Abstract
Total skin electron irradiation (TSEI) for patients with cutaneous lymphomas is technically challenging, and numerous approaches have been developed to overcome the many field matching problems associated with such a large and complex treatment volume. Since 1981 we have delivered TSEI using a rotational total skin electron irradiation (RTSEI) technique in conjunction with patch, treat and boost fields in order to provide complete skin and dose coverage. Initially we used a 6 MeV electron beam at an extended source-skin distance (SSD) on a modified linear accelerator. More recently we began using a high dose rate electron mode on a commercially available linear accelerator. The RTSEI technique allows the delivery of a seamless surface dose to the majority of the patient's skin surface in a single treatment. In this review paper we present our three-decade experience with the technical development, dosimetry, treatment delivery and clinical outcomes of our RTSEI technique.
Keywords: Total skin electron irradiation, Mycosis fungoides, Radiotherapy, Rotational radiotherapy
1. Background
Cutaneous T-cell and B-cell lymphomas are rare malignancies that account for only 2% of all lymphomas. They are the second most common site of extranodal non-Hodgkin's lymphoma. Treatment of patients with cutaneous lymphomas is challenging. There is no “ideal” or even standard treatment for patients with more extensive but still skin confined disease, and current recommendations and treatment guidelines typically suggest a number of possible options1,2 for treatment. One of these options is total skin electron beam irradiation (TSEI)3,4 which may provide long-term disease control when given alone or combined with adjuvant treatments with Psoralen and ultra violet A (PUVA) or one of a variety of topical or systemic agents.5 However, TSEI is technically difficult and generally not available even in large radiotherapy centers. Moreover it is also associated with acute and sub-acute side effects that may significantly affect patients’ quality of life during and for several months post treatment. At the McGill University Health Centre we have offered total skin electron irradiation since the early 1980s using a rotational technique that was developed in-house. In total 172 patients have been treated over the past 31 years. Our technique and clinical outcomes have been described in detail elsewhere. The purpose of this paper is to review the historical development of our technique, to discuss the technical challenges in setting up a total skin electron beam irradiation program, to explain some of the practical details of treatment delivery, and to describe acute and sub-acute side effects and care of the patients during and after treatment. In addition, our goal is to share our experience at a time when it is more feasible than in the past to consider offering TSEI using a commercially available linac.
2. Technical development of rotational total skin electron irradiation (RTSEI)
Our original technique was custom developed on an existing linear accelerator (Clinac-18, Varian Medical Systems, Palo Alto, CA, USA) without TSEI capabilities.6–10 In order to reduce the beam matching complications associated with delivering TSEI, an innovative technique delivering a large 6 MeV electron field with the patient rotating within a stationary electron beam at an extended source-surface distance (SSD) was developed. This technique is referred to as Rotational Total Skin Electron Irradiation (RTSEI). The original technique dating from 1981 is shown schematically in Fig. 1. During treatment the patient stood on a rotating platform, at a nominal SSD of 285 cm from the linear accelerator transmission ionization chamber. The X-ray collimator was set to the maximum opening to give a 35 cm × 35 cm field at 1 m from the X-ray target and the collimator head was rotated to the 135° position in order to make the vertical dimension of the electron field as large as possible. Both the X-ray target, which was used in the 10 MV X-ray mode, and the scattering foil, which was used in the 6 MeV normal electron mode, were removed so that the electron beam impinged directly onto the transmission ionization chamber which thus served as the primary electron beam scatterer. A custom-made scattering filter was mounted on the machine accessory plate 35 cm from the ionization chamber to both scatter and flatten the electron beam. A 4 ft × 8 ft sheet of Lucite of 1/4 in. (6 mm) thickness was placed 265 cm from the ionization chamber to further scatter the electron beam and degrade its energy. The electron beam thus passed through the ionization chamber, the custom-made scattering filter, and the Lucite scatterer before it impinged upon the patient. Two external ionization chambers were used for beam monitoring; both were mounted on the Lucite scatterer. One was used to monitor the patient dose and was placed on the horizontal field central axis with the sensitive volume about 20 cm from the field center. The other was used to monitor the beam flatness and the dose rate, and was placed 20 cm below the first chamber. These two external ionization chambers were critical to monitoring dose delivery, since the geometry of the electron pencil beam rendered the linac's transmission ionization chamber unreliable.
Fig. 1.
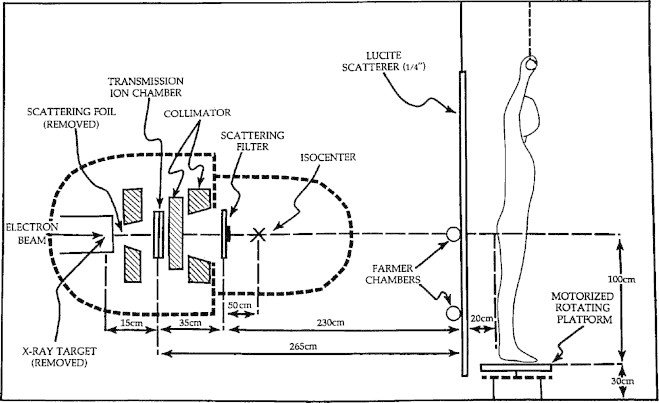
Schematic of the original RTSEI technique developed on the Varian Clinac-18.4
For our original technique, beam parameters were characterized in detail.6 Of particular interest were the vertical and horizontal off-axis ratios, the percent depth dose and the absolute dose and dose rate for both the stationary and the rotating beam. In fact the beam itself was not rotating, so that the reference to stationary and rotating electron beam refers to the patient's frame of reference. In addition, a considerable amount of effort was invested into the understanding of the complicated dosimetric effects of a patient rotating in a large stationary electron beam, especially upon surface dose and self shielding.8,9
Fig. 2 shows the percent depth dose for the original RTSEI technique. Previous TSEI techniques encountered difficulties not only with beam matching, but also with the location of the depth of maximum dose dmax. TSEI techniques aim to treat the superficial layer of the skin from the surface down to a specified depth. However, conventional stationary electron beams exhibit a percent depth dose buildup region and dose fall off that may not be desirable for TSEI. For example the percentage depth dose PDD of a typical 6 MeV electron beam at an SSD of 100 cm rises from about 78–100% over the first 1.2 g/cm2, and falls to 50% and 5% at about 2.3 g/cm2 and 3.0 g/cm2, respectively. Multiple stationary beams may overlap and raise the surface dose; however, as was found, and as shown in Fig. 2, there is a marked improvement in the 6 MeV electron beam PDD characteristics, first for the stationary beam at an extended SSD of 285 cm, and subsequently when the patient is rotating in the large field at an extended SSD. Under conditions of an extended SSD with the patient rotating in the beam, the PDD rises to 100% at the surface, falls to 50% at about 1.0–1.5 g/cm2 depending upon the presence of a Lucite scatterer, and falls to about 5% at about 3.0 g/cm2.
Fig. 2.
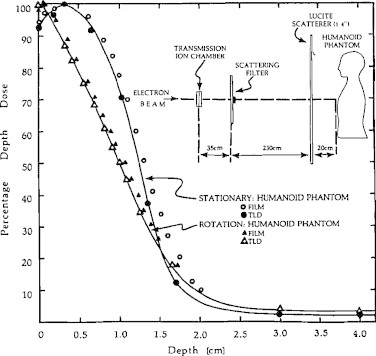
PDD for the stationary and rotational original RTSEI technique. Inset shows the room geometry.5
The use of an empirically designed custom scattering filter was important in producing a stationary electron beam at extended SSD with clinically acceptable flatness and symmetry. Details of these beam characteristics are shown in Fig. 3. In this figure the scatterer can be seen to produce a beam with flatness of ±5% over most of the field and ±10% over the entire region of clinical interest. For comparison, the consequence of not having a custom-made scattering filter can be seen on the right hand side of the sketch, with the open (unfiltered) beam clearly clinically unacceptable.
Fig. 3.
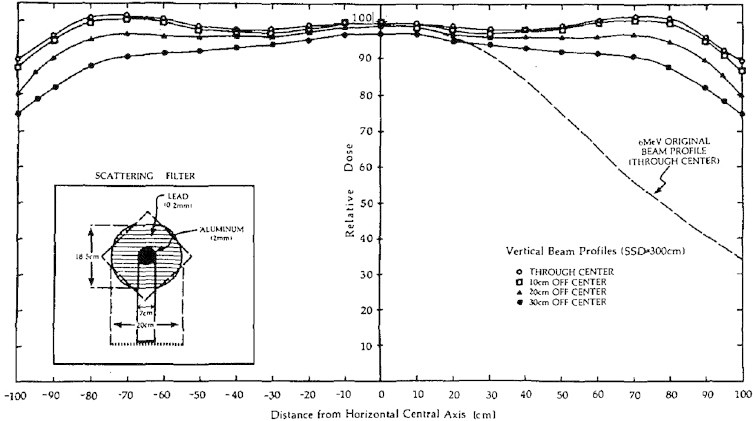
Off axis ratios of the original RTSEI technique in the vertical direction using the custom-made scattering filter. The clinically unsuitable open beam is shown on the right (dashed line) for comparison.4
The electron output of the original RTSEI technique was about 60 cGy/min for the stationary beam at extended SSD. The rotational surface dose was determined both experimentally and analytically to be about 45% of the stationary given dose. Thus, the rotational surface dose rate was about 25 cGy/min and typical treatment duration to deliver a tumor dose of 250 cGy was of the order of 10 min.
Treatment time per fraction varied slightly because of accelerator output variations. The expected reading on the external monitor chamber at the location of the Lucite plate was pre-calculated to determine the appropriate treatment time. The individual treatment was terminated at the nearest full revolution of the platform just after the monitor chamber reached its required reading. This ensured that there were always an integer number of patient revolutions during the beam-on time so that no body areas were over- or underdosed. During successive treatment fractions the patient arm positions were cycled through two orientations (right arm up or left arm up) and the patient feet positions were cycled through three possible orientations. Thus, six possible combinations of feet and arm locations were used so as to minimize self shielding. The patient used a rotating overhead handgrip attached to a chain in order to maintain balance throughout the 10-min rotation, and various combinations of eye and fingernail/toenail shields were used according to physician's prescription. From 1981 to 2006 a total of 155 patients were treated with our original RTSEI technique (see Fig. 4).
Fig. 4.
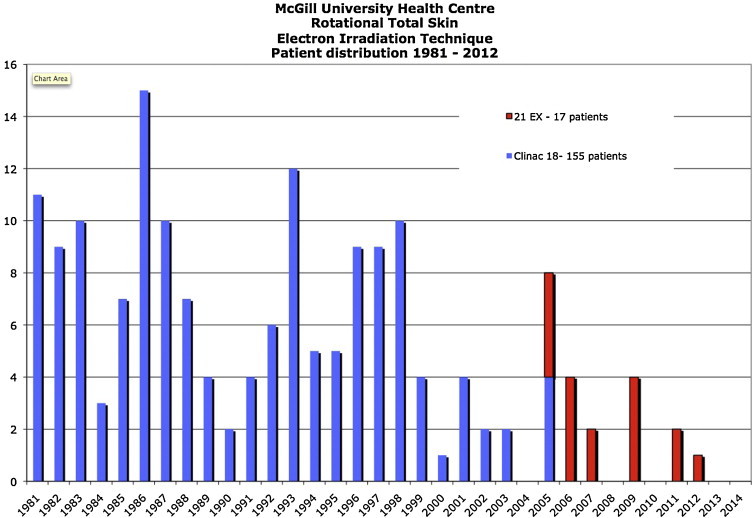
Distribution of patients treated with RTSEI from 1981 to 2012. 1992–2005: 155 patients treated with the Varian Clinac-18. 2005–2012: 17 patients treated with the Varian Clinac-21 EX.
In 2005, an upgrade to our radiotherapy department involved the decommissioning of the Varian Clinac-18 linear accelerator. In order to continue offering RTSEI treatments, we transferred the technique to a modern Varian Clinac-21EX (Varian Medical Systems, Palo Alto, CA, USA) linear accelerator equipped with an optional high dose total skin electron mode.11 This mode permits the delivery of a 6 MeV electron beam with a dose rate of the order of 27 Gy/min at an SSD of 100 cm. The 21EX linear accelerator is housed in a conventional radiation therapy treatment bunker. The distance from the isocenter (100 cm) to the wall near where the patient stands is greater than 300 cm, allowing sufficient space for both the patient and the rotating platform. With the rotating platform located a short distance from the wall, the treatment SSD from the linear accelerator target to the patient's umbilicus is 380 cm. A photograph of the treatment room showing the linear accelerator and the rotating platform is shown in Fig. 5.
Fig. 5.
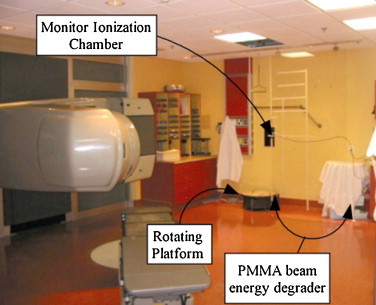
A photograph of the Clinac-21EX linac and treatment room used for RTSEI since 2005. The linac and couch positions, the rotating platform, the secondary ionization chamber, and the patient supports (wall hand grip and overhead hanging handlebar).9
As with our original RTSEI technique, a very large field, of the order of 200 cm in height by 90 cm in width, is required to cover the entire patient's skin surface. Again, this is achieved by rotating the gantry laterally to 270°, the collimator to 45°, and the couch to 45°. The precise couch position is not critical, but it must be positioned such that it is out of the beam's path. The photon collimator jaws are opened to their largest available setting of 40 cm by 40 cm to provide the largest possible field size. The 6 MeV electron beam used in our current technique lacks sufficient flatness to be used clinically and so in a manner similar to the original technique a custom-built flattening filter is added to the linac's coded accessory tray. The entire filter assembly is depicted in Fig. 6, with the left figure showing a schematic diagram of the filter and the right figure showing a photograph of the filter mounted on the linac's accessory tray.
Fig. 6.
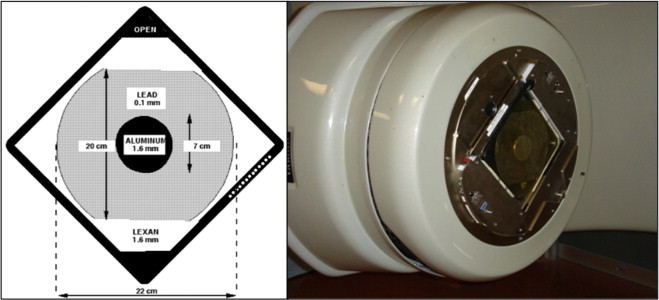
The custom-built flattening filter constructed of aluminum, lead, and PMMA mounted on the Varian supplied accessory tray. Left: a schematic diagram; right: a photograph of the filter mounted on the Varian-21EX linac service plate.9
The original custom-built rotating platform with a 60 cm diameter circular surface was retained for this current RTSEI technique. Unmodified, the top surface is 30 cm above the floor level. There is a hard foam spacer that can be added for shorter patients in order to increase the platform's height to 50 cm above the floor level. The platform has a small variable speed motor: typically, 3 rpm is selected during patient treatment. This setting is a compromise between the high speed required to minimize the dosimetric dependence on start and stop positions, and the low speed required for patient safety and comfort. As with the original technique, a grip bar to physically assist and support the patient is affixed to the wall to aid the patient during set-up, and a suspended rotating handlebar is centered midline above the axis of rotation to help the patient maintain balance during rotational treatment.
Given the extended SSD used in this technique, a higher nominal output is required from the linear accelerator. This is possible using the optional 6 MeV high dose rate electron mode provided by the manufacturer. Typically, in the standard linac electron modes, the nominal output of the linac is adjusted to yield 1 cGy per monitor unit (MU) at the depth of maximum dose at 100 cm SSD. In the 6 MeV high dose mode, on the other hand, the linac yields about 3.1 cGy per MU at the depth of maximum dose at 100 cm SSD. Furthermore, in contrast with the conventional machine output of 600 MU/min, the high dose rate electron mode runs at 888 MU/min. Consequently, the nominal output of the linac at SSD 100 cm for the 6 MeV high dose rate electron mode is approximately 2750 cGy per minute as opposed to the nominal linac output of 600 cGy per minute for the conventional 6 MeV electron mode.
Fig. 7 shows the standard 6 MeV PDD measured at an SSD of 100 cm, with a 10 cm by 10 cm electron applicator, along with the stationary and rotational PDDs at an SSD of 380 cm without applicator and with the custom-built flattening filter in place. In a similar manner to our original RTSEI technique on the Clinac-18 machine, the combination of extended SSD and patient rotation produce favorable beam characteristics for RTSEI: the rotational PDD has a surface dose at 100%, and an R50 of 1.5 g/cm2, and an Rp of 2.3 g/cm2. Of additional clinical importance in total skin treatments is the bremsstrahlung dose, as this represents the whole body dose that the patient will receive during treatment. For our current technique on the Clinac-21 EX the highest bremsstrahlung dose is delivered at the central axis and is of the order of 2.7% at a depth of 5 g/cm2, similar to the original technique.
Fig. 7.
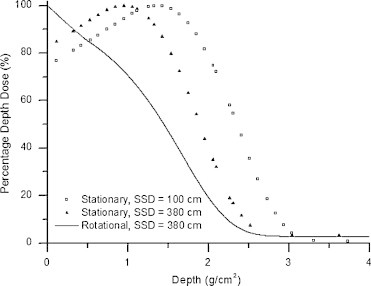
PDD for the Clinac-21 EX 6 MeV electron beam. The stationary PDD measured at an SSD of 100 cm (open box), the stationary PDD measured at an SSD of 380 cm (closed triangle), and the rotational PDD measured at an SSD of 380 cm (solid line).9
As in the original technique, beam flatness and symmetry for the open beam are clinically unacceptable. The use of an empirically designed flattening filter, as shown in Fig. 6, produces beam flatness and symmetry at the extended treatment SSD of 380 cm in both the vertical and the horizontal directions. The entire field flatness for the open field and the filtered field can be seen by the isodose contour plots in Fig. 8. The shaded body approximates the silhouette of an 80 kg man of 180 cm in height. The poor dose flatness in the unfiltered beam is shown on the left, the filtered beam on the right. In the case of the modified beam, we see that a typical patient would be entirely covered by the 90% level and, except for forearms and feet, would be irradiated to the 95% level.
Fig. 8.
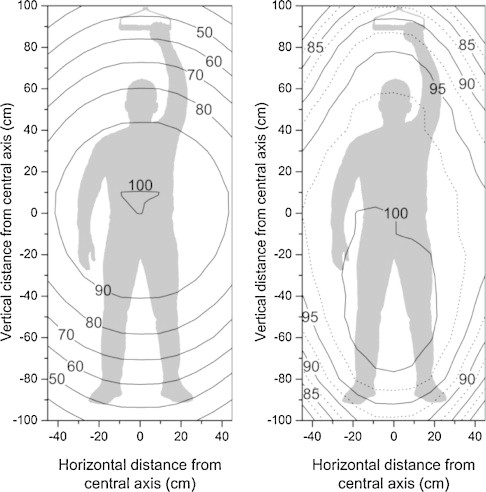
Beam contour plots, measured with and without the custom-made scattering filter, at an SSD of 380 cm, using the 6 MeV high dose electron- mode. The isodose contours represent a percentage dose relative to the dose on the central axis. The shaded body approximates the silhouette of an 80 kg man of 180 cm in height. Left: the clinically unsuitable open beam; right: the clinically acceptable filtered beam.9
Cross-sectional isodose distributions of the head, neck, and abdomen have been measured for the full rotation technique and are shown in Fig. 9. The absolute dose readings obtained when a 200 cGy dose is delivered under RTSEI conditions are shown.
Fig. 9.
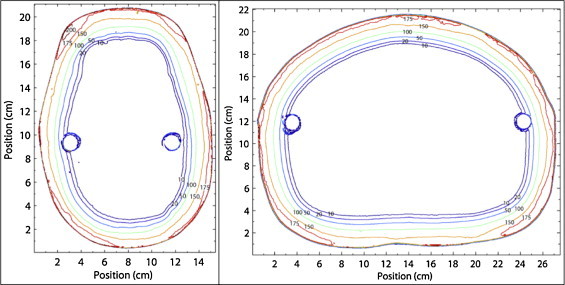
Isodose plots of the head (left), and abdomen (right) measured in a humanoid phantom using the 6 MeV RTSEI technique to deliver a 200 cGy dose. Lines represent the absolute dose values in cGy measured using radiochromic film.9
The static beam calibration was carried out for our RTSEI beam delivered with the gantry at 270°, the collimator set at 40 cm × 40 cm and rotated to 45°, the couch at 45°, the custom flattening filter in place, and a plane-parallel chamber embedded in water equivalent phantom at the depth of maximum dose. The details of the calibration procedure are described in detail elsewhere.11 In this manner, the absorbed dose delivered on the beam's central axis at an SSD of 380 cm per 1000 MU was determined to be about 54.0 cGy/1000 MU.
As in the original technique, a factor is required to relate the dose rate at the surface of the rotating patient to the stationary dose rate. For our current technique the ratio of the two dose rates was determined to be 0.448. This was in very close agreement with the previously determined ratio of 0.45 for the original RTSEI technique. Multiplying the stationary output of 54.0 cGy/1000 MU by the rotational factor of 0.45, results in the rotational dose rate at the patient surface of 24.3 cGy/1000 MU. At the high dose mode dose rate of 888 MU/min, this results in a treatment time of just shorter than 10 min, with about 28 revolutions, for a typical treatment fraction prescription of 200 cGy delivered with 8230 MU. As for all other therapeutic beams used at our institution, beam characteristics and output are monitored by an external auditing agency.
Since the custom made flattening filter is mounted downstream from the linac's transmission ionization chamber, there is a risk that treatment might be delivered without the correct filter in place, or that the filter might break and fall off during treatment resulting in a higher and non-uniform treatment dose. As a precaution against this unlikely event, a secondary dosimetry monitoring system consisting of an independent ionization chamber and electrometer combination has been installed. The chamber is mounted on a support system in the treatment beam slightly lateral to the patient near the nominal treatment SSD of 380 cm. During beam calibration, a relative factor is determined for this particular set-up with the secondary dosimetry system, and in this manner day-to-day treatments can be monitored for consistency and to provide increased safety. This system also allows us to carry out a set of pre-treatment checks prior to patient treatment in order to ensure that the technique is ready for proper beam delivery. The current technique has an additional advantage over our original technique, in that it is delivered within the scope of our current information management system (ARIA, Varian Medical Systems, Palo Alto, CA, USA). Thus, it can be scheduled, monitored, and recorded in the same manner as all other patients in the clinic. From 2005 to 2012 a total of 17 patients were treated with the RTSEI technique using the Varian 21 EX (see Fig. 4).
3. Clinical considerations and beam delivery
The delivery of the TSEI requires the expertise of a TSEI treatment team and close collaboration among the team members comprised of radiation oncologists, medical physicists, and the treating therapists. Our RTSEI technique, as with all TSEI techniques, leaves areas that are either completely untreated or underdosed. In terms of treatment delivery, there are generally two distinct treatment sessions. One session is dedicated to the delivery of the RTSEI component, and the second, to the delivery of direct static electron fields to undosed regions (treat fields), underdosed regions (patch fields), and regions requiring supplemental dose (boost fields). These direct static fields are delivered with the standard 6 MeV (non high dose rate mode) electron beam at the nominal SSD of 100 cm. A description of these considerations from the therapist's perspective is presented here.
Following initial consultation and the decision to commence total skin electron therapy, the patient is scheduled in our mold room in order to prepare custom shielding and accessories required for treatment. The patient also goes to the treatment machine in order to simulate the position for the RTSEI and perform planning for various direct electron fields requiring custom-made cutouts. The length of the handlebar that hangs from our treatment room ceiling is set according to the height of the patient. The radiation oncologist and therapists ensure that the patient is able to tolerate the rotating motion of the platform, and the treating radiation oncologist delineates the self-shielded areas requiring separate treatment with direct electron fields with the patient in the RTSEI treatment position.
All patients have eye shielding during RTSEI. This is preferably achieved using internal eye shields to ensure adequate irradiation of any disease on the eyelids and around the orbits. However, if there is no disease in the vicinity of the eyes, external eye shields may be used instead of internal eye shields. In our clinic, commercially available internal eye shields (Radiation Products Design, Albertville, MN, USA) made of aluminum and tungsten are available in 3 sizes in order to accommodate the patient's anatomy. External eye shields that are fabricated in-house are constructed with oval pieces of lead that are covered in a layer of wax and are taped over the closed eyelids. We have several different sizes and shapes of pre-made shields that we can select in order to best conform to the patient's eyes. Selected shields are labeled with the patient's name and positional orientation and used throughout the patient's course of RTSEI treatment.
A “custom” vertex shield is chosen from an assortment of ellipse-like shapes of wax-covered lead. The purpose of the vertex shield is to completely shield the top of the patient's head during RTSEI treatment. Since it is very difficult to estimate the dose gradient being delivered to the slope between the top of the head and the face of the patient, we aim to eliminate treatment to the larger part of the gradient during RTSEI and irradiate the vertex separately with a direct electron treat field. A vertex shield that best conforms to the patient's head shape is first chosen. Then a “halo”-like mark is drawn on the patient's scalp; this represents the area that requires separate treat irradiation and defines the location where the therapist must position the shield on the patient's vertex for RTSEI. This shield is identified with the patient's name and positional orientation and used throughout the course of the RTSEI treatment. A photo of a vertex shield (left image) and a patient with the vertex shield secured on his head (right image) for RTSEI treatment is shown in Fig. 10.
Fig. 10.
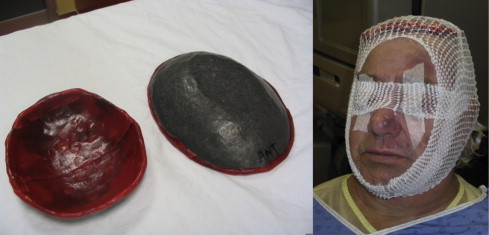
The vertex shield (left) and a patient with the vertex shield secured on his head (right) for RTSEI treatment.
For irradiation of the separate vertex electron treat field, an open 25 cm × 25 cm cutout is used with a standard 6 MeV stationary electron beam. The linac is setup with the gantry at 270°, collimator at 0°, couch at 270° and the patient lying supine, head toward gantry, with a cushion under the knees for comfort. We use a box made of Styrofoam that is covered with a sheet of lead on the side that is facing the machine, and the patient's head lies on the posterior “headrest”-like Styrofoam base of this box. We then appose two sheets of lead, each with a half-circle cut in the middle, on the machine side of this box in order to conform to the mark on the patient's head, leaving an open circle on the center area that requires treatment. Any radiation outside this area falls on these pieces of flexible lead when setup is done for this direct electron field. The SSD for the vertex field treatment is 100 cm with 1 cm bolus so that the SSD to the skin is 101 cm. The sheet of bolus is secured to the box and then taped again all around the exposed area in order to be in contact with the patient's head. A photo of the setup for vertex treatment is shown in Fig. 11.
Fig. 11.
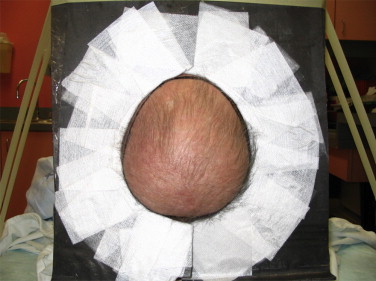
The setup for direct field treatment of the vertex. The area irradiated corresponds to the area shielded by the vertex shield in RTSEI shown in Fig. 10.
For treat irradiation of the soles of the feet, an open 25 cm × 25 cm cutout is used with a standard 6 MeV electron beam. The linac is setup with the gantry at 270°, collimator at 0°, couch at 270° with the patient lying supine with his feet toward the gantry and a pillow under the head for comfort. We again immobilize the patient using a box made of Styrofoam that is covered with a sheet of lead on the side facing the machine. The patient's heels lie on the posterior Styrofoam base of this box. On the day of planning, a malleable sheet of lead is customized to the shape and size of the patient's feet so as to allow the soles of the feet to be exposed while shielding the areas between, above and below completely. The lead is cut so that when lying supine on the treatment bed, the patient can point the toes and push through both “foot holes” in the sheet of lead. The patient then pushes the heels out and toes up so that the soles of the feet are upright and just projecting out from the mold. This custom-cut sheet of lead hangs from the top of the lead-lined Styrofoam box on the side of the linac. The patient side of the box also has a hanging full sheet of lead to counterbalance the weight of the opposite side and to shield the patient from any radiation in the case where there may be a small gap around one or both of the feet. The patient-side lead sheet hangs from the top of the box and reaches to just above the patient's ankles. The therapists also have access to some malleable lead paste which can be pushed around the soles of the feet in order to shield any gaps that may be leftover because of daily variations in positioning and the rigidity of the sheet of lead around the feet. Treatment is delivered in a similar manner to the vertex at SSD 100–1 cm bolus. Therapists set up with SSD 101 cm to the skin of one of the feet in an average depth area (not on the arch or ball of the foot but somewhere in the middle). The therapists must then displace the position of the treatment bed to ensure that the field light from the 25 cm × 25 cm cutout covers both soles of the feet completely while at the same time the area irradiated around the feet must still fall within the lead shielded area on the immobilization box. We then apply 1 cm bolus over the entirety of both soles. If the patient's feet are large, we may modify the treatment technique in one of two ways. If the feet are only slightly larger, we may be able to obtain complete coverage of both soles by using the same setup and simply using an extended SSD treatment distance. In this case a measured output at the extended treatment SSD is required. Alternatively, we immobilize the patient's feet in the same way but use a custom made cutout to treat both feet separately with individual treat fields. This cutout is basically an oval shape centered within a 25 cm × 25 cm cutout, made to be diagonal in orientation through the use of a 45° collimator angle. A photo of the setup for direct field treatment of the feet is shown in Fig. 12.
Fig. 12.
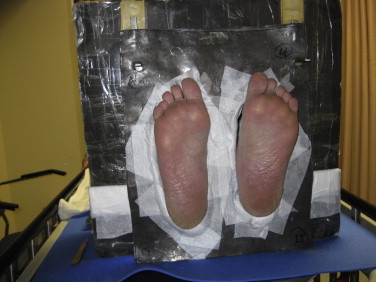
The setup for direct field treatment of the soles of the feet using the conventional 6 MeV electron beam. Set-up is at SSD 101 cm to the sole with 1 cm bolus (not shown).
In order to delineate the area around the perineum that is self-shielded by the patient's legs, we simulate RTSEI patient positions in the 3 different foot positions and mark the composite non-exposed area. The patient is then set up in a supine position on the treatment couch, head on a pillow with buttocks on the very end of the couch near the linac. The patient's legs are positioned either in stirrups that can be fixed to the couch side rails or with legs up and feet and heels resting on the horseshoe, knees bent and maintained at a reproducible distance from each other with a Styrofoam block inserted between the legs. Either of these positions allows reaching the patient's perineal area with an electron cone in place. At the time of planning, the marks delineating the self-shielded area are simply connected to set the limits of the perineum direct electron treat field. The projection of this mark is drawn onto a Plexiglas template inserted into a frame in an appropriately sized electron cone (typically a 15 cm × 15 cm cone) with the patient in treatment position. A custom cutout is required for these fields for all patients. A direct 6 MeV electron beam is used, with 1 cm bolus. As for other direct fields, setup is done at SSD 100 cm to bolus. The conditions for direct field treatment of the perineum are shown in Figs. 13 (setup) and 14 (area irradiated).
Fig. 13.
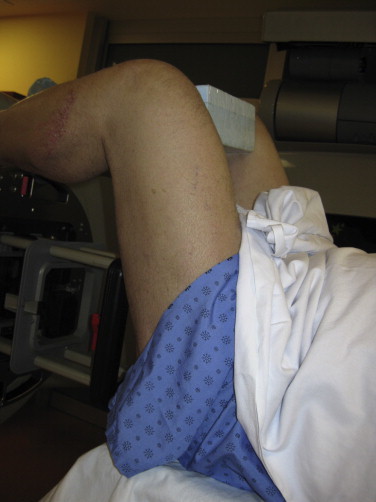
The setup for direct field treatment of perineum. Legs are supported by the linac “horseshoe” with a Styrofoam spacer between the knees for comfort. Nominal SSD to the skin is at SSD 101 cm with 1 cm bolus (not shown).
Fig. 14.
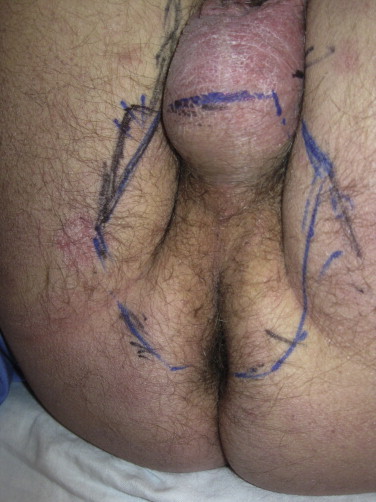
Typical irradiated area during direct field treatment of the perineum.
The patient's axillae usually require separate irradiation through patch fields. This is because each axilla receives only half of the RTSEI dose since the patient always has one arm down during treatment, resulting in self-shielding. Delineation of the area to be treated separately through a patch field is done by simulating RTSEI with both arm positions. The radiation oncologist determines the limits of the axillae area to be patch treated. Once these field limits have been marked on both sides, the patient is set up on the treatment couch, supine with a cushion under their knees for comfort and both arms up using either a wingboard, or with hands clasped above head so as to increase the ease of access to the axilla with the electron cone. The projection of these marks is then used to make custom cutouts for these fields for both the right and left sides. The position of the treatment couch, the gantry and the collimator is variable from patient to patient in order to have the incident electron beam as perpendicular as possible to the area to be treated. Extended SSD setups are sometimes necessary for larger fields or with patients that have limited arm mobility. 6 MeV electrons are used, typically at SSD 100 cm with 1 cm bolus. A photo of the setup for direct field patch of the axilla is shown in Fig. 15.
Fig. 15.
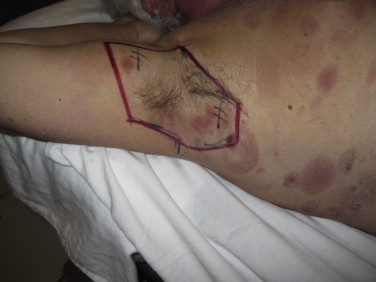
Typical setup for direct field patch of the axilla. Limits of the field to be patched are determined by the radiation oncologist with the patient standing on the platform, in the RTSEI position.
The palms of patient's hands require separate patch irradiation since the RTSEI delivery contributes only half the prescription dose given that the hand holding onto the handlebar is shielded. The other hand is at the patient's side, and open. We treat the palms of the hands using a 6 MeV electron beam and an open standard 25 cm × 25 cm cutout. The linac is setup with the gantry at 180°, collimator at 0° and couch at 0°. We use a see-through tabletop that allows the therapist to see the field light. The patient is treated in a standing position by aligning the limit of the palm of the hands that is exposed while holding the handlebar. This limit is delineated with a mark drawn by the treating therapist when the patient is holding onto the handlebar during RTSEI. We then ensure that both hands are within the irradiated area and that the hands lie flat on a 1 cm thick bolus with SSD set to 100 cm at the bolus. An additional 1 cm of bolus for backscatter is placed on the exit side of the patient's hands. A photo of the setup for direct field patch of the hands is shown in Fig. 16.
Fig. 16.
Setup for direct field patch of the hands. Hands are typically placed upon a 1 cm slab of bolus, with an additional 1 cm of bolus for backscatter.
Other areas that may require separate patch field irradiation due to self-shielding are the infra-mammary folds under pendulous breasts and/or under a lower abdominal fold in obese patients. These areas are verified by the radiation oncologist at the time of planning with the patient in RTSEI treatment position, and treatment field limits are defined if deemed necessary. Any patch treatment to these areas is usually delivered with a 6 MeV electron beam through a custom cutout that is made for the patient, in a treatment position that allows for reproducibility and accessibility to area, and is as parallel as possible to the electron cone. Bolus is used as required.
Shielding of the nails is considered an option for patients with no disease distal to the elbows and/or knees. Over the years we have assembled a large variety of shapes and sizes of nail shields, and similarly to the in-house external eye shields they are small round or oval pieces of lead covered in wax. A photo of the shielding technique for fingernails is shown in Fig. 17.
Fig. 17.
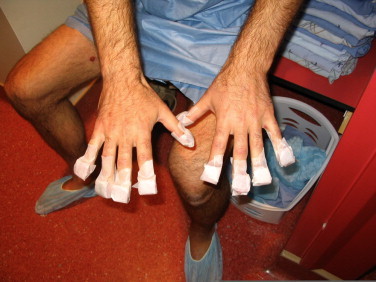
Typical setup for shielding the fingernails.
Prior to delivery of the RTSEI component of the treatment regime the therapists prepare the treatment room. The treatment couch is moved out of the way, to 45°, and the gantry is rotated to the lateral (270°) facing the rotating platform. The RTSEI filter is inserted into the collimator angled to 45°, and the field size is set to its maximum of 40 cm × 40 cm automatically after detecting the presence of the filter.
Our procedure currently involves a pre-treatment measurement of the dose delivered with our RTSEI beam. Once all pre-treatment checks have been performed, the therapists can proceed with the RTSEI patient preparations.
The therapists begin by assembling the eye shielding, the vertex shield, and any necessary nail shielding. The patient removes all clothing including underwear and changes into a thin, disposable paper hospital gown (originally intended for isolation patients), the sleeves of which have been cut off. The dosimetric effect of this gown has been determined to be negligible, and this gown provides the patient some measure of comfort and dignity during the treatment procedure.
The patient sits down for final preparation before assuming treatment positions. Toe and finger nail shields are taped and secured in place according to the physician's prescription.
A chair is setup inside the treatment room where technologists secure the vertex shield on top of the patient's head, and the eye shielding. External eye shields can be simply taped over the patient's closed eyes and secured into place with a mesh-like stretchy dressing that wraps around the patient's head and over the eyes. In patients requiring the use of internal eye shields the therapist first anaesthetizes the patient's eyes using xylocaine eye drops. This usually requires about 3 drops to each eye. By the time the third drop goes into the eye the pupil no longer reacts, which generally indicates that the eye shield can be safely and painlessly inserted. Once the internal eye shields are inserted into the eyes, the eyes are taped shut. The same mesh-like dressing is wrapped around the patient's head and over the eyes. Once the eyes are shielded, the vertex shield is positioned on the patient's head and secured into place with the mesh-like dressing that covers the top of the head and passes under the chin (Fig. 10).
Once prepared, the patient is helped onto the platform and one of the six available arm and feet combinations is chosen. The patient's chin is up, the patient's free arm is down and slightly away from body with elbow slightly bent and palm open. The rotating platform motor is set to 3 RPM, and patient balance and clearance from wall with arm is ensured. A physicist is present along with the therapist during delivery of the RTSEI treatment. A photo of the patient ready for RTSEI treatment is shown in Fig. 19, and the custom built rotating platform with the three color-coded feet position locations is shown in Fig. 20. RTSEI treatment is commenced once all staff have left the treatment room. The patient is monitored during treatment with intercom and closed circuit TV.
Fig. 19.
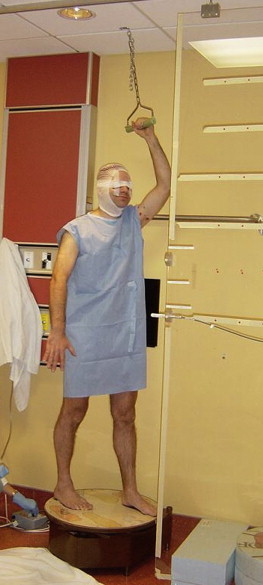
A photo of the patient ready for RTSEI treatment. The patient is standing on the rotating platform and is able to support himself with the rotating hand grip above his head. Note the presence of the vertex shield, eye shields, the arm and leg positions. The secondary ionization chamber used to monitor the beam may also be seen at the level of the waist, mounted on the Lexite sheet.
Fig. 20.

The custom built rotating platform with the three color-coded feet locations. A variable speed regulator is seen at the left of the platform.
Occasionally we treat patients with reduced mobility who are unable to tolerate the rotational nature of RTSEI. These patients are setup in a manner similar to other patients in terms of vertex, eye and fingernail shields. They are assisted to stand on the platform and a four-legged walker is placed on the platform in order to afford the patient more stability. The patient is rotated to six positions by having the platform rotate and then stop at every 60 degrees. At each of the six stop positions, the therapists leave the treatment room and deliver one sixth of the calculated MUs. The therapists then re-enter the treatment room and rotate the platform by another 60 degrees, leave the room and deliver another set of MUs, until the entire MU set has been delivered.
Another modification to the standard RTSEI delivery is encountered when patients only require a portion of their body to be treated. Sometimes upper body only, lower body only or head only are treated. A large sheet of plywood, two inches thick and suspended from the ceiling is used to delineate the treatment volume. The output for these RTSEI treatments are determined for each patient depending upon the location of the wood shield. Interestingly, we have noted that patients receiving lower body irradiation, having the upper part of their body shielded, become dizzy during RTSEI without the presence of eye shields. Thus, all patients have external eye shields, even when this is not clinically necessary.
4. Dose prescription
A typical dose prescription for a RTSEI patient with mycosis fungoides when TSEI is the sole treatment is 36 Gy given in 18 or 20 fractions of 1.8 or 2 Gy given 4 days each week. Untreated areas (treat volumes) are treated to a similar dose, while the prescribed dose to areas underdosed because of self-shielding (patch volumes) is typically half of that dose, namely 18 Gy given in 9 or 10 fractions of 1.8 or 2 Gy given on 2 days each week.
Thicker lesions or tumors in patients with more advanced disease may require treatment with boost fields (Fig. 18). This is usually given prior to RTSEI so that all disease at the time of start of RTSEI will be within the range of penetration of the electron beam. Treatment is delivered usually with direct electron fields but sometimes with photons to low doses such as 15 Gy in 5 daily fractions, depending on the depth and extent of the lesion(s).
Fig. 18.
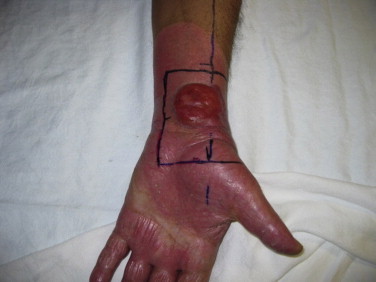
Typical field delineation for direct field boost of the wrist. Field limits are determined by the radiation oncologist.
Patients are typically given RTSEI treatments on Monday, Tuesday, Thursday and Friday. Boost, patch and treat fields to the vertex, soles and palms, perineum, inframammary folds and elsewhere are delivered as required throughout the five weekdays.
5. Care before, during, and after TSEI
TSEI is a difficult treatment for some, but not all, patients. Patients need to be prepared well for treatment, and preparation usually takes at least two visits to the radiation oncology department. The first visit includes the consultation and discussion of treatment options with the radiation oncologist and the second involves a session with a specialist therapist who shows the patient the treatment unit and explains the various aspects of treatment. Patients are allowed to shower and told that they can use a hydrating cream as is our standard practice for treatment to other areas. During treatment, patients are evaluated weekly initially. By week 3 many will have some erythema of the skin and by week 4 some may need special creams such as flamazine. By week 4 patients may have some swelling of the extremities. If swelling is severe and/or there is blistering (usually the feet, less frequently the hands) consideration is given to discontinuing the boosts to the soles of the feet at this point. Blood counts are monitored weekly during TSEI although unless patients have received prior chemotherapy it is unusual to see any significant change during treatment and very rare indeed to have to interrupt treatment.
Patients are followed very closely (Q 2–3 months) following treatment. Once the acute skin reaction and any edema has settled down (typically within the first 4–6 weeks) the degree of response is reasonably evident. However, sometimes there are residual lesions that if still present 6–9 months post TSEI should be biopsied and if positive can be boosted with local, low dose, radiotherapy with a good probability of permanent control.
6. Clinical outcomes
Clinical outcomes using our original RTSEI technique have been published twice previously.12,13 Fig. 21 shows cause-specific survival and Fig. 22 shows cause-specific survival by T stage as reported by Freeman et al.12 in 1992. Fig. 23 shows overall survival and Fig. 24 shows freedom from recurrence in complete responders as reported by Roberge et al.13
Fig. 21.
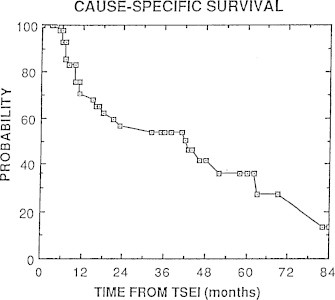
Cause-specific survival for 44 patients with mycosis fungoides treated with RTSEI as reported by Freeman et al.12
Fig. 22.
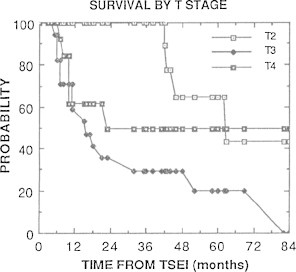
Cause-specific survival by T stage at RTSEI as reported by Freeman et al.12
Fig. 23.
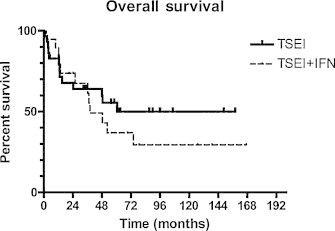
Overall survival as reported by Roberge et al.13 Patients with mycosis fungoides were treated either with RTSEI (TSEI) alone or RTSEI and alpha-interferon (IFN).
Fig. 24.
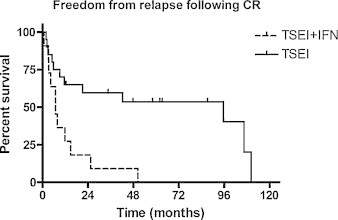
Freedom from recurrence in complete responders as reported by Roberge et al.13
At each review, acute toxicity was considered acceptable. Longer term sequelae were limited to permanent hair loss in a band-like distribution at the junction of the vertex and RTSEI fields in some patients, and dry skin and brittle nails which is a more universal finding. Telangectasia, which is common in patients treated with TSEI techniques using multiple stationary fields, is infrequently seen. Response to RTSEI was good, with a complete response rate of 73% in the 44 patients included in the 1992 review and 65% of the 31 patients treated with RTSEI alone included in the 2007 review. Overall 5-year survival was 38% and 50% respectively. In both reviews, long term relapse-free and overall survival were better for patients with earlier stage (T2, stages IA–IIa) disease than for more advanced disease.
Early results are quite similar for the patients treated since 2005 using our current technique (Table 1). A somewhat larger percentage (4/17) of patients treated since 2005 have diseases other than mycosis fungoides/CTCL and three of these received only low dose RTSEI (4–12 Gy in 2–5 fractions). Of the 12 patients (10 with mycosis fungoides/CTCL, one with a non-Hodgkin lymphoma blastic NK-cell type and one with a follicular lymphoma) that received RTSEI to doses =24 Gy, 9/12 had grade 2 or 3 dermatitis and 6/12 had swelling of the feet and/or hands, and treatment was discontinued at an RTSEI dose lower than planned in one of these patients because of this. Only one patient developed hematologic toxicity. She had been treated prior to RTSEI with chemotherapy and soon post RTSEI was found to have bone marrow involvement. Of the 7 patients with mycosis fungoides who received RTSEI to a dose =24 Gy, 5/7 of whom had advanced stage (=IIB) disease, a complete response was seen by the end of treatment in four and a good partial response in one. Only one patient, a patient with stage IVA T4 N3 mycosis fungoides, showed essentially no response to treatment by completion of treatment. For one of these 7 patients, no information regarding response to treatment was available.
Table 1.
Characteristics of 17 patients treated with current RTSEI technique.
| Age/sex | Diagnosis/stage | Prior/local RT | Dose/fx | Side effects | Response |
|---|---|---|---|---|---|
| 77/F | NHL blastic NK-cell type | No | 30 Gy/15 fx | Hematologic toxicity | GPR |
| 43/M | CTCL CD8+ | Yes | 36 Gy/18 fx | Gr 3 dermatitis, blistering of hands and feet | GPR |
| 72/M | Follicular lymphoma | No | 30.6 Gy/17 fx | None | CR |
| 75/M | MF stage IIB T3 N1 | Yes | 24 Gy/12 fx | Gr 3 dermatitis, swelling and blistering of hands and feet. Discontinued RTSEI early b/c of toxicity | CR |
| 74/M | CTCL, non MF | No | 32 Gy/16 fx | Gr 3 dermatitis | CR |
| 64/M | MF stage IIB T3 N0 | No | 16 Gy/8 fx | None. Discontinued RTSEI early b/c of deteriorating general condition | NR |
| 68/M | CLL | No | 4 Gy/2 fx | None | PD |
| 73/M | MF stage IVA T4 N3 | No | 36 Gy/20 fx | Pain and swelling of feet | NR |
| 67/F | MF stage IIA T2 N1 | No | 36 Gy/18 fx | Gr 2 dermatitis | GPR |
| 70/F | MF stage IA T1 N0 | No | 36 Gy/18 fx | Gr 2 dermatitis, swelling of hands and feet | Unknown, no follow up |
| 81/M | AML, lymphoma cutis | No | 12 Gy/5 fx | None | Unknown, no follow up |
| 43/F | HTLV-1 T-cell lymphoma | No | 5 Gy/2 fx | None | NR |
| 69/F | CTCL | Yes | 29 Gy/15 fx | Gr 2 dermatitis | GPR |
| 69/F | MALT lymphoma | Yes | 12 Gy/3 fx | None | |
| 66/M | MF stage IIB T3 N0 | Yes | 36 Gy/18 fx | Gr 3 dermatitis, swelling of hands and feet | CR |
| 25/F | MF stage IIB T3 Nx | Yes | 30.6 Gy/17 fx | Gr 2 dermatitis | CR |
| 64/M | MF stage IIB T3 N0 | Yes | 36 Gy/20 fx | Gr 3 dermatitis, swelling of hands and feet | CR |
GPR: good partial response, CR: complete response, NR no response, PD: progressive disease.
7. Conclusions
We have seen a fluctuation in the numbers of patients with mycosis fungoides referred for TSEI over the years as new topical and systemic agents have become available for treatment. However, as we and others have shown, TSEI is a useful treatment for this difficult disease, with a high complete response rate even in patients with advanced disease. Adjuvant treatment may be required to maintain response and an “ideal” management strategy has not yet been established.
TSEI is technically challenging due to the complex volume requiring treatment, and field matching remains the primary source of difficulty. At the McGill University Health Centre our 6 MeV rotational total skin electron irradiation technique (RTSEI) at an extended SSD seamlessly delivers a total skin dose while avoiding most of the beam matching problems associated with static electron field delivery. From 1981 to 2012, 172 patients have been treated, originally using a modified linear accelerator, and currently using a linear accelerator with a commercially available high dose electron mode. The dynamic nature of the RTSEI treatment has unique dosimetric aspects associated with the beam energy (PDD), beam flatness and absolute output. The treatment requires the close collaboration of radiation oncologists, treating therapists and medical physicists to deliver the RTSEI component of the treatment along with treat, patch, and boost fields to ensure complete volume and dose coverage.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Willemze R., Jaffe E., Burg G. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 2.Prince H.M., Whittaker S., Hoppe R.T. How I treat mycosis fungoides and Sézary syndrome. Blood. 2009;114:4337–4353. doi: 10.1182/blood-2009-07-202895. [DOI] [PubMed] [Google Scholar]
- 3.Piotrowski T., Malicki J. The rotary dual technique for total skin irradiation in the treatment of mycosis fungoides – a description of the applied method. Rep Pract Oncol Radiother. 2006;11(1):29–37. [Google Scholar]
- 4.Piotrowski T., Milecki P., Skorska M., Fundowicz D. Total skin electron irradiation techniques: a review. Postep Dermatol Alergol. 2013;XXX(1):50–55. doi: 10.5114/pdia.2013.33379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navi D., Riaz N., Levin Y., Sullivan N., Kim Y., Hoppe R.T. The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol. 2011;147:561–567. doi: 10.1001/archdermatol.2011.98. [DOI] [PubMed] [Google Scholar]
- 6.Podgorsak E.B., Pla C., Pla M., Lefebvre P.Y., Heese R. Physical aspects of a rotational total skin electron irradiation. Med Phys. 1983;10(2):159–168. doi: 10.1118/1.595296. [DOI] [PubMed] [Google Scholar]
- 7.Kim T.H., Pla C., Pla M., Podgorsak E.B. Clinical aspects of a rotational total skin electron irradiation. Br J Radiol. 1984;57:501–506. doi: 10.1259/0007-1285-57-678-501. [DOI] [PubMed] [Google Scholar]
- 8.Pla C., Heese R., Podgorsak E.B. Calculation of surface dose in rotational total skin electron irradiation. Med Phys. 1984;11(4):539–546. doi: 10.1118/1.595524. [DOI] [PubMed] [Google Scholar]
- 9.Pla C., Heese R., Pla M., Podgorsak E.B. A simple numerical method for surface dose calculation in rotational total skin electron irradiation. Proceedings Eighth International Conference on the Use of Computers in Radiation Therapy; IEEE Computer Society Press; 1984. pp. 145–150. [Google Scholar]
- 10.Gosselin M., Podgorsak E.B. The McGill rotational total skin electron irradiation technique. Med Dosim. 1985;10:29–32. [Google Scholar]
- 11.Reynard E., Evans M.D.C., Parker W. Rotational total skin electron irradiation (RTSEI) with a linear accelerator. J Appl Clin Med Phys. 2008;9:123–134. doi: 10.1120/jacmp.v9i4.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman C.R., Suissa S., Shenouda G. Clinical experience with a single field rotational total skin electron irradiation technique for cutaneous T-cell lymphoma. Radiother Oncol. 1992;24:155–162. doi: 10.1016/0167-8140(92)90374-4. [DOI] [PubMed] [Google Scholar]
- 13.Roberge D., Muanza T., Blake G., Shustik C., Vuong T., Freeman C.R. Does adjuvant alpha-interferon improve outcome when combined with total skin irradiation for mycosis fungoides? Br J Dermatol. 2007;156:57–61. doi: 10.1111/j.1365-2133.2006.07559.x. [DOI] [PubMed] [Google Scholar]


