Abstract
Neuropathic pain remains an area of considerable unmet clinical need. Research based on preclinical animal models has failed to deliver truly novel treatment options, questioning the predictive value of these models. This review addresses the shortcomings of rodent in vivo models commonly used in the field and highlights approaches which could increase their predictivity, including more clinically relevant assays, outcome measures and animal characteristics. The methodological quality of animal studies also needs to be improved. Low internal validity and incomplete reporting lead to a waste of valuable research resources and animal lives, and ultimately prevent an objective assessment of the true predictivity of in vivo models.
Keywords: animal model, translation, neuropathic pain, analgesic, preclinical model
Introduction
The International Association for the Study of Pain defines pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (Merksey and Bogduk, 1994). Pain is by definition a subjective multifaceted symptom which can only be ‘measured’ by self-report. Consequently, researchers and clinicians have to rely on subjective reports of presence, nature, location and intensity of pain in humans (Vierck et al., 2008). Because of this, in animals, the presence or absence of pain cannot be directly measured and can only be inferred from the observation of surrogate behaviours. The present review concentrates on animal models used in analgesic development, we specifically focus on neuropathic pain but all the concepts discussed here are relevant to other type of pain. The prime focus of this review is on the use of animal models to predict clinical efficacy to the extent required for justification for initiation of a clinical development programme. However, it is important to note that use of animal models is also critical for processes involved earlier in the drug development pipeline, such as understanding of disease mechanisms and drug target identification. Neuropathic pain as defined by the International Association for the Study of Pain is ‘pain caused by a lesion or disease of the somatosensory system’ (Treede et al., 2008; Jensen et al., 2011). It can be associated with a plethora of diseases or lesions affecting the sensory nervous system and is associated with heterogeneous mechanisms and clinical presentations. It is also linked with a broad spectrum of other symptoms and clinical signs associated with both sensory loss and sensory gain (Baron et al., 2010; Maier et al., 2010).
Translation problem
Neuropathic pain has a prevalence of 7–8% in north-western European populations (Torrance et al., 2006; Bouhassira et al., 2008) and currently available treatment options for neuropathic pain are characterized by limited efficacy and a narrow therapeutic window (Dworkin et al., 2007; Finnerup et al., 2010). It is also difficult to predict treatment responses at the individual patient level (Dworkin et al., 2007; Finnerup et al., 2010). Pharmacotherapy only provides clinically meaningful pain relief in no more than 50% of patients suffering from neuropathic pain, with number needed to treat (NNT) across neuropathic pain conditions ranging from two to 12 depending on drug class (Finnerup et al., 2010). The neuropathic pain associated with certain conditions, for example human immunodeficiency virus (HIV)-associated sensory neuropathy, responds to very few interventions (Phillips et al., 2010). Additionally, pain relief is almost always partial rather than complete (around 30% greater than placebo) and associated with many side effects including cardiac toxicity, nausea, sedation and physical dependence (Borsook and Becerra, 2006; Dworkin et al., 2010a,2010b).
Even though the rate of Food and Drug Administration (FDA) approval of new molecular entities (NMEs) has been constant over the last 60 years (Munos, 2009), with an average of 23.5 NMEs approved during 2002–2011 (FDA, 2012), the number of NME applications has been declining steadily over the last 15 years, from 45 in 1996 to 23 in 2010 (FDA, 2011). This suggests that the number of drugs coming out of the Research and Development (R&D) pipeline is declining, despite an increase in pharmaceutical R&D expenditure over the same period (EFPIA, 2013). Additionally, an analysis of the success rate from first-in-man to registration during the period 1991–2000 across therapeutic areas shows that the level of attrition was very high, with a success rate of only 11%, meaning that only one in nine compounds were approved (Kola and Landis, 2004). There is nothing to suggest that attrition has improved over the last decade as a success rate of 6% was observed in 2009–2010 (Khanna, 2012). The breakdown analysis per therapeutic area provided by Kola and Landis (2004) showed that attrition was only slightly lower for arthritis and pain drugs, with a success rate of about 17% from first-in-man clinical trials to registration. In the past few years, several large- and medium-sized pharmaceutical companies have considerably downscaled their drug development activity in the pain field and little success has come out of the analgesic R&D pipeline in recent years. An analysis of analgesic drugs developed over the last 20 years shows that the majority of new drug launches result either from the design of novel formulations or dosing forms to improve the efficacy or safety of existing medicines, from compounds directed against known mechanisms, or the combination of existing drugs which improve efficacy or reduce side effects (Burgess and Williams, 2010). Out of the 59 drugs developed since 1960 and still in clinical use for the treatment of pain, only two-thirds were specifically developed as analgesics and the vast majority of these compounds developed as analgesics were directed against mechanisms already known to be involved in pain (Kissin, 2010). Most of the drugs associated with some degree of efficacy in the treatment of neuropathic pain, such as opioids, anticonvulsants or tricyclic antidepressants were originally introduced for other therapeutic indications, rather than being rationally developed using the classic animal-based drug development process. Pregabalin is sometimes painted as an exemplar translational success story for the use of animal models in the neuropathic pain field. However, when the evidence of clinical efficacy of pregabalin is objectively scrutinized, the conclusion is one of rather modest efficacy. We have examined this evidence objectively in a recent meta-analysis (Wiffen et al., 2013). Firstly, there is no top tier clinical trial evidence that pregabalin is efficacious for the treatment of neuropathic pain. For second tier evidence, pregabalin 600 mg does have a degree of efficacy in some selected neuropathic pain conditions {painful diabetic neuropathy [NNT for 50% pain relief = 6.3 (95% CI 4.6 to 10)], postherpetic neuralgia [5.6 (3.5 to 14)]} and central neuropathic pain [4.0 (3.1 to 5.5)]. However, reflecting on these data, even for these conditions only a minority of people treated achieve acceptably good pain relief (50%) with pregabalin. Furthermore, there are certain neuropathic pain conditions, such as HIV-related neuropathy where pregabalin has been shown to be ineffective (Phillips et al., 2010). Hardly a shining beacon of clinical effectiveness, although, of course, pregabalin is a major marketing success. Animal models were employed in the preclinical development of pregabalin, but an area of potential bias is that the findings from these preclinical studies only started to appear (Field et al., 1997) at about the same time as the pivotal clinical trials of gabapentin (Backonja et al., 1998; Rowbotham et al., 1998; Rice and Maton, 2001).
The paucity of new analgesic drugs contrasts with the amount of preclinical research reported. A PubMed search using the MeSH terms ‘analgesic’ and ‘rodent’ identified over 35 000 publications, with an average of 1350 publications per year over the last two decades (Figure 1). This raises questions about the utility and predictivity of animal models of pain. Although this review has its focus on the use of animal models in preclinical drug development, it should be noted in passing that there is also scope for methodological improvement in the clinical development phase. The difficulties of conducting early stage clinical proof of concept studies in neuropathic pain are pertinent; because nerve damage is a necessary prerequisite of neuropathic pain, the human surrogate models useful in inflammatory pain drug development are more of a challenge in neuropathic pain phase 1 proof of concept. Essentially, these studies have to be conducted in highly profiled patients. Furthermore, the design, conduct and analysis of phase 2 and 3 trials in neuropathic pain is not perfect and are being carefully scrutinized as an element of translational failure (see http://www.acttion.org and http://www.immpact.org; for examples see Dworkin et al., 2008; 2010b; 2013). Notwithstanding this, one could also reflect that in the unlikely event of a very highly effective neuropathic pain drug emerging from preclinical development, then perhaps such ‘fine tuning’ of clinical trials would become redundant. Returning to the use of animal models, Kontinen and Meert (2003) attempted to quantify the predictive validity of several rat models of neuropathic pain in a systematic review. The models reviewed included the chronic constriction injury, partial sciatic ligation, spinal nerve ligation and streptozocin-induced diabetes models in rats. Pharmacological data on behavioural outcomes in awake rats were compared with the clinical effectiveness of the drugs tested. Overall, it was found that the models were poorly predictive of clinical efficacy, with a specificity ranging from 0 to 60% depending on the model. The low specificity numbers mean that animal studies detected an effect of drugs that had no efficacy in human clinical trials. These so called false positives lead to an unacceptable waste of resources, as ineffective drugs progress into further animal studies (e.g. toxicology, safety pharmacology) and clinical trials instead of being stopped at the efficacy stage. The sensitivity numbers were higher (61–88%), indicating that drugs, which displayed clinical efficacy usually also had efficacy in these animal models. The authors, however, recognized that their analysis had several limitations. First, the evidence used to determine the clinical efficacy of the drugs examined and used in the sensitivity calculations was not collected systematically and included weak evidence and clinical impression as well as evidence coming from systematic reviews. Drugs were considered effective if an effect had been observed in some kind of neuropathic pain; more rigid criteria might have decreased the sensitivity estimate for some of the models. Another issue was that of publication bias – very few animal studies reporting on ineffective drugs were identified in the systematic searches (Kontinen and Meert, 2003). Despite the limitations, this study provides a reminder that most commonly used animal models of neuropathic pain are not ideal and there is a real scope for improvement in the way pain research is currently carried out. An in vivo model is characterized by the characteristics of the animals used, the intervention or the disease resulting in neuropathic pain and the outcome(s) measured. Consideration should be given to the subject, the species and strain, as well as age and sex in relation to the clinical condition being modelled. Housing conditions can also influence the development of the condition and/or the outcomes measured. The assay – that is what is done to the animal to produce the condition of interest – can be either induced (surgically or via the injection of a chemical, a virus or immune mediators) or use animals presenting a naturally occurring disease. Chronicity is also an important part of the assay and consideration should be given to the duration of the disease and the timing of treatment and/or measurement of signs of pain. Again, the choice should be based on the human condition modelled. In terms of outcome measures, different tests can be used to infer signs of spontaneous pain, hypersensitivity and co-morbidities, which should provide a comprehensive picture of the pathophysiological condition developed by the animals and a complete assessment of the effect of putative analgesics with a spectrum of measures comparable with human clinical trials. Barriers to efficient clinical translation could arise either from issues with the models themselves – the subjects, the assays or the measures, and/or the way experiments are designed and reported. The present review explores potential refinements in each of these areas, which could improve the predictive validity of animal models of neuropathic pain.
Figure 1.
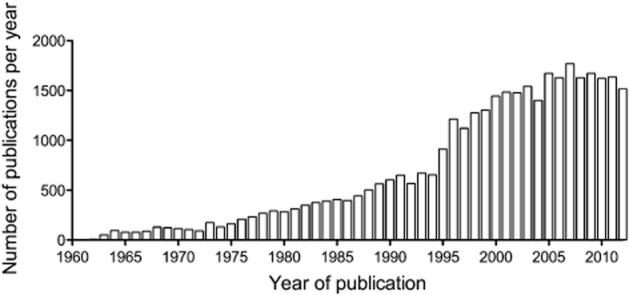
Number of articles published per year that reported experiments using rodents in the area of pain research. The graph was constructed based on a PubMed search using the MeSH terms: ‘analgesic’ and ‘rodent’.
Induction of neuropathic pain
In humans, neuropathic pain is a heterogeneous condition; patients present with various combinations of different signs and symptoms both within and between the different diseases and injuries associated with neuropathic pain (Baron et al., 2010; Maier et al., 2010). The incidence of sensory abnormalities (gain and loss) varies between, and within, pathophysiological conditions. For example, thermal hypersensitivity is observed in 39% of patients with peripheral nerve injury but only 8% of patients with polyneuropathy (Maier et al., 2010). In contrast, the industry standards for the preclinical assessment of novel analgesics targeted at neuropathic pain are peripheral nerve trauma models, which rely on injury to a single nerve, usually the sciatic (Berge, 2011). The predominance of traumatic nerve injury models does not match the clinical situation, as shown by an analysis of the randomized clinical trials included in a systematic review (Finnerup et al., 2005), which revealed that trials of patients with peripheral nerve injuries only represent 9% of the trials, while 53% and 21% of the trials were conducted in patients with peripheral polyneuropathies and post-herpetic neuralgia respectively (Rice et al., 2009).
It would thus seem logical to select preclinical models that reflect more closely the precise pathophysiological condition studied in humans, rather than generalizing models as ‘models of neuropathic pain’. A multitude of disease-specific models have been developed in recent years and, for example, include rodent models of peripheral nerve injury induced by anti-cancer (Authier et al., 2000; 2003a, b; 2009; Polomano et al., 2001; Flatters and Bennett, 2004; Ling et al., 2007; Zheng et al., 2011) or anti-retroviral therapy (Joseph et al., 2004; Wallace et al., 2007b; Huang et al., 2013), diabetic neuropathy (Malcangio and Tomlinson, 1998; D'Almeida et al., 1999), multiple sclerosis (Aicher et al., 2004; Lisi et al., 2012), lumbar radiculopathy (Olmarker et al., 2002), central post-stroke pain (Wasserman and Koeberle, 2009), varicella zoster infection (Fleetwood-Walker et al., 1999; Garry et al., 2005; Hasnie et al., 2007) or HIV-associated peripheral neuropathy (e.g. Wallace et al., 2007a; Cao et al., 2012). Although HIV neuropathy is difficult to replicate in rodents, as they are not a natural host for the virus, whereas the neuropathy can be induced in simian immunodeficiency virus infected non-human primates (Laast et al., 2011).
There are, however, issues with induced models in laboratory species in that the incidence of pain (at least pain erroneously inferred from changes in reflex hypersensitivity – see Outcome measures section) is much higher than in humans, most of whom will not develop neuropathic pain after an injury or disease of the somatosensory system. However, an induced model which would develop neuropathic pain with an incidence as low as seen in humans would waste resources and require an unethically high number of animals per experiment as animals not developing the disease would not be used. Additionally, the underlying pain processes in induced models are uncertain in relation to naturally occurring pain, which questions their relevance to the human condition. Naturally occurring disease models can be used in pain research; they have stronger face and construct validity than the induced models and can be used to investigate the effect of putative analgesic compounds and study the central and peripheral neurobiology of pain in a natural disease state. For example, the effect of putative analgesics can be investigated in canine osteoarthritis, where the disease process is similar to humans (Clements et al., 2006) or canine bone cancer (Brown et al., 2009). Complex behaviours can be used as an indication of spontaneous pain in companion cats and dogs, via the use of an accelerometer placed in the animal's collar to measure the impact of pain on spontaneous activity in the home environment or pressure sensitive walkways to record body weight distribution on each limb. Additionally, questionnaires filled by the dogs' owners enable a measure of global quality of life, including the affective component of pain and how the pain interferes with typical activities (Lascelles et al., 2010; Wernham et al., 2011). Neuropathic pain can be investigated using such an approach – for example, diseases such as feline diabetic neuropathy (Mizisin et al., 2002) might represent a more relevant model to study human diabetes than the streptozotocin rodent models, in which the resemblance to the human disease has been questioned (Tesch and Allen, 2007). Companion animals with spontaneous cancer could also be used in studies investigating cancer-related neuropathic pain or neuropathy induced by chemotherapy. Naturally occurring disease models do not circumvent the issue around outcome measures; there are no objective measures of pain. However, a salient feature is the comparability to human studies. Owners of companion animals are very familiar with their normal behaviour and approaches, so parental or caregiver questionnaires are used in the clinical assessment of non-verbal humans (e.g. children). There are disadvantages of using companion animals, which are mainly due to practicality and availability. Pet owners might not be willing to enrol their pets in a drug trial and with veterinary practices being broadly disseminated, it might be difficult to recruit these animals. These hurdles can, however, be overcome, as exemplified by the College of Veterinary Medicine at North Carolina State University which regularly advertises research programmes to recruit veterinary patients (http://www.cvm.ncsu.edu/docs/research.html). It would be unrealistic to expect naturally occurring disease studies to replace all rodent models but used in conjunction with some carefully chosen induced models in rodents in which the mode of induction and outcome measures are relevant to the clinical condition investigated, studies using companion animals could reduce the need for rodent studies and possibly help with the validation of some of the induced rodent models. Such studies can also be used to obtain more information and evaluate the efficacy of a putative analgesic before moving on to human clinical trials.
Reliance on existing analgesic drugs to investigate mechanisms and validate models can also be an issue. Animal models are often developed on the basis of mimicking clinical scenario and then optimized using known compounds which are sometimes incorrectly purported as ‘gold standards’, for example for a neuropathic pain model, gabapentin is often used as a comparative assay of analgesia. However, validating models with such compounds introduces a bias as known analgesic mechanisms will be preferred over novel mechanisms, which are not involved in the mode of action of gabapentin (Berge, 2011). Furthermore, gabapentin for that matter and other agents used clinically, such as tricyclic antidepressants or pregabalin, have limited effectiveness in the clinic – a recent meta-analysis estimated that the NNT with gabapentin or pregabalin for one patient to obtain 50% pain relief was over five across neuropathic pain conditions, and over 10 in patients with peripheral nerve injury (Finnerup et al., 2010). Still, these drugs are used to unravel the mechanisms involved in rodent models of peripheral nerve injury (Andrews et al., 2012; Morimoto et al., 2012; Yoshizumi et al., 2012) and such mechanisms might therefore not be clinically relevant. Another issue stemming from the lack of efficacious analgesics for neuropathic pain is the use of positive controls. Ideally, similar to the practice in clinical trials, when assessing the in vivo efficacy of a novel putative analgesic, a positive control group would be used. This is, however, difficult in neuropathic pain as no drug is unequivocally clinically effective enough to be used as such.
Outcome measures
Regardless of the species, there are no direct measures of pain. In humans, pain can be reported by the individual, but in animals, researchers rely on observing responses to presumed sources of pain. Historically, much emphasis has been placed on reflex withdrawal responses to sensory stimuli rather than measuring more complex, ethologically relevant behaviours from which the presence of spontaneous pain may perhaps be inferred. A review examining animal studies published in the journal Pain between 2000 and 2004 identified that the outcome most commonly assessed in preclinical studies is reflex hypersensitivity, with 90% of the papers exclusively reporting either thermal (48% of studies) or mechanical (42% of studies) hypersensitivity (Mogil and Crager, 2004). This finding is problematic for several reasons. First, there is a mismatch between animal studies and human trials. The predominant clinical feature of most neuropathic pain conditions is spontaneous (either continuous and/or paroxysmal) – as opposed to evoked – pain. While spontaneous pain is a symptom present in most neuropathic pain patients, sensory gain (as reflected in limb withdrawal measures) is much less frequent; mechanical hypersensitivity is seen in 57% of patients and thermal hypersensitivity in only 33% (Maier et al., 2010). Indeed, if paradoxical heat sensations were not included as a measure of sensory gain in such analyses, the prevalence of sensory gain would be much lower. Furthermore, while in humans these sensory gain features have some utility for sensory profiling, in animals they are incorrectly used as an efficacy parameter. In addition, there is limited correlation between thermal (hot and cold) and mechanical hypersensitivity and pain severity measured by questionnaires (Backonja and Stacey, 2004).
The majority of clinical trials include patients based on the level of pain intensity, rather than their sensory profile – although there is an increasing move to use sensory and symptom profiling as a stratification measure to predict analgesic responsiveness at the individual patient level (Attal et al., 2011; Freeman et al., 2014). In human trials, the primary efficacy measure is usually continuous spontaneous pain (see Table 1), which makes it difficult to draw any meaningful comparison with animal studies in which signs reflecting aspects of sensory gain are usually measured. It is likely that the difference in outcome measures partly contributes to the discrepancy between the findings of animal experiments and human clinical trials, as potential analgesics showing efficacy against hypersensitivity in animal models would have to be active under different neuropathological conditions to reduce spontaneous pain in patients (Lascelles and Flecknell, 2010).
Table 1.
Comparison of the generic outcome domains generally measured in animal studies of neuropathic pain and corresponding clinical trials (reproduced with permission from Rice, 2010)
| Outcome domain | Animal efficacy studies | Human randomized controlled trials |
|---|---|---|
| Evoked hypersensitivity | + | +/− (sensory profiling) |
| Spontaneous continuous pain | − | +a |
| Spontaneous paroxysmal pain | − | +/− |
| Co-morbidity | − | + |
| Physical function | − | + |
| Emotional function | − | + |
| Circadian rhythm disturbance | − | + |
| Adverse events | − | + |
| Global impression | − | + |
The usual primary efficacy measure.
Second, using reflex tests in animals presents an additional issue in that the mechanisms involved in the generation of reflexes do not include the cerebral cortex. Two conditions must be fulfilled to evaluate pain sensitivity: measures of pain should demonstrate transmission over nociceptive pathways to the cerebral cortex and require processing of sensory intensity in comparison with previous experiences (Vierck et al., 2008). Evidence from human fMRI studies shows that cortical regions are involved in both acute and chronic (including neuropathic) pain (Borsook and Becerra, 2006). Reflex tests such as paw withdrawal or tail flick may not involve cortical structures, but result in the activation of spinal and bulbospinal pathways (Lascelles and Flecknell, 2010). Behaviours, such as grooming of the injured paw or paw shaking following formalin injection and tail-flick upon thermal stimulation, have been observed in decerebrate animals (Matthies and Franklin, 1992). It is, however, possible to measure hypersensitivity using an operant testing paradigm rather than reflex measures. Operant responses, such as conditioned place preference, rely upon cerebral processing (Vierck et al., 2005) and yield findings that are more similar to the clinical impression than findings obtained using reflex testing (Vierck et al., 2008).
In recent years, novel measures have been investigated to infer signs of spontaneous pain in animals. Mogil et al. developed a method to assess pain using facial expression analysis. The mouse grimace scale (MGS) was adapted from a scale which is used in non-verbal human populations and consists in five facial features including three that are identical to those observed in humans – orbital tightening, nose bulge and cheek bulge – in addition to ear position and whisker change (Langford et al., 2010). The technique was useful to detect changes in a range of acute and inflammatory pain models, such as tail withdrawal from hot water or intraplantar capsaicin, but failed to detect changes in peripheral nerve injury models (chronic constriction injury and spared nerve injury). The analgesic effects of morphine and several NSAID analgesics could also be detected using the MGS as they reduced pain scores in mice challenged with cyclophosphamide-induced bladder cystitis (Langford et al., 2010) and in a postoperative pain assay (Matsumiya et al., 2012). Further work from the same laboratory showed that the MGS could be translated to the rat and enabled changes to be detected in inflammatory assays and following a laparotomy. These changes were also reversed by morphine (Sotocinal et al., 2011). Other groups have also looked at facial expression to assess pain in other species, for example rabbits (Keating et al., 2012).
Other analgesiometric tests have been developed based on the principle of looking at behaviours suppressed by pain – and therefore re-instated by analgesic drugs (Andrews et al., 2011). Andrews et al. used spontaneous burrowing as a behavioural endpoint, which is ethologically relevant to rodents. Burrowing activity (Deacon, 2006, 2009) in rats, measured as the amount of gravel displaced from a cylinder, was significantly reduced by three different techniques of peripheral nerve injury (tibial and L5 spinal nerve transections, and sciatic nerve ligation) and in an inflammatory model (intraplantar injection of complete Freund's adjuvant). This technique also detected the effect of analgesic drugs, and gabapentin and ibuprofen were shown to reverse the decrease in burrowing activity induced by peripheral nerve injury (up to 56 days post-injury) and complete Freund's adjuvant injection, respectively, at doses lower than those generally found effective in reflex response assays (Andrews et al., 2012). Burrowing is also perturbed in a clinically relevant model of antiretroviral-induced sensory neuropathy, which is not complicated by the potential confound of motor deficits (Huang et al., 2013). Burrowing has also been used as an indicator of postoperative pain in mice, measured as the amount of food pellets displaced; the decrease in burrowing activity following a laparotomy was reversed by the NSAID carprofen (Jirkof et al., 2010). In this study, anaesthesia on its own reduced the percentage of animals displaying the burrowing behaviour for up to 12 h, demonstrating that a reduction in burrowing activity is not specific to pain. However, in contrast to the burrowing decrease induced by surgery, this was not reversed by carprofen (Jirkof et al., 2010), indicating that pain is an important factor in burrowing reduction. The efficacy of analgesic drugs in this context does not, however, allow the distinction between whether this behaviour is an indicator of pain itself or the results of its co-morbidities, such as reduced well-being (Andrews et al., 2012). The utility of burrowing as an assay across time has been demonstrated in the detection of the behavioural effects of hippocampal scrapie infection in mice. Repeated measurement of this behaviour across time started to detect abnormalities at about 14 weeks following infection, corresponding with the clinical course of the disease (Deacon et al., 2005).
Other measures of spontaneous, voluntary activity, which have been investigated, include wheel running (Cobos et al., 2012) and rearing (Matson et al., 2007) in inflammatory models in mice and rats respectively. Some research groups have also used behavioural scores, recording behaviours such as arching of the back, pressing abdomen towards the floor or wobbling (Leach et al., 2012), but these measures have predominantly been investigated in models of postoperative pain rather than neuropathic pain. Behaviours such as hypomotility, licking, lifting or shaking of the affected paw and gait changes have also been used to assess spontaneous pain in rat and mice models of nerve injury and diabetic neuropathy (e.g. D'Almeida et al., 1999; Kontinen et al., 1999; Benbouzid et al., 2008). However, a recent, comprehensive study looking at over 20 strains of mice with two types of nerve injuries concluded that such behaviours could not reliably be used to measure spontaneous neuropathic pain (Mogil et al., 2010b).
The activity of C-nociceptive fibres can also be recorded as an indication of spontaneous pain. Microneurography provides an objective measure with high translational value, as the technique can be used in both in peripheral neuropathic pain patients and rodent models of neuropathic pain models such as peripheral nerve injury and diabetic neuropathy (Serra, 2012). It represents a direct approach to assess the efficacy of putative treatments.
Neuropathic pain also has a wide impact on quality of life, mental health and ability to function and is associated with a number of co-morbidities in humans, such as sleep disturbance, social withdrawal, cognitive dysfunction, anxiety or depression (Mogil et al., 2010a; Rice, 2010). In clinical trials, a broad range of measures and signs are usually measured as secondary outcomes and the IMMPACT group has provided recommendation on the domains that should be assessed (Dworkin et al., 2008). Co-morbidities are used to describe the multifactorial nature of pain and measuring such behaviours in animal models would provide a complete picture of the animal's experience of chronic pain (Blackburn-Munro, 2004). Many studies have attempted to measure a range of complex pain-related behaviours in animal models of neuropathic pain, including increased thigmotaxis (predator avoidance) in an open arena (e.g. Hasnie et al., 2007; Wallace et al., 2007a,b,c; 2008; Hu et al., 2009; Huang et al., 2013), and elevated plus-maze behaviours (Roeska et al., 2009a,b), sleep pattern disturbances (e.g. Andersen and Tufik, 2003) and various approaches to place preference (e.g. King et al., 2009). Studying in animal experiments the signs which are affected by pain and contribute to a reduced quality of life is important to understand the precise action of putative analgesic drugs and improve the relevance of these studies to humans. This requires preclinical researchers to have a detailed understanding of the ethology of rodents and how such behaviours are influenced by pain, especially as a prey species (Barnett et al., 2006; Barnett, 2009). What must be avoided is the temptation to anthropomorphize human emotions and behaviours to the rodent's world (Vasconcelos et al., 2012).
However, measuring complex behaviours is challenging and the stability of such techniques easily perturbed by subtle changes related to environmental events and minor protocol variations. For example, predator avoidance behaviours in rats – and consequently, effect of drugs – are modulated by illumination levels and height of the platform in the elevated plus-maze test (Roeska et al., 2009b). This variability can make consistent replication of these paradigms between laboratories and across time difficult. This is a particular challenge for preclinical drug development and a better understanding of these factors, and how to control them, is urgently required. Furthermore, there is considerable inter-animal variation in such behaviours, which can make group level effects difficult to interpret. More attention should be paid to within-subject changes in behaviours rather than group level averages – a straight parallel with the manner in which clinical trial analysis is moving away from group level effects to place more emphasis on the importance of individual patient responses (Moore et al., 2013).
Animal characteristics
In the vast majority of preclinical pain experiments, the animals used are previously healthy, young, male and genetically similar rodents, which contrasts with the clinical populations who develop chronic neuropathic pain (Rice, 2010). In addition, neuropathic pain often develops in association with a disease, such as HIV, diabetes or cancer, rather than in healthy individuals. The profile of human patients affected by neuropathic pain also varies based on the aetiology, for example, in a recent study including over 1200 patients, women represented 64% of postherpetic neuralgia patients and 58% of these patients were over 69 years old; whereas peripheral nerve injury patients were predominantly males (55%) and younger, with 58% under 50 years old (Maier et al., 2010). The human population is also heterogeneous in terms of pain tolerance based on gender (Mogil, 2012) and ethnicity (Alabas et al., 2013). Additionally, responses to analgesic drugs differ between sexes, for example morphine is more potent in women but has a slower onset of action (Niesters et al., 2010). Interestingly, most rodent studies show greater opioid analgesia in males compared with females (see Dahan et al., 2008 for review). Further heterogeneity has been observed in laboratory animals, and strain (Mogil et al., 2005) and age (Pickering et al., 2006) have been shown to impact on the development of signs of pain. This highlights the importance of considering carefully the characteristics of animals used in preclinical studies, as conclusions will be dependent on these variables.
It is also reasonable to question the choice of species in experimental pain studies. Since the 1980s an overwhelming majority of pain experiments have used rats, with the use of mice steadily increasing (with the advent of transgenic technology) and almost matching rat usage over the last 15 years (Mogil, 2009). The use of prey species to investigate pain behaviours is arguable; displaying overt pain behaviour would not represent a survival advantage for rodents as it would make them more vulnerable to predators. It is conceivable that rodents may not display any overt signs of chronic spontaneous pain or at least interpretable behaviours would be geared to increases in predator-avoidance behaviours rather than displays of pain behaviours. Furthermore, rodents cannot model the complex neuroanatomy of the human brain, nor complex human emotions and behaviours associated with pain, and pathways known to be involved in the human experience and emotionality of pain are not present in rodent brains (Craig, 2009), this limits the utility of rodent models to study central pathways and stresses the importance of exercising caution when predicting human outcomes based on rodent data.
Alternatively, some experiments might not necessitate the use of mammalian organisms and could be carried out in lower order vertebrates such as zebrafish, which are economically advantageous and low maintenance. In teleost fish, both mechanothermal and mechanochemical nociceptors are physiologically similar to mammalian receptors and the pathways to the CNS are conserved (Sneddon, 2004). Fish can be used to study the basic fundamental mechanism of nociception or, in appropriate conditions, for the screening of analgesic drugs. Zebrafish are easily amenable to the production of transgenic lines because of their high breeding rate (100 offspring every 3 months), external fertilization and the feasibility of high-throughput phenotyping (Gonzalez-Nunez and Rodriguez, 2009). Additionally, the transparency of the embryo and the developing larvae facilitate injections to the nervous system and reporter gene expression can be followed in vivo (Sneddon, 2004). Behavioural studies can also be carried out to look at signs of spontaneous pain (Sneddon, 2009). Models of acute nociception and pain sensitization have also been developed in Drosophila. This organism can play an important role in deciphering the genetic and molecular mechanisms of pain (see Milinkeviciute et al., 2012 for review). Heat and mechanical nociception assays have been developed in Drosophila and can be used for high-throughput genetic and pharmacological screening in vivo. For example, the entire fly genome has been screened for heat nociception, resulting in a library of ‘pain genes’, two-thirds of which (∼400 genes) conserved through to humans, including many that had never been linked to pain before (Neely et al., 2010). A model of nociceptive sensitization has also been described in Drosophila, where UV radiation results in thermal hypersensitivity. This model can be used to study signalling mechanisms and uncover novel potential therapeutic targets relevant to neuropathic pain. For instance, the importance of the Hedgehog signalling pathway in hypersensitivity identified in Drosophila was confirmed in rat models of inflammatory and neuropathic pain (Babcock et al., 2011).
Chronicity
In a recent trial of topical clonidine for the treatment of painful diabetic neuropathy, the average duration of diabetes was 10 years and the duration of pain, about 3 years (Campbell et al., 2012). In contrast, in animal studies, the effect of treatments is only tested a few weeks after diabetes induction (e.g. Schreiber et al., 2012; Sun et al., 2012). The same discrepancy is observed for pain of other aetiologies, for example post-herpetic neuralgia. While patients enter trials several years post-diagnosis (Rice and Maton, 2001), behavioural changes in rodent models of zoster-associated pain are only detected up to 10 weeks post-infection (Garry et al., 2005; Hasnie et al., 2007) and the effects of pharmacological treatments were investigated approximately 3 weeks post-infection (Hasnie et al., 2007). Thus, even considering the lifespan of laboratory rodents, which differ by a factor of about 35 (Rice et al., 2009), the duration of the disease tends to be shorter in animals compared with human patients. In preclinical models, signs of pain are measured during the initiation, or acute injury phase of the disease when inflammation may be a confounding factor and the neurobiology of pain – including mechanisms, co-morbidities and functional expression of drug targets – might differ from patients which would have had the painful condition for many years (Lascelles and Flecknell, 2010). Discrepancies in the duration of the measurement period post-treatment might also yield misleading results. For example, carbamazepine was found to be effective in a rat model of nerve injury 1 week post-surgery (Hahm et al., 2012), but in humans, it was shown to delay the onset of neuropathic pain following spinal cord injury, resulting in a lower incidence of neuropathic pain compared with the placebo-control group at the 1-month follow-up, but no difference were detected between the two groups after three months (Salinas et al., 2012). Preclinical and clinical studies also differ in terms of duration of treatment exposure. In preclinical models, drugs are usually given at high dose as a single administration, whereas in clinical settings they are titrated over several days for tolerability reasons (Berge, 2011). For example, in a clinical trial of patients suffering from post-herpetic neuralgia, gabapentin doses were increased over 2 weeks before reaching the test doses (Rice and Maton, 2001). This can lead to differences in plasma concentration and drug exposure. Whiteside et al. (2008) investigated five FDA-approved drugs for the treatment of neuropathic pain and compared drug exposure in humans with exposure in the rat spinal nerve ligation model. The minimal efficacious exposure in the rat model was between 1.2-fold (gabapentin) and 15-fold (duloxetine) greater than that measured in humans. These differences might reflect differences in drug distribution, especially across the blood–brain barrier, and differences in the antinociception mechanisms involved (Berge, 2011).
Design and reporting
The methodological quality and transparent reporting of animal studies is also a factor to consider when assessing the translational value of animal models (Rice et al., 2013). There is considerable scope for improving the standards to which animal research is conducted, especially with regard to means of addressing internal validity and experimental biases such as concealed allocation, randomization, observer blinding, reporting of withdrawals, declaration of conflicts of interest and details of sample size calculation (Kilkenny et al., 2009; Macleod et al., 2009). The field of pain research is not exempt from these issues (Rice et al., 2013). In a review of animal studies published in the journal Pain over a 6-month period in 2007, less than a third of studies were described as blinded or randomized (Rice et al., 2009) and only 15% reported both (Quessy, 2009). None of the 14 papers identified reported a power calculation to justify group sizes (Rice et al., 2009). In a more recent review looking at animal models of bone cancer pain, only 11% of the 150 publications identified reported random allocation to groups, a third mentioned blinded assessment of outcome and none reported sample size calculations (Currie et al., 2013).
Many studies have demonstrated the impact of bias on findings. Animal studies that do not report randomization and blinding are more likely to obtain a treatment effect (Bebarta et al., 2003). Systematic reviews in various domains of neuroscience, such as stroke, multiple sclerosis or Parkinson's disease, have demonstrated that studies which fail to report random allocation to experimental groups, blinded conduct of experiment or blinded assessment of outcome overestimate treatment efficacy and report an exaggerated effect size (Macleod et al., 2008; Vesterinen et al., 2010; Rooke et al., 2011). Failing to deal with subjective bias compromises the internal validity of an experiment and findings obtained are not robust and reproducible. Such studies are therefore wasting animals and other valuable resources. One could argue that these findings reflect incomplete reporting rather that poor methodological quality. However, if subjective bias was addressed in studies which failed to report it, we would not expect estimates of efficacy to be affected. Eisenach and Lindner (2004) also describe how findings in the rat spinal nerve ligation model of neuropathic pain have been influenced by the perceived clinical situation, with regard to the effects of intrathecal opioids and the role of the sympathetic system. The fact that rigorously blinded experiments were not able to replicate initial non-blinded studies indicates that the result of neuropathic pain studies can be affected by the expectations of the experimenter. In addition, studies are often not adequately powered, sample size calculation is virtually never reported in animal studies, including in the pain field (Rice et al., 2009; 2013; Currie et al., 2013). In the stroke field, it was estimated that preclinical studies only have a one in three chance of detecting a 20% difference in outcome (Macleod et al., 2009), which implies that two studies out of three are a waste of time, resources and animals. Although the face validity of animal models used in neuropathic pain research has improved over the recent years (see Induction section), the face validity of outcome measures remains a major hurdle (see Outcome measures section), in contrast with the stroke field in which a few objective outcome measures can be used and compared across species (e.g. infarct size). We would therefore argue that this estimation is conservative and is likely to be higher in neuropathic pain research.
Systematic review and meta-analysis are the method of choice to combine the results of multiple high-quality randomized controlled trials and to estimate the overall effectiveness of clinical interventions. The advantages of such an approach to ascertain efficiency of current models in predicting the clinical efficacy of putative analgesic drugs are obvious. In their review, Kontinen and Meert (2003) did not manage to assess the specificity of the models they investigated reliably, partly because the review only identified a limited number of studies reporting that the drugs tested were ineffective. This phenomenon is known as publication bias, when studies are less likely to be published if the data generated do not support rejection of the null hypothesis (i.e. for intervention studies, no difference between the treatment groups), or when an analysis or an outcome is selectively reported based on the direction of the effect. It has been widely recognized as a problem in clinical research, as it leads to incorrectly high expectations of efficacy and there have been calls to address this issue in the clinical pain field (Rowbotham, 2009). In animal research, a study investigating publication bias in the field of stroke estimated that in addition to the 1359 experiments identified, a further 214 experiments (16%) yielding negative results had been carried out but not reported; publication bias accounted for around one-third of the efficacy reported in systematic reviews (Sena et al., 2010). Additionally, an analysis of animal studies of neurological diseases, including over 4000 datasets, showed that there are too many animal studies with statistically significant results, which suggests strong selective analysis and outcome reporting biases (Tsilidis et al., 2013). There is no reason to believe preclinical pain studies are different in that respect and the true efficacy of putative analgesics in animal models cannot be reliably assessed if only some of the data generated by research teams around the world are made available.
There are also issues with the information included in studies that are published. Many have raised concerns about the underreporting of crucial aspects of the design and conduct of animal studies in general (Kilkenny et al., 2009; Muhlhausler et al., 2013) and in the field of pain specifically (Rice et al., 2009; 2013; Mogil et al., 2010a). The ARRIVE guidelines (Kilkenny et al., 2010a,b) were developed to address this issue and ensure that manuscripts contain the minimum information necessary to reproduce the experiments described or to assess their methodological quality, an essential step in meta-analysis. They have now been adopted by over 300 journals and major funding bodies, universities and learned societies (http://www.nc3rs.org.uk/ARRIVE). Implementation of the ARRIVE guidelines is essential to increase methodological transparency, which will in turn facilitate systematic reviews and meta-analyses of the animal literature and ensure that high-quality studies are recognized as robust, regardless of the direction of the outcome. Additionally, animal pain studies have been shown to be influenced by a plethora of experimental conditions, ranging from the animals' characteristics (Mogil, 2009) and housing conditions (Langford et al., 2006) to room temperature (Hole and Tjolsen, 1993) and bedding texture (Robinson et al., 2004). This information needs to be included in publications to inform interpretation of the findings and ensure that they can be replicated.
Research priorities
Neuropathic pain and pain in general are complex, multifactorial conditions which remain poorly understood and an unmet clinical need. In the last few decades, research has predominantly relied on in vivo animal models and will realistically continue to do so in the foreseeable future, but so far, despite a crucial need for more effective and safer analgesic drugs, little has translated to the clinic. In this review, we have discussed many ways by which the translational value of in vivo models could be improved. Tailoring models to the human condition investigated is essential. Recent efforts have focused on developing more clinically and ethologically relevant assays and outcome measures. Careful consideration should be given to the characteristics of the animal used and the design of the study to ensure that it matches the clinical situation. This will improve face and construct validity, but predictive validity should be assessed carefully. However, the environmental factors which govern variability in such measures, especially those that account for inter-laboratory variability, and how best to control these require elucidation. A systematic review is underway to assess objectively animal models of neuropathic pain and tease out the variables that improve their predictive value and reduce the burden on experimental animals (http://www.nc3rs.org.uk/researchportfolio-Sena). Issues related to subjective bias and selective or incomplete reporting also need to be addressed urgently
Acknowledgments
The authors thank Vicky Robinson and Anthony Holmes (NC3Rs) for help and useful comments on the manuscript.
Glossary
- MGS
mouse grimace scale
- NME
new molecular entity
- NNT
number needed to treat
Conflict of interest
NPdS is employed by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs). The authors state no other conflicts of interest.
References
- Aicher SA, Silverman MB, Winkler CW, Bebo BF., Jr Hyperalgesia in an animal model of multiple sclerosis. Pain. 2004;110:560–570. doi: 10.1016/j.pain.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Alabas OA, Tashani OA, Johnson MI. Effects of ethnicity and gender role expectations of pain on experimental pain: a cross-cultural study. Eur J Pain. 2013;17:776–786. doi: 10.1002/j.1532-2149.2012.00229.x. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Tufik S. Sleep patterns over 21-day period in rats with chronic constriction of sciatic nerve. Brain Res. 2003;984:84–92. doi: 10.1016/s0006-8993(03)03095-6. [DOI] [PubMed] [Google Scholar]
- Andrews N, Harper S, Issop Y, Rice AS. Novel, nonreflex tests detect analgesic action in rodents at clinically relevant concentrations. Ann N Y Acad Sci. 2011;1245:11–13. doi: 10.1111/j.1749-6632.2011.06342.x. [DOI] [PubMed] [Google Scholar]
- Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, et al. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain. 2012;16:485–495. doi: 10.1016/j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Attal N, Bouhassira D, Baron R, Dostrovsky J, Dworkin RH, Finnerup N, et al. Assessing symptom profiles in neuropathic pain clinical trials: can it improve outcome? Eur J Pain. 2011;15:441–443. doi: 10.1016/j.ejpain.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res. 2000;887:239–249. doi: 10.1016/s0006-8993(00)02910-3. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. An animal model of nociceptive peripheral neuropathy following repeated cisplatin injections. Exp Neurol. 2003a;182:12–20. doi: 10.1016/s0014-4886(03)00003-7. [DOI] [PubMed] [Google Scholar]
- Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. A new animal model of vincristine-induced nociceptive peripheral neuropathy. Neurotoxicology. 2003b;24:797–805. doi: 10.1016/S0161-813X(03)00043-3. [DOI] [PubMed] [Google Scholar]
- Authier N, Balayssac D, Marchand F, Ling B, Zangarelli A, Descoeur J, et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics. 2009;6:620–629. doi: 10.1016/j.nurt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, Galko MJ. Hedgehog signaling regulates nociceptive sensitization. Curr Biol. 2011;21:1525–1533. doi: 10.1016/j.cub.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Barnett SA. The Rat: A Study in Behaviour. London: Aldine Transaction; 2009. [Google Scholar]
- Barnett SA, Whishaw IQ, Kolb B. The Behaviour of the Laboratory Rat. 1. Oxford: Oxford University Press; 2006. [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Bebarta V, Luyten D, Heard K. Emergency medicine animal research: does use of randomization and blinding affect the results? Acad Emerg Med. 2003;10:684–687. doi: 10.1111/j.1553-2712.2003.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Benbouzid M, Pallage V, Rajalu M, Waltisperger E, Doridot S, Poisbeau P, et al. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain. 2008;12:591–599. doi: 10.1016/j.ejpain.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol. 2011;164:1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn-Munro G. Pain-like behaviours in animals – how human are they? Trends Pharmacol Sci. 2004;25:299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Borsook D, Becerra LR. Breaking down the barriers: fMRI applications in pain, analgesia and analgesics. Mol Pain. 2006;2:30. doi: 10.1186/1744-8069-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Brown DC, Boston R, Coyne JC, Farrar JT. A novel approach to the use of animals in studies of pain: validation of the canine brief pain inventory in canine bone cancer. Pain Med. 2009;10:133–142. doi: 10.1111/j.1526-4637.2008.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G, Williams D. The discovery and development of analgesics: new mechanisms, new modalities. J Clin Invest. 2010;120:3753–3759. doi: 10.1172/JCI43195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, Kipnes MS, Stouch BC, Brady KL, Kelly M, Schmidt WK, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815–1823. doi: 10.1016/j.pain.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Butler MB, Tan L, Draleau KS, Koh WY. Murine immunodeficiency virus-induced peripheral neuropathy and the associated cytokine responses. J Immunol. 2012;189:3724–3733. doi: 10.4049/jimmunol.1201313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements DN, Carter SD, Innes JF, Ollier WE, Day PJ. Analysis of normal and osteoarthritic canine cartilage mRNA expression by quantitative polymerase chain reaction. Arthritis Res Ther. 2006;8:R158. doi: 10.1186/ar2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. A rat is not a monkey is not a human: comment on Mogil (Nature Rev. Neurosci. 10, 283–294 (2009)) Nat Rev Neurosci. 2009;10:466. doi: 10.1038/nrn2606-c1. [DOI] [PubMed] [Google Scholar]
- Currie GL, Delaney A, Bennett M, Dickenson AH, Egan K, Vesterinen HM, et al. Animal models of bone cancer pain: systematic review and meta-analyses. Pain. 2013;154:917–926. doi: 10.1016/j.pain.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Dahan A, Kest B, Waxman AR, Sarton E. Sex-specific responses to opiates: animal and human studies. Anesth Analg. 2008;107:83–95. doi: 10.1213/ane.0b013e31816a66a4. [DOI] [PubMed] [Google Scholar]
- D'Almeida JA, de Castro-Costa CM, Frota CH, Severo JF, Rocha TD, Nogueira TF. Behavioral changes of Wistar rats with experimentally-induced painful diabetic neuropathy. Arq Neuropsiquiatr. 1999;57(3B):746–752. doi: 10.1590/s0004-282x1999000500004. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protoc. 2006;1:118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Burrowing: a sensitive behavioural assay, tested in five species of laboratory rodents. Behav Brain Res. 2009;200:128–133. doi: 10.1016/j.bbr.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Reisel D, Perry VH, Nicholas J, Rawlins P. Hippocampal scrapie infection impairs operant DRL performance in mice. Behav Brain Res. 2005;157:99–105. doi: 10.1016/j.bbr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpaa ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010a;85(3 Suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Peirce-Sandner S, McDermott MP, Farrar JT, Hertz S, et al. Placebo and treatment group responses in postherpetic neuralgia versus painful diabetic peripheral neuropathy clinical trials in the REPORT database. Pain. 2010b;150:12–16. doi: 10.1016/j.pain.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Peirce-Sandner S, He H, McDermott MP, Farrar JT, et al. Assay sensitivity and study features in neuropathic pain trials: an ACTTION meta-analysis. Neurology. 2013;81:67–75. doi: 10.1212/WNL.0b013e318297ee69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFPIA. 2013. The pharmaceutical industry in figures. Available at: http://www.efpia.eu/uploads/Figures_Key_Data_2013.pdf (accessed 1/10/2013)
- Eisenach JC, Lindner MD. Did experimenter bias conceal the efficacy of spinal opioids in previous studies with the spinal nerve ligation model of neuropathic pain? Anesthesiology. 2004;100:765–767. doi: 10.1097/00000542-200404000-00003. [DOI] [PubMed] [Google Scholar]
- FDA. 2011. Is it true FDA is approving fewer new drugs lately? Available at: http://www.fda.gov/downloads/AboutFDA/Transparency/Basics/UCM247465.pdf (accessed 21/1/2013)
- FDA. 2012. Novel new drugs 2011. Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm293663.pdf (accessed 21/1/2013)
- Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305. doi: 10.1016/j.pain.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Fleetwood-Walker SM, Quinn JP, Wallace C, Blackburn-Munro G, Kelly BG, Fiskerstrand CE, et al. Behavioural changes in the rat following infection with varicella-zoster virus. J Gen Virol. 1999;80((Pt 9)):2433–2436. doi: 10.1099/0022-1317-80-9-2433. [DOI] [PubMed] [Google Scholar]
- Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–376. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Garry EM, Delaney A, Anderson HA, Sirinathsinghji EC, Clapp RH, Martin WJ, et al. Varicella zoster virus induces neuropathic changes in rat dorsal root ganglia and behavioral reflex sensitisation that is attenuated by gabapentin or sodium channel blocking drugs. Pain. 2005;118:97–111. doi: 10.1016/j.pain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nunez V, Rodriguez RE. The zebrafish: a model to study the endogenous mechanisms of pain. ILAR J. 2009;50:373–386. doi: 10.1093/ilar.50.4.373. [DOI] [PubMed] [Google Scholar]
- Hahm TS, Ahn HJ, Ryu S, Gwak MS, Choi SJ, Kim JK, et al. Combined carbamazepine and pregabalin therapy in a rat model of neuropathic pain. Br J Anaesth. 2012;109:968–974. doi: 10.1093/bja/aes306. [DOI] [PubMed] [Google Scholar]
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus-associated pain: relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole K, Tjolsen A. The tail-flick and formalin tests in rodents: changes in skin temperature as a confounding factor. Pain. 1993;53:247–254. doi: 10.1016/0304-3959(93)90220-J. [DOI] [PubMed] [Google Scholar]
- Hu B, Doods H, Treede RD, Ceci A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 2009;143:206–212. doi: 10.1016/j.pain.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Huang W, Calvo M, Karu K, Olausen H, Bathgate G, Okuse K, et al. A clinically relevant rodent model of HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain. 2013;154:560–575. doi: 10.1016/j.pain.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, et al. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci. 2010;4:165. doi: 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Khasar SG, Levine JD. Novel mechanism of enhanced nociception in a model of AIDS therapy-induced painful peripheral neuropathy in the rat. Pain. 2004;107:147–158. doi: 10.1016/j.pain.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Keating SC, Thomas AA, Flecknell PA, Leach MC. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS ONE. 2012;7:e44437. doi: 10.1371/journal.pone.0044437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna I. Drug discovery in pharmaceutical industry: productivity challenges and trends. Drug Discov Today. 2012;17:1088–1102. doi: 10.1016/j.drudis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Parsons N, Kadyszewski E, Festing MF, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE. 2009;4:e7824. doi: 10.1371/journal.pone.0007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010a;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010b;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, et al. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissin I. The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg. 2010;110:780–789. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, Meert TF. Predictive validity of neuropathic pain models in pharmacological studies with behavioural outcome in the rat: a systematic review. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Proceedings of the 10th World Congress on Pain. Seattle: IASP Press; 2003. pp. 489–498. [Google Scholar]
- Kontinen VK, Kauppila T, Paananen S, Pertovaara A, Kalso E. Behavioural measures of depression and anxiety in rats with spinal nerve ligation-induced neuropathy. Pain. 1999;80:341–346. doi: 10.1016/s0304-3959(98)00230-9. [DOI] [PubMed] [Google Scholar]
- Laast VA, Shim B, Johanek LM, Dorsey JL, Hauer PE, Tarwater PM, et al. Macrophage-mediated dorsal root ganglion damage precedes altered nerve conduction in SIV-infected macaques. Am J Pathol. 2011;179:2337–2345. doi: 10.1016/j.ajpath.2011.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Lascelles BD, DePuy V, Thomson A, Hansen B, Marcellin-Little DJ, Biourge V, et al. Evaluation of a therapeutic diet for feline degenerative joint disease. J Vet Intern Med. 2010;24:487–495. doi: 10.1111/j.1939-1676.2010.0495.x. [DOI] [PubMed] [Google Scholar]
- Lascelles BDX, Flecknell PA. Do animal models tell us about human pain? Pain Clin Updates. 2010;18:1–6. [Google Scholar]
- Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE. 2012;7:e35656. doi: 10.1371/journal.pone.0035656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling B, Authier N, Balayssac D, Eschalier A, Coudore F. Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain. 2007;128:225–234. doi: 10.1016/j.pain.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Lisi L, Navarra P, Cirocchi R, Sharp A, Stigliano E, Feinstein DL, et al. Rapamycin reduces clinical signs and neuropathic pain in a chronic model of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2012;243:43–51. doi: 10.1016/j.jneuroim.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39:2824–2829. doi: 10.1161/STROKEAHA.108.515957. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke. 2009;40:e50–e52. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PubMed] [Google Scholar]
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Tomlinson DR. A pharmacologic analysis of mechanical hyperalgesia in streptozotocin/diabetic rats. Pain. 1998;76:151–157. doi: 10.1016/s0304-3959(98)00037-2. [DOI] [PubMed] [Google Scholar]
- Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. Inflammation-induced reduction of spontaneous activity by adjuvant: a novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther. 2007;320:194–201. doi: 10.1124/jpet.106.109736. [DOI] [PubMed] [Google Scholar]
- Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, et al. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci. 2012;51:42–49. [PMC free article] [PubMed] [Google Scholar]
- Matthies BK, Franklin KB. Formalin pain is expressed in decerebrate rats but not attenuated by morphine. Pain. 1992;51:199–206. doi: 10.1016/0304-3959(92)90261-9. [DOI] [PubMed] [Google Scholar]
- Merksey H, Bogduk N. Classification of Chronic Pain. 2nd edn. Seattle: IASP Press; 1994. [Google Scholar]
- Milinkeviciute G, Gentile C, Neely GG. Drosophila as a tool for studying the conserved genetics of pain. Clin Genet. 2012;82:359–366. doi: 10.1111/j.1399-0004.2012.01941.x. [DOI] [PubMed] [Google Scholar]
- Mizisin AP, Shelton GD, Burgers ML, Powell HC, Cuddon PA. Neurological complications associated with spontaneously occurring feline diabetes mellitus. J Neuropathol Exp Neurol. 2002;61:872–884. doi: 10.1093/jnen/61.10.872. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, et al. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci U S A. 2005;102:12938–12943. doi: 10.1073/pnas.0503264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Davis KD, Derbyshire SW. The necessity of animal models in pain research. Pain. 2010a;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, et al. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain. 2010b;6:34. doi: 10.1186/1744-8069-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ. 2013;346:f2690. doi: 10.1136/bmj.f2690. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Ito M, Oda S, Sugiyama A, Kuroda M, Adachi-Akahane S. Spinal mechanism underlying the antiallodynic effect of gabapentin studied in the mouse spinal nerve ligation model. J Pharmacol Sci. 2012;118:455–466. doi: 10.1254/jphs.11102fp. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Bloomfield FH, Gillman MW. Whole animal experiments should be more like human randomized controlled trials. PLoS Biol. 2013;11:e1001481. doi: 10.1371/journal.pbio.1001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8:959–968. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- Neely GG, Kuba K, Cammarato A, Isobe K, Amann S, Zhang L, et al. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell. 2010;141:142–153. doi: 10.1016/j.cell.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters M, Dahan A, Kest B, Zacny J, Stijnen T, Aarts L, et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151:61–68. doi: 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Olmarker K, Storkson R, Berge OG. Pathogenesis of sciatic pain: a study of spontaneous behavior in rats exposed to experimental disc herniation. Spine (Phila Pa 1976) 2002;27:1312–1317. doi: 10.1097/00007632-200206150-00013. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomised controlled trials. PLoS ONE. 2010;5:e14433. doi: 10.1371/journal.pone.0014433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering G, Jourdan D, Millecamps M, Chapuy E, Alliot J, Eschalier A. Age-related impact of neuropathic pain on animal behaviour. Eur J Pain. 2006;10:749–755. doi: 10.1016/j.ejpain.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US, Bennett GJ. A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain. 2001;94:293–304. doi: 10.1016/S0304-3959(01)00363-3. [DOI] [PubMed] [Google Scholar]
- Quessy SN. Comment on: animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and a call for uniform reporting standards. Pain. 2009;142:284–285. doi: 10.1016/j.pain.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Rice AS. Predicting analgesic efficacy from animal models of peripheral neuropathy and nerve injury: a critical view from the clinic. In: Mogil JS, editor. Pain 2010 – An Updated Review: Refresher Course Syllabus. Seattle: IASP Press; 2010. pp. 415–426. [Google Scholar]
- Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–224. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, et al. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: a critical appraisal and call for uniform reporting standards. Pain. 2009;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Rice AS, Morland R, Huang W, Currie GL, Sena ES, Macleod M. Transparency in the reporting of in vivo preclinical pain research: the relevance and implications of the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Scand J Pain. 2013;4:58–62. doi: 10.1016/j.sjpain.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Robinson I, Dowdall T, Meert TF. Development of neuropathic pain is affected by bedding texture in two models of peripheral nerve injury in rats. Neurosci Lett. 2004;368:107–111. doi: 10.1016/j.neulet.2004.06.078. [DOI] [PubMed] [Google Scholar]
- Roeska K, Ceci A, Treede RD, Doods H. Effect of high trait anxiety on mechanical hypersensitivity in male rats. Neurosci Lett. 2009a;464:160–164. doi: 10.1016/j.neulet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2009b;139:349–357. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Rooke ED, Vesterinen HM, Sena ES, Egan KJ, Macleod MR. Dopamine agonists in animal models of Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17:313–320. doi: 10.1016/j.parkreldis.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC. The case for publishing ‘negative’ clinical trials. Pain. 2009;146:225–226. doi: 10.1016/j.pain.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Salinas FA, Lugo LH, Garcia HI. Efficacy of early treatment with carbamazepine in prevention of neuropathic pain in patients with spinal cord injury. Am J Phys Med Rehabil. 2012;91:1020–1027. doi: 10.1097/PHM.0b013e3182643c85. [DOI] [PubMed] [Google Scholar]
- Schreiber AK, Neufeld M, Jesus CH, Cunha JM. Peripheral antinociceptive effect of anandamide and drugs that affect the endocannabinoid system on the formalin test in normal and streptozotocin-diabetic rats. Neuropharmacology. 2012;63:1286–1297. doi: 10.1016/j.neuropharm.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J. Microneurography: towards a biomarker of spontaneous pain. Pain. 2012;153:1989–1990. doi: 10.1016/j.pain.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Sneddon LU. Evolution of nociception in vertebrates: comparative analysis of lower vertebrates. Brain Res Brain Res Rev. 2004;46:123–130. doi: 10.1016/j.brainresrev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Sneddon LU. Pain perception in fish: indicators and endpoints. ILAR J. 2009;50:338–342. doi: 10.1093/ilar.50.4.338. [DOI] [PubMed] [Google Scholar]
- Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Miao B, Wang XC, Duan JH, Ye X, Han WJ, et al. Gastrodin inhibits allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing excitability of nociceptive primary sensory neurons. PLoS ONE. 2012;7:e39647. doi: 10.1371/journal.pone.0039647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 2007;12:261–266. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, et al. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol. 2013;11:e1001609. doi: 10.1371/journal.pbio.1001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos M, Hollis K, Nowbahari E, Kacelnik A. Pro-sociality without empathy. Biol Lett. 2012;8:910–912. doi: 10.1098/rsbl.2012.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterinen HM, Sena ES, ffrench-Constant C, Williams A, Chandran S, Macleod MR. Improving the translational hit of experimental treatments in multiple sclerosis. Mult Scler. 2010;16:1044–1055. doi: 10.1177/1352458510379612. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Mauderli AP, Wiley RG. Assessment of pain in laboratory animals: a comment on Mogil and Crager (2004) Pain. 2005;114:520–523. doi: 10.1016/j.pain.2005.01.021. author reply 523–524. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, et al. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007a;133:47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, et al. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007b;130(Pt 10):2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Segerdahl AR, Lambert DM, Vandevoorde S, Blackbeard J, Pheby T, et al. The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. Br J Pharmacol. 2007c;151:1117–1128. doi: 10.1038/sj.bjp.0707326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Segerdahl AR, Blackbeard J, Pheby T, Rice AS. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci Lett. 2008;448:153–156. doi: 10.1016/j.neulet.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JK, Koeberle PD. Development and characterization of a hemorrhagic rat model of central post-stroke pain. Neuroscience. 2009;161:173–183. doi: 10.1016/j.neuroscience.2009.03.042. [DOI] [PubMed] [Google Scholar]
- Wernham BG, Trumpatori B, Hash J, Lipsett J, Davidson G, Wackerow P, et al. Dose reduction of meloxicam in dogs with osteoarthritis-associated pain and impaired mobility. J Vet Intern Med. 2011;25:1298–1305. doi: 10.1111/j.1939-1676.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Adedoyin A, Leventhal L. Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology. 2008;54:767–775. doi: 10.1016/j.neuropharm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice AS, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia – an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;(11) doi: 10.1002/14651858.CD010567.pub2. CD010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizumi M, Parker RA, Eisenach JC, Hayashida K. Gabapentin inhibits gamma-amino butyric acid release in the locus coeruleus but not in the spinal dorsal horn after peripheral nerve injury in rats. Anesthesiology. 2012;116:1347–1353. doi: 10.1097/ALN.0b013e318254e6fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng FY, Xiao WH, Bennett GJ. The response of spinal microglia to chemotherapy-evoked painful peripheral neuropathies is distinct from that evoked by traumatic nerve injuries. Neuroscience. 2011;176:447–454. doi: 10.1016/j.neuroscience.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


