Abstract
Cosmeceuticals are the fastest growing segment of the personal care industry, and a number of topical cosmeceutical treatments for conditions such as photoaging, hyperpigmentation, wrinkles, and hair damage have come into widespread use. In the cosmeceutical arena nanotechnology has played an important role. Using new techniques to manipulate matter at an atomic or molecular level, they have been at the root of numerous innovations, opening up new perspectives for the future of cosmeceutical industry. Nanotechnology-based cosmeceuticals offer the advantage of diversity in products, and increased bioavailability of active ingredients and increase the aesthetic appeal of cosmeceutical products with prolonged effects. However increased use of nanotechnology in cosmeceuticals has raised concern about the possible penetration of nanoparticles through the skin and potential hazards to the human health. This review outlines the different nanoparticles used in various classes of cosmeceuticals, nanotechnology-based cosmeceutical products present in the market, and the potential risk caused by nanoparticles on exposure and recent regulatory steps taken to overcome them.
1. Introduction
Cosmetics are defined by the FDA as “articles intended to be applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance” [1]. FDA does not have the legal authority to approve cosmetics before they go on the market. However, cosmetics must be safe for consumers and properly labeled. Companies and individuals who market cosmetics have a legal responsibility for the safety and labeling of their products [2]. The word “cosmeceutical” is used to define a product that fits the niche between a drug and cosmetics [3]. It is used in the professional skin care arena to describe a product that has measurable biological action in the skin, like a drug, but is regulated as a cosmetic since it claims to affect appearance [4]. Cosmeceuticals are not categorized by the FDA, but this term is used by skin scientists, physicians, and skin care professionals, to encourage the consumers to continue buying cosmetic products especially antiaging and sunscreen products, marketed by many manufacturers with scientific claims and natural positioning as a way to emphasize that using these products is not only necessary but also natural. Cosmeceuticals are the fastest growing segment of the personal care industry [5]. Cosmeceutical formulations now have expanded from skin to body to hair and a number of topical cosmeceutical treatments for conditions such as photoaging, hyperpigmentation, wrinkles, and hair damage have come into widespread use [6]. Recent researches focusing on cosmeceutical products highlighted strong growth perspectives in the coming years. According to them expanding at a rapid compound annual growth rate of 7.7%, the global cosmeceutical market will reach $31.84 billion by 2016 [7]. The global cosmeceutical market offers huge potential among the Asian countries, such as Japan, China, and India which are set to attract major players in the future. Japan has already made a remarkable position in the global cosmetics market and its position in the cosmeceutical segment is effectively improving [7]. A report, “Cosmeceuticals market to 2018,” forecasted that the global cosmeceuticals market will reach $42.4 billion by 2018 [8].
Among the technologies used to develop elegant and effective cosmeceuticals, nanotechnology finds special place. In the cosmetic arena it is believed that the smaller particles are readily absorbed into the skin and repair damage easily and more efficiently [9]. Incorporation of nanotechnology in cosmeceuticals is aimed at making incense of perfumes last longer, sunscreens to protect the skin, antiaging creams to fight back the years, and moisturizers to maintain the hydration of skin. Some of the nanotechnology-based innovations are nanoemulsions (which are transparent and have unique tactile and texture properties), nanocapsules (which are used in skin care products), nanopigments (that are transparent and increase the efficiency of sunscreen products), liposome formulations (which contain small vesicles consisting of conventional cosmetic materials that protect oxygen or light sensitive cosmetic ingredients), niosomes, nanocrystals, solid lipid nanoparticles, carbon nanotubes, fullerenes, and dendrimers. The primary advantages of using nanoparticles in cosmeceuticals include improvement in the stability of cosmetic ingredients (e.g., vitamins, unsaturated fatty acids, and antioxidants) by encapsulating within the nanoparticles; efficient protection of the skin from harmful ultraviolet (UV) rays; aesthetically pleasing products (e.g., in mineral sunscreens, using smaller particles of active mineral allows them to be applied without leaving a noticeable white cast); targeting of active ingredient to the desired site and controlled release of active ingredients for prolonged effect [10, 11].
2. Nanoparticles in Cosmeceuticals
2.1. Liposomes
Bangham published the first paper on liposomes in 1963, and it was in the early 1980s that Mezei and Gulasekharam reported the efficacy of liposomes in topical drug delivery [14, 15]. Liposomes are spherical, self-closed vesicles of colloidal dimensions, in which phospholipid bilayers sequester part of the solvent, in which they freely float, into their interior (Figure 1). Liposomes typically vary in size between 20 nm and a few hundred micrometers [16]. Liposomes are used in a variety of cosmeceuticals because they are biocompatible, biodegradable, nontoxic, and flexible vesicles and can encapsulate active ingredients easily. Liposomes have an ability to protect the encapsulated drug from external environment and are suitable for delivery of hydrophobic and hydrophilic compounds [16]. These characteristics make them ideal candidate for the delivery of vitamins and other essential molecules to regenerate the epidermis [17]. One of the main ingredients of liposome is Phosphatidylcholine which has been used in skin care products (moisturizer, lotions, creams, etc.) and hair care products (shampoo, conditioner) due to its softening and conditioning properties. Several active ingredients (e.g., vitamins A, E, and K) and antioxidants (e.g., Carotenoids, lycopene, and CoQ10) have been incorporated into liposomes which increases their physical and chemical stability when dispersed in water. Lipophilic compounds such as cholesterol and ceramides have been used in topical skin creams for many years, because they are the lipids found in normal skin tissue, and are easily incorporated into liposomes to improve skin hydration and to make the skin texture softer and smoother. “Capture” was the first liposomal antiageing cream launched by Dior in 1986 [18].
Figure 1.
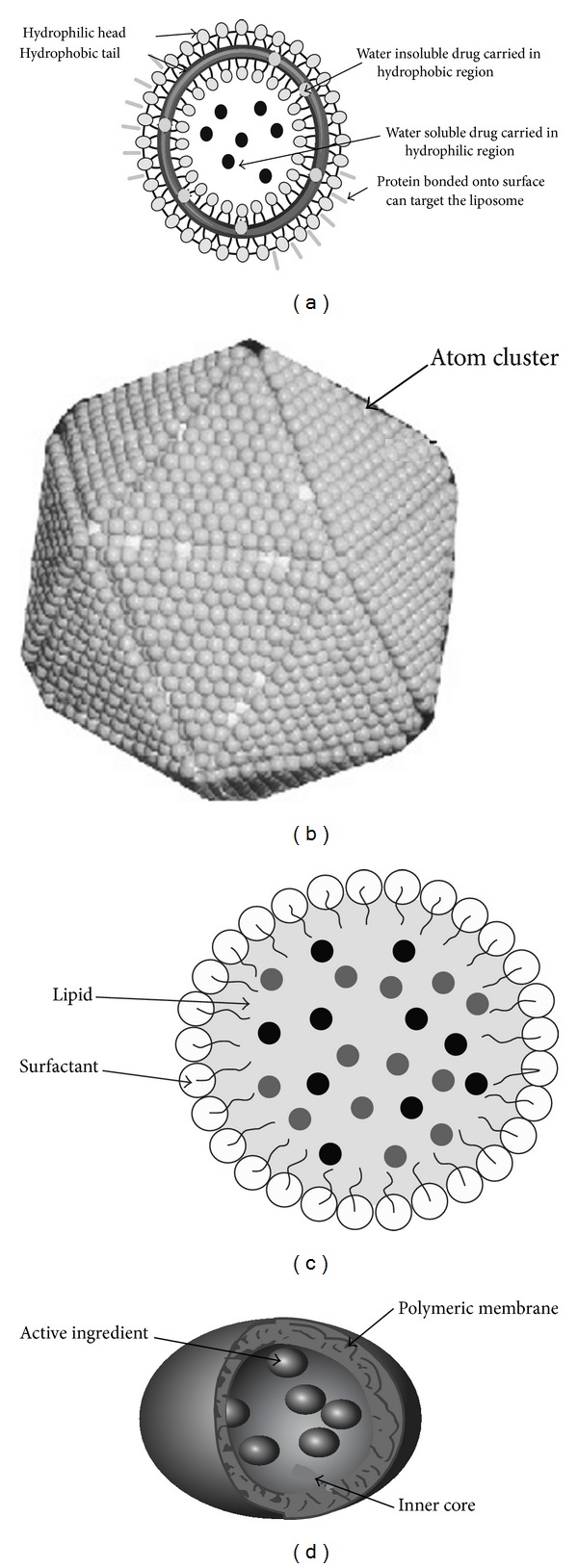
Different types of nanoparticles. (a): liposome showing a phospholipid bilayer surrounding an aqueous interior, (b): nanocrystal, (c): solid lipid nanoparticle [12], and (d): nanocapsule with different drug-loading modalities [13].
2.2. Nanocapsule
The potential dermatological use of nanocapsules was investigated when the first nanocapsule-based cosmetic product was launched by the French company L'Oreal in 1995 in order to improve the impact of their cosmetics [19]. The term nanocapsule is used for vesicular systems that are made up of a polymeric membrane in which an inner liquid core is encapsulated at the nanoscale level (10 nm to 1000 nm) (Figure 1) [20].
2.3. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) (Figure 1) are submicron colloidal carriers whose size ranges from 50 to 1000 nm and are composed of physiological lipid, dispersed in water or in aqueous solution of surfactant [12]. SLNs are popular in cosmeceuticals because of various advantages: these are composed of physiological and biodegradable lipids that exhibit low toxicity; the small size of SLNs ensures close contact with the stratum corneum and increases the penetration of active ingredients through the skin; SLNs provide occlusive properties that result in increased skin hydration [21]. The products Nano Repair Q10 cream and Nano Repair Q10 Serum (Dr. Kurt Richter Laboratorien GmbH, Berlin, Germany) introduced to the cosmetic market in October 2005 revealed the success of lipid nanoparticles in the antiageing field [22]. It has been found that SLNs possess characteristics of physical UV blockers on their own, thus offering the choice for developing a more effective sunscreen system with reduced side effects [23]. In an in vivo study it has been shown that skin hydration increases by 31% after 4 weeks by the addition of 4% SLNs to a conventional cream [24]. SLNs are also advantageous as topical vehicle for perfumes. By incorporating perfumes/fragrances in SLNs, the release can be slowed down to provide prolonged effect [25].
2.4. Nanocrystals
Nanocrystals are aggregates composed of several hundreds to thousands of atoms that combine into a cluster and are in the size range of 10–400 nm used for the delivery of poorly soluble actives (Figure 1) [26]. Nanocrystals appeared first in the cosmeceutical market in 2000 by Juvena with the product Juvedical having rutin [27]. In a study it was observed that, compared to the water-soluble rutin glucoside (rutin with attached glucose), the nanocrystal formulation of original rutin molecule possesses 500 times higher bioactivity [28]. A rutin nanosuspension with 5% rutin as nondissolved nanocrystals was applied to the skin of human volunteers and compared to a 5% solution of a water-soluble rutin glucoside regarding photoprotection of the skin. In the aqueous nanosuspension, the solubility of rutin was 500 times lower as compared to the water-soluble derivative. It was observed that, despite the 500 times lower concentration of dissolved rutin in the water phase of the nanocrystal suspension, the nanosuspension was about 25% more effective in photoprotection and the concentration of actives formulated as nanocrystals in the skin were much higher compared to water-soluble derivative or using the active in normal powder form.
2.5. Dendrimers
Dendrimers are organic chemical entities with a semipolymeric tree-like structure (Figure 2). The terminals of the branches provide a rich source of nanoparticles surface functionality. Their dimensions are extremely small, having diameters in the range of 2 to 10 nm [29]. Dendrimers are an exciting new class of macromolecular architecture and an important component in the area of nanotechnology-based cosmeceuticals to treat varieties of skin conditions. L'Oreal, Unilever, and The Dow Chemical Company have several patents for the application of dendrimers in hair care, skin care, and nail care products [30]. A patent on cosmetic formulation containing carbosiloxane dendrimer claimed that it can provide good water resistance, sebum resistance, glossiness, tactile sensation, and/or adhesive properties to the hair and/or skin [31].
Figure 2.
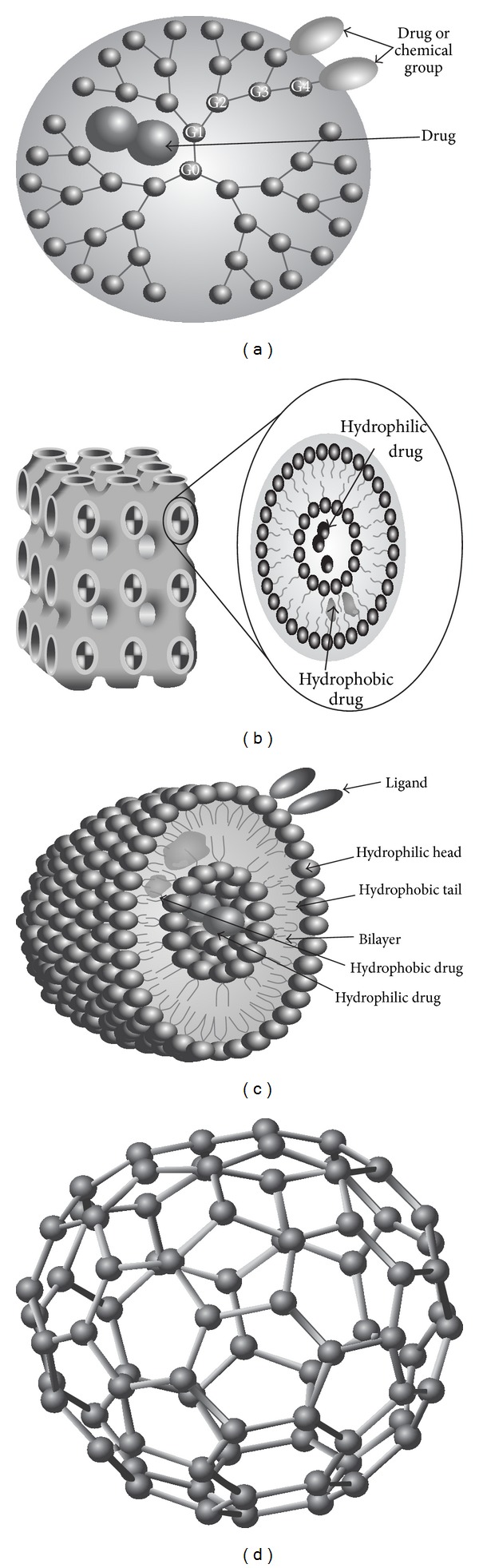
Different types of nanoparticles. (a): dendrimer with its different drug-loading modalities, (b): cubosome and its membrane composition with different drug-loading modalities. (c): niosome and its internal synthetic surfactant surrounding drug, and (d): fullerene [13].
2.6. Nanogold and Nanosilver
Gold and silver nanoparticles have been studied as a valuable material in cosmeceutical industry for their strong antibacterial and antifungal properties. These particles are widely used in cosmeceutical products like deodorant, face pack, antiaging cream, and so forth. An ointment containing silver nanoparticle was claimed to have antibacterial activity and can be used for skin inflammation and skin wound disinfection [32]. A study conducted by French scientist Dr. Philippe Walter and his team, published in ACS Nanoletters, describes the synthesis of fluorescent gold nanoparticles inside human hair. It involved soaking white hairs in a solution of a gold compound. The hairs turned pale yellow and then darkened to a deep brown. Using an electron microscope, the scientists confirmed that the particles were forming inside the hair's central core cortex. The color remained even after repeated washings [33].
2.7. Cubosomes
Cubosomes are discrete, submicron, nanostructured particles of bicontinuous cubic liquid crystalline phase (Figure 2) [34]. Recent research activities on the use of cubosome in personal care product areas varied from skin care to hair care and antiperspirants. The number of researches in association with cosmetic companies like L'Oreal and Nivea is trying to use cubosome particles as oil-in-water emulsion stabilizers and pollutant absorbents in cosmeceuticals [35–38].
2.8. Niosomes
Niosomes are nonionic surfactant vesicles devised by using nonionic surfactants (Figure 2) [39]. These vesicles possess high entrapment efficiency, improved chemical stability, and enhanced penetration, as well as lower production cost as compared to liposomes. In morphology, a niosome is a nanostructure with 100 nm to 2 μm in diameter, whose center is an aqueous cavity enveloped by layers of nonionic surfactant in lamellar phase [13]. These have been evaluated as vesicular carriers for variety of drugs and cosmetics topically. Niosomes are found to be efficient in topical delivery of active ingredients as they can enhance residence time of the active ingredients in the stratum corneum as well as epidermis and also reduce the system absorption [39]. By using niosomes, targeted delivery can also be achieved as the active ingredient is directly delivered to the specific site where therapeutic effect is desired [40].
2.9. Fullerene
Other nanoscale materials such as carbon fullerene have been used in some cosmetic products because of their antioxidative properties. They display potent scavenging capacities against radical oxygen species and they have been considered for their use in the preparation of skin rejuvenation cosmeceutical formulations [41]. These structures are comprised of carbon rings and contain odd-numbered (like Pentagon and heptagon) carbon rings, conferring a three dimensional spherical shape [42]. These structures have thus been called fullerenes or “Bucky Balls” (Figure 2). Fullerenes are highly hydrophobic and thus are not soluble in aqueous solutions, which initially limited their applications, but the use of surfactants or surface modifications has increased the ability of fullerenes to solubilize in water and brought more attention to their potential pharmaceutical uses [43].
3. Major Classes of Nanocosmeceuticals
3.1. Moisturizers
Stratum corneum is the primary barrier of the skin whose main purpose is to keep inside in and outside out. Water from the stratum corneum gets evaporated quickly leading to dehydration. This dehydration of skin can be averted by using moisturizers which provide flexibility to the skin. When moisturizers are applied to the skin, a thin film of humectant is formed which retains moisture and gives better appearance to the skin. Liposomes, nanoemulsions, SLNs are widely used moisturizing formulations because of their prolonged effects. These are considered to be the most useful product for the management of various skin conditions (e.g., atopic dermatitis, psoriasis, and pruritus).
3.2. Sunscreens
Sunscreens are widely used to protect the skin from harmful effects of sun rays on exposure. Zinc oxide (ZnO) and titanium dioxide (TiO2) are the most effective approved mineral-based ingredient which protects the skin from sun damage. This mineral forms a materialistic barrier on the skin, reflects UVA and UVB rays from penetrating down to the deeper layers of skin, and is less irritating [45]. The main drawback of traditional or conventional sunscreen is that, when applied, it leaves a white chalky layer on the skin [46]. This is where nanoparticles come in. Improved sunscreens are just one of the many innovative uses of nanotechnology. Sunscreen products using nanoparticles of ZnO or TiO2 are transparent, less greasy, and less smelly and have increased aesthetic appeal.
3.3. Antiaging Products
Chemical products, pollution, stress, irradiation from infrared (IR) and ultraviolet (UV) sources, and abrasion are involved in skin aging. Collagen plays an important role in skin rejuvenation and wrinkle reversal effect. The quantity of collagen in the skin decreases along with age. The aging of the skin manifests itself in many ways: drying out, loss of elasticity and texture, thinning, damaged barrier function, appearance of spots, modification of surface line isotropy, and, finally, wrinkles. Most of the cosmeceuticals have been developed with claims of antiwrinkle and firming, moisturizing and lifting, and skin toning and whitening activity. Antiaging products are the main cosmeceuticals in the market currently being made using nanotechnology. L'Oreal has employed nanotechnology in products such as Revitalift antiwrinkle cream which contains nanosomes of Pro-Retinol A, and claims that it instantly retautens the skin and reduces the appearance of wrinkles [47]. Application of retinol can increase epidermal water content, epidermal hyperplasia, and cell renewal while enhancing collagen synthesis [48]. Retinol also interferes with melanogenesis and inhibits matrix metalloproteinases, which are involved in collagen breakdown. The clinical benefits include a reduction in the appearance of fine lines and wrinkles and lightening of lentigines [49]. Lancôme introduces Hydra Zen Cream to renew the skin's healthy look which contains nanoencapsulated Triceramide [50].
3.4. Hair Care
Hair care is another promising field for nanotechnology. Companies are using nanotechnology in hair care products and research is ongoing to discover the ways of how nanoparticles can be used to prevent hair loss and to maintain shine, silkiness, and health of hairs. Unlike ordinary hair straightening products nanoemulsion in hair cosmetics does not destroy the outer structure of the hair fibers, called cuticles, to penetrate into the hair strands [51]. Sericin (composed of cationic sericin nanoparticles) is an active area of hair cosmeceuticals. Studies have shown that sericin nanoparticles in hair cosmeceuticals easily adhere to the surface of hair seal and treat the damaged cuticles (Figure 3) [44].
Figure 3.
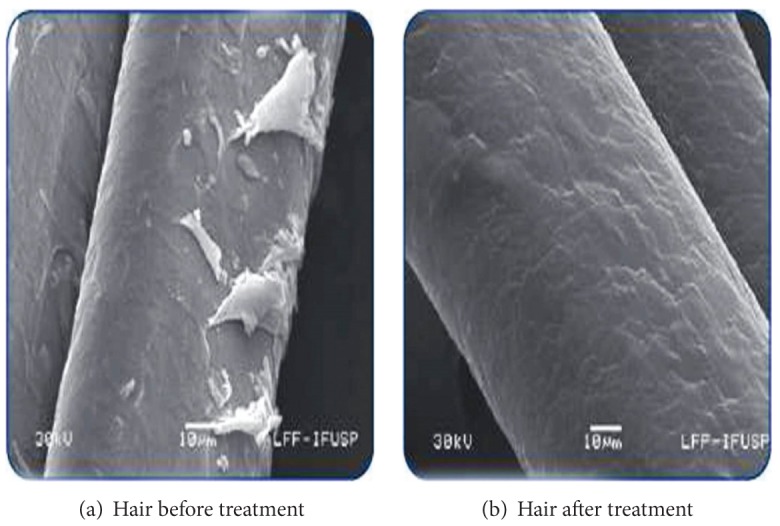
Effect of sericin nanoparticles on hair cuticle. Increased hair gloss (b) obtained in damaged hair (a) after treating with sericin nanoparticles [44].
3.5. Skin Cleanser
The skin is covered with a hydrolipid film that, depending on the area of the body, comprises secretions from sebaceous glands and from apocrine and eccrine sweat glands. Decomposition products from corneocytes and cornification (cellular debris and stratum corneum lipids) in the process of being shed are also present. This film provides a natural defense against pathogenic organisms but also attracts dirt and pollutants from the environment. Sometimes the microorganisms present on the skin surface act on components of the surface film and create undesirable by-products, such as those resulting from the metabolism of compounds found in apocrine sweat that create body odor [52]. Thus, periodic cleansing to remove debris, dirt, and odor is essential to maintain skin health. Cleansing is also necessary to remove soil (which may include bacteria) from the skin surface that is acquired by incidental contact or by intentional application (medications or makeup and other cosmetic products). Silver nanoparticles are used as skin disinfectant and decontamination. Nano Cyclic Inc. produces Nano Cyclic cleanser pink soap which is a scientifically balanced blend of nanosilver and natural ingredients and claims that it kills harmful bacteria and fungi, fights acne, and diminishes age spots and sun damaged skin [53].
3.6. Lip Care
Lip care is another promising class of cosmeceuticals. Different nanoparticles can be incorporated into lipstick and lip gloss which will soften or soothe the lips by preventing transepidermal water loss. Korea Research Institute of Bioscience and Biotechnology holds a patent that described that it is possible to prepare pigments exhibiting wide range of colors using gold or silver nanoparticles by mixing in various compositional ratios and whose color can be maintained for a long period of time [54]. Silica nanoparticles used in lipsticks improve the homogenous distribution of pigments. Once applied, they prevent the pigments from migrating or bleeding into the fine line of lips [55].
3.7. Nail Care
Nanotechnology-based nail cosmeceuticals have various advantages over conventional products. A study revealed that nail paints having nanosized particles improve toughness, mar resistance, and impact resistance of the mammalian nails [56]. Nano Labs Corp. (a nanotechnology research and development company) was awarded a provisional patent for its original nanonail polish and lacquer having advantages that it dries to a very hard state, resists shock, cracking, scratching, and chipping and its elasticity offers superior ease of application without cracking [57]. One of the new strategies which may have great potential in the cosmeceuticals is the incorporation of nanoparticles having antifungal activity (like silver and metal oxide nanoparticles) in nail polish to treat fungal toenail infections.
A review on various nanotechnology-based cosmeceutical products in the market and patents have been tabulated in Tables 1 and 2.
Table 1.
Various nanotechnology-based cosmeceutical products in the market.
| Product | Proposed use | Manufacturer | Marketing claims |
|---|---|---|---|
| Hydra Flash Bronzer Daily Face moisturizer | Moisturizer | Lancôme | Nanocapsules of pure vitamin E provide powerful antioxidant protection. A light touch of self-tanner ensures a natural, healthy glowing skin. |
|
| |||
| Hydra Zen Cream | Moisturizer | Lancôme | Containing Nanoencapsulated Triceramides, Hydra Zen helps restore perfect comfort and softness and renew skin's healthy look. Protected from signs of daily stress and fully hydrated, your skin is beautifully soft and smooth all day long. |
|
| |||
| Nano-In Hand and Nail Moisturizing Serum and Foot Moisturizing Serum | Moisturizer | Nano-Infinity Nanotech | Fine crystals of ZnO nanoparticles will go straight into skin tissue to prevent hand and nails from being hurt and restore skin health |
|
| |||
| Lancôme Renergie Microlift | Antiwrinkle | Lancôme | Formulated with colloidal silica and soy protein nanoparticles to provide the closest possible face-lift effect. |
|
| |||
| RevitaLift Anti-Wrinkle and Firming Face and Neck Contour Cream | Antiwrinkle | L'Oreal | The Revitalift formula is enriched with Pro-Retinol A, a powerful antiwrinkle agent, which is encapsulated in nanosomes. Nanosomes penetrate deep into the epidermis to work at the heart of wrinkles. |
|
| |||
| Revitalift Double Lifting | Antiwrinkle | L'Oreal | It contains nanosomes of Pro-Retinol A. RevitaLift Double Lifting is a unique dual action treatment that instantly retightens skin and effectively fights wrinkles. |
|
| |||
| Eye Tender | Antiwrinkle | Kara Vita | It contains nanospheres, delivers 13 bioactives including proven, wrinkle-reducing peptides to stimulate fibroblasts, build collagen, brighten skin, and reduce inflammation for a younger, healthier appearance. |
|
| |||
| Eye Contour Nanolift | Antiwrinkle Antiaging | Euoko | It is based on nanocapsules technology. Lifting nanocapsules join seven other immediate and long-term fighters of fine lines, wrinkles, and puffiness. It provides instant and long-term smoothness, gives the eye area more radiance, and diminishes the appearance of dark circles and puffiness. |
|
| |||
| Soleil Soft-Touch Anti-Wrinkle Sun Cream SPF 15 | Antiwrinkle sunscreen | Lancôme | It contains vitamin nanocapsules which help to preserve skin's youth effectively. SPF 15 offers optimal protection against the sun. It contains exclusive ingredients to guarantee a long-lasting effect. |
|
| |||
| Nano Gold Firming Treatment | Antiaging | Chantecaille | Infinitely small nanoparticles of pure gold are bound to silk microfibers to firm and tone skin, while delivering incredible anti-inflammatory, healing, and age defying power. |
|
| |||
| Nanosphere Plus | Antiaging | DermaSwiss | A stem cells revolutionary antiaging therapy Nanosphere Plus serum has been specially formulated to allow natural stem cells to preserve and protect skin cells. Using the cells from a rare Swiss apple (Uttwiler Spatlauber), Nanosphere Plus protects longevity and combats chronological aging. |
|
| |||
| Zelens Fullerene C-60 Night Cream | Antiaging | Zelens | Fullerene C-60 is a naturally occurring microscopic form of carbon which was found to have remarkable antioxidant properties. |
|
| |||
| Clearly It! Complexion Mist | Antiacne | Kara Vita | This nanosphere technology-based product tackles acne conditions and balances sebum production. Nanosphere time-released bioactives stimulate capillary activity for all-day detoxifying results. |
|
| |||
| DiorSnow Pure UV Base SPF 50 | Sunscreen | Dior | Contains nano-UV filters for ultraprotection against the damaging effects of UVA and UVB rays. |
|
| |||
| Soleil Instant Cooling Sun Spritz SPF 15 | Sun protection spray | Lancôme | Contains vitamin nanocapsule. Instant cooling sun spray SPF 15 immediately offers a sensation of freshness. SPF 15 provides optimal protection against the sun. |
|
| |||
| Fresh As A Daisy Body Lotion | Body lotion | Kara Vita | This lotion uses nanospheres to quickly penetrate, moisturize, and nourish all types of skin. |
|
| |||
| Cosil Nano Beauty Soap | Cleanser | Natural Korea | Silver nanoparticles are highly effective as disinfectant and guarantee protection of skin. |
|
| |||
| Cosil Whitening Mask | Face mask | Natural Korea | Made with nanocolloidal silver used for the effect of getting rid of germs from your face, compressing pores, soothing the skin condition, and keeping your skin radiant and soft. |
|
| |||
| Nanorama—Nano Gold Mask Pack | Face mask | LEXON NanoTech | It contains pure nanosized gold that is highly effective in penetrating small pores and disinfecting skin, helps to reduce pore size, and prevents and treats acne. It is well known that nanogold is very effective disinfectants. |
|
| |||
| Primordiale Optimum Lip | Lip treatment | Lancôme | Delivers 100% botanically pure vitamin E via nanocapsule technology to reduce lip bleeding and feathering due to fine lines and wrinkles. |
|
| |||
| Lip Tender | Lip moisturizer | Kara Vita | Ten bioactive ingredients are precisely calculated to work within lyphazomes, delivering a 4-in-1 formula and bringing long-lasting hydration for fast and dramatic lip repair. |
|
| |||
| Nano Cyclic Cleanser Silver | Cleanser | Nano Cyclic | Cyclic cleanser is a scientifically balanced blend of nanosilver and natural ingredients. It kills harmful bacteria and fungi, treats acne, exfoliates dead skin on all parts of the body, diminishes age spots, deodorizes the body, and fights wrinkles. |
|
| |||
| LifePak Nano | Face gel | Pharmanex | LifePak Nano is a nutritional antiaging program formulated to nourish and protect cells, tissues, and organs in the body with the specific purpose of guarding against the ravages of aging. Nanoencapsulation increases bioavailability coenzyme Q10 by 5–10 times. |
Table 2.
Patent review on nanotechnology-based cosmeceuticals.
| Title | Publication number | Publication date | Applicant |
|---|---|---|---|
| Cosmetic composition containing retinol stabilized by porous polymer beads and nanoemulsion | EP 2583665A2 | April 24, 2013 | Act Co., Ltd |
| Multiactive microtargeted antiaging skincream polymer technology | EP 20110798597 | April 17, 2013 | NY Derm LLC |
| Semipermanent mascara and method of applying | US 20130068242A1 | March 21, 2013 | Cry Baby Culture |
| Topically administered, skin-penetrating glycosaminoglycan formulations suitable for use in cosmetic and pharmaceutical applications | US 20130059769A1 | March 7, 2013 | Eva Turley |
| Biodegradable, biocompatible, and nontoxic material sheets consisting of said material and the use thereof in food, pharmaceutical, cosmetic, and cleaning products | US 20130034638A1 | February 7, 2013 | Inis Biotech LLC |
| Metal oxide nanocomposites for UV protection | US 20130022655A1 | January 24, 2013 | BASF SE |
| Oil-in-water-type emulsion sunscreen cosmetic composition | US 20130011348A1 | January 10, 2013 | Tomiko Takakura |
| Synthetic collagen threads for cosmetic uses including skin wrinkle treatments and associated methods | US 20130018415A1 | January 17, 2013 | Rebeccah Brown |
| Deodorant composition | WO 201210122A1 | August 2, 2012 | Ilios Srl |
| Preparation of cationic nanoparticles and personal care compositions comprising said nanoparticles | EP 2254545A2 | December 1, 2010 | BASF SE |
| Gel technology suitable for use in cosmetic compositions | US 20100266649A1 | October 21, 2010 | Avon Products, Inc. |
| Nanocrystals for use in topical cosmetic formulations and thereof method of production | EP 2099420A1 | September 16, 2009 | Abbott GmbH & Co.KG |
| Nanodiamond UV protectant formulations | US 20090220556A1 | September 3, 2009 | International Technology Center |
| Nanoparticle compositions providing enhanced color for cosmetic formulations | US20090175915A1 | July 3, 2009 | Avon Products, Inc. |
| Nanocomposite pigments in a topical cosmetic application | WO 2008079758A1 | July 3, 2008 | Avon Products, Inc. |
| Cosmetic pigment composition containing gold or silver nanoparticles | EP 1909745A1 | April 16, 2008 | Korea Research Institute of Bioscience and Biotechnology |
| Antimicrobial silver compositions | WO 2006026026A2 | March 9, 2006 | Acry Med, Inc. |
| Long-lasting coatings for modifying hard surfaces and processes for applying the same | US 6955834B2 | October 18, 2005 | The Proctor & Gamble Company |
| Nail polish compositions comprised of nanoscale particles free of reactive groups | US 20050220730A1 | October 6, 2005 | Martinez Francisco |
| Use of nanoscale deodorants | EP 1239823B1 | June 16, 2004 | Cognis Deutschland GmbH & Co.KG |
| Cosmetic compositions comprising nanoparticles and processes for using the same | US 20030064086A1 | March 13, 2003 | Danuvio Carrion |
| A controlled delivery system for hair care products | WO 2002060399A1 | August 8, 2002 | Salvona LLC |
| Antimicrobial body care product | WO 2000078281A1 | December 28, 2000 | Bernhard Hanke |
4. Exposure to Nanoparticles
Industrial use of nanoparticles has created new opportunities, but it also presents some risks and uncertainties. Increasing production and use of nanomaterials results in an increasing number of workers and consumers exposed to nanomaterials. This shows that there is greater need for information on their exposure routes. Human routes of exposure to nanoparticles are inhalation, ingestion, and dermal routes [58]. Inhalation is the most common route of exposure to airborne nanoparticles [59]. Workers may inhale nanoparticles while production or consumers may inhale on the use of aerosolized cosmeceuticals (deodorant, perfumes, etc.). The deposition of nanoparticles in the respiratory system depends on their interactions with respiratory epithelium membrane. Nanoparticles may travel via the nasal nerves to the brain (transsynaptic transport after inhalation through the olfactory epithelium) and gain access to the nervous system [60]. Because of their size, these nanoparticles can easily gain access to the blood stream inhalation or skin and from there they are transported to the various organs [58]. Ingestion may occur from unintentional hand to mouth transfer of nanoparticles or from those cosmeceuticals that are applied near mouth or lips (e.g., lip color, lip gloss). Large fractions of nanoparticles rapidly pass out of the body after ingestion, but a small fraction may be taken up by the body which migrates into the different organs [61]. The other route of exposure of nanoparticles into the systemic circulation is dermal absorption. Majority of cosmeceuticals are applied to the skin. Three pathways of penetration across the skin have been identified: intercellular, transfollicular, and transcellular [62].
5. Skin Penetration of Nanoparticles
The skin is the largest organ of the body. Human skin is made up of three layers (Figure 4): the epidermis (the outermost layer of skin), the dermis (contains tough connective tissue, hair follicles, and sweat glands), and the hypodermis (made up of fat and connective tissue). The epidermis is divided into several layers and its outermost layer, the stratum corneum, is responsible for the barrier function of the skin due to its lipophilicity and high cohesion between cells [63]. Passive routes by which a molecule can cross the stratum corneum are intercellular, transcellular, and appendageal routes (Figure 5) [64].
Figure 4.
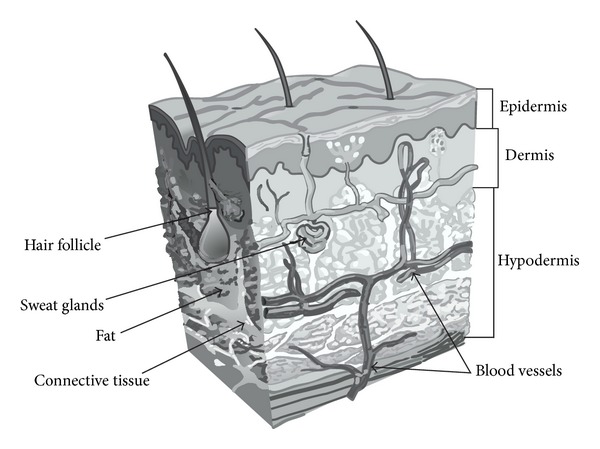
Human skin layers.
Figure 5.
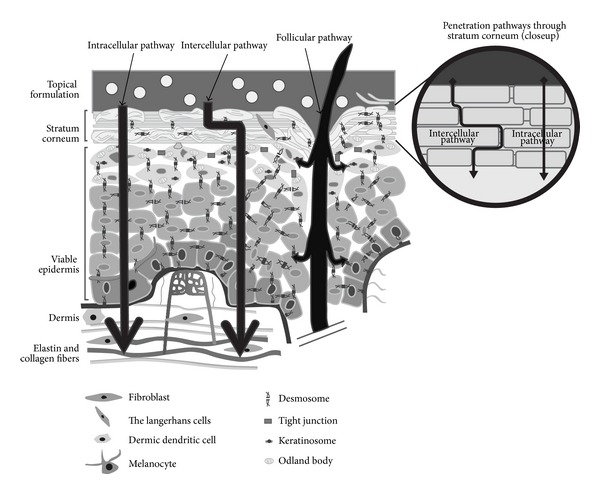
Skin penetration pathways (intracellular, intercellular, and follicular) by which a molecule can cross the stratum corneum [64].
A variety of cosmeceutical products having nanoparticles are in the market that are applied to the skin and concerns have been raised regarding the potential dangers which may occur on their skin penetration. The transport of nanoparticles through the skin is related to the nature and physicochemical properties of the nanoparticles and vehicles, the nature of the substance, and the conditions of the skin [65]. Nanoparticles can be divided into two groups: (1) soluble and/or biodegradable nanoparticles (e.g., liposomes and nanoemulsion); (2) insoluble and/or nonbiodegradable nanoparticles (e.g., TiO2, fullerenes, and quantum dots). Dermal absorption of nanoparticles does not occur readily but can take place under certain conditions. Although cosmetic products are meant to be used on normal skin, it is known that they are also applied on nonhealthy skin. In such conditions the barrier properties of skin may be impaired. Most of the study reported that nanoproducts applied to the skin only penetrate through hair follicular openings and skin pores, with minimal amount being found below the stratum corneum [66].
Research on the fate of these nanoparticles when applied to mammalian skin by employing laser scanning confocal microscopy to see whether fluorescently tagged particles (20 to 200 nm) were absorbed into the skin showed that nanoparticles contacting intact or partially damaged skin cannot penetrate skin barrier and do not reach the viable cells of the epidermis or beyond and hence proved that the nanotopical delivery systems are useful and safe for cosmeceuticals [67]. In a study on dermal absorption of ZnO nanoparticles from sunscreen applied to humans at the beach, Gulson et al. revealed that zinc from ZnO particles in sunscreen penetrates healthy skin and is observed in blood and urine. Whether the Zn was present as particles or soluble Zn ion was unknown at that stage [68]. A review on the use of nanoparticles in personal care products done by the Environmental Working Group, a US-based NGO, concluded after peer review of more than 400 documents that: “zinc and titanium-based formulations are among the safest, most effective sunscreens on the market based on available evidence” and of 16 studies on skin absorption, “nearly all showing no absorption of smallscale zinc and titanium sunscreen ingredients through healthy skin” [69]. The controversy has intensified, with numerous studies reporting that they do not cross the skin barrier, whilst others lead us to suspect new risks to humans, though without any of them providing a definitive answer. A review of percutaneous absorption studies of TiO2 and ZnO nanoparticles has been shown in Table 3 [70–77].
Table 3.
Research studies done on percutaneous absorption of TiO2 and ZnO nanoparticles.
| Test material | Skin model | Particle size | Results | Reference |
|---|---|---|---|---|
| TiO2 | Dermatomed skin | 1–35 nm, (noncoating), 2–35 nm (alumina/silica/silicon coated), and 3–10 × 100 nm (mixture of alumina and silicon coated) | No penetration was observed regardless of TiO2 type in intact and stripped skin. SEM-EDS observation showed that Ti penetrated into vacant hair follicles (greater than 1 mm below the skin surface); however it did not penetrate into dermis and viable epidermis. | [70] |
|
| ||||
| TiO2 | Human skin in vitro | 20 nm | Penetration in restricted to the topmost corneocyte layers in the stratum corneum. No penetration into living skin was observed. | [71] |
|
| ||||
| TiO2 alone or in combination with ZnO | Human skin (biopsy) | 20 nm | TiO2 or ZnO nanoparticles are absent or their concentration is too low to be tested under the stratum corneum in human viable epidermis. Therefore, significant penetration towards the underlying keratinocytes is unlikely. | [72] |
|
| ||||
| TiO2 in a sunscreen formulation | Human skin in vitro and human subjects | 20 nm | Results showed penetration is limited to upper layers of stratum corneum. No penetration in skin furrows or follicular opening may be mistaken for penetration in the epidermal compartment. | [73] |
|
| ||||
| TiO2 | Human skin in vitro | 10 nm to 100 nm | Results showed penetration of particles into the upper layers of stratum corneum. No penetration into living skin. | [74] |
|
| ||||
| TiO2 in various formulations | Pig skin in vitro | Needles: 45 to 150 nm × 17 to 35 | Particles on/in the stratum corneum; minimal penetration into stratum granulosum. No penetration into living skin. | [75] |
|
| ||||
| TiO2 | Human subjects (biopsy) | 150 nm to 170 nm | Results showed particles in the upper layers of stratum corneum. About 1% of particles in the follicle ostium. No penetration into living skin. | [76] |
|
| ||||
| TiO2 and ZnO | Human skin in vitro | TiO2: 50 to 100 nm ZnO: 20 to 200 nm | Results showed that penetration is limited to upper layers of stratum corneum. | [77] |
Continued research is required to evaluate the behavior of nanoparticles, including whether the nanoparticles remain on the surface of skin and/or stratum corneum or absorbed into the blood stream to reach different organs.
6. Toxicity of Nanoparticles
Nanoparticles from various cosmeceutical products applied on skin can have toxic effects if reaching to blood stream. A research on toxicity of TiO2 nanoparticles demonstrated that when nano-sized TiO2 administered subcutaneously to pregnant mice, they transferred to the offspring and result in brain damage and reduced sperm production in male offspring [78]. Various researches have shown that TiO2 nanoparticles can produce free radicals and cause cell toxicity in test tube studies, when exposed to UV light [79, 80]. Studies have shown that cobalt-chromium nanoparticles (29.5 nm in diameter) can destroy human fibroblast cells across an intact cellular barrier. If nanoparticles are inhaled and eaten accidentally or absorbed through skin, they could cause skin and lung damage and organ toxicity or can harm unborn children [81]. Silver nanoparticles are used in cosmeceuticals for their antimicrobial activity. Concentration of silver that is lethal for bacteria is also lethal for both keratinocytes and fibroblasts [82]. The cosmeceutical industry debates that consumer risks are low, as there is no evidence that nanoparticles from the product penetrate healthy, intact adult skin.
7. Recent Advances in Nanoproduct Regulation
Recently USFDA has published an Import Alert 66-38, for skin care products labeled as antiaging creams [83]. This is because there are numerous skin care products in the market which claim that the products counteract, retard, or control the aging process. According to USFDA, A claim such as “molecules absorb and expand, exerting upward pressure to lift wrinkles upward” is a claim for an inner structural change that would usually cause a product to be a drug. FDA has stated such claims are illegal on cosmetic labeling.
In the European Union (EU), the new Cosmetic Products Regulation 1223/2009 attempts to go some way in addressing concerns over nanomaterials. According to this regulation all ingredients present as nanomaterials must be indicated on the package, from July 11, 2013, with the word “nano” [84]. The format distinguishes a nanoparticle with the suffix “nano,” so TiO2 becomes TiO2-nano [85, 86]. The regulation also requires that all marketed cosmetics and sunscreens using nanoparticles be individually tested for safety. Cosmetic products containing nanomaterials must be notified by electronic means to the commission, providing data on identification, specification, quantity, toxicological profile, safety data, and foreseeable exposure conditions. Such notification must occur six months before a cosmetic product containing nanomaterials is placed on the market [84].
8. Conclusion
Growth of cosmeceutical industry is increasing day by day as the cosmeceuticals market is highly diversified, with products coming from major and small manufacturers and local companies around the world. Nanotechnology represents the key technologies of the twenty-first century, offering excellent opportunities for both research and business. The rapid spread and commercialization of nanotechnology in cosmeceuticals have given rise to great technical and economic aspirations but also question about the emerging risks to health and safety of consumers. Thus, cosmeceutical products based on nanotechnology should be designed and sold in a way that fully respects the health of consumers and the environment.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.U.S. Food and Drug Administration. Is it a cosmetic, a drug, or both? (Or is it soap?) http://www.fda.gov/cosmetics/guidancecomplianceregulatoryinformation/ucm074201.htm.
- 2.U.S. Food and Drug Administration. Cosmetics Q&A: FDA’s Authority. http://www.fda.gov/Cosmetics/ResourcesForYou/Consumers/CosmeticsQA/ucm135709.htm.
- 3.Fulekar MH. Nanotechnology: Importance and Application. New Delhi, India: IK International Publishing House; 2010. [Google Scholar]
- 4.Mukta S, Adam F. Cosmeceuticals in day-to-day clinical practice. Journal of Drugs in Dermatology. 2010;9(5):s62–s66. [PubMed] [Google Scholar]
- 5.Cosmeceuticals: Products and Global Markets. http://www.bccresearch.com/market-research/advanced-materials/cosmeceuticals-global-markets-avm099a.html.
- 6.Brandt FS, Cazzaniga A, Hann M. Cosmeceuticals: current trends and market analysis. Seminars in Cutaneous Medicine and Surgery. 2011;30(3):141–143. doi: 10.1016/j.sder.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.RNCOS E-Services Pvt. Ltd. Global cosmeceuticals market outlook 2016. http://www.giiresearch.com/report/rnc263147-global-cosmeceuticals-market outlook.html.
- 8.GBI Research. Cosmeceuticals market to 2018—Technological advances and consumer awareness boost commercial potential for innovative and premium-priced products. http://www.researchandmarkets.com/reports/2393091/cosmeceuticals_market_to_2018_technological.
- 9.Singh R, Tiwari S, Tawaniya J. Review on nanotechnology with several aspects. International Journal of Research in Computer Engineering and Electronics. 2013;2(3):1–8. [Google Scholar]
- 10.Padamwar MN, Pokharkar VB. Development of vitamin loaded topical liposomal formulation using factorial design approach: drug deposition and stability. International Journal of Pharmaceutics. 2006;320(1-2):37–44. doi: 10.1016/j.ijpharm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Mu L, Sprando RL. Application of nanotechnology in cosmetics. Pharmaceutical Research. 2010;27(8):1746–1749. doi: 10.1007/s11095-010-0139-1. [DOI] [PubMed] [Google Scholar]
- 12.Ekambaram P, Sathali AAH, Priyanka K. Solid lipid nanoparticles: a review. Scientific Reviews & Chemical Communications. 2012;2:80–102. [Google Scholar]
- 13.Bei D, Meng J, Youan B-BC. Engineering nanomedicines for improved melanoma therapy: progress and promises. Nanomedicine. 2010;5(9):1385–1399. doi: 10.2217/nnm.10.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangham AD. Physical structure and behavior of lipids and lipid enzymes. Advances in Lipid Research. 1963;64:65–104. doi: 10.1016/b978-1-4831-9937-5.50008-9. [DOI] [PubMed] [Google Scholar]
- 15.Mezei M, Gulasekharam V. Liposomes - a selective drug delivery system for the topical route of administration. I. Lotion dosage form. Life Sciences. 1980;26(18):1473–1477. doi: 10.1016/0024-3205(80)90268-4. [DOI] [PubMed] [Google Scholar]
- 16.Kaur IP, Agrawal R. Nanotechnology: a new paradigm in cosmeceuticals. Recent Patents on Drug Delivery & Formulation. 2007;1(2):171–182. doi: 10.2174/187221107780831888. [DOI] [PubMed] [Google Scholar]
- 17.Lasic DD. Novel applications of liposomes. Trends in Biotechnology. 1998;16(7):307–321. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Goymann CC. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58(2):343–356. doi: 10.1016/j.ejpb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Poletto FS, Beck RCR, Guterres SS, Pohlmann AR. Polymeric nanocapsule: concepts and applications. In: Beck R, Guterres S, Pohlmann A, editors. Nanocosmetics and Nanomedicines: New Approaches for Skin Care. Berlin, Germany: Springer; 2011. pp. 47–51. [Google Scholar]
- 20.Kothamasu P, Kanumur H, Ravur N, et al. Nanocapsules: the weapons for novel drug delivery systems. BioImpacts. 2012;2(2):71–81. doi: 10.5681/bi.2012.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. International Journal of Pharmaceutics. 2009;366(1-2):170–184. doi: 10.1016/j.ijpharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Müller RH, Petersen RD, Hommoss A, Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Advanced Drug Delivery Reviews. 2007;59(6):522–530. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Wissing SA, Mader K, Muller RH. Solid lipid nanopartices (SLN) as a novel carrier system offering prolonged release of the perfume Allure (Chanel). Proceedings of the International Symposium on Controlled Release of Bioactive Materials; 2000; Paris, France. pp. 311–312. [Google Scholar]
- 24.Mei Z, Wu Q, Hu S, Li X, Yang X. Triptolide loaded solid lipid nanoparticle hydrogel for topical application. Drug Development and Industrial Pharmacy. 2005;31(2):161–168. doi: 10.1081/ddc-200047791. [DOI] [PubMed] [Google Scholar]
- 25.Souto EB, Müller RH. Cosmetic features and applications of lipid nanoparticles (SLN, NLC) International Journal of Cosmetic Science. 2008;30(3):157–165. doi: 10.1111/j.1468-2494.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- 26.Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. European Journal of Pharmaceutics and Biopharmaceutics. 2006;62(1):3–16. doi: 10.1016/j.ejpb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto J, Annapragada A, Decuzzi P, Ferrari M. Antibiological barrier nanovector technology for cancer applications. Expert Opinion on Drug Delivery. 2007;4(4):359–369. doi: 10.1517/17425247.4.4.359. [DOI] [PubMed] [Google Scholar]
- 28.Petersen R. Nanocrystals for use in topical cosmetic formulations and method of production thereof. US Patent US 20100047297A1. February 2010.
- 29.Dendrimers & Dendrons: Facets of Pharmaceutical Nanotechnology. Drug-Dev Newsletter, http://www.kellerfoundation.com/ME2/dirmod.asp?sid=4306B1E9C3CC4E07A4D64E23FBDB232C&nm=Back+Issues&type=Publishing&mod=Publications%3A%3AArticle&mid=8F3A7027421841978F18BE895F87F791&tier=4&id=9B9BA1DAA5BE455A85A81D97382FE885.
- 30.Tournihac F, Simon P. Cosmetic or dermatological topical compositions comprising dendritic polyesters. U.S. Patent 6,287,552, September 2001.
- 31.Furukawa H, Limura T. Copolymer having carbosiloxane dendrimer structure, and composition and cosmetic containing the same. U.S. Patent 20120263662A1, October 2012.
- 32.Lin Y, Yan L. Broad spectrum anti-bactericidal ointment nano. CN Patent. CN 1480045 A. March 2004.
- 33.First synthesis of gold nanoparticles inside human hair for dyeing and much more. http://www.nanowerk.com/news2/newsid=28260.php.
- 34.Hyde S, Andersson A, Larsson K, et al. The Language of Shape. 1st edition. New York, NY, USA: Elsevier; 1997. [Google Scholar]
- 35.Kimmes SC, Feltin C. Cosmetic composition comprising an oil and a polymer both bearing a hydrogen-bond-generating joining group, and cosmetic treatment process. European Patent 2575751A1, April 2013.
- 36.Ribier A, Biatry B. Cosmetic or dermatologic oil/water dispersion stabilized with cubic gel particles and method of preparation. European Patent 0711540B1, May 2000.
- 37.Albrecht H, Schreiber J. Hair care products with disperse liquid crystals exhibiting the cubic phases. W.O. Patent 2002041850A1, May 2002.
- 38.Simonnet JT, Sonneville O, Legret S. Nanoemulsion based on phosphoric acid fatty acid esters and its uses in the cosmetics, dermatological, pharmaceutical, and/or ophthalmological fields. U.S. Patent 6274150 B1, August 2001.
- 39.Anisha S, Kumar SP, Kumar GV, Garima G. Approaches used for penetration enhancement in transdermal drug delivery system. International Journal of Pharmaceutical Sciences. 2010;2(3):708–716. [Google Scholar]
- 40.Sankhyan A, Pawar P. Recent trends in noisome as vesicular drug delivery system. Journal of Applied Pharmaceutical Science. 2012;2:20–32. [Google Scholar]
- 41.Lens M. Use of fullerenes in cosmetics. Recent Patents on Biotechnology. 2009;3(2):118–123. doi: 10.2174/187220809788700166. [DOI] [PubMed] [Google Scholar]
- 42.Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318(6042):162–163. [Google Scholar]
- 43.Cusan C, Da Ros T, Spalluto G, et al. A new multi-charged C60 derivative: synthesis and biological properties. European Journal of Organic Chemistry. 2002;(17):2928–2934. [Google Scholar]
- 44.Carmen MD, Pereda V, Polezel A, et al. Sericin cationic nanoparticles for application in products for hair and dyed hair. U.S. Patent 20120164196, June 2012.
- 45.Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnology, Science and Applications. 2011;4(1):95–112. doi: 10.2147/NSA.S19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faunce T. Exploring the safety of nanoparticles in Australian Sunscreens. International Journal of Biomedical Nanoscience and Nanotechnology. 2010;1:87–94. [Google Scholar]
- 47.L'Oreal Paris. http://www.lorealparisusa.com/en/Products/Skin Care/Moisturizers/RevitaLift-Anti-Wrinkle-Firming-Day-Cream-SPF-18.aspx.
- 48.Draelos ZD. Retinoids in cosmetics. Cosmetic Dermatology. 2005;18(1):3–5. [Google Scholar]
- 49.Choi CM, Berson DS. Cosmeceuticals. Seminars in Cutaneous Medicine and Surgery. 2006;25(3):163–168. doi: 10.1016/j.sder.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 50.The project on emerging nanotechnologies. http://www.nanotechproject.org/inventories/consumer/browse/products/5043/
- 51.Ereno D. Well-grounded Beauty. http://revistapesquisa.fapesp.br/en/2008/04/01/wellgrounded-beauty/
- 52.Ertel K. Personal cleansing products: properties and use. In: Draelos ZD, Thaman LA, editors. Cosmetic Formulation of Skin Care Products. New York, NY, USA: Taylor & Francis; 2006. pp. 32–36. [Google Scholar]
- 53.Nanocyclic cleanser pink. http://www.nanocyclic.com/ProductDetails.asp?ProductCode=CY-40P.
- 54.Ha TH, Jeong JY, Jung BTYH, Kim JK. Cosmetic pigment composition containing gold or silver nano-particles. European Patent 1909745A1, April 2008.
- 55.Viladot PJL, Delgado GR, Fernandez BA. Lipid nanoparticle capsules. European Patent 2549977A2, January 2013.
- 56.Amato SW, Farer A, Hoyte WM, Pavlovsky M, et al. Coatings for mammalian nails that include nanosized particles. U.S. Patent 2007/002207, August 2007.
- 57.NanoLabs. http://nanolabs.us/press-releases/green-chemistry-and-new-thinking-at-playas-nano-labs-ctle-receives-provisional-patent-for-unique-nanotech-nail-polish/
- 58.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yah CS, Simate G, Iyuke SE. Nanoparticles toxicity and their routes of exposures. Pakistan Journal of Pharmaceutical Sciences. 2012;25(2):477–491. [PubMed] [Google Scholar]
- 60.Paul JAB, Roel PFS. Toxicological characterization of engineered nanoparticles. In: Gupta RB, Kompella UB, editors. Nanoparticle Technology for Drug Delivery. New York, NY, USA: Taylor & Francis; 2006. pp. 161–170. [Google Scholar]
- 61.Raj S, Jose S, Sumod US, Sabitha M. Nanotechnology in cosmetics: opportunities and challenges. Journal of Pharmacy and Bioallied Sciences. 2012;4(3):186–193. doi: 10.4103/0975-7406.99016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buzea C, Blandino IIP, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;4:MR17–MR172. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 63.Benson HAE. Transdermal drug delivery: penetration enhancement techniques. Current Drug Delivery. 2005;2(1):23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- 64.Bolzinger M-A, Briançon S, Pelletier J, Chevalier Y. Penetration of drugs through skin, a complex rate-controlling membrane. Current Opinion in Colloid and Interface Science. 2012;17(3):156–165. [Google Scholar]
- 65.Cevc G, Vierl U. Nanotechnology and the transdermal route. A state of the art review and critical appraisal. Journal of Controlled Release. 2010;141(3):277–299. doi: 10.1016/j.jconrel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 66.Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. Journal of Investigative Dermatology. 2004;123(1):168–176. doi: 10.1111/j.0022-202X.2004.22717.x. [DOI] [PubMed] [Google Scholar]
- 67.Christopher SJ, Campbell L, Contreras-Rojas LR, et al. Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. Journal of Controlled Release. 2012;162(1):201–207. doi: 10.1016/j.jconrel.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 68.Gulson B, Mccall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicological Sciences. 2010;118(1):140–149. doi: 10.1093/toxsci/kfq243. [DOI] [PubMed] [Google Scholar]
- 69.Gulson B, McCall M, Gomez L, Korsch M, et al. Dermal absorption of ZnO particles from sunscreens applied to humans at the beach. International Conference on Nanoscience and Nanotechnology; February 2010; Sydney, Australia. [Google Scholar]
- 70.Senzui M, Tamura T, Miura K, Ikarashi Y, Watanabe Y, Fujii M. Study on penetration of titanium dioxide (TiO2) nanoparticles into intact and damaged skin in vitro. Journal of Toxicological Sciences. 2010;35(1):107–113. doi: 10.2131/jts.35.107. [DOI] [PubMed] [Google Scholar]
- 71.Butz T. Dermal penetration of nanoparticles: what we know and what we don't. Cosmetic. Science Conference Proceedings, Munich. SÖFW Journal. 2009;135(4):8–10. [Google Scholar]
- 72.Filipe P, Silva JN, Silva R, et al. Stratum corneum is an effective barrier to TiO2 and ZnO nanoparticle percutaneous absorption. Skin Pharmacology and Physiology. 2009;22(5):266–275. doi: 10.1159/000235554. [DOI] [PubMed] [Google Scholar]
- 73.Mavon A, Miquel C, Lejeune O, Payre B, Moretto P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacology and Physiology. 2006;20(1):10–20. doi: 10.1159/000096167. [DOI] [PubMed] [Google Scholar]
- 74.Pflücker F, Wendel V, Hohenberg H, et al. The human stratum corneum layer: an effective barrier against dermal uptake of different forms of topically applied micronised titanium dioxide. Skin Pharmacology and Applied Skin Physiology. 2001;14(1):92–97. doi: 10.1159/000056396. [DOI] [PubMed] [Google Scholar]
- 75.Menzel F, Reinert T, Vogt J, Butz T. Investigations of percutaneous uptake of ultrafine TiO2 particles at the high energy ion nanoprobe LIPSION. Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms. 2004;219-220(1-4):82–86. [Google Scholar]
- 76.Lademann J, Weigmann H-J, Rickmeyer C, et al. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacology and Applied Skin Physiology. 1999;12(5):247–256. doi: 10.1159/000066249. [DOI] [PubMed] [Google Scholar]
- 77.Dussert AS, Gooris E. Characterisation of the mineral content of a physical sunscreen emulsion and its distribution onto human stratum corneum. International Journal of Cosmetic Science. 1997;19:119–129. doi: 10.1046/j.1467-2494.1997.171707.x. [DOI] [PubMed] [Google Scholar]
- 78.Takeda K, Suzuki K-I, Ishihara A, et al. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. Journal of Health Science. 2009;55(1):95–102. [Google Scholar]
- 79.Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Letters. 1997;418:87–90. doi: 10.1016/s0014-5793(97)01356-2. [DOI] [PubMed] [Google Scholar]
- 80.Arora S, Rajwade JM, Paknikar KM. Nanotoxicology and in vitro studies: the need of the hour. Toxicology and Applied Pharmacology. 2012;258(2):151–165. doi: 10.1016/j.taap.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 81.De Jong WH, Borm PJA. Drug delivery and nanoparticles: applications and hazards. International Journal of Nanomedicine. 2008;3(2):133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poon VKM, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns. 2004;30(2):140–147. doi: 10.1016/j.burns.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 83.U.S. Food and Drug Administration. Import Alert 66-38. http://www.accessdata.fda.gov/cms_ia/importalert_188.html.
- 84.Nanomaterials and the EU Cosmetics Regulation: Implications for Your Company. http://www.gcimagazine.com/business/management/regulation/143553126.html?pa.
- 85.New EU Cosmetics Regulations:A Quick Guide for Busy Formulators. http://chemistscorner.com/new-eu-cosmetics-regulations-a-quick-guide-for-busyformulators/
- 86.Stafford N. New nano rule for EU cosmetics. Royal society of Chemistry. http://www.rsc.org/chemistryworld/News/2009/November/27110901.asp.


