Abstract
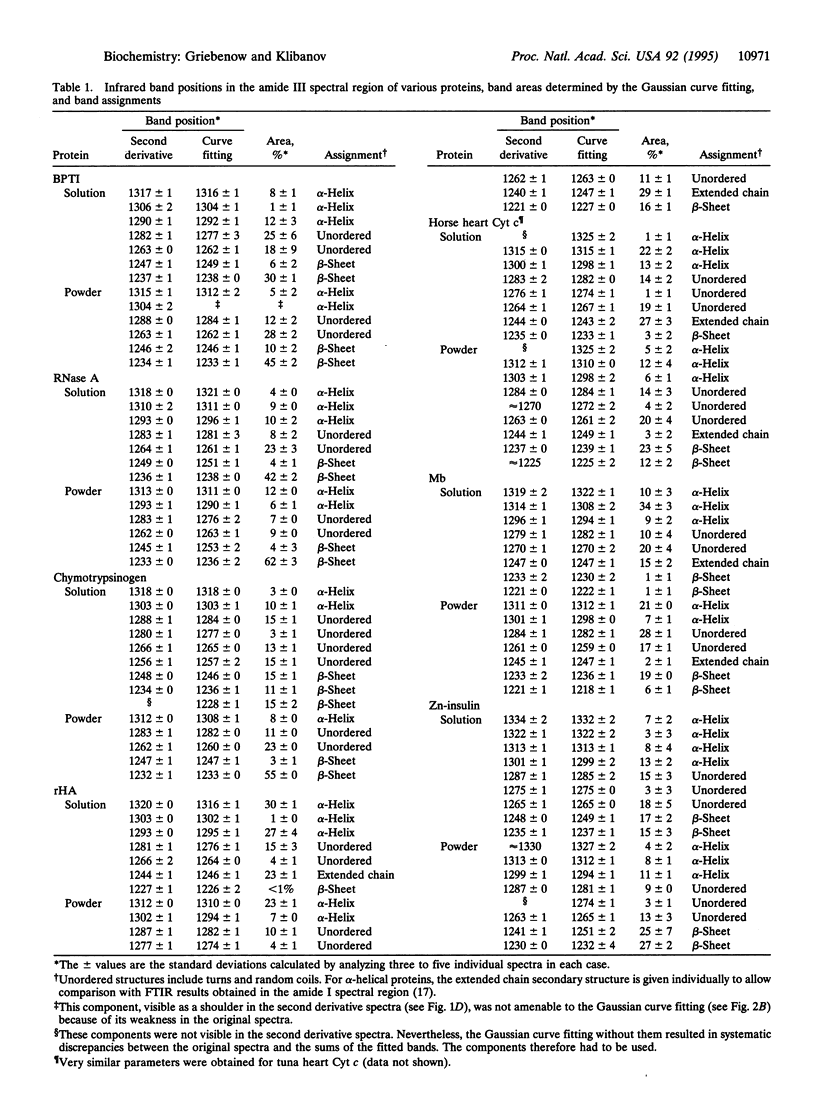
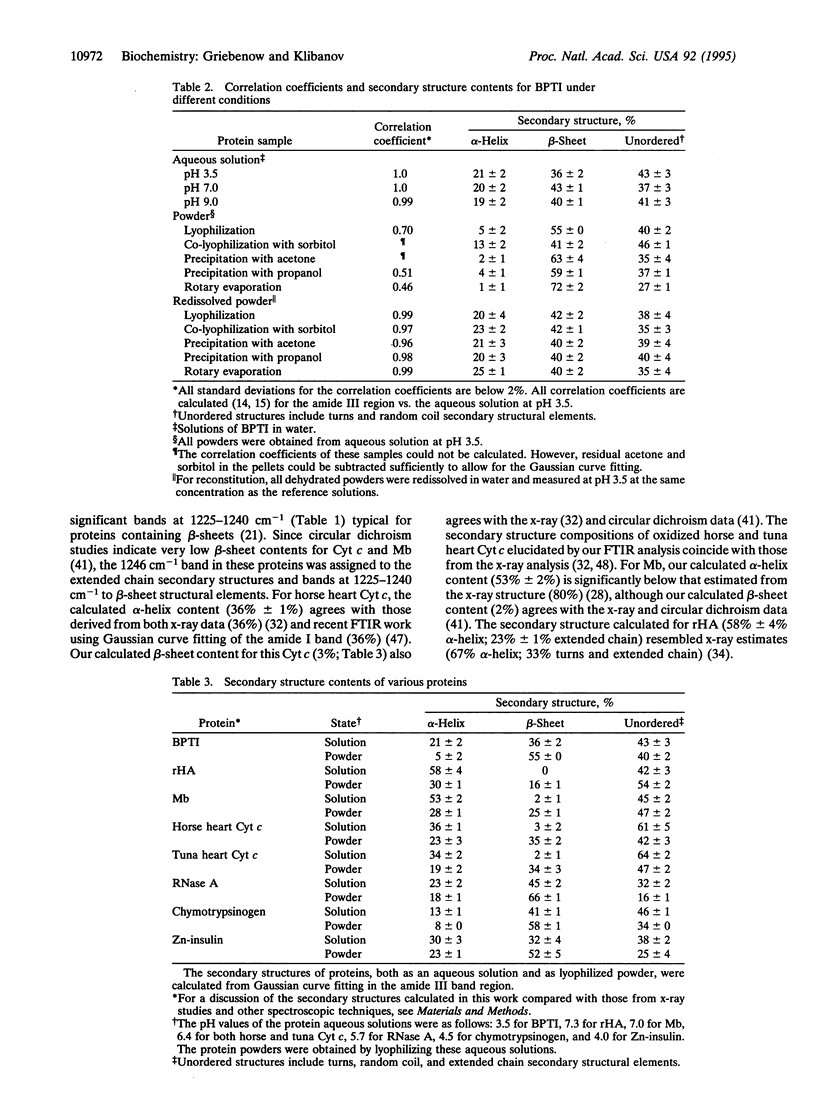
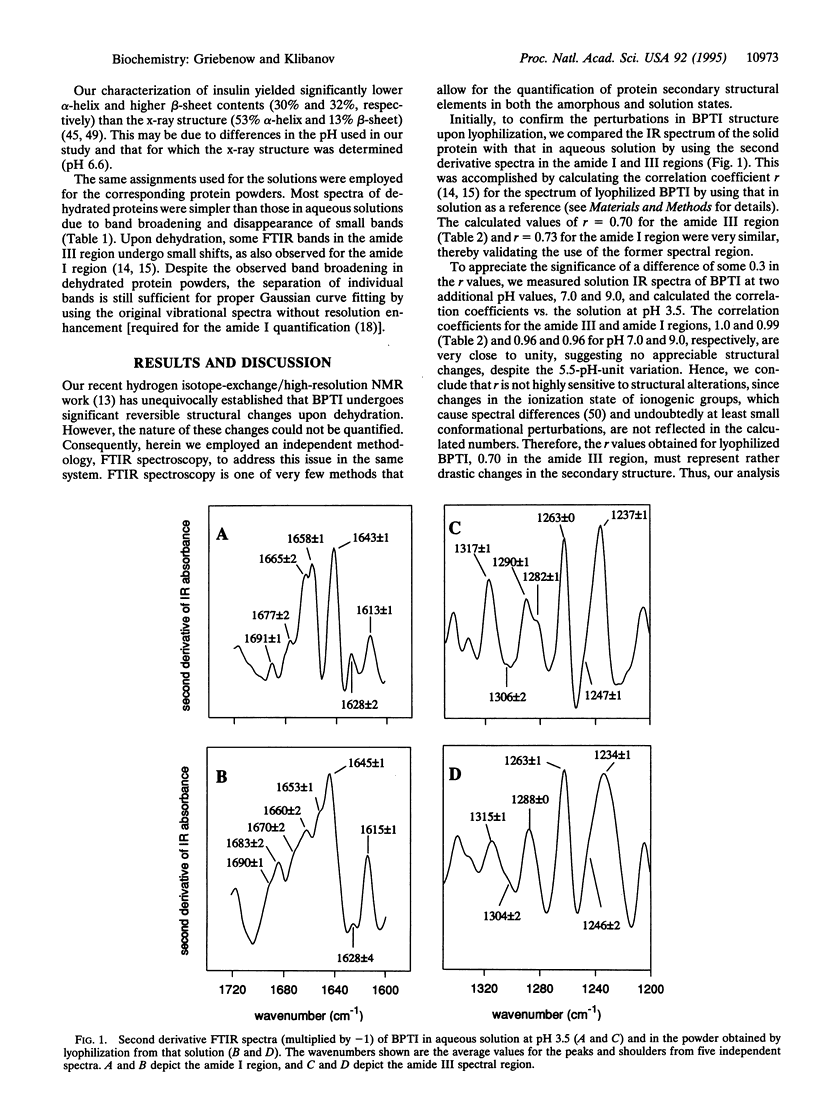
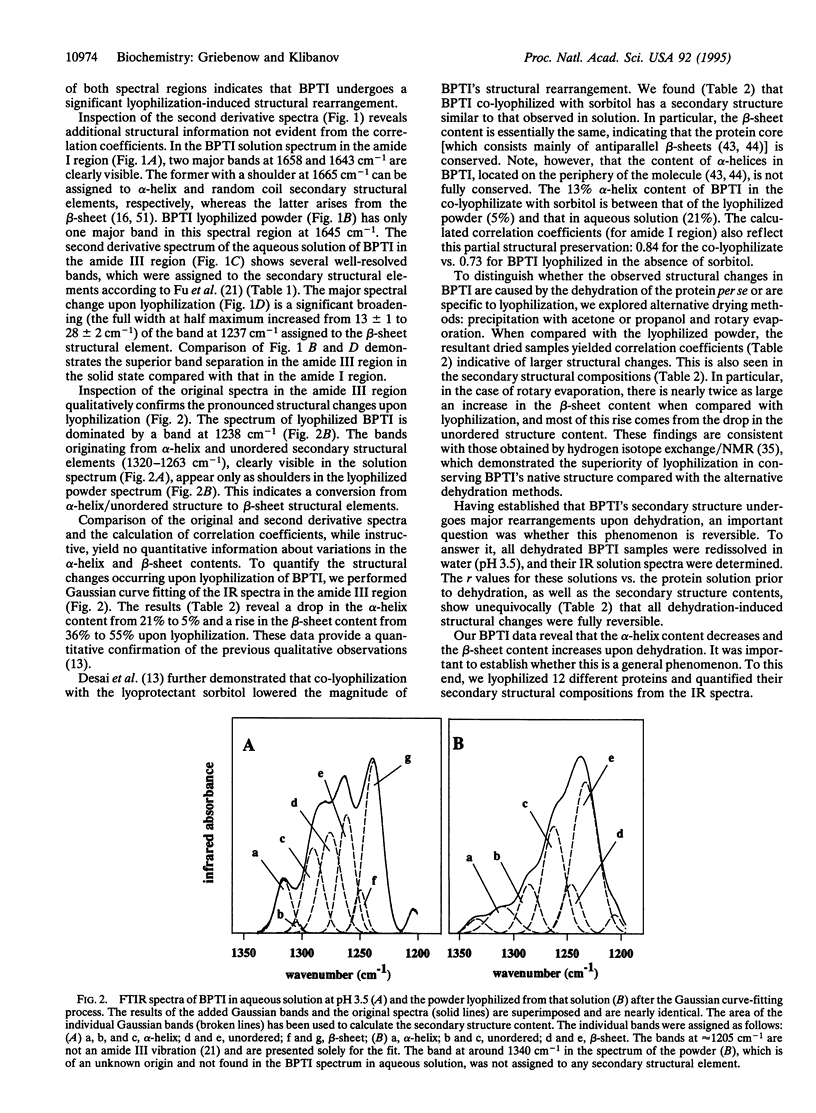
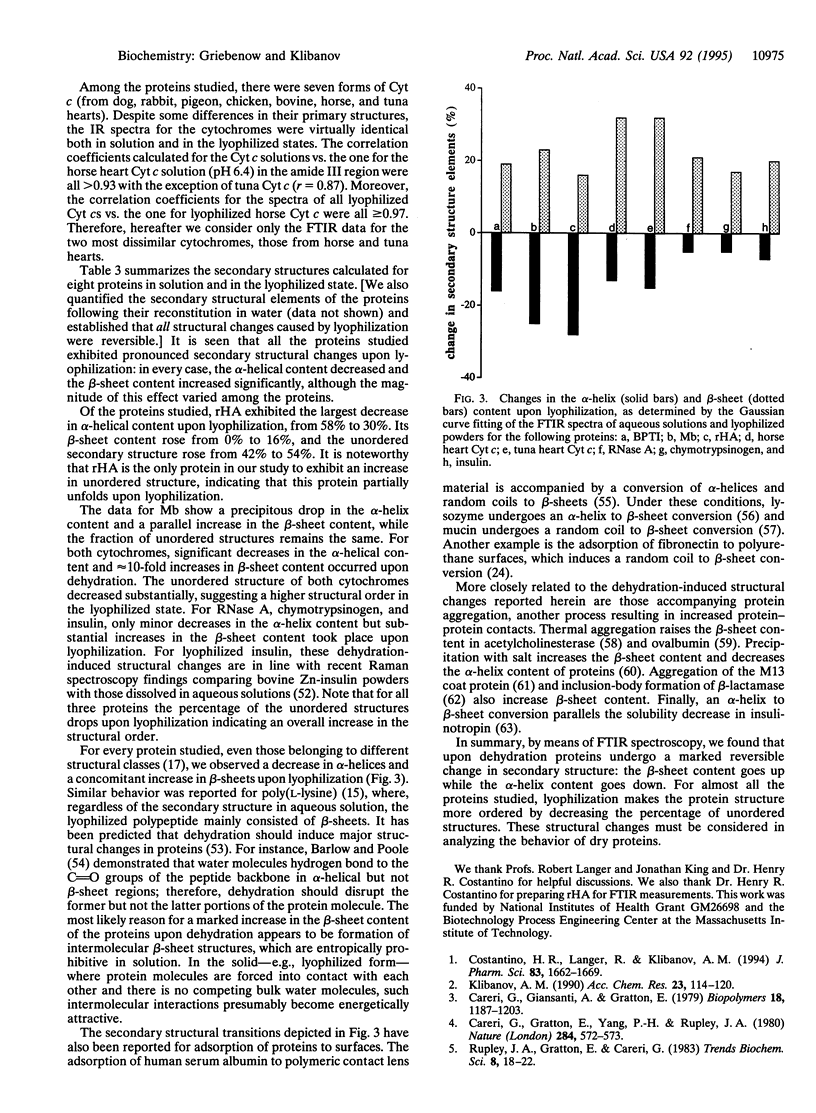
Changes in the secondary structure of some dozen different proteins upon lyophilization of their aqueous solutions have been investigated by means of Fourier-transform infrared spectroscopy in the amide III band region. Dehydration markedly (but reversibly) alters the secondary structure of all the proteins studied, as revealed by both the quantitative analysis of the second derivative spectra and the Gaussian curve fitting of the original infrared spectra. Lyophilization substantially increases the beta-sheet content and lowers the alpha-helix content of all proteins. In all but one case, proteins become more ordered upon lyophilization.
Full text
PDF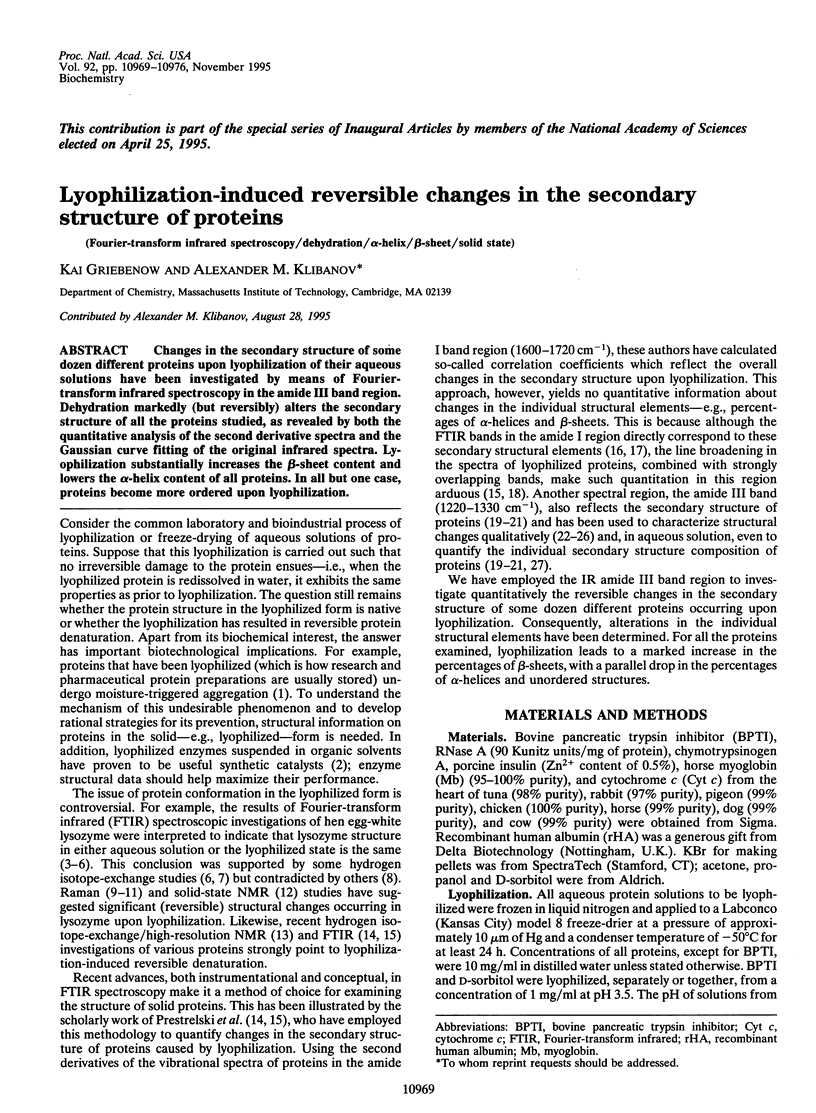


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L. J., Hansen A. M., Rao P. B., Bryan W. P. Effects of the presence of water on lysozyme conformation. Biopolymers. 1983 Jul;22(7):1637–1640. doi: 10.1002/bip.360220703. [DOI] [PubMed] [Google Scholar]
- Barlow D. J., Poole P. L. The hydration of protein secondary structures. FEBS Lett. 1987 Mar 23;213(2):423–427. doi: 10.1016/0014-5793(87)81535-1. [DOI] [PubMed] [Google Scholar]
- Bushnell G. W., Louie G. V., Brayer G. D. High-resolution three-dimensional structure of horse heart cytochrome c. J Mol Biol. 1990 Jul 20;214(2):585–595. doi: 10.1016/0022-2836(90)90200-6. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Careri G., Giansanti A., Gratton E. Lysozyme film hydration events: an ir and gravimetric study. Biopolymers. 1979 May;18(5):1187–1203. doi: 10.1002/bip.1979.360180512. [DOI] [PubMed] [Google Scholar]
- Careri G., Gratton E., Yang P. H., Rupley J. A. Correlation of IR spectroscopic, heat capacity, diamagnetic susceptibility and enzymatic measurements on lysozyme powder. Nature. 1980 Apr 10;284(5756):572–573. doi: 10.1038/284572a0. [DOI] [PubMed] [Google Scholar]
- Carlisle C. H., Palmer R. A., Mazumdar S. K., Gorinsky B. A., Yeates D. G. The structure of ribonuclease at 2-5 Angström resolution. J Mol Biol. 1974 May 5;85(1):1–18. doi: 10.1016/0022-2836(74)90125-9. [DOI] [PubMed] [Google Scholar]
- Carpenter J. F., Crowe J. H. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry. 1989 May 2;28(9):3916–3922. doi: 10.1021/bi00435a044. [DOI] [PubMed] [Google Scholar]
- Castillo E. J., Koenig J. L., Anderson J. M., Jentoft N. Protein adsorption on soft contact lenses. III. Mucin. Biomaterials. 1986 Jan;7(1):9–16. doi: 10.1016/0142-9612(86)90081-5. [DOI] [PubMed] [Google Scholar]
- Castillo E. J., Koenig J. L., Anderson J. M., Lo J. Characterization of protein adsorption on soft contact lenses. I. Conformational changes of adsorbed human serum albumin. Biomaterials. 1984 Nov;5(6):319–325. doi: 10.1016/0142-9612(84)90029-2. [DOI] [PubMed] [Google Scholar]
- Castillo E. J., Koenig J. L., Anderson J. M., Lo J. Protein adsorption on hydrogels. II. Reversible and irreversible interactions between lysozyme and soft contact lens surfaces. Biomaterials. 1985 Sep;6(5):338–345. doi: 10.1016/0142-9612(85)90089-4. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Martinez H. M. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 1972 Oct 24;11(22):4120–4131. doi: 10.1021/bi00772a015. [DOI] [PubMed] [Google Scholar]
- Costantino H. R., Langer R., Klibanov A. M. Aggregation of a lyophilized pharmaceutical protein, recombinant human albumin: effect of moisture and stabilization by excipients. Biotechnology (N Y) 1995 May;13(5):493–496. doi: 10.1038/nbt0595-493. [DOI] [PubMed] [Google Scholar]
- Costantino H. R., Langer R., Klibanov A. M. Solid-phase aggregation of proteins under pharmaceutically relevant conditions. J Pharm Sci. 1994 Dec;83(12):1662–1669. doi: 10.1002/jps.2600831205. [DOI] [PubMed] [Google Scholar]
- Dong A., Prestrelski S. J., Allison S. D., Carpenter J. F. Infrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation. J Pharm Sci. 1995 Apr;84(4):415–424. doi: 10.1002/jps.2600840407. [DOI] [PubMed] [Google Scholar]
- Evans S. V., Brayer G. D. High-resolution study of the three-dimensional structure of horse heart metmyoglobin. J Mol Biol. 1990 Jun 20;213(4):885–897. doi: 10.1016/S0022-2836(05)80270-0. [DOI] [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- Gregory R. B., Gangoda M., Gilpin R. K., Su W. The influence of hydration on the conformation of lysozyme studied by solid-state 13C-NMR spectroscopy. Biopolymers. 1993 Apr;33(4):513–519. doi: 10.1002/bip.360330402. [DOI] [PubMed] [Google Scholar]
- Görne-Tschelnokow U., Naumann D., Weise C., Hucho F. Secondary structure and temperature behaviour of acetylcholinesterase. Studies by Fourier-transform infrared spectroscopy. Eur J Biochem. 1993 May 1;213(3):1235–1242. doi: 10.1111/j.1432-1033.1993.tb17874.x. [DOI] [PubMed] [Google Scholar]
- He X. M., Carter D. C. Atomic structure and chemistry of human serum albumin. Nature. 1992 Jul 16;358(6383):209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- Heimburg T., Marsh D. Investigation of secondary and tertiary structural changes of cytochrome c in complexes with anionic lipids using amide hydrogen exchange measurements: an FTIR study. Biophys J. 1993 Dec;65(6):2408–2417. doi: 10.1016/S0006-3495(93)81299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Kukla D., Rühlmann A., Epp O., Formanek H. The basic trypsin inhibitor of bovine pancreas. I. Structure analysis and conformation of the polypeptide chain. Naturwissenschaften. 1970 Aug;57(8):389–392. doi: 10.1007/BF00599976. [DOI] [PubMed] [Google Scholar]
- Kim Y., Rose C. A., Liu Y., Ozaki Y., Datta G., Tu A. T. FT-IR and near-infrared FT-Raman studies of the secondary structure of insulinotropin in the solid state: alpha-helix to beta-sheet conversion induced by phenol and/or by high shear force. J Pharm Sci. 1994 Aug;83(8):1175–1180. doi: 10.1002/jps.2600830819. [DOI] [PubMed] [Google Scholar]
- Kuntz I. D., Jr, Kauzmann W. Hydration of proteins and polypeptides. Adv Protein Chem. 1974;28:239–345. doi: 10.1016/s0065-3233(08)60232-6. [DOI] [PubMed] [Google Scholar]
- Levitt M., Greer J. Automatic identification of secondary structure in globular proteins. J Mol Biol. 1977 Aug 5;114(2):181–239. doi: 10.1016/0022-2836(77)90207-8. [DOI] [PubMed] [Google Scholar]
- Olinger J. M., Hill D. M., Jakobsen R. J., Brody R. S. Fourier transform infrared studies of ribonuclease in H2O and 2H2O solutions. Biochim Biophys Acta. 1986 Jan 17;869(1):89–98. doi: 10.1016/0167-4838(86)90314-6. [DOI] [PubMed] [Google Scholar]
- Poole P. L., Finney J. L. Sequential hydration of a dry globular protein. Biopolymers. 1983 Jan;22(1):255–260. doi: 10.1002/bip.360220135. [DOI] [PubMed] [Google Scholar]
- Prestrelski S. J., Arakawa T., Carpenter J. F. Separation of freezing- and drying-induced denaturation of lyophilized proteins using stress-specific stabilization. II. Structural studies using infrared spectroscopy. Arch Biochem Biophys. 1993 Jun;303(2):465–473. doi: 10.1006/abbi.1993.1310. [DOI] [PubMed] [Google Scholar]
- Prestrelski S. J., Tedeschi N., Arakawa T., Carpenter J. F. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys J. 1993 Aug;65(2):661–671. doi: 10.1016/S0006-3495(93)81120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Przybycien T. M., Bailey J. E. Structure-function relationships in the inorganic salt-induced precipitation of alpha-chymotrypsin. Biochim Biophys Acta. 1989 May 1;995(3):231–245. doi: 10.1016/0167-4838(89)90041-1. [DOI] [PubMed] [Google Scholar]
- Przybycien T. M., Dunn J. P., Valax P., Georgiou G. Secondary structure characterization of beta-lactamase inclusion bodies. Protein Eng. 1994 Jan;7(1):131–136. doi: 10.1093/protein/7.1.131. [DOI] [PubMed] [Google Scholar]
- Rupley J. A., Careri G. Protein hydration and function. Adv Protein Chem. 1991;41:37–172. doi: 10.1016/s0065-3233(08)60197-7. [DOI] [PubMed] [Google Scholar]
- Schinkel J. E., Downer N. W., Rupley J. A. Hydrogen exchange of lysozyme powders. Hydration dependence of internal motions. Biochemistry. 1985 Jan 15;24(2):352–366. doi: 10.1021/bi00323a018. [DOI] [PubMed] [Google Scholar]
- Sherwood C., Mauk A. G., Brayer G. D. Crystallization and preliminary diffraction data for horse heart metmyoglobin. J Mol Biol. 1987 Jan 5;193(1):227–227. doi: 10.1016/0022-2836(87)90641-3. [DOI] [PubMed] [Google Scholar]
- Singh B. R., Fu F. N., Ledoux D. N. Crystal and solution structures of superantigenic staphylococcal enterotoxins compared. Nat Struct Biol. 1994 Jun;1(6):358–360. doi: 10.1038/nsb0694-358. [DOI] [PubMed] [Google Scholar]
- Singh B. R., Fuller M. P., Schiavo G. Molecular structure of tetanus neurotoxin as revealed by Fourier transform infrared and circular dichroic spectroscopy. Biophys Chem. 1990 Jul;36(2):155–166. doi: 10.1016/0301-4622(90)85019-3. [DOI] [PubMed] [Google Scholar]
- Spruijt R. B., Wolfs C. J., Hemminga M. A. Aggregation-related conformational change of the membrane-associated coat protein of bacteriophage M13. Biochemistry. 1989 Nov 14;28(23):9158–9165. doi: 10.1021/bi00449a030. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta. 1988 Jan 29;952(2):115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Protein structure by Fourier transform infrared spectroscopy: second derivative spectra. Biochem Biophys Res Commun. 1983 Aug 30;115(1):391–397. doi: 10.1016/0006-291x(83)91016-1. [DOI] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Methods Enzymol. 1986;130:290–311. doi: 10.1016/0076-6879(86)30015-6. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Venyaminov SYu, Kalnin N. N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino acid residue absorption bands. Biopolymers. 1990;30(13-14):1243–1257. doi: 10.1002/bip.360301309. [DOI] [PubMed] [Google Scholar]
- Wang D., Bode W., Huber R. Bovine chymotrypsinogen A X-ray crystal structure analysis and refinement of a new crystal form at 1.8 A resolution. J Mol Biol. 1985 Oct 5;185(3):595–624. doi: 10.1016/0022-2836(85)90074-9. [DOI] [PubMed] [Google Scholar]
- Wasacz F. M., Olinger J. M., Jakobsen R. J. Fourier transform infrared studies of proteins using nonaqueous solvents. Effects of methanol and ethylene glycol on albumin and immunoglobulin G. Biochemistry. 1987 Mar 10;26(5):1464–1470. doi: 10.1021/bi00379a038. [DOI] [PubMed] [Google Scholar]
- Yeo S. D., Debendetti P. G., Patro S. Y., Przybycien T. M. Secondary structure characterization of microparticulate insulin powders. J Pharm Sci. 1994 Dec;83(12):1651–1656. doi: 10.1002/jps.2600831203. [DOI] [PubMed] [Google Scholar]
- Yu N. T., Jo B. H. Comparison of protein structure in crystals and in solution by laser raman scattering. I. Lysozyme. Arch Biochem Biophys. 1973 Jun;156(2):469–474. doi: 10.1016/0003-9861(73)90296-8. [DOI] [PubMed] [Google Scholar]



