques, “shadowed” areas of the body were routinely supplemented with electrons or orthovoltage irradiation (median dosage, 20 Gy) for compensation. The overall response rate was 100%; 60% of patients achieved a complete clinical response (T2 cases, 75%; T3 cases, 47%). The 5- and 10-year OS rates of the entire cohort were 59% and 40%, respectively. There were no significant differences in freedom from relapse, overall survival, and progression-free survival disease between TSEI monotherapy and TSEI with adjuvant HN2. Authors stated that a TSEI of 30 Gy or greater is highly effective in treating T2-T3 MF, with better outcomes in T2 disease. Patients with stage III disease have diffuse erythroderma involving the entire skin surface. The rate of remission with TSEI is about 75%, although all patients experience relief of major symptoms. The overall 5-year progression-free survival is 26%. However, there are two subgroups of patients: those with and those without blood involvement. Patients without blood involvement (i.e. those who are B0) may be treated with TSEI to prolong progression-free survival because two thirds of them remain disease-free at 10 years after TSEI.125 TSEI may instead be offered for palliation only to B1-patients. The 10-year disease-free survival for this subgroup is 15%. TSEI may be repeated if necessary. The addition of extracorporeal photoimmune chemotherapy or PUVA to TSEI remains experimental.
6.6. TSEI palliative setting
TSEI may be proposed for patients with stage IVA (N2-3) and stage IVB (M1) disease with palliative intent. The chance of complete remission in the skin is around 70%. Some patients with stage IVA disease may have complete cutaneous remissions for 5–10 years, but the 10-year progression-free survival is low. In contrast, all patients with stage IVB disease die of MF within 5 years from TSEI, but this treatment is very effective for palliation and improves quality of life. TSEI can be used as a second line treatment after the failure of other therapies and patients typically experience disease clearing.113 Introcaso et al.127 employed TSEI in four patients with SS obtaining significant improvement in their blood burden of malignant cells in addition to complete cutaneous responses to total skin electron beam therapy. They point out the potential for TSEI as both a skin and blood tumor debulking agent, and not merely to a palliation for skin symptoms.
Hauswald et al.128 recently reported treatment results of 25 patients with cutaneous manifestations of advanced and therapy-refractory cutaneous lymphoma (17/25 patients T-cell lymphomas) stage IIB–IV or leukemia treated between 1993 and 2010. All patients were symptomatic. The median total dose was 29 Gy, applied in 29 fractions of median 1 Gy each. The median follow-up time was 10 months. Treatment response regarding palliation with symptom relief, especially of pruritus and regression of cutaneous lesions, was achieved in 23 patients (92%). A clinical complete response was documented in 13 (52%) and a partial response in 10 patients (40%).
6.7. TSEI repeating and low dose TSEI
Investigators at Yale University129,130 and, subsequently, at Stanford University,128 reported the successful administration of multiple courses of TSEI. Occasionally, it is also possible to administer a second complete course of “high” dose TSEI. However, most patients receive lower dose TSEI during the second or third course. The goal is to relieve symptoms while avoiding long-term adverse effects. With minimal applications of TSEI, consisting of several fractions to a dose of 4–8.0 Gy, administered every 3–18 months, the treatment may be safely given to control disease with minimal toxicity.
The Stanford update reports on 14 patients retreated with a second course of TSEI to a dose of 24 Gy. All 14 patients responded and two patients had a complete response.126
Interest in the CTCL community is now focusing on low-dose TSEI after a recent Stanford review131 on 102 patients treated with less than 30 Gy. The overall response rate was 90–98%. The relapse-free period after TSEI was 22.2 months with more than 30 Gy, 29.3 months in the 20–30 Gy group, 25.7 months in the 10–20 Gy group and 12 months in the 5–10 Gy group.
6.8. Association of TSEI with other treatment modalities
A trial randomizing patients to observation or vorinostat after low-dose TSEI has recently opened to accrual in the USA.132 The use of adjuvant PUVA and topical mechlorethamine after TSEI has been reported, but the data are limited and retrospective. The data on PUVA are based on only 14 patients133 and it is suggested that PUVA can maintain remission after TSEI. Initial results from Stanford in 1999 reported higher response rates and longer freedom from relapse using mechlorethamine as an adjuvant after TSEI,134 but their more recent data update with TSEI in the modern era do not confirm this finding.126 In patients with blood, lymph node or visceral involvement, TSEI can be used to control disease in the skin sequentially with other treatments such as chemotherapy. The response rates in the skin are high, but the duration of response is short at 2–3 months. In selected patients who are fit for a reduced intensity allogeneic hematopoietic stem cell transplantation (HSCT), TSEI can be considered as debulking treatment. Duvic et al.135 reported the successful use of TSEI in 18 patients with advanced stage IIB–IVB mycosis fungoides before allogeneic HSCT. Among these patients, 14 had stage IV disease with involved lymph nodes or bone marrow, and 11 were erythrodermic with B2 blood involvement. All patients had received prior treatments (median 4): with a median follow-up of 19 months (range 1.3–8.3 years) 11 patients remain in complete remission.
6.9. New radiotherapy technologies opportunities
New RT technologies such as Tomotherapy may open new treatment approaches to treat patients with MF. Several papers in the literature report on the use of image-guided-radiation-therapy (IGRT) to treat large skin volumes saving organs at risk with palliative intent.90,136 In Modena we have recently treated with tomotherapy a 38-year-old female patient affected with a refractory MF who was already treated with multiple chemotherapy and biological approaches and who was affected with mental retardation. She was referred to our center because of a symptomatic (itch and pain) large involvement of the skin face, which was covered by multiple large tumor lesions (Fig. 1b). Our choice was driven by the need to give a homogeneous dose to a target volume located under the skin surface at a depth larger than that we could adequately cover with an electron beam, particularly in a highly irregular anatomic region like the face and the neck. The patient was treated with a hypofractionated regimen (3 Gy/die, 15 Gy total dose), with a very good response. During the treatment she experienced acute erythema and edema of the skin and after two weeks a complete response of all irradiated lesions was observed, with complete regression of the acute symptoms.
7. Conclusions
Treatment of MF and SS is still a challenge, and these diseases should be managed by a skilled multidisciplinary team. An overview of all the treatment strategies available for each stage of the disease has been reported in Table 2: radiotherapy remains a valid treatment option for all the stages of the disease.
The extreme radiosensitivity of these disorders offers a good therapeutic ratio for curative or palliative setting for most patients, and the newest RT technologies may offer new possibilities, taking into account technical and dosimetric problems related to electron beam irradiation.
Some priorities for clinical research are suggested by the rarity of the disease, the variety of potentially effective treatment modalities that are currently available (from different physical agents, to many drugs, or a combination of the two), and the challenging radiotherapy technical problems to be solved. Multicenter clinical trials aimed at defining the best first-line treatment stage by stage, the minimum effective radiotherapy dose both for palliation and for cure, and the possible need of elective multimodality therapy, as well as the role of the novel irradiation technologies are strongly warranted and should be pursued by experienced reference centers. Moreover, in order to develop and share adequate National and International guidelines, perspective multicenter accumulation of clinical data in nationwide repositories, including detailed data relative to radiotherapy-treated patients, should also be encouraged.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Girardi M., Heald P.W., Wilson L.D. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350:1978–1988. doi: 10.1056/NEJMra032810. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe E., Harris N., Stein H. IARC Press; Lyon, France: 2001. World Health Organization classification of tumours: pathology and genetics of tumours of haematopoietic and lymphoid tissues. [Google Scholar]
- 3.Willemze R., Kerl H., Sterry W. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997;90:354–371. [PubMed] [Google Scholar]
- 4.Willemze R., Jaffe E.S., Burg G. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow S., Campo E., Harris N. IARC; Lyon: 2008. WHO classification of tumors of haematopoietic and lymphoid tissues. [Google Scholar]
- 6.Bradford P.T., Devesa S.S., Anderson W.F. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064–5073. doi: 10.1182/blood-2008-10-184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaf C., Gellrich S., Steinhoff M. Cutaneous lymphomas in Germany: an analysis of the Central Cutaneous Lymphoma Registry of the German Society of Dermatology (DDG) J Dtsch Dermatol Ges. 2007;5:662–668. doi: 10.1111/j.1610-0387.2007.06337.x. [DOI] [PubMed] [Google Scholar]
- 8.Criscione V.D., Weinstock M.A. Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Arch Dermatol. 2007;143:854–859. doi: 10.1001/archderm.143.7.854. [DOI] [PubMed] [Google Scholar]
- 9.Heald P., Yan S.L., Edelson R. Profound deficiency in normal circulating T cells in erythrodermic cutaneous T-cell lymphoma. Arch Dermatol. 1994;130:198–203. [PubMed] [Google Scholar]
- 10.Huang K.P., Weinstock M.A., Clarke C.A. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sezary syndrome: evidence from population-based and clinical cohorts. Arch Dermatol. 2007;143:45–50. doi: 10.1001/archderm.143.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Mallo-Garcia S., Coto-Segura P., Suarez-Casado H. Fatal outcome due to bacterial superinfection of eczema herpeticum in a patient with mycosis fungoides. Dermatol Online J. 2008;14:21. [PubMed] [Google Scholar]
- 12.Lansigan F., Foss F.M. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70:273–286. doi: 10.2165/11532190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Kim E.J., Hess S., Richardson S.K. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vergier B., de Muret A., Beylot-Barry M. Transformation of mycosis fungoides: clinicopathological and prognostic features of 45 cases. French Study Group of Cutaneious Lymphomas. Blood. 2000;95:2212–2218. [PubMed] [Google Scholar]
- 15.Diamandidou E., Colome-Grimmer M., Fayad L. Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood. 1998;92:1150–1159. [PubMed] [Google Scholar]
- 16.Pimpinelli N., Olsen E.A., Santucci M. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053–1063. doi: 10.1016/j.jaad.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 17.Olsen E., Vonderheid E., Pimpinelli N. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 18.Tsai E.Y., Taur A., Espinosa L. Staging accuracy in mycosis fungoides and Sezary syndrome using integrated positron emission tomography and computed tomography. Arch Dermatol. 2006;142:577–584. doi: 10.1001/archderm.142.5.577. [DOI] [PubMed] [Google Scholar]
- 19.Olsen E.A., Whittaker S., Kim Y.H. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arulogun S.O., Prince H.M., Ng J. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112:3082–3087. doi: 10.1182/blood-2008-05-154609. [DOI] [PubMed] [Google Scholar]
- 21.Yen A., McMichael A., Kilkenny M. Mycosis fungoides: an Australian experience. Australas J Dermatol. 1997;38(Suppl. 1):S86–S90. doi: 10.1111/j.1440-0960.1997.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 22.Agar N.S., Wedgeworth E., Crichton S. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–4739. doi: 10.1200/JCO.2009.27.7665. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.H., Liu H.L., Mraz-Gernhard S. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 24.van Doorn R., Van Haselen C.W., van Voorst Vader P.C. Mycosis fungoides: disease evolution and prognosis of 309 Dutch patients. Arch Dermatol. 2000;136:504–510. doi: 10.1001/archderm.136.4.504. [DOI] [PubMed] [Google Scholar]
- 25.Zackheim H.S., Amin S., Kashani-Sabet M. Prognosis in cutaneous T-cell lymphoma by skin stage: long-term survival in 489 patients. J Am Acad Dermatol. 1999;40:418–425. doi: 10.1016/s0190-9622(99)70491-3. [DOI] [PubMed] [Google Scholar]
- 26.Diamandidou E., Cohen P.R., Kurzrock R. Mycosis fungoides and Sezary syndrome. Blood. 1996;88:2385–2409. [PubMed] [Google Scholar]
- 27.Kaye F.J., Bunn P.A., Jr., Steinberg S.M. A randomized trial comparing combination electron-beam radiation and chemotherapy with topical therapy in the initial treatment of mycosis fungoides. N Engl J Med. 1989;321:1784–1790. doi: 10.1056/NEJM198912283212603. [DOI] [PubMed] [Google Scholar]
- 28.Hymes K.B. Choices in the treatment of cutaneous T-cell lymphoma. Oncology (Williston Park) 2007;21:18–23. [PubMed] [Google Scholar]
- 29.Rosen S.T., Querfeld C. Primary cutaneous T-cell lymphomas. Hematology Am Soc Hematol Educ Program. 2006;323–330:513. doi: 10.1182/asheducation-2006.1.323. [DOI] [PubMed] [Google Scholar]
- 30.Zackheim H.S. Treatment of patch-stage mycosis fungoides with topical corticosteroids. Dermatol Ther. 2003;16:283–287. doi: 10.1111/j.1396-0296.2003.01639.x. [DOI] [PubMed] [Google Scholar]
- 31.Zackheim H.S., Kashani-Sabet M., Amin S. Topical corticosteroids for mycosis fungoides. Experience in 79 patients. Arch Dermatol. 1998;134:949–954. doi: 10.1001/archderm.134.8.949. [DOI] [PubMed] [Google Scholar]
- 32.Zackheim H.S. Topical carmustine (BCNU) in the treatment of mycosis fungoides. Dermatol Ther. 2003;16:299–302. doi: 10.1111/j.1396-0296.2003.01641.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y.H. Management with topical nitrogen mustard in mycosis fungoides. Dermatol Ther. 2003;16:288–298. doi: 10.1111/j.1396-0296.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 34.Vonderheid E.C., Tan E.T., Kantor A.F. Long-term efficacy, curative potential, and carcinogenicity of topical mechlorethamine chemotherapy in cutaneous T cell lymphoma. J Am Acad Dermatol. 1989;20:416–428. doi: 10.1016/s0190-9622(89)70051-7. [DOI] [PubMed] [Google Scholar]
- 35.Breneman D., Duvic M., Kuzel T. Phase 1 and 2 trial of bexarotene gel for skin-directed treatment of patients with cutaneous T-cell lymphoma. Arch Dermatol. 2002;138:325–332. doi: 10.1001/archderm.138.3.325. [DOI] [PubMed] [Google Scholar]
- 36.Coors E.A., Schuler G., Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16:391–393. [PubMed] [Google Scholar]
- 37.Deeths M.J., Chapman J.T., Dellavalle R.P. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275–280. doi: 10.1016/j.jaad.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Gonzalez M.C., Verea-Hernando M.M., Yebra-Pimentel M.T. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18:148–152. doi: 10.1684/ejd.2008.0352. [DOI] [PubMed] [Google Scholar]
- 39.Diederen P.V., van Weelden H., Sanders C.J. Narrowband UVB and psoralen-UVA in the treatment of early-stage mycosis fungoides: a retrospective study. J Am Acad Dermatol. 2003;48:215–219. doi: 10.1067/mjd.2003.80. [DOI] [PubMed] [Google Scholar]
- 40.Querfeld C., Rosen S.T., Kuzel T.M. Long-term follow-up of patients with early-stage cutaneous T-cell lymphoma who achieved complete remission with psoralen plus UV-A monotherapy. Arch Dermatol. 2005;141:305–311. doi: 10.1001/archderm.141.3.305. [DOI] [PubMed] [Google Scholar]
- 41.Ponte P., Serrao V., Apetato M. Efficacy of narrowband UVB vs. PUVA in patients with early-stage mycosis fungoides. J Eur Acad Dermatol Venereol. 2010;24:716–721. doi: 10.1111/j.1468-3083.2009.03500.x. [DOI] [PubMed] [Google Scholar]
- 42.Gathers R.C., Scherschun L., Malick F. Narrowband UVB phototherapy for early-stage mycosis fungoides. J Am Acad Dermatol. 2002;47:191–197. doi: 10.1067/mjd.2002.120911. [DOI] [PubMed] [Google Scholar]
- 43.Olsen E.A. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16:311–321. doi: 10.1111/j.1396-0296.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan E.H., Rosen S.T., Norris D.B. Phase II study of recombinant human interferon gamma for treatment of cutaneous T-cell lymphoma. J Natl Cancer Inst. 1990;82:208–212. doi: 10.1093/jnci/82.3.208. [DOI] [PubMed] [Google Scholar]
- 45.Kuzel T.M., Roenigk H.H., Jr., Samuelson E. Effectiveness of interferon alfa-2a combined with phototherapy for mycosis fungoides and the Sezary syndrome. J Clin Oncol. 1995;13:257–263. doi: 10.1200/JCO.1995.13.1.257. [DOI] [PubMed] [Google Scholar]
- 46.Stadler R., Otte H.G., Luger T. Prospective randomized multicenter clinical trial on the use of interferon −2a plus acitretin versus interferon −2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood. 1998;92:3578–3581. [PubMed] [Google Scholar]
- 47.Zhang C., Duvic M. Treatment of cutaneous T-cell lymphoma with retinoids. Dermatol Ther. 2006;19:264–271. doi: 10.1111/j.1529-8019.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 48.Duvic M., Hymes K., Heald P. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II–III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 49.Duvic M., Martin A.G., Kim Y. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Arch Dermatol. 2001;137:581–593. [PubMed] [Google Scholar]
- 50.Duvic M., Talpur R., Ni X. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olsen E.A., Kim Y.H., Kuzel T.M. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 52.Olsen E., Duvic M., Frankel A. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 53.Prince H.M., Duvic M., Martin A. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:1870–1877. doi: 10.1200/JCO.2009.26.2386. [DOI] [PubMed] [Google Scholar]
- 54.Zackheim H.S., Kashani-Sabet M., Hwang S.T. Low-dose methotrexate to treat erythrodermic cutaneous T-cell lymphoma: results in twenty-nine patients. J Am Acad Dermatol. 1996;34:626–631. doi: 10.1016/s0190-9622(96)80062-4. [DOI] [PubMed] [Google Scholar]
- 55.Zackheim H.S., Kashani-Sabet M., McMillan A. Low-dose methotrexate to treat mycosis fungoides: a retrospective study in 69 patients. J Am Acad Dermatol. 2003;49:873–878. doi: 10.1016/s0190-9622(03)01591-3. [DOI] [PubMed] [Google Scholar]
- 56.Aviles A., Nambo M.J., Neri N. Interferon and low dose methotrexate improve outcome in refractory mycosis fungoides/Sezary syndrome. Cancer Biother Radiopharm. 2007;22:836–840. doi: 10.1089/cbr.2007.0402. [DOI] [PubMed] [Google Scholar]
- 57.Zinzani P.L., Venturini F., Stefoni V. Gemcitabine as single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 1998;21:860–863. doi: 10.1093/annonc/mdp508. [DOI] [PubMed] [Google Scholar]
- 58.Duvic M., Talpur R., Wen S. Phase II evaluation of gemcitabine monotherapy for cutaneous T-cell lymphoma. Clin Lymphoma Myeloma. 2006;7:51–58. doi: 10.3816/CLM.2006.n.039. [DOI] [PubMed] [Google Scholar]
- 59.Zinzani P.L., Baliva G., Magagnoli M. Gemcitabine treatment in pretreated cutaneous T-cell lymphoma: experience in 44 patients. J Clin Oncol. 2000;18:2603–2606. doi: 10.1200/JCO.2000.18.13.2603. [DOI] [PubMed] [Google Scholar]
- 60.Tani M., Fina M., Alinari L. Phase II trial of temozolomide in patients with pretreated cutaneous T-cell lymphoma. Haematologica. 2005;90:1283–1284. [PubMed] [Google Scholar]
- 61.Zinzani P.L., Musuraca G., Tani M. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:4293–4297. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]
- 62.Wollina U., Dummer R., Brockmeyer N.H. Multicenter study of pegylated liposomal doxorubicin in patients with cutaneous T-cell lymphoma. Cancer. 2003;98:993–1001. doi: 10.1002/cncr.11593. [DOI] [PubMed] [Google Scholar]
- 63.O’Connor O.A., Pro B., Pinter-Brown L. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edelson R., Berger C., Gasparro F. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 65.Zic J.A. The treatment of cutaneous T-cell lymphoma with photopheresis. Dermatol Ther. 2003;16:337–346. doi: 10.1111/j.1396-0296.2003.01646.x. [DOI] [PubMed] [Google Scholar]
- 66.Trowell O.A. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. 1952;64:687–704. doi: 10.1002/path.1700640403. [DOI] [PubMed] [Google Scholar]
- 67.Qasim M.M. Blood and bone marrow response following total body irradiation in patients with lymphosarcomas. Eur J Cancer. 1977;13:483–487. doi: 10.1016/0014-2964(77)90107-4. [DOI] [PubMed] [Google Scholar]
- 68.Hoppe R.T. Mycosis fungoides: radiation therapy. Dermatol Ther. 2003;16:347–354. doi: 10.1111/j.1396-0296.2003.01647.x. [DOI] [PubMed] [Google Scholar]
- 69.Scholtz W. Ueber den einfluss der rontgenstrahlen auf die haut in gesunden und krankem zustande. Arch Dermat U Syph. 1902;59:421. [Google Scholar]
- 70.Jamieson W. Mycosis fungoides, and its treatment by the X-rays. Br J Dermatol. 1903;15:1–10. [PMC free article] [PubMed] [Google Scholar]
- 71.Marsh J. A case of mycosis fungoides symptomatically cured by means of X-rays. Am J Med Sci. 1903;126:314–318. [Google Scholar]
- 72.Carrier A. A case of mycosis fungoides treated by the X-ray. J Cutaneous Dis. 1904;22:73–77. [Google Scholar]
- 73.Kim J.H., Nisce L.Z., D’Anglo G.J. Dose-time fractionation study in patients with mycosis fungoides and lymphoma cutis. Radiology. 1976;119:439–442. doi: 10.1148/119.2.439. [DOI] [PubMed] [Google Scholar]
- 74.Cotter G.W., Baglan R.J., Wasserman T.H. Palliative radiation treatment of cutaneous mycosis fungoides – a dose response. Int J Radiat Oncol Biol Phys. 1983;9:1477–1480. doi: 10.1016/0360-3016(83)90321-8. [DOI] [PubMed] [Google Scholar]
- 75.Chan D.V., Aneja S., Honda K. Radiation therapy in the management of unilesional primary cutaneous T-cell lymphomas. Br J Dermatol. 2012;166:1134–1137. doi: 10.1111/j.1365-2133.2011.10728.x. [DOI] [PubMed] [Google Scholar]
- 76.Piccinno R., Caccialanza M., Percivalle S. Minimal stage IA mycosis fungoides. Results of radiotherapy in 15 patients. J Dermatolog Treat. 2009;20:165–168. doi: 10.1080/09546630802516571. [DOI] [PubMed] [Google Scholar]
- 77.Micaily B., Miyamoto C., Kantor G. Radiotherapy for unilesional mycosis fungoides. Int J Radiat Oncol Biol Phys. 1998;42:361–364. doi: 10.1016/s0360-3016(98)00218-1. [DOI] [PubMed] [Google Scholar]
- 78.Wilson L.D., Kacinski B.M., Jones G.W. Local superficial radiotherapy in the management of minimal stage IA cutaneous T-cell lymphoma (mycosis fungoides) Int J Radiat Oncol Biol Phys. 1998;40:109–115. doi: 10.1016/s0360-3016(97)00553-1. [DOI] [PubMed] [Google Scholar]
- 79.Oliver G.F., Winkelmann R.K. Unilesional mycosis fungoides: a distinct entity. J Am Acad Dermatol. 1989;20:63–70. doi: 10.1016/s0190-9622(89)70008-6. [DOI] [PubMed] [Google Scholar]
- 80.Heald P.W., Glusac E.J. Unilesional cutaneous T-cell lymphoma: clinical features, therapy, and follow-up of 10 patients with a treatment-responsive mycosis fungoides variant. J Am Acad Dermatol. 2000;42:283–285. doi: 10.1016/S0190-9622(00)90140-3. [DOI] [PubMed] [Google Scholar]
- 81.Hodak E., Phenig E., Amichai B. Unilesional mycosis fungoides: a study of seven cases. Dermatology. 2000;201:300–306. doi: 10.1159/000051542. [DOI] [PubMed] [Google Scholar]
- 82.Palmer R.A., Keefe M., Slater D. Case 4: pagetoid reticulosis (Woringer–Kolopp type) or unilesional mycosis fungoides (MF) Clin Exp Dermatol. 2002;27:345–346. doi: 10.1046/j.1365-2230.2002.01044.x. [DOI] [PubMed] [Google Scholar]
- 83.Yoo S.S., Viglione M., Moresi M. Unilesional mycosis fungoides mimicking Bowen's disease. J Dermatol. 2003;30:417–419. doi: 10.1111/j.1346-8138.2003.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 84.Alsaleh Q.A., Nanda A., Baker H. Unilesional (segmental) mycosis fungoides presenting in childhood. Pediatr Dermatol. 2004;21:558–560. doi: 10.1111/j.0736-8046.2004.21507.x. [DOI] [PubMed] [Google Scholar]
- 85.Wilson L.D., Jones G.W., Smith B.D. Cutaneous lymphomas – radiotherapeutic strategies. Front Radiat Ther Oncol. 2006;39:1–15. doi: 10.1159/000090798. [DOI] [PubMed] [Google Scholar]
- 86.NCCN Clinical Practice Guidelines in Oncology . 2013. Non-Hodgikin's Lymphomas, Version 1.2013. www.nccn.org. [Google Scholar]
- 87.Lichter M.D., Karagas M.R., Mott L.A. Therapeutic ionizing radiation and the incidence of basal cell carcinoma and squamous cell carcinoma. The New Hampshire Skin Cancer Study Group. Arch Dermatol. 2000;136:1007–1011. doi: 10.1001/archderm.136.8.1007. [DOI] [PubMed] [Google Scholar]
- 88.Litvinov I.V., Jones D.A., Sasseville D. Transcriptional profiles predict disease outcome in patients with cutaneous T-cell lymphoma. Clin Cancer Res. 2010;16:2106–2114. doi: 10.1158/1078-0432.CCR-09-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whittaker S.J., Foss F.M. Efficacy and tolerability of currently available therapies for the mycosis fungoides and Sezary syndrome variants of cutaneous T-cell lymphoma. Cancer Treat Rev. 2007;33:146–160. doi: 10.1016/j.ctrv.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Morris S.L. Skin lymphoma. Clin Oncol (R Coll Radiol) 2012;24:371–385. doi: 10.1016/j.clon.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Allen C. Lea Bros; New York, NY: 1904. Radiotherapy and phototherapy; pp. 257–264. [Google Scholar]
- 92.Sommeroille J. Mycosis fungoides treated with general X-ray bath. Br J Dermatol. 1939;51:323. [Google Scholar]
- 93.Karzmark C.J., Loevinger R., Steele R.E. A technique for large-field, superficial electron therapy. Radiology. 1960;74:633–644. doi: 10.1148/74.4.633. [DOI] [PubMed] [Google Scholar]
- 94.Trump J.G., Wright K.A., Evans W.W. High energy electrons for the treatment of extensive superficial malignant lesions. Am J Roentgenol Radium Ther Nucl Med. 1953;69:623–629. [PubMed] [Google Scholar]
- 95.Podgorsak E. I.A.E. Agency; Vienna, Austria: 2003. Review of radiation oncology physics: a handbook for teachers and students. [Google Scholar]
- 96.Karzmack C. 1987. AAPM report No. 23, total skin electron therapy: technique and dosimetry. Report of group 30 radiation therapy committee AAPM. [Google Scholar]
- 97.Diamantopoulos S., Platoni K., Dilvoi M. Clinical implementation of total skin electron beam (TSEB) therapy: a review of the relevant literature. Phys Med. 2011;27:62–68. doi: 10.1016/j.ejmp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 98.Page V., Gardner A., Karzmark C.J. Patient dosimetry in the electron treatment of large superficial lesions. Radiology. 1970;94:635–641. doi: 10.1148/94.3.635. [DOI] [PubMed] [Google Scholar]
- 99.Bjarngard B.E., Chen G.T., Piontek R.W. Analysis of dose distributions in whole body superficial electron therapy. Int J Radiat Oncol Biol Phys. 1977;2:319–324. doi: 10.1016/0360-3016(77)90090-6. [DOI] [PubMed] [Google Scholar]
- 100.Hoppe R.T., Fuks Z., Bagshaw M.A. Radiation therapy in the management of cutaneous T-cell lymphomas. Cancer Treat Rep. 1979;63:625–632. [PubMed] [Google Scholar]
- 101.Reynard E.P., Evans M.D., Devic S. Rotational total skin electron irradiation with a linear accelerator. J Appl Clin Med Phys. 2008;9:2793. doi: 10.1120/jacmp.v9i4.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Funk A., Hensley F., Krempien R. Palliative total skin electron beam therapy (TSEBT) for advanced cutaneous T-cell lymphoma. Eur J Dermatol. 2008;18:308–312. doi: 10.1684/ejd.2008.0394. [DOI] [PubMed] [Google Scholar]
- 103.Piotrowski T., Malicki J. The rotary dual technique for total skin irradiation in the treatment of mycosis fungoides – a description of the applied method. Rep Pract Oncol Radiother. 2006;11(1):29–37. [Google Scholar]
- 104.Williams P.C., Hunter R.D., Jackson S.M. Whole body electron therapy in mycosis fungoides – a successful translational technique achieved by modification of an established linear accelerator. Br J Radiol. 1979;52:302–307. doi: 10.1259/0007-1285-52-616-302. [DOI] [PubMed] [Google Scholar]
- 105.Kuten A., Stein M., Mandelzweig Y. Total-skin electron irradiation for cutaneous T-cell lymphoma: the Northern Israel Oncology Center experience. Strahlenther Onkol. 1991;167:392–396. [PubMed] [Google Scholar]
- 106.Rosenblatt E., Kuten A., Leviov M. Total skin electron irradiation in mycosis fungoides dose and fractionation considerations. Leuk Lymphoma. 1998;30:143–151. doi: 10.3109/10428199809050937. [DOI] [PubMed] [Google Scholar]
- 107.Anacak Y., Arican Z., Drumea K. Total skin electron irradiation in mycosis fungoides: comparison between a modified Christie Hospital translational technique and the Stanford technique. Leuk Lymphoma. 2002;43:2093–2097. doi: 10.1080/1042819021000016177. [DOI] [PubMed] [Google Scholar]
- 108.Shouman T., El-Taher Z. Total skin electron therapy: a modified technique for small room linear accelerator. J Egypt Natl Cancer Inst. 2004;16:202–209. [PubMed] [Google Scholar]
- 109.Chen Z., Agostinelli A.G., Wilson L.D. Matching the dosimetry characteristics of a dual-field Stanford technique to a customized single-field Stanford technique for total skin electron therapy. Int J Radiat Oncol Biol Phys. 2004;59:872–885. doi: 10.1016/j.ijrobp.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 110.Freeman C.R., Suissa S., Shenouda G. Clinical experience with a single field rotational total skin electron irradiation technique for cutaneous T-cell lymphoma. Radiother Oncol. 1992;24:155–162. doi: 10.1016/0167-8140(92)90374-4. [DOI] [PubMed] [Google Scholar]
- 111.Ravichandran R., Kirshnamurthy K., Sivakumar S. Clinical electron beam configuration and physical parameters for total skin electron treatment (Test) J Med Phys. 2005;30:60–65. [Google Scholar]
- 112.Hoppe R.T., Fuks Z., Bagshaw M.A. The rationale for curative radiotherapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 1977;2:843–851. doi: 10.1016/0360-3016(77)90182-1. [DOI] [PubMed] [Google Scholar]
- 113.Jones G.W., Kacinski B.M., Wilson L.D. Total skin electron radiation in the management of mycosis fungoides: consensus of the European Organization for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Project Group. J Am Acad Dermatol. 2002;47:364–370. doi: 10.1067/mjd.2002.123482. [DOI] [PubMed] [Google Scholar]
- 114.Bittoun J., Saint-Jalmes H., Querleux B.G. In vivo high-resolution MR imaging of the skin in a whole-body system at 1.5 T. Radiology. 1990;176:457–460. doi: 10.1148/radiology.176.2.2367660. [DOI] [PubMed] [Google Scholar]
- 115.International Commission on Radiation Units and Measurements; Bethesda (MD): 1993. Phantoms and computational models in therapy, diagnosis and protection. Publication No. 48. [Google Scholar]
- 116.Jones G.W., Hoppe R.T., Glatstein E. Electron beam treatment for cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 1995;9:1057–1076. [PubMed] [Google Scholar]
- 117.Niroomand-Rad A., Gillin M.T., Komaki R. Dose distribution in total skin electron beam irradiation using the six-field technique. Int J Radiat Oncol Biol Phys. 1986;12:415–419. doi: 10.1016/0360-3016(86)90361-5. [DOI] [PubMed] [Google Scholar]
- 118.Kumar P.P., Patel I.S. Comparison of dose distribution with different techniques of total skin electron beam therapy. Clin Radiol. 1982;33:495–497. doi: 10.1016/s0009-9260(82)80156-6. [DOI] [PubMed] [Google Scholar]
- 119.Desai K.R., Pezner R.D., Lipsett J.A. Total skin electron irradiation for mycosis fungoides: relationship between acute toxicities and measured dose at different anatomic sites. Int J Radiat Oncol Biol Phys. 1988;15:641–645. doi: 10.1016/0360-3016(88)90306-9. [DOI] [PubMed] [Google Scholar]
- 120.Antolak J.A., Cundiff J.H., Ha C.S. Utilization of thermoluminescent dosimetry in total skin electron beam radiotherapy of mycosis fungoides. Int J Radiat Oncol Biol Phys. 1998;40:101–108. doi: 10.1016/s0360-3016(97)00585-3. [DOI] [PubMed] [Google Scholar]
- 121.Wilson L.D. Delivery and sequelae of total skin electron beam therapy. Arch Dermatol. 2003;139:812–813. doi: 10.1001/archderm.139.6.812. [DOI] [PubMed] [Google Scholar]
- 122.Yu J.B., Khan A.M., Jones G.W. Patient perspectives regarding the value of total skin electron beam therapy for cutaneous T-cell lymphoma/mycosis fungoides: a pilot study. Am J Clin Oncol. 2009;32:142–144. doi: 10.1097/COC.0b013e3181841f5c. [DOI] [PubMed] [Google Scholar]
- 123.Ysebaert L., Truc G., Dalac S. Ultimate results of radiation therapy for T1–T2 mycosis fungoides (including reirradiation) Int J Radiat Oncol Biol Phys. 2004;58:1128–1134. doi: 10.1016/j.ijrobp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 124.Smith B.D., Wilson L.D. Cutaneous lymphomas. Semin Radiat Oncol. 2007;17:158–168. doi: 10.1016/j.semradonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 125.Jones G.W., Rosenthal D., Wilson L.D. Total skin electron radiation for patients with erythrodermic cutaneous T-cell lymphoma (mycosis fungoides and the Sezary syndrome) Cancer. 1999;85:1985–1995. doi: 10.1002/(sici)1097-0142(19990501)85:9<1985::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 126.Navi D., Riaz N., Levin Y.S. The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol. 2011;147:561–567. doi: 10.1001/archdermatol.2011.98. [DOI] [PubMed] [Google Scholar]
- 127.Introcaso C.E., Micaily B., Richardson S.K. Total skin electron beam therapy may be associated with improvement of peripheral blood disease in Sezary syndrome. J Am Acad Dermatol. 2008;58:592–595. doi: 10.1016/j.jaad.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 128.Hauswald H., Zwicker F., Rochet N. Total skin electron beam therapy as palliative treatment for cutaneous manifestations of advanced, therapy-refractory cutaneous lymphoma and leukemia. Radiat Oncol. 2012;7:118. doi: 10.1186/1748-717X-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilson L.D., Quiros P.A., Kolenik S.A. Additional courses of total skin electron beam therapy in the treatment of patients with recurrent cutaneous T-cell lymphoma. J Am Acad Dermatol. 1996;35:69–73. doi: 10.1016/S0190-9622(96)90499-5. [DOI] [PubMed] [Google Scholar]
- 130.Becker M., Hoppe R.T., Knox S.J. Multiple courses of high-dose total skin electron beam therapy in the management of mycosis fungoides. Int J Radiat Oncol Biol Phys. 1995;32:1445–1449. doi: 10.1016/0360-3016(94)00590-H. [DOI] [PubMed] [Google Scholar]
- 131.Harrison C., Young J., Navi D. Revisiting low-dose total skin electron beam therapy in mycosis fungoides. Int J Radiat Oncol Biol Phys. 2011;81:e651–e657. doi: 10.1016/j.ijrobp.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 132.Kim Y. Low dose (12Gy) TSEB + Vorinostat versus low-dose TSEB monotherapy in mycosis fungoides. 2013, NCT01187446.
- 133.Quiros P.A., Jones G.W., Kacinski B.M. Total skin electron beam therapy followed by adjuvant psoralen/ultraviolet-A light in the management of patients with T1 and T2 cutaneous T-cell lymphoma (mycosis fungoides) Int J Radiat Oncol Biol Phys. 1997;38:1027–1035. doi: 10.1016/s0360-3016(97)00127-2. [DOI] [PubMed] [Google Scholar]
- 134.Chinn D.M., Chow S., Kim Y.H. Total skin electron beam therapy with or without adjuvant topical nitrogen mustard or nitrogen mustard alone as initial treatment of T2 and T3 mycosis fungoides. Int J Radiat Oncol Biol Phys. 1999;43:951–958. doi: 10.1016/s0360-3016(98)00517-3. [DOI] [PubMed] [Google Scholar]
- 135.Duvic M., Donato M., Dabaja B. Total skin electron beam and non-myeloablative allogeneic hematopoietic stem-cell transplantation in advanced mycosis fungoides and Sezary syndrome. J Clin Oncol. 2010;28:2365–2372. doi: 10.1200/JCO.2009.25.8301. [DOI] [PubMed] [Google Scholar]
- 136.Samant R.S., Fox G.W., Gerig L.H. Total scalp radiation using image-guided IMRT for progressive cutaneous T cell lymphoma. Br J Radiol. 2009;82:e122–e125. doi: 10.1259/bjr/61338036. [DOI] [PubMed] [Google Scholar]


