Abstract
BACKGROUND AND PURPOSE
Insulin secretion from isolated pancreatic islets is a pivotal assay in developing novel insulin secretagogues, given its good correlation with in vivo efficacy. Because the supply of human islets is limited, this assay is typically run with rodent islets, which do not address species differences and are low-throughput, because of the size matching or volume normalization required. Here we have evaluated the suitability of human re-aggregated islets for this assay.
EXPERIMENTAL APPROACH
We generated re-aggregated human islets of a consistent size, using micromolds and compared their responses with those of native human and rat islets, to known secretagogues and inhibitors of insulin release.
KEY RESULTS
Insulin secretion from rat islets, human islets and human re-aggregated cell clusters was concentration-dependently increased by glucose. The calcium channel agonist, Bay K 8644, stimulated insulin secretion in native rat islets and human re-aggregated islets, but not native human islets. Glibenclamide and tolbutamide were more effective and potent in re-aggregated human clusters compared with the other two preparations. Rat islets outperformed both human preparations of islets in response to caffeine, carbachol and glucagon-like peptide-1. Re-aggregated human islet clusters were more sensitive to somatostatin, diazoxide and sodium azide, but rodent islets were more sensitive to nifedipine.
CONCLUSIONS AND IMPLICATIONS
Human re-aggregated clusters of islet cells, of a constant size were more responsive to all compounds tested than native human islets. Importantly, the assay variability was less in the re-aggregated cluster preparations, which suggests that such re-aggregated cells could be useful for drug development.
Keywords: Insulin, islets, re-aggregate, somatostatin, glibenclamide, tolbutamide, GLP-1, elisa, rat, human
Introduction
Diabetes affects 8.3% of the population within the United States and over 346 million individuals worldwide (King et al., 1998; Unwin et al., 2013). The Centers for Disease Control predicts that by 2050, 20–33% of the Americans will have diabetes, either diagnosed or undiagnosed (Boyle et al., 2010). The economic burden within the United States alone exceeded $200 billion in 2007 and continues to grow. While significant effort has been put into the discovery of new therapies for people with diabetes, few new therapeutic agents have reached the market in the past 10 years.
Drug discovery on targets that modulate insulin secretion relies heavily on the use of insulinoma cell lines cultured in monolayers as the primary tool for assessing efficacy. These cell lines represent the pancreatic beta cells, the insulin-producing cells of the pancreas, which do not normally replicate in adults. In the body, beta cells are clustered into islets, together with other types of cells including glucagon-producing alpha cells and somatostatin-producing delta cells. Beta cell lines, transformed for replication, are not a suitable substitute for human islets, and do not offer the best approach to identify lead compounds during drug discovery (Hohmeier and Newgard, 2004; Walpita et al., 2012).
In drug discovery, compounds that target insulin secretion in the primary screen are typically advanced to secondary screens that are performed using rodent islets. While the rodent islets are more predictive of eventual outcomes on insulin secretion in vivo, they too have shortcomings. First, rodent islets (like human) come in a wide range of sizes from 40 to 400 μm (MacGregor et al., 2006). The great variation in cell number per islet makes analysis of the results complicated. Current methods of normalization based on islet equivalency have been shown to alter results, depending on the size of the islets being tested (Huang et al., 2012; Farhat et al., 2013). Second, there are physiological differences between rodent and human islets, including the fact that rodent islets are less bioenergetically efficient than humans (Wikstrom et al., 2012). Lastly, there is a significant risk that important species differences will be overlooked unless human islets are used early in drug discovery.
In order to circumvent the limitations of native islets and cell culture, and to make human islet material of a consistent size and at larger volumes available for early drug discovery, re-aggregated islets were produced from human islets using a previously published method (Ramachandran et al., 2013). This process results in miniature human islet cell clusters of a uniform size, with an average diameter of 40 μm (Ramachandran et al., 2013). These clusters are responsive to glucose, have the same cellular composition as native islets, and have improved diffusion properties (Ramachandran et al., 2013). The uniform re-aggregated islet clusters are small enough to be used in pharmaceutical industry robotics. However, their response to known insulin secretagogues and inhibitors remains unknown. The purpose of this study was to compare the response of native rat islets, used as secondary screens in the pharmaceutical industry, with human re-aggregated clusters, to a variety of compounds known to either stimulate or block insulin secretion in vitro. In certain cases, human native islets were included in the comparisons.
Methods
Rat islet isolation and testing
All animal care and experimental procedures complied with AAALAC and AVMA guidelines and were approved by an internal Lilly ethical committee (IACUC) and in accordance with U.S. and Indiana laws and regulations. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 104 animals were used in the experiments described here.
Male Sprague-Dawley rats (250–280 g; Harlan, Indianapolis, IN) were anaesthetised with CO2 and killed by cervical dislocation. After the common bile duct was cannulated, the pancreas was distended in situ with Hank's buffer (10 mL, containing 2% BSA and 1 mg·mL–1 Sigma Type V collagenase or 0.15 mg·mL–1 liberase; Roche). Subsequently, within 2-3 minutes, tissues were moved to a 37 °C water bath and digested in Hank's buffer + collagenase at 37°C for 10–12 min (or for 19–21 min, using liberase). Islets were purified by centrifugation of the digest on a Histopaque-1100 solution (100 ml Histopaque-1077 + 120 ml Histopaque-1119; Sigma-Aldrich, St. Louis, MO) for 18 min at 750× g. The islets migrate to the interface between the digestion medium and the Histopaque solution. After isolation the islets were cultured overnight in RPMI-1640 medium (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA) containing 10% FBS, 100 U·mL–1 penicillin and 100 μg·mL–1 streptomycin, before the insulin secretion assay. Purity was determined with dexamethasone staining.
Human islet procurement and cluster production
Human islets were obtained from the Integrated Islet Distribution Program (City of Hope Hospital, Duarte, CA). The characteristics of the 23 adult donors are provided in Table 1. Head trauma was the most common cause of death (when reported) at 39%. Other causes included cerebral vascular injury (24%), followed by anoxia (19%). Isolated islets were maintained in CMRL (Mediatech Inc, Manassas, VA, USA) 1066 medium with 2 mM L-glutamine, 10% FBS and 1% antibiotic/antimycotic at 37°C in a culture chamber containing 5% CO2.
Table 1.
Donor characteristics
| Donor characteristics | Age (years) | Body mass index | Sex | Race | Cold ischemia time (min) |
|---|---|---|---|---|---|
| 23 Human donors | 40.2 ± 2.2 | 31.0 ± 1.5 | 63% male | 86% Caucasian | 528 ± 46 |
For dispersion, islets were washed twice with calcium- and magnesium-free HBSS (cmf-HBSS), before the addition of digestion medium consisting of cmf-HBSS supplemented with papain (10 units·mL–1; Worthington, Lakewood, NJ, USA). Suspensions were incubated on a rotator at 37°C for 10–15 min. Islets were dispersed by trituration using a pipette until the cell suspension contained mostly single cells. The cells were then washed to remove residual papain and transferred to an aggregate culture medium, consisting of CMRL 1066 supplemented with 2 mM L-glutamine, 10% FBS and 100 U penicillin, 100 μg streptomycin, and 25 μg amphotericin. Samples were removed for cell counts and yield using a hemocytometer.
The suspension of single cells was then plated onto sterile glass micromolds as described by Ramachandran et al., (2013). Within 30 min, cells began to settle into the recesses of the micromold and were in close proximity to each other, allowing cell-to-cell re-adhesion. Cells in the micromolds were incubated for 3–5 days at 37°C and 5% CO2. Cells re-aggregated while limiting the size to no bigger than the recess allowed (100 μm). Aggregate culture medium was changed every 24–48 h until re-agregated islets were formed. Re-aggregated islet clusters were removed by washing the micromold several times with culture medium until they were dislodged and were aspirated with a pipette to be plated in CMRL-based medium for long-term culture.
Compound testing for all groups
To test the secretion of insulin stimulated by glucose or exogenous compounds, intact islets and re-aggregated islet clusters were washed repeatedly with Earle's balanced salt solution (EBSS, Invitrogen) supplemented with 0.1% BSA and low (2.8 mM) glucose. The EBSS was specifically produced to contain no glucose, allowing for the addition of glucose at varying levels. Cultured, size-matched (150–200 μm diameter), intact rat and human islets were loaded into 96-well culture plates (four per well) and preconditioned with a 30 min starvation in EBSS. Re-aggregated cell clusters were treated identically but without size matching or counting the number of clusters per well. On average, 10–12 clusters were dispensed per well. Subsequently, the solutions were changed to 2.8–22.4 mM glucose and/or increasing levels of test compound (four to six replicates). Rat insulin was measured over 90 min static incubation at 37°C and 5% CO2 using the Mesoscale Insulin Assay (Meso Scale, Gaithersburg, MD, USA). Human islets and re-aggregated clusters were subjected to a 60-min static incubation at 37°C and 5% CO2. The conditioned medium from human islets or re-aggregated cells was collected and kept frozen at −80°C for later determination of insulin content by elisa (ALPCO, Windham, NH, USA or Mercodia, Uppsala, Sweden). The re-aggregated human islets require 60 min incubation to release quantifiable levels of insulin. Although 60 min would also have been sufficient for rat islets, we chose to stay with our standard incubation time of 90 min. Comparing 60 and 90 min incubation times indicate that there are no qualitative changes in responses from rat islets (K. Bokvist and X. Peng, unpubl. obs.).
Stock solutions of compounds were made in either DMSO or H2O. Compounds, premixed at a double concentration in EBSS, were added to each well to achieve desired compound – and a final concentration of 11.2 mM glucose (unless otherwise noted). Serial dilutions were prepared to achieve half-log concentrations for testing. A range of control glucose concentrations were included on each plate as a quality control measure. Concentration response curves were determined, and assay quality was assigned based on the signal window as defined in NIH Assay Guidance Manual (Iversen et al., 2004). The robustness of each response was determined based on the Z-factor (Iversen et al., 2004).
Data analysis
The purpose of this study was to use industry standard methods for drug screening with the re-aggregated islets. Large-scale screens are often conducted once with subsequent follow-up tests to determine validity and reproducibility. Thus, these screens were conducted on one preparation of islets, unless those islet cells failed to respond to increasing concentrations of glucose with greater release of insulin. Unless otherwise noted, all results were tested for significance using a one-way anova with P < 0.05. For all-pairwise comparisons, a Holm–Sidak test was used. Where appropriate, figures illustrate the mean of one preparation ± SE, comprised of four replicates.
Materials
Sigma-Aldrich supplied α-ketoisocaproic acid, Bay K8644, caffeine, carbachol, diazoxide, glibenclamide, nifedipine, sodium azide, somatostatin and tolbutamide; GLP-1 and GRP were supplied by Bachem US, Torrance, CA.
Results
Glucose sensitivity
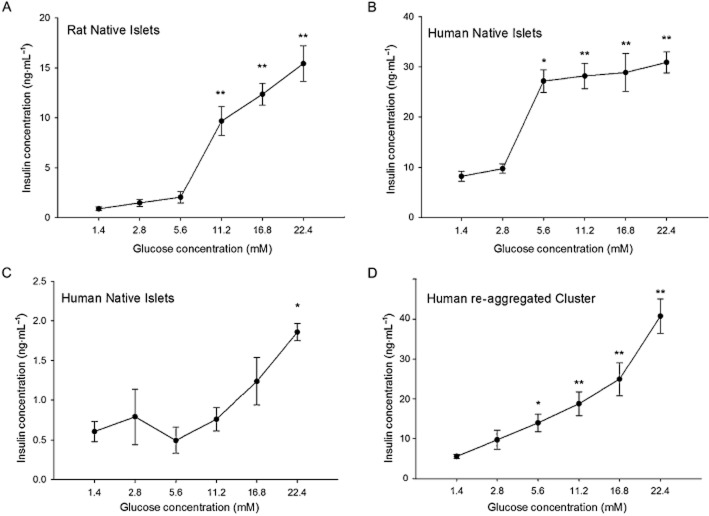
Glucose-dependent insulin secretion is the physiological function of the pancreatic islet and the response to glucose is a key indicator of islet health. As a consequence, the response to glucose was used as a measure of the quality of different batches of islets. Figure 1A shows the response to glucose in native rat islets, compared with native human islets (Figure 1B). The human islets appeared to be more sensitive to glucose and respond to lower glucose levels as compared with the rat islets. This may be in line with the fact that euglycaemia is slightly higher in rodents than humans (Kohn and Clifford, 2002). However, some human islet preparations, of high purity and viability, still secreted insufficient amounts of insulin, as illustrated in Figure 1C. Interestingly, small re-aggregated islets from the same islet preparation had improved responses to 5.6 mM glucose (Figure 1D) when compared with native human islets. Similarly concentration-dependent responses to glucose were found in several preparations of re-aggregated islet cell clusters (n = 6 different donor preparations).
Figure 1.
Glucose sensitivity of islet groups. (A) Static incubation with different glucose concentrations illustrates the responsiveness of rat islets to glucose. (B) Native human islets exposed to same glucose concentration showed significant increases in insulin secretion at lower glucose levels compared with rat islets. (C) Islets from a different human donor were less sensitive to high-glucose concentrations, and released significantly less insulin (see Y axis scale bar). (D) Human re-aggregated cell clusters made from the same donor shown in (C) were more responsive to glucose. *P < 0.05. **P < 0.01, significantly above baseline.
The stimulation index (SI) of the response was calculated by dividing the maximum response by the baseline average (in 2.8 mM glucose). The SI for rat islets was 8.33, for human native islets it was 2.35 and for human re-aggregated clusters, it was 4.20. One problem inherent in cells from human donors is the variability between donors as shown by the donor characteristics in Figure 1. We compared the SI of the re-aggregated clusters from donors from different body mass index categories and stratified for age (when known). Table 2 shows that there were no donors in the normal weight range. There was no statistical difference in the SI values between the overweight and obese categories (P < 0.73). We also examined the effect of age by categorizing the SI for donors under or over 50 years of age (Table 2). Again there was no statistical difference between the groups (P < 0.44).
Table 2.
Effects of body mass index and age on glucose stimulation index (SI) for re-aggregated clusters
| Donor characteristics | SI |
|---|---|
| Overweight (n ± 9) | 2.58 ± 0.81 |
| Obese (n ± 11) | 3.24 ± 0.92 |
| 20–50 yo (n ± 12) | 2.60 ± 0.72 |
| 50–75 yo (n ± 7) | 3.35 ± 1.09 |
Insulin-secretion stimulation
Calcium channel agonists
Exocytosis in the beta cell, and the associated insulin secretion, is a Ca2+-dependent process, and compounds that increase intracellular Ca2+ concentrations enhance vesicle fusion and the release of insulin in vitro (Rorsman et al., 2012). Bay K 8644 is a Ca2+ channel agonist, specifically acting on L-type voltage-gated Ca2+ channels (CaV1.x; channel nomenclature follows Alexander et al., 2013) in the plasma membrane. Exposure of native rat islets to Bay K 8644 in the presence of 11.2 mM glucose increased insulin secretion more than twofold (Figure 2A). A significant increase in insulin release was obtained at 3 μM Bay K 8644 in rat islets, resulting in an EC50 of 0.08 ± 0.04 μM. The native human islet EC50 was 1.9 ± 1.6 μM, with very little response to the drug (Figure 2B). Human re-aggregated clusters responded to Bay K 8644 (Figure 2C) with an EC50 of 0.26 ± 0.20 μM.
Figure 2.
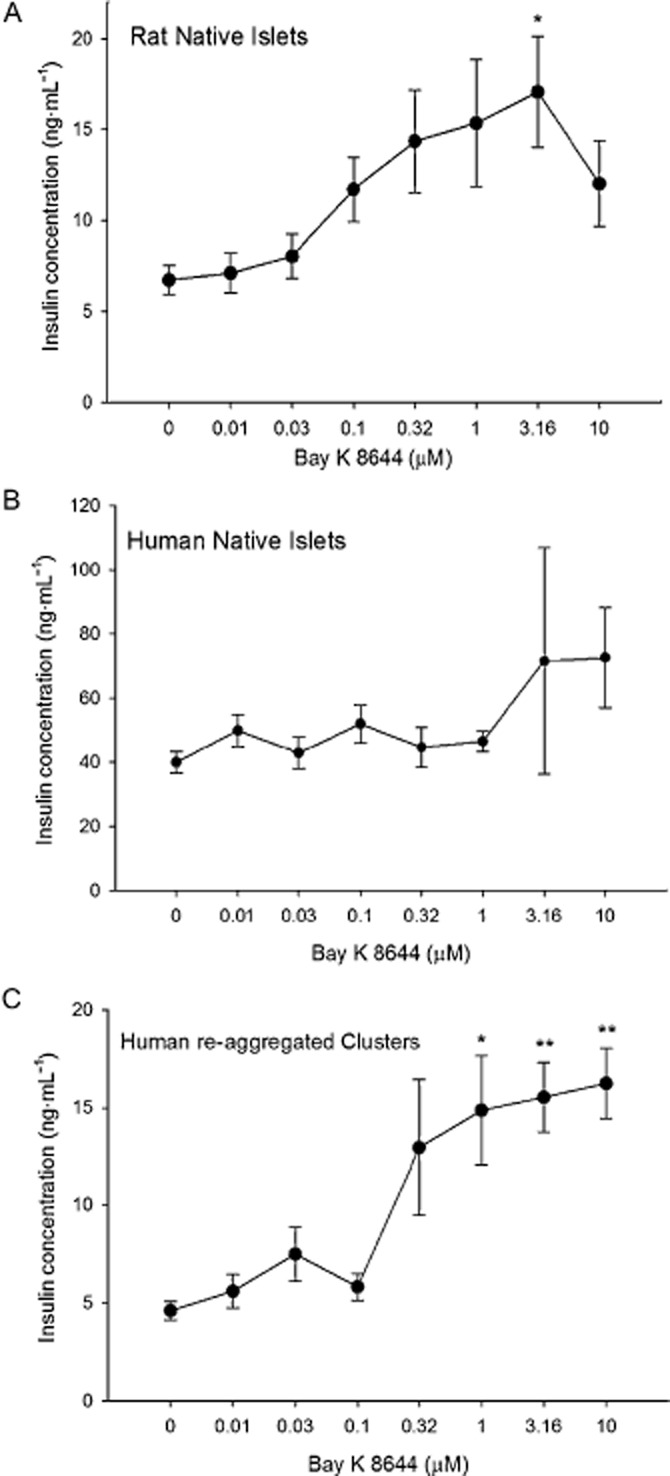
Islet and re-aggregated cluster responses to Bay K 8644. (A) Rat islets exhibited a steady increase in insulin secretion in response to higher concentrations of Bay K 8644, until 10 μM. (B) Human native islets did not respond with an increase in insulin secretion to Bay K 8644. (C) Re-aggregated islet cell clusters had significant responses to 1 μM and all higher concentrations of Bay K 8644. *P < 0.05. **P < 0.01, significantly above baseline.
One possible explanation for the decreased sensitivity in the human islets was that 11.2 mM glucose alone was a near-maximal stimulation for insulin levels from the human islet (Figure 1B), and therefore, little additional stimulation could have been elicited by Bay K 8644, explaining the high insulin secretion at low Bay K 8644 concentrations in the human islets (Figure 2B). This possibility was investigated by reducing the glucose level to 5.6 mM and calculating the percentage increase by determining the difference between the basal insulin secretion and the maximal response. The percentage increase of the response was greater for human islets and the re-aggregated clusters in 5.6 mM, than that in 11.2 mM glucose (Table 3).
Table 3.
Effect of glucose concentrations on responses to other compounds affecting insulin secretion
| Native rat islets (%) | Native human islets (%) | Human reaggreagates (%) | |
|---|---|---|---|
| Bay K 8644 (11.2 mM glucose) | 153 | 79 | 236 |
| Bay K 8644 (5.6 mM glucose) | – | 83 | 325 |
| Glibenclamide (11.2 mMglucose) | 533 | 41 | 164 |
| Glibenclamide (5.6 mMglucose) | – | 10 | 106 |
| Tolbutamide (11.2 mM glucose) | 244 | 88 | 168 |
| Carbachol (11.2 mM glucose) | 376 | 74 | 145 |
| Carbachol (5.6 mM glucose) | – | – | 106 |
| Caffeine (11.2 mM glucose) | 561 | 111 | 220 |
| GLP-1 (11.2 mM glucose) | 255 | 26 | 38 |
| α-Ketoisocaproic acid (2.8 mM glucose) | 2764 | – | 185 |
| GRP (11.2 mM glucose) | 19 | – | 31 |
| Somatostatin (11.2 mM glucose) | −63 | – | −71 |
| Nifedipine (11.2 mM glucose) | −88 | −36 | −70 |
| Nifedipine (5.6 mM glucose) | −80 | – | −88 |
| Diazoxide (11.2 mM glucose) | −83 | – | −65 |
| Diazoxide (5.6 mM glucose) | – | – | −87 |
| Sodium azide (11.2 mM glucose) | −93 | – | −64 |
The concentration-response curve was calculated in half-log concentrations for each of the compounds listed at either 5.6 or 11.2 mM glucose, and the percentage increase or decrease (negative number) compared with basal (no compound) conditions was calculated. A dash indicates that a concentration–response curve was not generated for that compound at that glucose concentration. GRP, gastrin-releasing peptide.
Sulfonylurea drugs
Glibenclamide is an anti-diabetic drug that belongs to the sulfonylurea class. Its primary mechanism of action is to block the ATP-regulated potassium channel (KATP, KIR6.x) and induce and/or stimulate electrical activity in the beta cell, which in turn enhances insulin secretion (Garcia-Barrado et al., 1996). Figure 3A summarizes the concentration–secretion curve of rat islets in response to glibenclamide. Native rat islets showed a predictable increase in insulin secretion with a significant insulin response at 31.6 nM glibenclamide, and an EC50 of 37.9 ± 30.6 nM. Human native islets did not respond with increased insulin secretion at any concentration tested (Figure 3B). However, human re-aggregated islet clusters released more insulin when exposed to 10 nM glibenclamide (Figure 3C), resulting in an EC50 of 13.1 ± 20.6 nM. The rat islets had the greatest percentage increase in insulin secretion when normalized to baseline (Table 3). Reducing glucose to 5.6 mM failed to uncover improved glibenclamide efficacy in native human islets or in re-aggregated clusters (Table 3).
Figure 3.
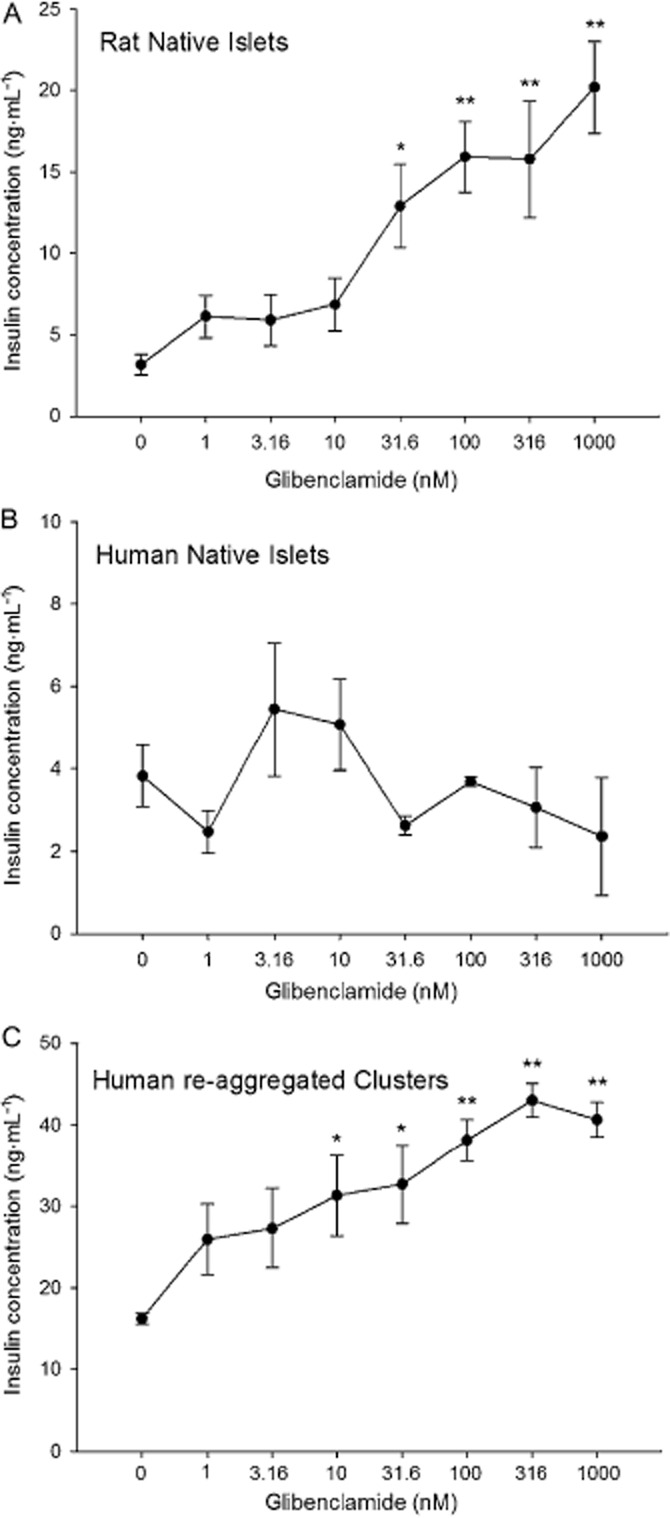
Islet and re-aggregated cluster responses to glibenclamide. (A) Native rat islets were exposed to increasing concentrations of glibenclamide with greater release of insulin. (B) Native human islets showed no response to glibenclamide. (C) Human re-aggregated clusters responded with increasing insulin secretion from 10 nM glibenclamide. *P < 0.05. **P < 0.01, significantly above baseline.
Another sulfonylurea drug that has been widely used in humans is tolbutamide (Schöfl et al., 2000). Like glibenclamide, tolbutamide induced the expected increase in insulin secretion from native rat islets and from human re-aggregated islet clusters, but not from native human islets (Figure 4). None of the concentrations of tolbutamide caused insulin secretion that was higher than the basal level in the native human islets (Figure 4B). In contrast, increases above baseline were identified at 3.16 μM in human re-aggregated clusters (Figure 4C) and at 10 μM in rat islets (Figure 4A). The EC50 for the rat islet preparation was 11.8 ± 3.6 μM. Human re-aggregated clusters responded to tolbutamide with an EC50 of 3.1 ± 1.3 μM. The magnitude of the response as measured by percentage increase to tolbutamide was higher in the native rat islets than the human re-aggregated clusters (Table 3).
Figure 4.
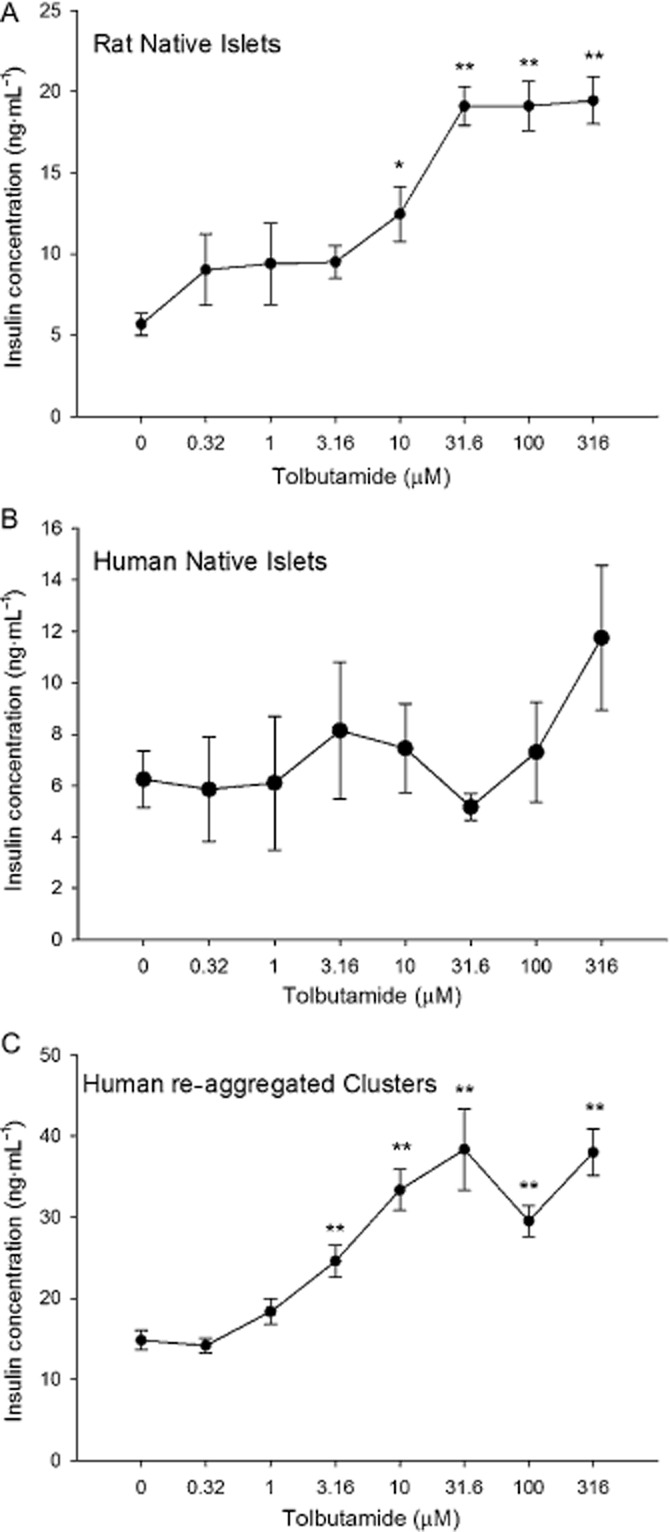
Islet and re-aggregated cluster responses to tolbutamide. (A) Native rat islets were exposed to increasing concentrations of tolbutamide with increased release of insulin at 10 μM tolbutamide and higher. (B) Native human islets showed no response to tolbutamide. (C) Human re-aggregated clusters responded with increasing insulin secretion from 3 μM tolbutamide. *P < 0.05. **P < 0.01, significantly above baseline.
Mitochondrial metabolism
The mitochondrial stimulator, α-ketoisocaproic acid (as sodium 4-methyl-2-oxovalerate) is known as a mitochondrial fuel that induces the release of insulin from dispersed beta cells (Hutton et al., 1980; Lenzen et al., 1982; Takei et al., 2013), and native rodent islets by generating oxaloacetate (Panten et al., 2013). In rat islets, there was a steady increase in insulin secretion with concentrations of α-ketoisocaproic acid of 5.6 mM and higher (Figure 5A). Human re-aggregated islets had a response with a lower magnitude, but with significant increases over baseline at 22.4 mM α-ketoisocaproic acid (Figure 5B).
Figure 5.
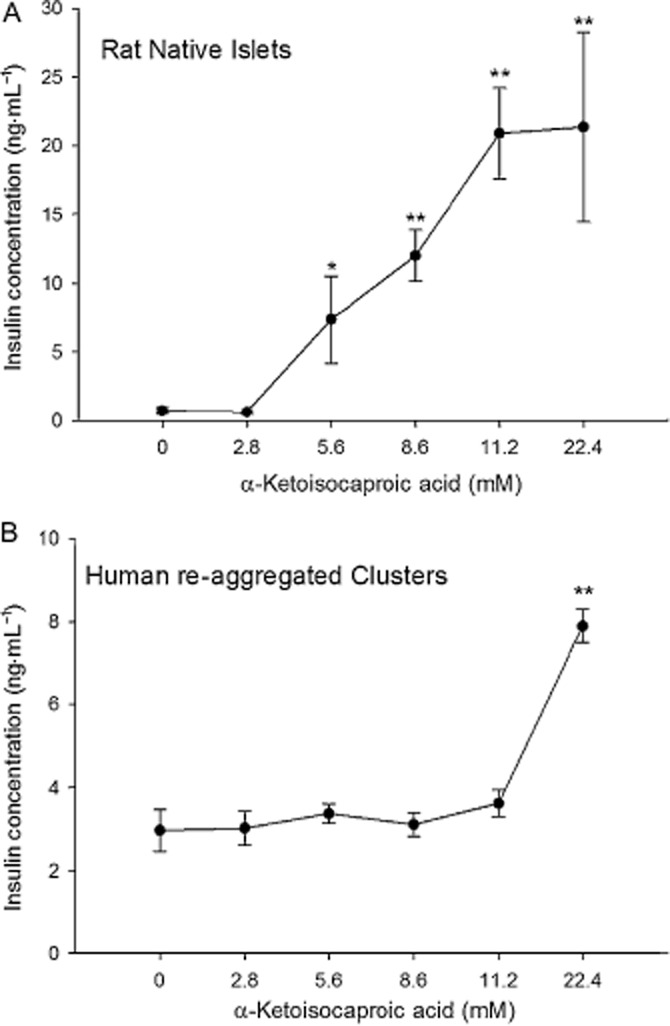
Islet and re-aggregated cluster responses to α-ketoisocaproic acid. (A) Native rat islets were exposed to increasing concentrations of α-ketoisocaproic acid with increased release of insulin at concentrations of 5.6 mM and higher. (B) Human re-aggregated clusters responded with increasing insulin secretion, but only at the highest concentration of 22.4 mM. *P < 0.05. **P < 0.01, significantly above baseline.
Cholinergic stimulation
Carbachol has long been used to study the muscarinic stimulation of beta cells by activating the second messenger, phospholipase C (Konrad et al., 1992). Both the native rat islets and the human re-aggregated clusters showed significant differences from baseline in response to 3.16 μM carbachol (Figure 6A) in the presence of 11.2 mM glucose. The EC50 for rat islets was 2.4 ± 1.1 μM and 7.1 ± 1.9 μM for the human re-aggregated islets. Native human islets failed to respond to carbachol in a concentration-dependent manner. Lowering the glucose concentration to 5.6 mM had little effect on the responses to carbachol in re-aggregated human islet cell clusters (Table 3).
Figure 6.
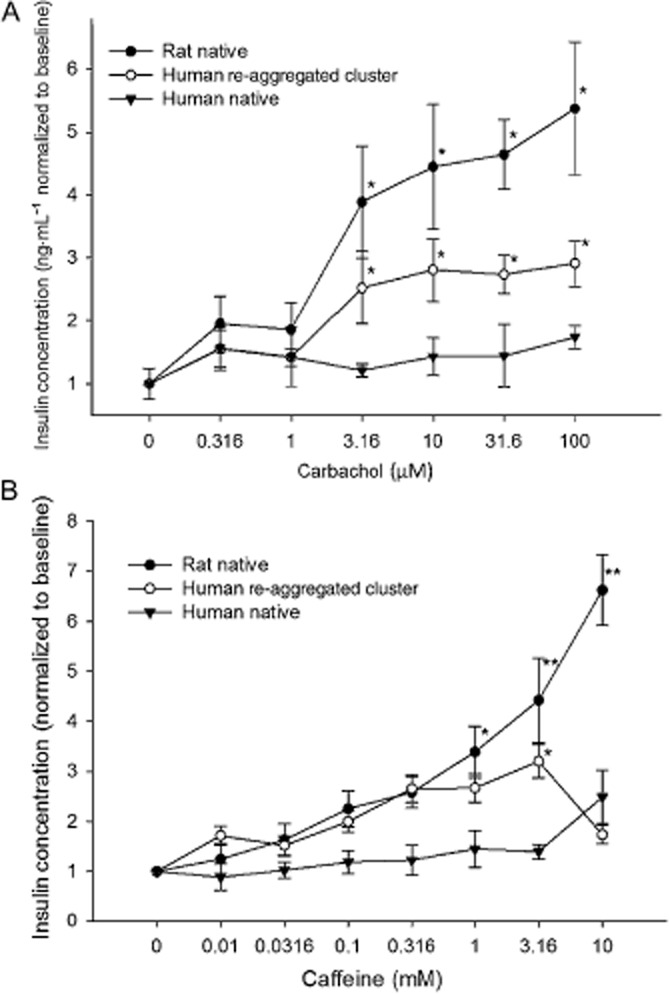
Islet and re-aggregated cell cluster responses to insulin secretagogues. In order to illustrate the dynamic range of the responses, data were normalized to the baseline (0 drug) level. (A) Carbachol elicited a strong response from rat islets and human re-aggregated cell clusters with a threshold at 3.16 nM. There was no measurable response from native human islets. (B) Caffeine stimulated a similar response in rat islets and human islet clusters with thresholds at 1-3mM. Secretion from human re-aggregated clusters declined at 10 mM caffeine. *P < 0.05. **P < 0.01, significantly below baseline.
Intracellular Ca2+ release
Caffeine acts to increase intracellular Ca2+ by inducing release through the Ca2+ channels of the endoplasmic reticulum. A strong sensitivity to caffeine was identified in the rat islets, with significantly increased secretion above 1mM caffeine (Figure 6B). The EC50 value for the human re-aggregated clusters was 0.04 ± 0.06 mM, but an EC50 value could not be calculated for rat islets, as the effect had not reached a plateau at the highest concentration tested (10 mM). The human re-aggregated islet clusters showed signs of possible caffeine toxicity at 10 mM caffeine. In native human islets, the magnitude of the response was clearly less than that of the rat islets (Table 3). In fact, there was no significant increase in insulin release from native human islets at any concentration of caffeine tested, although there was a trend towards increased secretion at the highest (10 mM) concentration (P < 0.2).
Gut hormones and neuropeptides
Glucagon-like peptide-1 (GLP-1) is a gut hormone that stimulates insulin secretion from beta cells in conditions of high glucose. Rat islets responded with a significantly higher release of insulin at 0.3 nM GLP-1 and an EC50 value of 0.14 ± 0.12 nM (Figure 7). Native human islets failed to respond to increasing concentrations of GLP-1. Human re-aggregated islet clusters showed the same lack of sensitivity to GLP-1 as native human islets, below 100 nM GLP-1.
Figure 7.
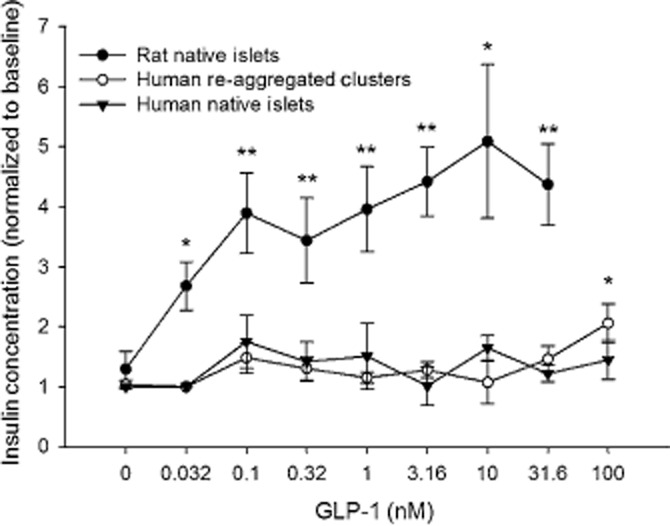
Islet and re-aggregated islet cell clusters response to GLP-1. Only rat islets responded robustly to GLP-1 with increasing concentrations. *P < 0.05. **P < 0.01, significantly below baseline.
Gastrin-releasing peptide is a neurotransmitter released by nerves within and around islets during activation of the vagus nerve (Knuhtsen et al., 1985). There was little effect on either native rat islets or re-aggregated human cell clusters when tested repeatedly with different donor tissue (Table 3).
Insulin secretion inhibitors
Somatostatin
Somatostatin was first identified as a hypothalamic growth hormone inhibiting factor. Later it was identified as an inhibitor of both growth hormone and insulin release. In fact, it is used clinically to treat a variety of neoplasms (Youos, 2011). In this study, exposure to somatostatin reduced insulin secretion in both native rat islets and in human re-aggregated islet clusters (Figure 8A). However, human re-aggregated clusters were more sensitive than native rat islets because insulin was lower than basal levels for all concentrations of the drug tested on the clusters. The maximal percentage change was nearly the same for the rat and human re-aggregated cluster groups (Table 3).
Figure 8.
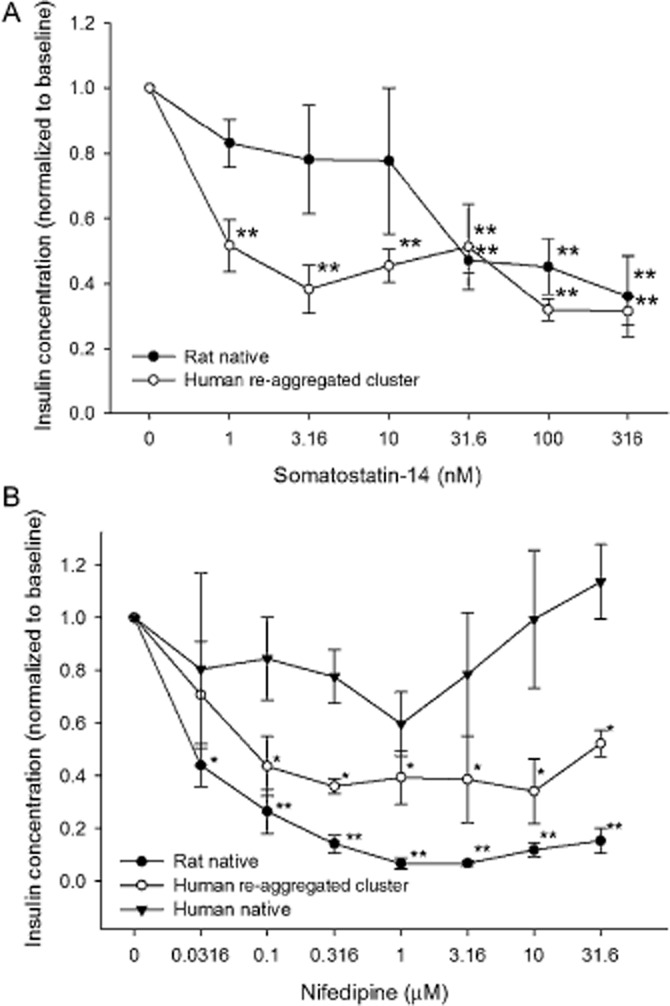
Islet and re-aggregated islet clusters response to inhibitors of insulin secretion. (A) Somatostatin induced a concentration-dependent reduction in insulin secretion in native islets and in human re-aggregated islet cell clusters. (B) Nifedipine was tested in all three groups, but was effective only in the rat islets and human re-aggregated clusters. *P < 0.05. **P < 0.01, significantly below baseline.
Calcium channel blockers
Calcium influx during action potentials is essential for the triggering of exocytosis and the consequent insulin release (Pento et al., 1974). Blocking influx through voltage-dependent Ca2+ channels should reduce insulin secretion at stimulatory glucose levels (Al-Mahmood et al., 1986). Exposure of rat islets demonstrated a strong sensitivity to the Ca2+ channel blocker, nifedipine (Figure 8B). In the native rat islets, every concentration tested was significantly lower than basal levels with an EC50 of 0.027 ± 0.004 μM. In human re-aggregated islet clusters significance was reached at 0.1 μM nifedipine, but with a lower dynamic range than rat islets (Table 3) and an EC50 of 0.014 ± 0.014 μM. In contrast, native human islets did not respond to nifedpine.
ATP-dependent K+ channel (KATP) activators
Diazoxide directly activates KATP channels, repolarizes the beta cell, inhibits electrical activity and subsequently inhibits insulin release (Jijakli and Malaisse, 1998b; Wajchenberg, 2007). Diazoxide blocked insulin release in the rat islets and human re-aggregated islet clusters in a concentration-dependent manner in 11.2 mM glucose (Figure 9A). The EC50 for rat islets was 142. ± 312 μM and for human re-aggregated clusters was 8.09 ± 4.22 μM. When glucose was reduced to 5.6 mM, the secretion of insulin was still inhibited (Table 3).
Figure 9.
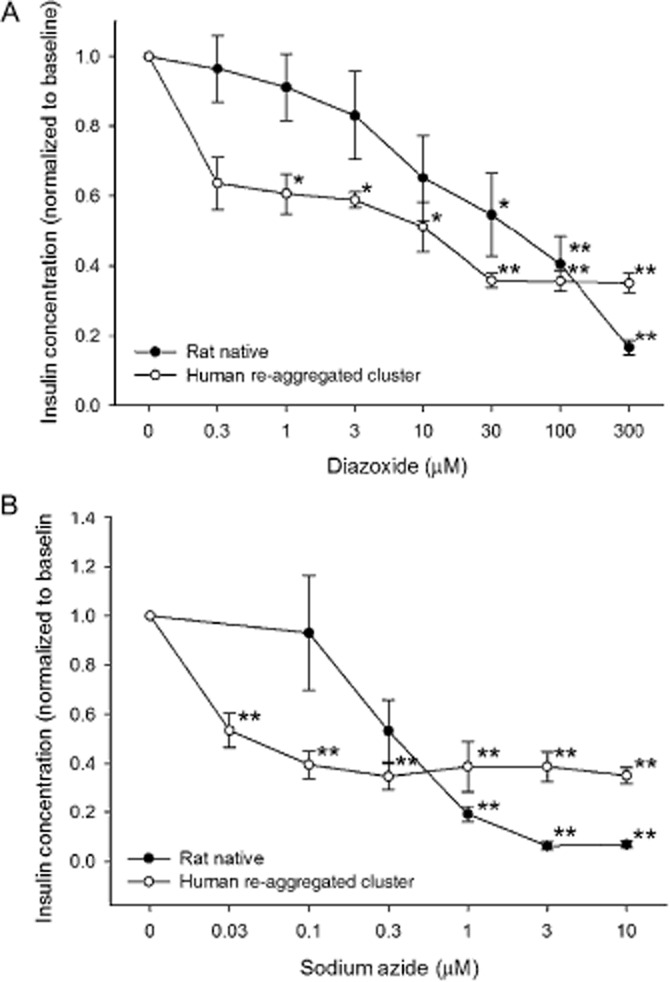
Islet and re-aggregated clusters response to mitochondrial inhibition. (A) Diazoxide reduced insulin secretion in both groups. (B) Sodium azide affected the human re-aggregated islet clusters at lower concentrations than those affecting rat islets. *P < 0.05. **P < 0.01, significantly below baseline.
Mitochondrial cytochrome c oxidase inhibitor
Sodium azide inhibits mitochondrial metabolism raising intracellular ADP levels, which in turn activate KATP channels and decrease insulin secretion from the beta cell (Misler et al., 1992). When islets were exposed to sodium azide, insulin secretion declined first in the human re-aggregated clusters with an EC50 of 0.016 ± 0.007 μM, and at higher concentrations in the rat islets with an EC50 of 0.085 ± 0.006 μM (Figure 9B). At each concentration tested, the re-aggregated islet cell clusters had a significant decline in the insulin released. The reduction in rat islets was only significant at the highest three concentrations, although the maximal magnitude of the response was greater in the rat islets (Table 3). Some caution should be taken when interpreting the role of mitochondria in insulin release, as sodium azide may have a role in increasing the level of reactive oxygen species, which can also potentiate insulin secretion.
Quality Control
Assays used in support of drug development need to provide consistent and predictable results for a large number of compounds that are being tested. Even secondary or tertiary assays can see more than a hundred different molecules. Therefore, any assay used needs to pass certain quality criteria, which are nicely outlined in the NIH Assay Guidance Manual (Iversen et al., 2004). The first pass quality test used is the Z-factor, which calculates the statistical effect size of the test compound (Zhang et al., 1999). Z-factor values were calculated for each assay and are provided in Table 4. While the Z-factor for glucose stimulation of the rat islets scored in the ‘excellent’ range, none of the other drugs tested scored in that category. In fact, all of the Z-factor values for the drugs tested on rat islets were in the negative range, even though each rat assay had six replications and a large percentage change (Table 3). Likewise, native human islets had Z-factor scores that were all in the negative range, indicating very poor drug assays. The Z-factor calculated for glucose stimulation was 0.11, indicating a marginal assay. For human re-aggregated clusters, 30% of the responses were in the excellent assay category (Table 4).
Table 4.
Quality of responses: Z-factor
| Native rat islets | Native human islets | Human re-aggregated clusters | |
|---|---|---|---|
| Bay K 8644 | −3.11 | −162.74 | −0.03 |
| Glibenclamide | −0.48 | −5.32 | 0.51* |
| Tolbutamide | −0.09 | −2.04 | 0.37* |
| Carbachol | −0.70 | −99.85 | −2.26 |
| Caffeine | −0.26 | −2.25 | −6.31 |
| α-Ketoisocaproic acid | −1.32 | – | 0.03 |
| GLP-1 | −0.56 | −8.01 | −2.91 |
| GRP | −9.88 | – | −15.19 |
| IBMX | −0.23 | −0.79 | −1.69 |
| Somatostatin | −1.50 | – | 0.54* |
| Nifedipine | −0.55 | −41.95 | −0.89 |
| Diazoxide | −0.35 | – | −1.45 |
| Sodium azide | −0.80 | – | 0.53* |
The statistical effect size was calculated as described by Iversen et al. (2004).
Z-factor score, indicating a positive response by the compound >0.4 is rated as excellent. Only the human re-aggregated islet clusters demonstrated any assays scoring in the excellent category.
The Z-factor indicates the strength of the response to the test compound but it does not indicate the strength of the assay, independent of the test compound. Another parameter, the signal window, can be calculated from the negative and positive controls, and thus does not involve the potency of the test compound. Signal window values were calculated for the human re-aggregated islets and human native islets only, based on the basal conditions (insulin secretion in 11.2 mM glucose) and maximal stimulation with 30 mM K+ (20 mM glucose) to depolarize the cells leading to maximal insulin secretion (Jijakli and Malaisse, 1998a; Hatlapatka et al., 2009). According to the NIH Assay Guidance Manual, the threshold signal window for an ‘excellent’ assay is greater than 2 (Iversen et al., 2004). Over 60% of the time, the human re-aggregated cluster assay fell in the excellent range based on the signal window (Table 5). In contrast, none of the tests completed on the native human islets fell in the excellent or moderate categories.
Table 5.
Quality of assays: signal window
| Native human islets | Human re-aggregated clusters | |
|---|---|---|
| Bay K 8644 | −0.25 | 3.89* |
| Glibenclamide | −7.67 | 5.40* |
| Tolbutamide | 0.18 | 2.19* |
| Carbachol | −6.5 | 1.67 |
| Caffeine | −3.54 | 0.73 |
| GLP-1 | −29.01 | 0.29 |
| IBMX | −3.3 | 6.68* |
| Nifedipine | −4.07 | 6.78* |
The signal window is a based on the positive and negative controls used for each plate. In this case the negative control was 11.2 mM glucose with no added drug. The positive control was 20 mM glucose + 30 mM K+.
Signal windows >2 are rated as excellent (Iversen et al., 2004)
Discussion
The procedure of reducing large numbers of compounds to a few good leads is an inherently risky process with many chances for incorrect decisions. New methods that allow primary screening using systems that are more predictive of the in vivo response are vitally important when searching for the next class of drugs to treat diabetes. Native human islets could be used for screening, but they vary greatly in size and quality so that different compounds are ultimately tested in an assay with very high intrinsic variability as demonstrated by the large error bars shown in Figures 3B and 4B, unless an extra step of size separation is included, either manually or using a size-sorting instrument (Steffen et al., 2011). In addition, diffusion limitations in the large human islets block many drugs from reaching the cells at the core of the islet (Williams et al., 2012).
One of the most important observations in this study is that the use of human islets to generate re-aggregated islet cell clusters provided an assay system with improved sensitivity to many of the well-validated pathways we examined in this study, thereby making re-aggregation the system of choice for generating data from human in vitro assays. Specifically, Figures 1C and D illustrate the ability to take intact human islets, disperse them into single cell suspensions and then to re-aggregate them into clusters that release significantly more insulin at lower glucose concentrations. In fact, the robust responses of the re-aggregated clusters to the commonly used diabetic drugs, glibenclamide and tolbutamide, suggest that the re-aggregated islets could yield results predictive of the in vivo (clinical) responses. Moreover, re-aggregated clusters can respond with an increase in insulin secretion to repeated glucose stimulations. Specifically, re-aggregated human islets were perifused with 2.8 mM glucose for 1 h, followed by a high-glucose challenge for 30 min (16 mM), returned to low glucose (30 min), and a second challenge with high glucose and return to the low-glucose condition.
During the first challenge, insulin secretion rose to a level 25 times above baseline. The subsequent increase was significantly higher than baseline, but only 60% as much as the first insulin peak (S. Rawal and L. Stehno-Bittel, unpubl. obs.).
The generation of re-aggregated islet clusters also allows for maximal use of the donor tissue as all live endocrine cells are used to generate clusters of uniform size (Ramachandran et al., 2013). This uniformity of size in the re-aggregated clusters eliminated the need for post-assay normalization to tissue volume. For the tests using native islets, approximately the same number and size of islets were manually placed in each well. Contrastingly, the clusters were automatically dispensed without regard to numbers. Yet, the variation between repetitions was small enough to discern drug effects using the clusters with acceptable quality control results. In addition, re-aggregated islet clusters have a lower diffusion barrier that allows large molecules to reach even the core cells (Ramachandran et al., 2013). The native rat islets had a greater percentage change for nearly every drug (Table 3), yet the Z scores were all negative, even with six replications. This indicates that the SD for each replicate was high (Zhang et al., 1999). The same was true for the native human islets. In contrast, the human reaggregates had some Z scores that were in the positive range. More importantly, the signal window was never acceptable for the native human islets, but fell in the acceptable range for the majority of assays using re-aggregated islet cell clusters.
Another important question is which of these preparations (native rat islet, human clusters or native human islet) was more predictive of the human responses in vivo. The sulfonylureas stimulate insulin secretion in vivo, comprising a common category of drugs prescribed for the treatment of diabetes. Both sulfonylureas tested, glibenclamide and tolbutamide, induced a robust increase in insulin secretion in rat islets and human re-aggregates, but not in native human islets. One possible explanation for the increased insulin secretion in re-aggregated islet clusters compared with native islets is that, for unknown reasons, the re-aggregated islets produced and secreted more glucagon. However, we have already shown that there was no difference in the percentage of glucagon-positive alpha-cells in the re-aggregated islets, compared with the native islets (Ramachandran et al., 2013). In addition, we conducted preliminary studies using elisa and found no difference in the amount of glucagon released (when normalized to islet volume) between native and re-aggregated islet clusters.
Initially, the lack of response to GLP-1 in the human islets or re-aggregated islet clusters indicated a poor prediction of the outcomes of the drug in humans. GLP-1 analogues are widely used for the treatment of diabetes and are known to enhance insulin secretion in humans (Dalle et al., 2013). The underlying reason for the lack of a response to GLP-1 is unclear, but could be dependent on the duration of GLP-1 exposure. It is important to note that, whereas groups working with rodent islets (Dachicourt et al., 1997; Schwasinger-Schmidt et al., 2013), dissociated human islet cells (Huypens et al., 2000) or cultured beta cell lines (Ding et al., 2011) have clearly shown an enhancement of glucose sensitivity to insulin secretion by GLP-1 or its analogues with short-term exposures, studies using isolated human islets have been less conclusive. In the few articles on responses of isolated human islets exposed to GLP-1, the exposure was typically long term. In isolated human islets, a 24 h exposure to GLP-1 is not uncommon (Park et al., 2013). When a timed study was conducted, the greatest increase in insulin secretion occurred with a 3 day exposure to GLP-1 (Farilla et al., 2003). Our own experience with GLP-1 responses in native human islets suggested that with a 90 min exposure, insulin secretion was rarely enhanced more than 50% in 11.2 mM glucose (K. Bokvist and X. Peng, unpubl. obs.), whereas that same exposure caused a robust enhancement of insulin release in native rat islets and in re-aggregated rat islet cell clusters (K. Ramachandran and L. Stehno-Bittel, unpubl. obs.). Thus, the lack of effect in human re-aggregated islets to GLP-1 may reflect more the duration of stimulation than the ability to respond. However, given that GLP-1 when administered to humans rapidly increases insulin secretion (see Nauck et al., 1993), this is a topic that would require additional studies both in native and re-aggregated human islets.
Of the inhibitors, somatostatin decreases insulin secretion in humans. In fact, somatostatin injections reduced basal insulin levels (Alberti et al., 1973). In this study, both rat islets and human re-aggregated cell clusters demonstrated a marked inhibition of insulin secretion that was greater than previously reported levels of inhibition in rat islets (Csernus et al., 1998). Nifedipine has been studied in patients with insulin resistance, and no effect on basal or oral glucose tolerance was noted (Maccario et al., 1998). However, another study reported a slight inhibition in the early insulin response in the presence of nifedipine (Valensi et al., 1991). Two weeks of treatment with nifedipine in non-diabetic subjects reduced the response of beta cells to glucose (Jasik et al., 1996). Thus, the less robust response to nifedipine in the human reaggregates compared with native rat islets may be more predictive of the human in vivo response to the drug. Overall, our findings in re-aggregated islet clusters combined with in vivo observations in humans suggest that the re-aggregated islet cluster is representative of human outcomes for the target classes mentioned earlier. While rat islets remain a good indicator, they are apparently overly sensitive to calcium blockers like nifedipine.
In summary, re-aggregated human islet cell clusters offer some benefits over working with rat islets as a secondary screening tool for new compounds, acting on insulin release. The small diameter re-aggregated clusters have a lower variability, resulting in improved assay quality and general predictability of the in vivo response.
Acknowledgments
This work was supported by the Lilly Research Laboratories, and a Lilly Research Award Program (LRAP) grant to KB and LSB.
Glossary
- EBSS
Earle's balanced salt solution
- GLP-1
glucagon like peptide 1
- SI
stimulation index
Conflict of Interest
KR and LSB have licensed the patents for the micromolds from the University of Kansas and started a private company, Likarda. KB and XP are employees of Eli Lilly and Co. KB owns common stock or options in Eli Lilly and Co.
References
- Al-Mahmood H, el Khatim M, Gumaa K, Thulesius O. The effect of calcium-blockers nicardipine, darodipine, PN-200-110 and nifedipine on insulin release from isolated rat pancreatic islets. Acta Physiol Scand. 1986;126:295–298. doi: 10.1111/j.1748-1716.1986.tb07817.x. [DOI] [PubMed] [Google Scholar]
- Alberti K, Christensen N, Christensen S, Hansen A, Iversen J, Lundbaek K, et al. Inhibition of insulin secretion by somatostatin. Lancet. 1973;2:1299–1301. doi: 10.1016/s0140-6736(73)92873-0. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmar M. New physiological effects of the incretin hormones GLP-1 and GIP. Dan Med Bull. 2011;58:B4248. [PubMed] [Google Scholar]
- Boyle J, Thompson T, Gregg E, Barker L, Williamson W. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernus V, Hammer T, Peschke D, Peschke E. Dynamic insulin secretion from perifused rat pancreatic islets. Cell Mol Life Sci. 1998;54:733–743. doi: 10.1007/s000180050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dachicourt N, Serradas P, Bailbe D, Kergoat M, Doare L, Portha B. Glucagon-like peptide-1(y-36)- amide confers glucose sensitivity to previously glucose-incompetent beta-cells in diabetic rats: in vivo and in vitro studies. J Endocrinol. 1997;155:369–376. doi: 10.1677/joe.0.1550369. [DOI] [PubMed] [Google Scholar]
- Dalle S, Burcelin R, Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic β-cell impairments in type 2 diabetes. Cell Signal. 2013;25:570–579. doi: 10.1016/j.cellsig.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Ding SY, Nkobena A, Kraft CA, Markwardt ML, Rizzo MA. Glucagon-like peptide 1 stimulates post-translational activation of glucokinase in pancreatic beta cells. J Biol Chem. 2011;286:16768–16774. doi: 10.1074/jbc.M110.192799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat B, Almelkar A, Ramachandran K, Williams SJ, Huang HH, Zamierowksi D, et al. Small human islets comprised of more β-cells with higher insulin content than large islets. Islets. 2013;5:87–94. doi: 10.4161/isl.24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- Garcia-Barrado M, Jonas J, Gilon P, Henquin J. Sulphonylureas do not increase insulin secretion by a mechanism other than a rise in cytoplasmic Ca2+ in pancreatic beta-cells. Eur J Pharmacol. 1996;298:279–286. doi: 10.1016/0014-2999(95)00806-3. [DOI] [PubMed] [Google Scholar]
- Hatlapatka K, Willenborg M, Rustenbeck I. Plasma membrane depolarization as a determinant of the first phase of insulin secretion. Am J Physiol Endocrinol Metab. 2009;297:E315–E322. doi: 10.1152/ajpendo.90981.2008. [DOI] [PubMed] [Google Scholar]
- Hohmeier H, Newgard C. Cell lines derived from pancreatic islets. Mol Cell Endocrinol. 2004;228:121–128. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Huang H, Ramanchadran K, Stehno-Bittel L. A replacement for islet equivalents with improved reliability and validity. Acta Diabetol. 2012;50:687–696. doi: 10.1007/s00592-012-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J, Sener A, Malaisse W. Interaction of branched chain amino acids and keto acids upon pancreatic islet metabolism and insulin secretion. J Biol Chem. 1980;255:7340–7346. [PubMed] [Google Scholar]
- Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–1019. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- Iversen P, Benoit B, Chen Y, Dere W, Devanarayan V, Eastwood B, et al. Sciences ELCatNCfAT HTS assay validation. 2004. pp. 1–31. Assay Guidance Manual [Internet]. Bethesda, MD. Available at: http://www.ncbi.nlm.nih.gov/books/NBK53196 (accessed 06/26/2013)
- Jasik M, Kasperska-Dworak A, Czyzyk A. Effect of nifedipine, captopril and prazosin on secretory function of pancreatic beta-cells in hypertensive patients with type-2 (non-insulin-dependent) diabetes and in hypertensive non-diabetics. Diabetes Res Clin Pract. 1996;33:59–66. doi: 10.1016/0168-8227(96)01258-2. [DOI] [PubMed] [Google Scholar]
- Jijakli H, Malaisse W. Glucose-induced mobilisation of intracellular Ca2+ in depolarised pancreatic islets. J Physiol (Paris) 1998a;92:31–35. doi: 10.1016/S0928-4257(98)80020-X. [DOI] [PubMed] [Google Scholar]
- Jijakli H, Malaisse W. Verapamil- and cadmium-resistant stimulation of calcium uptake and insulin release by D-glucose in depolarised pancreatic islets exposed to diazoxide. Cell Signal. 1998b;10:661–665. doi: 10.1016/s0898-6568(98)00009-6. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H, Aubert R, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- Knuhtsen S, Holst JJ, Jensen SL, Knigge U, Nielsen OV. Gastrin-releasing peptide: effect on exocrine secretion and release from isolated perfused porcine pancreas. Am J Physiol. 1985;248:G281–G286. doi: 10.1152/ajpgi.1985.248.3.G281. [DOI] [PubMed] [Google Scholar]
- Kohn D, Clifford C. Biology and diseases of rats. In: Fox J, Anderson L, Lowe F, editors. Laboratory Animal Medicine. 2nd edn. Vol. 2. New York: Academic Press; 2002. pp. 121–167. [Google Scholar]
- Konrad R, Jolly Y, Major C, Wolf B. Carbachol stimulation of phospholipase A2 and insulin secretion in pancreatic islets. Biochem J. 1992;287:283–290. doi: 10.1042/bj2870283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S, Formanek H, Panten U. Signal function of metabolism of neutral amino acids and 2-keto acids for initiation of insulin secretion. J Biol Chem. 1982;257:6631–6633. [PubMed] [Google Scholar]
- Maccario M, Oleandri S, Avogadri E, Rossetto R, Grottoli S, Procopio M, et al. Effects of 3-month nifedipine treatment on endocrine-metabolic parameters in patients with abdominal obesity and mild hypertension. J Endocrinol Invest. 1998;21:56–63. doi: 10.1007/BF03347287. [DOI] [PubMed] [Google Scholar]
- MacGregor RR, Williams SJ, Tong PY, Kover K, Moore WV, Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab. 2006;290:E771–E779. doi: 10.1152/ajpendo.00097.2005. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S, Barnett D, Falke L. Effects of metabolic inhibition by sodium azide on stimulus-secretion coupling in B cells of human islets of Langerhans. Pflugers Arch. 1992;421:289–291. doi: 10.1007/BF00374842. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panten U, Willenborg M, Schumacher K, Hamada A, Ghaly H, Rustenbeck I. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metabolism. 2013;62:1375–1378. doi: 10.1016/j.metabol.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Park YJ, Ao Z, Kieffer TJ, Chen H, Safikhan N, Thompson DM, et al. The glucagoin-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: implications in type 2 diabetes and islet transplantation. Diabetologia. 2013;56:508–519. doi: 10.1007/s00125-012-2802-z. [DOI] [PubMed] [Google Scholar]
- Pento J, Kagan A, Glick S. Influence of altered states of calcium homeostasis on insulin secretion in rats and rabbits. Horm Metab Res. 1974;6:177–180. doi: 10.1055/s-0028-1093867. [DOI] [PubMed] [Google Scholar]
- Ramachandran K, Williams S, Huang H, Novikova L, Stehno-Bittel L. Engineering islets for improved performance by optimized reaggregation in a micromold. Tissue Eng Part A. 2013;19:18–26. doi: 10.1089/ten.TEA.2012.0553. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium. 2012;51:300–308. doi: 10.1016/j.ceca.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöfl C, Börger J, Mader T, Waring M, von zur Mühlen A, Brabant G. Tolbutamide and diazoxide modulate phospholipase C-linked Ca(2+) signaling and insulin secretion in beta-cells. Am J Physiol Endocrinol Metab. 2000;278:E639–E647. doi: 10.1152/ajpendo.2000.278.4.E639. [DOI] [PubMed] [Google Scholar]
- Schwasinger-Schmidt T, Robbins DC, Williams SJ, Novikova L, Stehno-Bittel L. Long-term liraglutide treatment is associated with increased insulin content and secretion in β-cells, and a loss of α-cells in ZDF rats. Pharmacol Res. 2013;76:58–66. doi: 10.1016/j.phrs.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Steffen A, Ludwig B, Krautz C, Bornstein S, Solimena M. Functional assessment of automatically sorted pancreatic islets using large particle flow cytometry. Islets. 2011;3:267–270. doi: 10.4161/isl.3.5.15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei M, Dezaki K, Ishii H, Nishio S, Sato Y, Suzuki S, et al. A new experimental model of ATP-sensitive K(+) channel-independent insulinotropic action of glucose: a permissive role of cAMP for triggering of insulin release from rat pancreatic β-cells. Endocr J. 2013;60:599–607. doi: 10.1507/endocrj.ej12-0388. [DOI] [PubMed] [Google Scholar]
- Unwin N, Whiting D, Guariguata L, Hennis A, Husseini A, Ji L. Chapter 2, The global burden. In: Unwin N, Whiting D, Guariguata L, Ghyoot G, Gan D, et al., editors. Diabetes Atlas. 5th edn. Vol. 2013. Brussels, Belgium: International Diabetes Federation; 2013. pp. 32–35. [Google Scholar]
- Valensi P, Uzzan B, Vassy R, Attali J, Perret G. Effect of nifedipine and nitrendipine on insulin release in non-diabetic obese patients: a double-blind, placebo-controlled study. Methods Find Exp Clin Pharmacol. 1991;13:557–563. [PubMed] [Google Scholar]
- Wajchenberg B. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- Walpita D, Hasaka T, Spoonamore J, Vetere A, Takane K, Fomina-Yadlin D, et al. A human islet cell culture system for high-throughput screening. J Biomol Screen. 2012;17:509–518. doi: 10.1177/1087057111430253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom J, Sereda S, Stiles L, Elorza A, Allister E, Neilson A, et al. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS ONE. 2012;7:e33023. doi: 10.1371/journal.pone.0033023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Schwasinger-Schmidt T, Zamierowski D, Stehno-Bittel L. Diffusion into human islets is limited to molecules below 10 kDa. Tissue Cell. 2012;44:332–341. doi: 10.1016/j.tice.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Youos J. The role of α-, δ- and F cells in insulin secretion and action. Diabetes Res Clin Pract. 2011;93(Suppl. 1):S25–S26. doi: 10.1016/S0168-8227(11)70009-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chung T, Oldenburg K. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]