Abstract
Background
Osteopontin (OPN) is a Hedgehog (Hh)-regulated cytokine that is up-regulated during chronic liver injury, and directly promotes fibrosis. We reported that Hh-signaling enhances viral permissiveness and replication in HCV-infected cells. Hence, we hypothesized that OPN directly promotes HCV replication, and that targeting OPN could be beneficial in HCV.
Methods
We compared expression of OPN mRNA and protein in HCV (JFH1)-infected Huh7 and Huh7.5 cells, and evaluated if modulating OPN levels using exogenous OPN ligands (upregulate OPN) or OPN-specific RNA-aptamers (neutralize OPN), leads to changes in HCV expression. Sera and livers from patients with chronic HCV were analyzed to determine if OPN levels were associated with disease severity or response to therapy.
Results
Compared with Huh7, Huh7.5 support higher levels of HCV replication (15-fold), and expressed significantly more OPN mRNA (30-fold) and protein. Treating Huh7 with OPN ligands led to dose-related increase in HCV (15-fold) and OPN (8-fold) mRNA. Conversely, treating Huh7.5 with OPN-specific RNA-aptamers inhibited HCV RNA and protein by >50% and repressed OPN mRNA to basal levels. Liver OPN expression was significantly higher (3-fold) in patients with advanced fibrosis. Serum OPN positively correlated with fibrosis-stage (p=0.009), but negatively correlated with end-of-treatment (ET) biochemical-response (BCR), ET virological-response (VR), sustained (S)BCR, and SVR (p=0.007). The OPN-Fibrosis Score (serum OPN and presence of fibrosis ≥F2) may be a predictor of SVR.
Conclusions
OPN is upregulated in the liver and serum of patients with chronic HCV, and supports increased viral replication. OPN neutralization may be a novel therapeutic strategy in chronic HCV.
Keywords: Fibrosis, Hedgehog, Hepatitis, Osteopontin, Replication
Introduction
Hepatitis C virus (HCV) infection is a leading cause of liver disease, affecting up to 3% of the world’s population (1). Up to 85% of HCV-infected subjects become chronically infected, and are at risk of developing progressive liver fibrosis, liver cancer and liver failure (2). Conventional combination therapy (i.e. interferon and ribavirin) is limited by host and viral factors, which lead to adverse drug side-effects and reduced efficacy among those infected with less responsive HCV genotypes or with more advanced fibrosis (3).
The progression to liver fibrosis and cirrhosis occurs in context of chronic inflammation, as part of a conserved repair response to chronic injury(4). Previously, we reported that resurrection of the Hedgehog (Hh) morphogenic pathway occurs during chronic liver disease (5). Hh pathway activation promotes the accumulation of collagen-producing myofibroblasts (i.e. more fibrosis) (6), and amplifies the chronic inflammatory response by stimulating ductular cells to secrete chemokines and cytokines that drive immune cell recruitment into the liver (i.e. more inflammation) (7).
Osteopontin (OPN) is a pro-inflammatory cytokine and a matrix molecule that is a downstream target and effector of the Hh pathway (8). Like Hh, OPN is highly up-regulated in response to chronic tissue injury and plays a critical role in the wound healing response. In humans, over-expression of liver OPN occurs in NASH, alcoholic liver disease, chronic biliary disease and chronic viral hepatitis, and directly stimulates hepatic stellate cells into collagen-producing myofibroblasts (8–11). High levels of serum or plasma OPN have also been detected in patients with liver disease and liver cancer (12–14). In mice, over-expression of tissue OPN leads to more fibrosis, while genetic deficiency of OPN leads to attenuated fibrogenesis, outcomes that resemble Hh activity (8).
Recently, we showed that activation of the Hh pathway also occurs during chronic HCV (15, 16). Intriguingly, Hh signaling could enhance viral permissivity and replication in HCV-infected liver cells. Treating HCV-infected cells with a Hh pathway antagonist, on the other hand, led to inhibition of HCV replication. Although no study has yet explored the potential role that OPN may play in HCV replication, polymorphisms in the promoter region of the OPN gene have been proposed to predict efficacy of interferon-based therapies (17).
We hypothesized that OPN could promote HCV replication, and targeting OPN could be of benefit in HCV infection. We further analyzed liver and serum samples from patients undergoing treatment for chronic HCV to determine if OPN levels are associated with disease severity and / or response to therapy. This study is of particular clinical value therapeutically as small molecular inhibitors and compounds that neutralize OPN are currently being developed to treat cancer and inflammatory disease.
Methods
A) In vitro studies
HCV replication
Human Huh7 and Huh7.5 cells (a gift from C. Rice, Rockefeller University) were cultured in DMEM supplemented with 10% fetal bovine serum, streptomycin and penicillin (18). JFH cDNA was kindly provided by T. Wakita, National Institute of Infectious Diseases, Tokyo, Japan. Infectious JFH1 virus was obtained by transfection of Huh7.5 cells with in vitro transcribed RNA and harvesting of cell supernatant as described (19, 20). To generate viral stocks, clarified supernatant was used to infect naive Huh7.5 cells, supernatants were recovered 7 days post-infection, concentrated using an Amicon 100k device and titered by focus-forming assay using α-Core antibody (20).
Huh7 and Huh7.5 cells were treated with Osteopontin (OPN) ligand (10–1000 μg/mL) (R&D Systems, Minneapolis, MN) or vehicle (control) for 24 h prior to infection with JFH1 virus or mock infection (control). For OPN inhibition studies, OPN-specific RNA aptamers (OPN-R3), sham aptamers (OPN-R3-2) (both synthesized by Abgene, Thermo Fisher Scientific), or vehicle (control) were added to cultures at the time of infection with JFH1 virus. 100 nmol/L of sham or OPN aptamers were used, as this concentration of aptamers were found to inhibit adhesion, migration and invasion in MDA-MB-231 breast cancer cell line (which highly expresses OPN and is a standard tool for evaluating OPN actions), and were shown to inhibit hepatic stellate cell activation (21–23). RNA and protein were harvested at 48 h after infection.
In separate experiments, Huh7.5 cells were treated with the Hh agonist SAG (0.3uM) or the Hh antagonist GDC-0449 (5uM) at the time of infection with JFH1 virus or mock infection (control), and RNA harvested as previously described (15).
Messenger RNA quantification by real-time reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cells using TRIzol (Life Technologies, Carlsbad, CA), followed by RNase-free DNase I treatment (Qiagen, Valencia, CA). RNA was reverse transcribed to cDNA templates using random primer and Superscript RNase H-reverse transcriptase (Life Technologies) and amplified.
For semi-quantitative qRT-PCR, 1.5% of the first-strand reaction was amplified using StepOne Plus real-time PCR platform (ABI/Life Technologies), and specific oligonucleotide primers for target sequences, as well as the β-Actin housekeeping gene. qRT-PCR parameters were as follows: denaturation at 95°C for 3 min, followed by 40 cycles of denaturing at 95°C for 10 s and annealing-extension at the optimal primers temperatures for 60 s. Threshold cycles (Ct) were automatically calculated by the StepOne Plus Real-Time Detection System. Target gene levels in the cells are presented as a ratio to levels detected in the corresponding control cells according to the 2−ΔΔCt method. Primer sequences are listed as follows: JFH1 (AB237837) Forward: TGGGTTCGCATGGTCCTAATGACA, Reverse: TGGAAGGTCCAAAGGATTCACGGA; OPN (NM_000582) Forward: TGAAATTCATGGCTATGGAA, Reverse: TGAAACGAG TCAGCTGGATG; CD44 (NM_000610) Forward: AGCAACCAAGAGGCAAGAAA, Reverse: GTGTGGTTGAAATGGTGCTG; β-Actin (NM_001101) Forward: TGGCATCCACGAAACTACCT, Reverse: ACGGAGTACTTGCGCTCAG.
Western blot
Whole cell proteins were homogenized using standard RIPA buffer (Tris-buffered saline [TBS], 1% NP-40, 0.1% SDS) containing Protease Inhibitor Cocktail Tablets from Roche (Indianapolis, IN). Protein concentration was measured using BCA Protein Assay Kit from Pierce Biotechnology (Rockford, IL). 20 to 40 μg of protein were separated by polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (0.45μm; Invitrogen). After blocking with 5% non-fat milk (Carnation, Swampscott, MA) in TBS (20mmol/L Tris, pH 7.5, 150 mmol/L NaCl) containing 0.1% Tween-20 (TBS-T), nitrocellulose membranes were incubated with primary antibodies. Primary antibodies used were: α-HCV Core (C7-50, Abcam), and α-tubulin (Sigma-Aldrich). Secondary antibodies used were: ECL sheep anti-mouse, IgG HRP-conjugated (GE Healthcare, Amersham, UK). SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) was used to detect specific antibody-HRP complexes. The band density was measured using the Alphalmager 3400 Analysis System (Alpha Innotech, San Leandro, CA).
B) Clinical Studies
Patient Recruitment and Demographics (for Serum OPN and Luminex studies)
Human studies were conducted in accordance with the Declaration of Helsinki (2008), and in accordance with NIH and respective Institutional guidelines for human subject research. Informed consent was obtained from participating subjects.
Serum samples from patients (n = 41) with Chronic Hepatitis C (CHC) were selected from Duke Hepatology Clinical Research Database and bio-repository (Table 1). CHC was defined as the presence of liver disease and detectable hepatitis C virus (HCV) RNA in the serum (other causes of liver disease were excluded by a full liver screen on admission). Serum samples were obtained before and after combination therapy with Pegylated interferon and Ribavirin, and used in ELISA and Luminex assays as described below.
Table 1.
Characteristics of Clinical Cohort
| Age (years) | 44±10 |
| Gender (M/F) | 29/12 |
| BMI (kg/m2) | 27±4 |
| Genotype 1 (n) | 28 |
| Log(OPN) | 3.46±0.03 |
| Log(IL6) | 1.40±0.08 |
| Log(TNF) | 2.04±0.10 |
| Log(Leptin) | 4.23±0.06 |
| Log(ARCP30) | 7.54±0.06 |
| Log(APOA1) | 7.99±0.03 |
| Log(CRP) | 5.80±0.08 |
| Log(E-Selectin) | 4.77±0.03 |
| Log(L-Selectin) | 6.31±0.02 |
| Log(PAI1) | 4.10±0.03 |
| Steatosis (0/1/2/3) | (25/10/2/4) |
| HAI | 8.5±4.8 |
| Fibrosis (0/1/2/3/4) | (6/21/6/7/1) |
| ETBCR (n) | 24 |
| ETVR (n) | 25 |
| SBCR (n) | 11 |
| SVR (n) | 13 |
Note: serum analytes were presented as log values
OPN-ELISA and Luminex array
Serum were taken from patients before and after CHC treatment, and stored at −80°C till analysis. Serum OPN was measured using the commercially available OPN ELISA kit (R&D; DY1433) and in accordance with the manufacturer’s instructions. All samples were run in duplicate, and expressed as pg/ml.
Selected biomarkers were assayed without access to clinical or demographic data using a mulitiplex ultrasensitive SearchLight Chemiluminescent Protein Array platform (Pierce Biotechnology, c/o Thermo Fisher Scientific Inc., Rockford, IL). Briefly, this array includes a 96 well-plate allowing upto16 capture antibodies per well. Addition of ≤10 μL of serum to the well results in capture of the arrayed antibody. This is followed by addition of biotinylated antibodies that bind to captured antibodies, streptavidin conjugated to horseradish peroxidase, and a chemiluminescent substrate. Signal intensity is captured by SearchLight Plus CCD Imaging and analyzed through the SearchLight Array Analyst software for standard curve comparisons and custom data reporting. Rapid throughput was facilitated by using Tecan Genesis (Tecan group Inc, RTP, NC), Caliper Mini-Staccato (Caliper Life Sciences, Hopkinton, MA) and rapid plate transfer pre-analytical automated sample processing.
Immunohistochemistry
FFPE liver sections of de-identified subjects with CHC from the Departments of Pathology of Duke University (n=25) and the Universidade Federal do Espirito Santo (Brazil) (n= 72) were used. Primary antibodies used were: OPN (R&D, AF1433, 1:40), Gli2 (Genway; 18–732; 1:4500), αSMA (1:500; Abcam 5694). Polymer-HRP anti-rabbit (Dako; K4003) and anti-goat (Santa Cruz; sc-2768; 1:250) were used as secondary antibodies. Antigens were demonstrated by diaminobenzidine (DAB) (DAKO). Omitting primary antibodies eliminated staining, demonstrating specificity.
Picrosirius red staining with quantification by morphometric analysis was performed as previously described (26). For OPN quantification, 15 randomly selected x40 fields (excluding the major bile duct in each portal tract from consideration) were analyzed with the MetaView software.
Liver biopsies from CHC patients were scored by an expert histopathologist (from respective Institutions) using the METAVIR fibrosis stage (F0–F4) (24), HAI inflammation score (25), (mild/0 = 0–5, moderate/ 1 = 6–10, severe/2 = 11–18) and steatosis grade according to the percentage of hepatocytes containing fat droplets (0 < 3%, 1 = 3–30%, 2 = 31–59% and 3 > 59%).
Statistical Analysis
Statistical analyses were carried out using Graphpad Prism (v 6.0b) or SPSS (v 20). Data are shown as mean ± SEM unless otherwise stated. Mann Whitney U test was performed for comparison between two groups and the Kruskall-Wallis test with Dunn’s multiple comparison test was performed for analysis of multiple groups. Spearman’s rank correlation coefficient was used for univariate correlation analysis. Multiple linear regression with backward elimination was performed to identify the best set of independently associated variables. Significance was set at p<0.05.
Results
Huh7.5 support increased HCV replication and have greater Osteopontin (OPN) expression relative to Huh7 cells
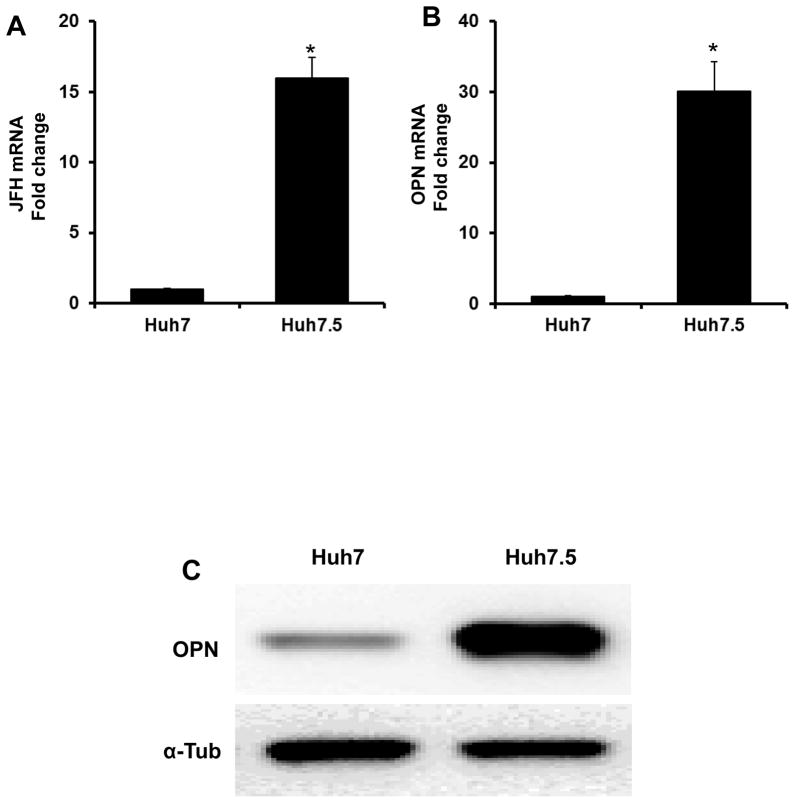
Huh7.5 cells are more permissive for HCV viral replication and support higher levels of HCV replication than Huh7 (Fig 1A). We had previously reported that permissiveness of HCV replication was associated with increased Hedgehog (Hh) activity (15), and that inhibition of Hh signaling led to a reduction in cell-associated HCV RNA levels. Consistently, we find that basal expression of OPN, a Hh-regulated target gene and a proximal effector of the Hh pathway (8), is 30-fold higher in Huh7.5 than Huh7 cells (Fig 1B–C).
Figure 1. Huh7.5 cells support increased HCV (JFH1) replication and express higher levels of Osteopontin (OPN) than Huh7 cells.
JFH1-infected Huh7 and Huh7.5 cells were harvested after 48 h, and analyzed for OPN and JFH gene and protein expression. (A) JFH mRNA. (B) OPN mRNA. (C) OPN protein by western blot. Experiments were performed in triplicate; results are expressed as fold change relative to Huh7 expression; mean ± SEM; *p<0.05
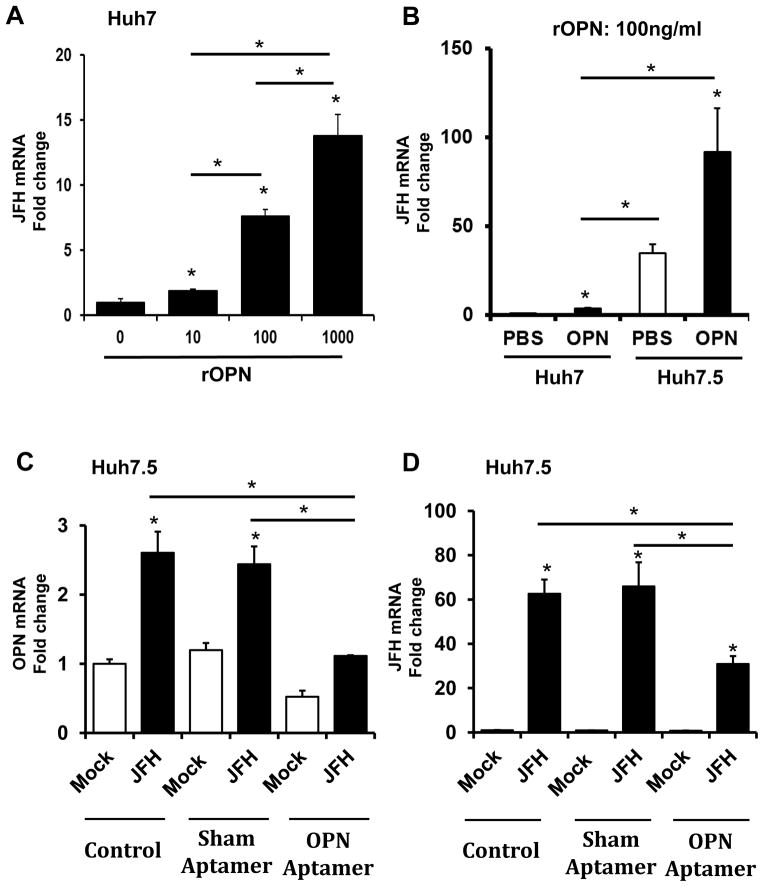
Next, we evaluated if the addition of OPN ligand could enhance HCV viral titers. Incubating Huh7 cells with exogenous OPN for 48 h led to an up-regulation of HCV RNA levels in a dose-dependent fashion, by up to 15-fold (Fig 2A). This was mirrored by the further induction of Hh-regulated genes, OPN, Gli1 and Ptc (Supplemental Fig 1). Infected-Huh7.5 cells exhibited even greater responsiveness to OPN, and upregulated JFH mRNA expression by an additional 30-fold compared with vehicle treated Huh7.5 cells (Fig 2B). OPN treatment also resulted in a feed-forward increase (~2 fold) in OPN, and CD44, a putative OPN receptor and an integral component of the cytoskeletal / migratory complex (Supplemental Fig 2).
Figure 2. OPN promotes HCV expression in Huh7 and Huh7.5 cells.
(A–B) Huh7 and Huh7.5 cells were cultured with OPN ligand for 24 h prior to infection with JFH1 virus for a further 48 h. (C–D) In separate experiments, JFH1-infected Huh7.5 cells were treated with (OPN-neutralizing) OPN-specific RNA aptamers or sham aptamers or control (vehicle) for 48 h. RNA was harvested at the end of treatment for qRT-PCR analysis. (A) JFH mRNA in Huh7 cells treated with rOPN (0–1000ng/ml). (B) JFH mRNA in Huh7 and Huh7.5 cells treated with 100ng/ml rOPN. (C) OPN mRNA and (D) JFH mRNA in JFH1-infected Huh7.5 cells. Experiments were performed in triplicate; results are expressed as fold change relative to respective baseline; mean ± SEM; *p<0.05
We further evaluated OPN expression in HCV-infected cells treated with either the Hh agonist (SAG) or antagonist (GDC-0449) (Supplemental Figure 3). Treatment with SAG (associated with increased HCV RNA (15)) led to a 2-fold increase in OPN mRNA, while inhibiting the Hh pathway (associated with reduced HCV RNA) repressed OPN mRNA to basal (control) levels. The aggregate data supports our hypothesis that OPN supports HCV replication in vitro.
Inhibition of OPN expression in Huh7.5 cells decreases HCV expression
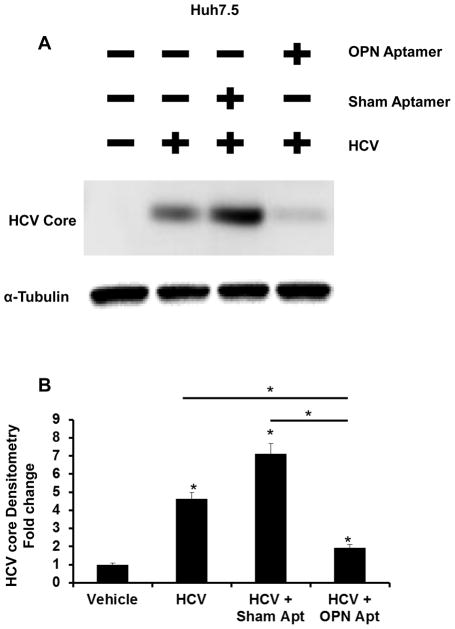
Given that OPN expression is significantly up-regulated in Huh7.5 cells, and that the addition of OPN ligands could enhance HCV replication (Fig 1, 2A–B), we next examined if neutralizing OPN in conditioned media could inhibit HCV RNA replication. Huh7.5 cells infected with JFH HCV were treated with OPN-specific aptamers, sham aptamers, or vehicle (control) for 48 h. Compared with control or sham, treatment with OPN-specific aptamers resulted in 2.5-fold reduction in OPN expression (Fig 2C), and repressed HCV RNA expression by greater than 50% (Fig 2D). Similarly, OPN neutralization reduced HCV-Core content by over 3-fold (Fig 3A–B).
Figure 3. OPN neutralization reduces HCV-core protein expression in Huh7.5 cells.
JFH1-infected Huh7.5 cells were treated (OPN-neutralizing) OPN-specific RNA aptamers or sham aptamers for 48 h. Whole cell protein was harvested for western blot analysis. (A) HCV core protein and α–tubulin (loading control). (B) Protein densitometry. Experiments were performed in duplicate; densitometry results are expressed as fold change relative to vehicle (un-infected Huh7.5 cells); mean ± SEM; *p<0.05
Up-regulated expression of Liver OPN in Human Chronic Hepatitis C (CHC)
Previously, we had reported that Hh pathway activation occurs in patients with CHC, and that Hh pathway activity mirrors liver disease stage (16). We therefore examined if tissue and blood levels of OPN, a downstream target of the Hh pathway, would be similarly induced during different stages of CHC.
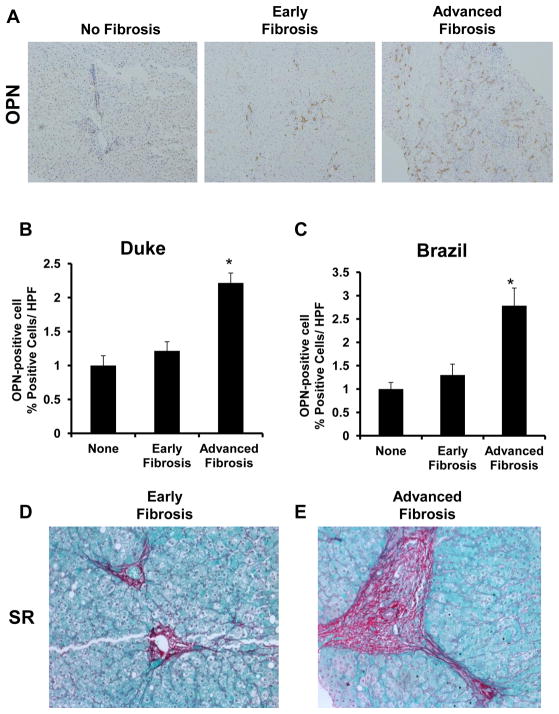
Coded liver sections from Duke with well characterized CHC (no fibrosis: n= 5; early fibrosis (F1–2): n= 10; advanced fibrosis (F3–4): n= 10) were stained to demonstrate OPN, and then analyzed by computer-assisted morphometry. Expression of OPN was highest in patients with advanced CHC and lowest in those without fibrosis or with early stages of fibrosis (Fig 4A–B, 4D–E). We confirm that the OPN regulator, Gli2 (a Hh-transcription factor) exhibited a similar pattern of expression, with increasing numbers of nuclear Gli2-positive cells with advancing fibrosis (Suppl Fig 4A–B). Consistently, the highest expression of the HSC activation marker α-SMA was also seen in those with advanced fibrosis (Suppl Fig 4C–D).
Figure 4. Liver OPN is over-expressed in advanced HCV-fibrosis.
Coded and de-identified paraffin embedded liver sections from HCV-infected patients were used for immunohistochemistry. Duke Cohort: no fibrosis: n=5; early fibrosis: n=10; advanced fibrosis: n=10; Brazil Cohort: no fibrosis: n=5; early fibrosis: n=46; advanced fibrosis: n=21). (A) Representative immunostaining for OPN. (B–C) OPN densitometry from Duke and Brazil Cohorts, respectively; results are expressed as fold change relative to non-fibrotic livers; mean ± SEM; *p<0.05. (D–E) Representative Sirius Red staining from patients with early or advanced HCV-fibrosis
To further validate this association between CHC and OPN expression, OPN immuno-reactivity was evaluated in a second cohort of patients with CHC (Brazil) (no fibrosis: n= 5; early fibrosis F1–2): n= 46; advanced fibrosis (F3–4): n= 21). At early stage fibrosis, liver OPN expression was comparable to those without fibrosis. In contrast, liver OPN expression was significantly up-regulated with advanced fibrosis (by nearly 3 fold; p<0.05) (Fig 4C). These findings support our previous observations that OPN over-expression can enhance HSC transition into collagen-producing, myofibroblasts (8).
Serum OPN levels are elevated with CHC- Fibrosis and are associated with Biochemical and Virological Response to Anti-viral therapy
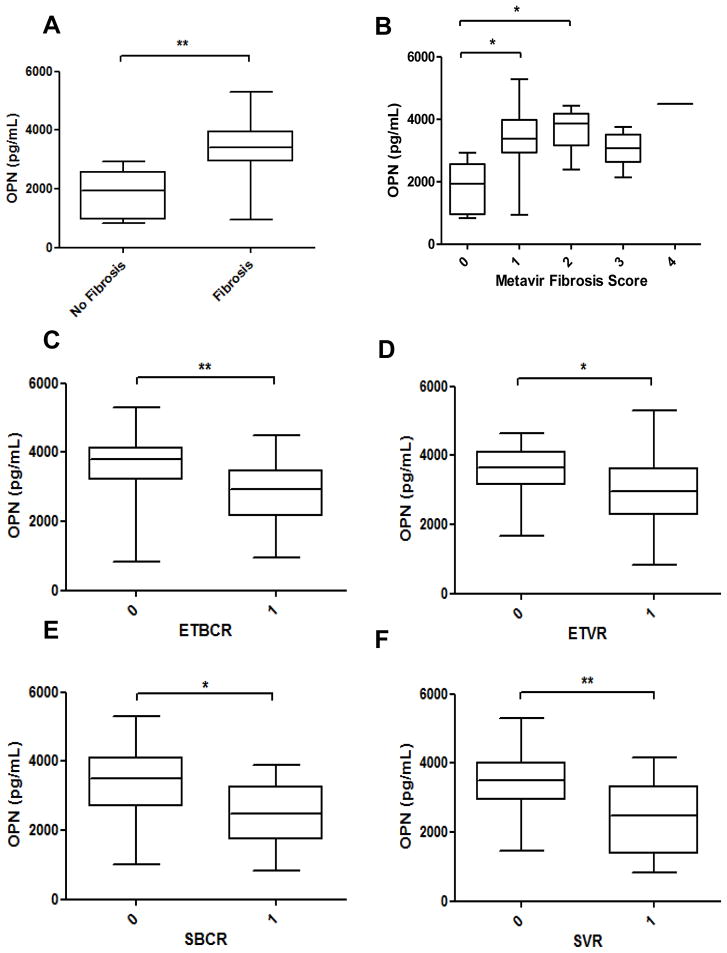
OPN is a highly modified glycoprotein that may be released into the circulation. As such, elevated plasma and serum OPN levels have been detected among individuals with alcoholic liver disease (8), nonalcoholic fatty liver disease (8), and chronic viral hepatitis (9). Indeed, from this cohort of patients with CHC (n=41), we observed that serum OPN levels were significantly higher in those with liver fibrosis (p=0.001) (compared to those without fibrosis) (Fig 5A). Consistent with previous reports (9, 23), serum OPN levels also correlated with CHC fibrosis stage (OPN vs. Metavir score; p=0.009) (Fig 5B).
Figure 5. Serum OPN levels are increased in HCV-fibrosis and correlate with Biochemical and Virological response to anti-HCV therapy.
Pre-treatment serum samples from patients (n = 41) with chronic HCV were measured for OPN by ELISA (R&D). OPN levels (pg/ml) were analyzed by the presence or absence of fibrosis, and compared between patients who responded (1) and those who did not respond (0) to treatment. (A) Increased serum OPN with fibrosis. (B) Serum OPN correlates with HCV-fibrosis stage. (C–F) Serum OPN negatively correlates with end-of-treatment biochemical response (ETBR; p=0.003) (C), end-of-treatment virological response (ETVR; p=0.021) (D), sustained biochemical response (SBCR; p=0.009) (E), and sustained virological response (SVR; p=0.007) (F), respectively. Data are shown as median ± range; Mann Whitney U test was used; *p<0.05; **p<0.01
Identifying patients who are more likely to respond successfully to anti-viral therapy is important to avoid unnecessary treatment and the risk of treatment side effects. It is well recognized that individuals with advanced fibrosis stage are less likely to respond successfully to anti-viral therapy (27). Here, we confirm that the presence of fibrosis was associated with reduced ETVR (p=0.034), SBCR (p=0.036), as well as SVR (p=0.003) (Suppl Fig 5). Interestingly, levels of serum OPN similarly demonstrated significant negative correlation with ETBR (p=0.003), ETVR (p=0.021), SBCR (p=0.009), and SVR (p=0.007) (Fig 5C–F). No statistically significant correlation was observed between age (p=1.0, 0.16, 0.61, 0.43 respectively) or BMI (p=0.05, 0.15, 0.53, 0.45 respectively) with ETBR, ETVR, SBCR or SVR. While the presence of fibrosis was strongly associated with male gender (p=0.029), male gender itself did not show correlation with SVR (p=0.56) (Tables 2 and 3).
Table 2.
Factors associated with Liver Fibrosis
| A: Univariate Correlations with the Presence of Fibrosis.
| ||
|---|---|---|
| Variable | rs Value | p Value |
| Log(OPN) | 0.49 | 0.0010 |
| SVR | −0.46 | 0.0030 |
| Male | 0.34 | 0.029 |
| Log(E-Selectin) | 0.26 | 0.097 |
| Age | −0.23 | 0.15 |
| Log(L-Selectin) | 0.21 | 0.19 |
| Steatosis | 0.20 | 0.20 |
| HAI | 0.18 | 0.28 |
| Log(CRP) | −0.16 | 0.31 |
| Log(IL6) | 0.16 | 0.33 |
| Log(ACRP30) | −0.15 | 0.36 |
| Log(Leptin) | −0.13 | 0.41 |
| Log(APOA1) | −0.099 | 0.54 |
| Log(TNF) | −0.97 | 0.55 |
| Log(PAI1) | 0.093 | 0.56 |
| Genotype 1 | −0.082 | 0.61 |
| BMI | 0.006 | 0.97 |
| B: Factors Independently Associated with the Presence of Fibrosis
| ||
|---|---|---|
| Variable | Beta Coefficient | p Value |
| Male | 0.35 | 0.014 |
| SVR | −0.35 | 0.027 |
| Log(OPN) | 0.34 | 0.028 |
Note: serum analytes were presented as log values
Table 3.
Factors associated with Sustained Virological Response (SVR)
| A: Univariate Correlations with SVR
| ||
|---|---|---|
| Variable | rs | p Value |
| Log(OPN) | −0.41 | 0.0070 |
| F ≥2 | −0.38 | 0.014 |
| Log(E-Selectin) | −0.38 | 0.015 |
| Log(HAI) | −0.25 | 0.12 |
| Log(L-Selectin) | −0.22 | 0.17 |
| Genotype 1 | −0.17 | 0.30 |
| Log(IL6) | −0.14 | 0.39 |
| Log(Leptin) | −0.14 | 0.40 |
| Age | −0.13 | 0.43 |
| BMI | −0.12 | 0.45 |
| Log(CRP) | 0.097 | 0.54 |
| Male | 0.093 | 0.56 |
| Log(PAI1) | −0.075 | 0.64 |
| Steatosis | 0.053 | 0.74 |
| Log(TNF) | −0.007 | 0.97 |
| Log(ACRP30) | 0.004 | 0.98 |
| Log(APOA1) | −0.004 | 0.98 |
| B: Factors Independently Associated with SVR
| ||
|---|---|---|
| Variable | Beta Coefficient | p Value |
| Log(OPN) | −0.38 | 0.01 |
| F≥2 | −0.31 | 0.034 |
Note: serum analytes were presented as log values
(Adipo-) cytokines and growth factors have been recognized to participate in the liver repair and inflammatory process, and can directly modulate liver disease (fibrosis) progression (28). Consistently, we found that serum OPN levels positively correlated with serum adiponectin (rs = 0.32, p=0.042) and ApoA1 (rs = 0.36, p=0.021). Furthermore, serum leptin correlated with serum IL6 (p=0.047) and TNFα(p=0.016), while serum adiponectin correlated serum HAI (p=0.028), TNFα(p=0.039), and ApoA1 (p=0.00021). However, neither serum leptin (rs = 0.11, p=0.49) nor adiponectin (rs = 0.10, p=0.54) were shown to correlate with liver fibrosis stage. Surprisingly, levels of serum E-selectin was found to correlate positively with SVR (rs = −0.38, p=0.015) on univariate analysis (Table 3).
Fibrosis score incorporating blood OPN levels predicts SVR
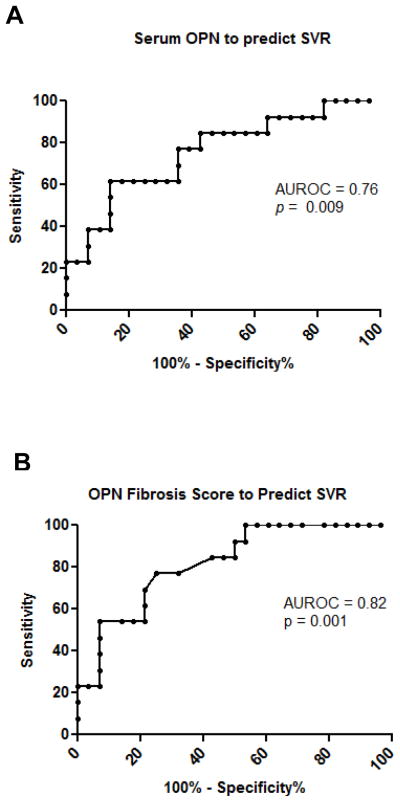
As shown in Fig 6A, the area under the ROC curve in patients with CHC to predict SVR with serum OPN levels was 0.76 (95% CI 0.59–0.92). This result is consistent with a recent study in CHC and ALD (9), and indicates that elevated circulating OPN is a conserved response to chronic liver injury.
Figure 6. Pre-treatment serum OPN levels predict SVR.
Pre-treatment serum samples were measured for OPN by ELISA (R&D), and selected biomarkers using a ultrasensitive multiplex platform. (A) The area under the ROC curve for the performance of serum OPN in predicting SVR (p=0.009). (B) The area under the ROC curve for the performance of the OPN-Fibrosis Score (p=0.001) (which incorporates serum OPN and presence of fibrosis ≥F2, factors independently associated with SVR).
Multiple linear regression with backward elimination found that only serum OPN and the presence of Significant Fibrosis (stage ≥F2) were independent variables when SVR was the judgment criterion (OPN - β Coefficient −0.381, p=0.01; Significant Fibrosis- β Coefficient −0.308, p=0.034) (Table 3). A score combining these two factors, calculated from the regression equation (OPN Fibrosis Score = 0.96 – [0.00017 x log(OPN)] – [0.30 x ≥F2 (1=yes, 0=no)]) improved the area under the ROC curve to 0.82 (95% CI 0.69–0.95) (Fig 6B).
Given the small size of the study cohort, and the possibility of inter-observer variations in the staging of F2 and F3 disease, additional analyses were performed. The exclusion of F3 samples did not significantly alter AUROC values (Supplemental Figure 6).
Discussion
We present a novel association between OPN and HCV infection in liver cells. Huh7.5 cells which support higher levels of HCV replication expressed significantly higher levels of OPN compared with Huh7 cells. Provision of exogenous OPN ligand to both types of liver cells led to enhanced HCV expression, while OPN neutralization using RNA specific aptamers led to repression of HCV levels. Furthermore, we show that upregulated liver and serum OPN levels correlate with HCV-stage, and may be a useful predictor of SVR prior to anti-viral therapy.
In addition to host and viral factors, recent studies suggest that soluble mediators such as (adipo-) cytokines and morphogens are important regulators of HCV replication (15, 29, 30). For example, liver sinusoidal endothelial cells secrete bone morphogenic protein 4 (BMP4) (a member of the TGFβ superfamily), which increases permissiveness for HCV replication in a dose-dependent fashion. Similarly, we had reported that the morphogen, hedgehog (Hh), could directly enhance HCV replication in liver cells, while blocking Hh pathway activity led to reduction of HCV expression. This is supported by observations that fetal hepatocytes, which express high levels of Hh signaling, are capable of supporting HCV replication in vitro compared with adult hepatocytes (31). Nevertheless, the underlying mechanisms by which Hh or BMP4 could have led to changes in HCV expression (or replication) remain unclear, and are likely to involve multiple downstream mediators. For example, activation of the Hh pathway induces hepatic stellate cell activation (i.e. fibrogenesis), and reprogramming of epithelial cells to a mesenchymal phenotype (i.e. epithelial-mesenchymal transition, EMT). In vivo, livers from viral-infected patients exhibit EMT gene expression changes. The shape change and loss of cellular polarity during EMT may expose or alter gap junction complex proteins important for HCV entry or replication. Indeed, this hypothesis is supported by observations that the cytoskeletal protein, ezrin-moesin-radixin (ERM), can efficiently modulate HCV infection (32).
Like BMP4, OPN is a pro-fibrogenic cytokine that is highly upregulated during tissue injury, and regulates repair (8, 33). It is a downstream mediator of the Hh pathway, and thus, exhibits similar functions to Hh signaling (Suppl Fig 3). Furthermore, intracellular OPN is an integral component of the CD44-ERM cytoskeletal complex (i.e. involved in migration and EMT) (33); thus, changes to cellular OPN expression may also alter susceptibility to HCV infection. Huh7.5 cells which exhibit greater permissiveness express higher levels of OPN and CD44 than Huh7 cells. Treating infected-Huh7.5 cells with exogenous OPN ligand led to further induction of OPN (~2 fold), and CD44 (~ 2 fold), a cytoskeletal protein and marker of mesenchymal stem cells, mirroring increases in JFH mRNA expression. In support of our previous report, increase in HCV expression was paralleled by Hh pathway activity (i.e. Gli and Ptc expression; Suppl Fig 1 and 3). Future studies will be needed to understand if, and how OPN regulates Hh in an auto-regulatory loop, and to identify specific signaling pathways by which OPN exerts its effects.
The progression of chronic liver disease is characterized by changes in the cytokine milieu in blood and liver. Consistent with published reports, liver OPN expression was significantly higher among individuals with advanced liver fibrosis (8, 9). Surprisingly, among serum analytes measured, only OPN was found to be independently associated with fibrosis stage and SVR. Liver biopsy sampling and observer variability for intermediate disease stages are well established. Furthermore, due to the small sample size (F2–3 n=13, F4 n=1), we were unable to show differences in OPN between stage 2 and 3, and which would have allowed us to further delineate differences in OPN and virologic response according to disease stage. The inclusion of all patients with significant fibrosis (i.e. F>=2) in the predictive modeling, however, corrects for any inaccuracies in assignment between F2 and F3. Furthermore, multiple logistic-regression modeling suggests that OPN and the presence of significant fibrosis (i.e. F>=2) are both independently associated with SVR rather than OPN simply acting as a surrogate marker of fibrosis. These findings in relation to virologic response have to be interpreted with caution given the limited size of our study cohort and absence of IL28B data for these patients that received peg-IFN and RBV prior to availability of IL28B genotyping assays (34, 35). Levels of serum adiponectin have been reported to correlate with HCV-fibrosis stage (35). Although we noted a positive correlation between serum OPN and adiponectin (p=0.042), serum adiponectin was not found to be independently associated with either fibrosis stage or SVR. The independent association between OPN and fibrosis in this study also indicates an important role for OPN in HCV-related fibrosis progression (p=0.028), as it does in other chronic liver diseases such as NAFLD (8). This will require longitudinal assessment, and further evaluation of OPN in relation to clinical outcomes in patients with advanced stage disease.
In conclusion, we have described a novel association between OPN and HCV, which may represent a new therapeutic target in HCV infection. We further show that serum levels of OPN may be useful in predicting virological response to HCV therapy. Identification of potential host targets such as OPN will remain of importance in the era of direct acting antiviral therapy for HCV given the potential for viral resistance and concerns regarding availability of emerging therapy. Importantly, the potential anti-fibrogenic role of targeted OPN therapy would represent an important therapeutic advance. Future studies in a larger, prospective cohort will be necessary to ascertain if OPN polymorphisms can predict serum or liver OPN expression, and to validate if serum OPN levels could be a useful biomarker of disease progression or change in fibrosis with antiviral or anti-fibrotic therapy.
Supplementary Material
Clinical Perspective.
We need to understand if and how host proteins may modulate HCV infection, and thereby, identify novel therapeutic targets. We show, for the first time, that OPN, a pro-fibrogenic cytokine, may enhance HCV replication in vitro, and that neutralizing OPN leads to repression of HCV RNA and protein expression. Importantly, pre-treatment, serum OPN levels appear to correlate with fibrosis-stage and may be used to predict biochemical or virological response to anti-viral therapy.
Summary Statement.
Osteopontin is upregulated in patients with chronic hepatitis C, and directly promotes hepatitis C replication. Reducing Osteopontin represses hepatitis C levels. Serum Osteopontin levels correlate with disease severity and may predict response to anti-viral treatment.
Acknowledgments
Funding: This work was supported by the following grants: BRET grant (WKS), CORE-UK (WKS), National Institute of Health 5K08DK080980 (SSC), R03DK084120 (RJ).
Footnotes
Conflict of Interest:
All authors declare no conflict of interest in relation to this study
Author contributions:
Steve S Choi and Wing-Kin Syn performed the majority of experimental and human studies. All other authors contributed intellectually, and / or were involved in the recruitment of patients with CHC at Duke or Brazil. Wing-Kin Syn is the senior author and guarantor for the paper.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlotsky JM. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology; 53:1742–1751. doi: 10.1002/hep.24262. [DOI] [PubMed] [Google Scholar]
- 4.Diehl AM, Chute J. Underlying potential: cellular and molecular determinants of adult liver repair. J Clin Invest; 123:1858–1860. doi: 10.1172/JCI69966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol; 43:238–244. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syn WK, Choi SS, Liaskou E, Karaca GF, Agboola KM, Oo YH, Mi Z, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology; 53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patouraux S, Bonnafous S, Voican CS, Anty R, Saint-Paul MC, Rosenthal-Allieri MA, Agostini H, et al. The osteopontin level in liver, adipose tissue and serum is correlated with fibrosis in patients with alcoholic liver disease. PLoS One. 7:e35612. doi: 10.1371/journal.pone.0035612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F, Moles A, et al. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology; 56:1108–1116. doi: 10.1002/hep.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X, Xie H, et al. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int J Clin Pract. 2008;62:1056–1062. doi: 10.1111/j.1742-1241.2007.01368.x. [DOI] [PubMed] [Google Scholar]
- 12.Nagoshi S. Osteopontin: Versatile modulator of liver diseases. Hepatol Res. doi: 10.1111/hepr.12166. [DOI] [PubMed] [Google Scholar]
- 13.Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, Hainaut P, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology; 55:483–490. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber GF, Lett GS, Haubein NC. Categorical meta-analysis of Osteopontin as a clinical cancer marker. Oncol Rep; 25:433–441. doi: 10.3892/or.2010.1106. [DOI] [PubMed] [Google Scholar]
- 15.Choi SS, Bradrick S, Qiang G, Mostafavi A, Chaturvedi G, Weinman SA, Diehl AM, et al. Up-regulation of Hedgehog pathway is associated with cellular permissiveness for hepatitis C virus replication. Hepatology; 54:1580–1590. doi: 10.1002/hep.24576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Pereira TA, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, Agboola KM, et al. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest; 90:1690–1703. doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaker OG, Sadik NA, El-Dessouki A. Single-nucleotide polymorphism in the promoter region of the osteopontin gene at nucleotide −443 as a marker predicting the efficacy of pegylated interferon/ribavirin-therapy in Egyptians patients with chronic hepatitis C. Hum Immunol; 73:1039–1045. doi: 10.1016/j.humimm.2012.07.329. [DOI] [PubMed] [Google Scholar]
- 18.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791–796. doi: 10.1038/nm1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato T, Date T, Murayama A, Morikawa K, Akazawa D, Wakita T. Cell culture and infection system for hepatitis C virus. Nat Protoc. 2006;1:2334–2339. doi: 10.1038/nprot.2006.395. [DOI] [PubMed] [Google Scholar]
- 21.Mi Z, Guo H, Russell MB, Liu Y, Sullenger BA, Kuo PC. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol Ther. 2009;17:153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbot LJ, Mi Z, Bhattacharya SD, Kim V, Guo H, Kuo PC. Pharmacokinetic characterization of an RNA aptamer against osteopontin and demonstration of in vivo efficacy in reversing growth of human breast cancer cells. Surgery; 150:224–230. doi: 10.1016/j.surg.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syn WK, Agboola KM, Swiderska M, Michelotti GA, Liaskou E, Pang H, Xie G, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in non-alcoholic fatty liver disease. Gut; 61:1323–1329. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 25.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 26.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. e1478. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petta S, Craxi A. How to optimize HCV therapy in genotype 1 patients: predictors of response. Liver Int. 33(Suppl 1):23–29. doi: 10.1111/liv.12053. [DOI] [PubMed] [Google Scholar]
- 28.Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- 29.Kukla M, Mazur W, Buldak RJ, Zwirska-Korczala K. Potential role of leptin, adiponectin and three novel adipokines--visfatin, chemerin and vaspin--in chronic hepatitis. Mol Med; 17:1397–1410. doi: 10.2119/molmed.2010.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowe IA, Galsinh SK, Wilson GK, Parker R, Durant S, Lazar C, Branza-Nichita N, et al. Paracrine signals from liver sinusoidal endothelium regulate hepatitis C virus replication. Hepatology. doi: 10.1002/hep.26571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazaro CA, Chang M, Tang W, Campbell J, Sullivan DG, Gretch DR, Corey L, et al. Hepatitis C virus replication in transfected and serum-infected cultured human fetal hepatocytes. Am J Pathol. 2007;170:478–489. doi: 10.2353/ajpath.2007.060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukong TN, Kodys K, Szabo G. Human ezrin-moesin-radixin proteins modulate hepatitis C virus infection. Hepatology. doi: 10.1002/hep.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsukita S, Yonemura S. ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci. 1997;22:53–58. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- 34.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 35.Corbetta S, Redaelli A, Pozzi M, Bovo G, Ratti L, Redaelli E, Pellegrini C, et al. Fibrosis is associated with adiponectin resistance in chronic hepatitis C virus infection. Eur J Clin Invest; 41:898–905. doi: 10.1111/j.1365-2362.2011.02498.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.