Abstract
BACKGROUND AND PURPOSE
Recent and ongoing clinical studies have indicated that topiramate (Topamax®) could be effective in treating ethanol or cocaine abuse. However, the effects of topiramate on the co-administration of ethanol and cocaine remain largely unknown.
EXPERIMENTAL APPROACH
We studied the effects of topiramate, in Wistar rats, on operant ethanol self-administration with the co-administration of cocaine (i.p.). The psychomotor effects of topiramate were examined before ethanol self-administration and cocaine exposure. Blood samples were collected to analyse ethanol and cocaine metabolism (blood ethanol levels and benzoylecgonine). Quantitative real-time PCR was used to characterize the gene expression in the prefrontal cortex.
KEY RESULTS
Topiramate prevented the cocaine-induced increased response to ethanol in a dose-dependent manner without causing any motor impairment by itself. This effect was observed when topiramate was administered before ethanol access, but not when topiramate was administered before the cocaine injection. Topiramate did not block cocaine-induced psychomotor stimulation. Topiramate reduced blood ethanol levels but did not affect cocaine metabolism. Ethanol increased the gene expression of DNA methyltransferases (Dnmt1 and Dnmt3a), the corepressor Dnmt1-associated protein 1 (Dmap1), and the RNA methyltransferase Trdmt1. These effects were prevented by topiramate or cocaine. Gene expression of histone deacetylase-2 and glutamate receptor kainate-1 were only increased by cocaine treatment. Topiramate and cocaine co-administration caused an up-regulation of dopamine (Drd1, Th) and opioid (Oprm1) receptor genes. Topiramate showed a tendency to alter episodic-like memory.
CONCLUSIONS AND IMPLICATIONS
Topiramate is an effective inhibitor of the cocaine-induced increase in operant ethanol self-administration.
Keywords: operant ethanol self-administration, Dnmt1 corepressors, histone deacetylase-2, dual dependence, DNA methyltransferases, addiction, gene expression
Introduction
Topiramate (Topamax, Janssen-Cilag S.A., Madrid, Spain) is a sulphamate that was discovered by Maryanoff and Gardocki through a model-based screening procedure, and was patented by McNeil Laboratories, Inc. in 1983 (Maryanoff and Gardocki, 1985; Tatum et al., 2009). Work in animal models (mice and cats) revealed that topiramate has potent anticonvulsant activity, and this drug was approved by the Food and Drug Administration (FDA) for the treatment of epilepsy in 1996 (Maryanoff et al., 1987; Nakamura et al., 1993; FDA, 1996). Topamax has also been approved for the prevention of migraine headaches in adults (FDA, 2012). Although the mechanism of action of topiramate is not fully understood, many results have indicated that this drug inhibits carbonic anhydrase enzymes types II and IV, which interact with diverse ion channels by either enhancing ion channel activity, as in GABAA receptors (for receptor nomenclature see Alexander et al., 2013a), or reducing ion channel activity, as in voltage-activated Na+ channels, Ca2+ channels and AMPA/kainate (but not at NMDA) receptors (see Johnson, 2005, for a review). Recently, it has been demonstrated that topiramate binds selectively to kainate receptors containing the GluK1 subunit (Braga et al., 2009).
Currently, there are ongoing clinical trials to determine whether topiramate is effective in the treatment of ethanol abuse, cocaine abuse, or the dual dependence of ethanol and cocaine (ClinicalTrials.gov, US NIH, 2009). Topiramate has been shown to reduce ethanol consumption in Wistar rats using two-bottle choice tests (Knapp et al., 2007); it reduced the motivation to lick for beer (Hargreaves and McGregor, 2007) and attenuated the withdrawal signs after chronic intermittent ethanol treatment (Cagetti et al., 2004). Effects of topiramate on ethanol intake have also been demonstrated in rats that were selectively bred for high ethanol preference and in mice (Nguyen et al., 2007; Breslin et al., 2010; Zalewska-Kaszubska et al., 2013). However, little is known about the effects of topiramate on operant ethanol self-administration in rats. Most of the evidence about the effects of topiramate on cocaine abuse comes from clinical studies in which topiramate reduced the reinforcing effects and cravings induced by cocaine in non-treatment-seeking research volunteers (Johnson et al., 2013); reduced the craving intensity and duration during outpatient treatment for cocaine dependence (Reis et al., 2008); and increased the probability of cocaine abstinence compared with placebo-treated subjects (Kampman et al., 2004). These results contrast with previous preclinical studies in which rats and mice treated with topiramate did not exhibit a decrease in the response to cocaine (Le Foll et al., 2008). Also topiramate was found to be ineffective at preventing cocaine-induced clonic seizures (Gasior et al., 1999).
Currently, many authors are considering epigenetic explanations for drug addiction (see Robison and Nestler, 2011, for a review). The hypothesis is that drugs of abuse, such as ethanol and cocaine, alter the two key epigenetic mechanisms that control gene expression: histone modifications (such as acetylation, methylation and phosphorylation) and DNA methylation patterns. Methyltransferases are enzymes that transfer methyl groups onto DNA or RNA, and in many cases, methyltransferases recruit corepressor complexes for repressing gene transcription. For example, DNA methyltransferase DNMT1 binds to histone deacetylase-2 (HDAC2) and DNA methyltransferase 1-associated protein (DMAP1), which act as corepressors of gene transcription (Rountree et al., 2000). In addition, the maintenance of genomic methylation patterns are related to the ability of DNMT1 to bind to PCNA and the ability of ubiquitin-like Pleckstrin homology domain and ring finger domains 1 (UHRF1) to target DNMT1 (Schermelleh et al., 2007; Hervouet et al., 2010; Liu et al., 2013; Schneider et al., 2013). Recent studies have shown that DNMT1 gene expression is associated with alcohol-related behaviours (Botia et al., 2012; Warnault et al., 2013), but the association of this gene with cocaine remains unknown.
The aims of this study were (i) to determine the effects of topiramate on operant ethanol self-administration with the co-administration of cocaine and (ii) to investigate whether these effects were paralleled with changes in the expression of DNA/RNA methyltransferases, of key Dnmt1 corepressor complexes, and of topiramate proteins targets. Additionally, we assessed the expression of key genes involved in dopaminergic (Drd1, Drd2 and Th) and opioid neurotransmission (Oprm1), which are related directly to the rewarding effects of ethanol and cocaine.
Methods
Subjects
Ninety-six male Wistar rats (Harlan, Barcelona, Spain), weighing 275–325 g at the start of the experiments, were housed in groups of four per cage in a temperature- and humidity-controlled environment (21 ± 2ºC, 60% relative humidity) on a 12 h reverse light/dark cycle (lights off at 0700 h). The same animals were used throughout the experiments. Seventy-one rats had access to ethanol and 25 only had access to saccharin in the operant self-administration procedures. An additional group of 32 rats was used to evaluate the effects of topiramate on episodic-like memory. That is, a total of 128 rats were used in this study. Experimental sessions were performed during the dark phase. Food and water were available ad libitum except as specified later. All research was conducted in strict adherence to the European Directive 2010/63/EU (EU 2010/63/EU) on the protection of animals used for scientific purposes. The Ethics Committee of the Faculty of Psychology of the Complutense University of Madrid approved the study. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs and general procedures for pharmacological treatments
Each day, a 10% ethanol v v-1 solution was prepared from 99% ethanol (Alcoholes Aroca, S.L., Madrid, Spain). Cocaine hydrochloride (Sigma-Aldrich, S.L., Madrid, Spain) was dissolved in physiological saline and injected i.p. at a volume of 1 mL·kg−1. Cocaine-control animals were injected with saline. Cocaine doses are expressed as the salt. Topiramate (2,3:4,5-bis-O-(1-methylethylidene)-beta-D-fructopyranose sulphamate; prepared as Topamax dispersible capsules, Janssen-Cilag, S.A., Madrid, Spain) was dissolved in 25% β-cyclodextrin (Sigma-Aldrich) and was administered p.o. at a volume of 3 mL·kg−1. Topiramate-control animals received a solution of 25% β-cyclodextrin in equivalent proportions to the animals treated with topiramate.
Throughout all of the experiments, cocaine was injected i.p. 6 h after the operant ethanol self-administration sessions. We followed this schedule to avoid cocaine-induced place-conditioned motor sensitization and motor hyperactivity in the operant ethanol chambers (Antoniou et al., 1998; Stromberg and Mackler, 2005). The dose of cocaine was chosen based on previous studies done under similar experimental conditions, where 20 mg·kg−1 cocaine resulted in a robust increase in operant ethanol self-administration (Echeverry-Alzate et al., 2012).
Topamax was administered (p.o.) at different times depending on the experiment, but was generally administered 120 min before ethanol/cocaine administration because the peak plasma concentration occurs approximately 2 h after an oral dose [FDA, 1995; AEMPS (Spanish Agency for Medicines and Health Products), 2012 ]. The rats were not deprived of food before Topamax treatment because the absorption of topiramate is independent of food intake (FDA, 1995; AEMPS, 2012). The doses of Topamax were chosen based on previous studies in rats that investigated the effects of topiramate on ethanol-related behaviours (Hargreaves and McGregor, 2007; Zalewska-Kaszubska et al., 2007; Breslin et al., 2010; Lynch et al., 2011). The administration route and the use of a marketed drug (Topamax) were chosen to strengthen the ecological validity of the study, because these most closely resemble the situation for human patients.
Ethanol self-administration and motor experiments
Apparatus and procedure
The operant ethanol sessions were conducted in eight modular chambers enclosed in sound-proof cubicles (Med Associates, Inc., St. Albans, VT, USA). The exhaust fans were inactivated because the fans increased the rate of ethanol evaporation. The chambers were equipped with two retractable levers located 7 cm above a grid floor on either side of a drinking reservoir positioned in the centre of the front panel of the chamber and 4 cm above the grid floor. The levers were counterbalanced to respond as the active lever (delivering 0.1 mL) or as the inactive lever. As far as some animals press the levers two/three times to obtain the rewarding solution, the contents of the stainless dipper were accessible to the animal until the next lever press, at least 2.5 s later, to avoid measuring dipper presentations (rewards) as lever presses. It did not use light or sound as stimuli.
Training was conducted using a modification of the methods described by Alén et al. (2009). Briefly, the rats were placed on a restricted water intake schedule for 11 h ranging from 2 to 4 days to facilitate the training in lever pressing. The length of the water restriction was dependent upon the rate of learning of the animal, 4 days being the highest number of days water intake was restricted. After this period, for the rest of the experiment, the animals had access to food and water ad libitum. During the first 4 days of training, animals received a 1% w v-1 saccharin solution (Sigma-Aldrich) in the dipper. Thereafter, the following sequence was used on a fixed-ratio 1 schedule of reinforcement: 0.2% saccharin for three sessions, 0.2% saccharin and 0.2% ethanol for three sessions, 0.16% saccharin and 2% ethanol for three sessions, 0.12% saccharin and 4% ethanol for three sessions, 0.08% saccharin and 6% ethanol for three sessions, 0.04% saccharin and 8% ethanol for three sessions, 0.02% saccharin and 10% ethanol for three sessions, and 10% ethanol for the remaining sessions. Twenty animals that had access to saccharin only and did not receive any pharmacological treatment during the study were used as the control group for the genetic expression experiments (the calibrator group – i.e. the non-ethanol-treated group). The baseline corresponded to the average number of ethanol responses obtained on the final 3 days before the first experiment in which the number of responses varied by 15% or less. Animals that did not reach at least 20 ethanol responses during the baseline period were omitted for the experiments (n = 11). All the operant ethanol sessions lasted 30 min under a fixed-ratio 1 schedule 7 days a week for the entire study.
The locomotor activity of the rats was assessed using six custom-made 40 × 35 × 35 cm rectangular boxes, and the boxes were equipped with eight photocells arranged in two lines (4 and 8 cm above the floor) that detected the locomotor activity as beam breaks.
Ethanol and benzoylecgonine analysis
To determine blood ethanol concentrations, 250 μL of blood was collected from the rat tail vein into a capillary tube (Microvette® CB 300 K2E, Sarstedt AG & Co, Nümbrecht, Germany) that contained EDTA dipotassium salt. The whole blood was centrifuged for 15 min at 1500× g using a refrigerated centrifuge, and the plasma was stored at −20°C until use. The ethanol concentration was measured using the EnzyChrom ethanol assay kit following the protocol recommended by the manufacturer (Bioassay Systems, Hayward, CA, USA). All measurements were performed in duplicate. See experiment 3 later for further details.
Benzoylecgonine, a main metabolite of cocaine, was measured in blood using the Cocaine Metabolite Direct elisa Benzoylecygonine Assay Kit, following the manufacturer's instructions (Bio-Quant, Heidelberg, Germany). Approximately 400–450 μL of blood from the rat trunk at the moment of killing (by decapitation) was collected in VACUTEST tubes (Vacutest Kima S.r.l., Arzergrande, Italy) that contain K3 EDTA. Following, plasma was obtained as described earlier and stored at −20°C until use. See experiment 3 later for further details.
Test of novel object recognition
Object recognition memory was assessed in the six custom-made 40 × 35 × 35 cm rectangular boxes described earlier located in a dimly lit room (20 luxes). All the sessions were monitored by a video camera above the apparatus. One set of test objects was made of dark red glass (18 × 8 × 4.5 cm) and the second set of objects was made of steel (22 × 6 × 6 cm). Sessions were carried out following the protocol described by Ennaceur et al. (2005). On day 1 (habituation session), each rat was exposed to one box individually for 10 min to habituate to the test environment. On day 2 (sample session), rats were placed in the box and given 3 min to explore two identical sample objects. Following, the rats were removed from the box and returned to their cages for 15 min (retention interval). Then, rats were placed in the same box with one familiar and one novel object (counterbalanced across rats) and given 3 min to explore the objects (test session). An experimenter who was blind to the experimental treatments scored the time the rats spent exploring each object, the latency of first approach to explore them and the frequency of approach. It was considered a valid object approach any directed contact with the mouth, nose or paw not including accidental contacts such as backing into the object (Bevins and Besheer, 2006).
Real-time quantitative PCR experiments
Real-time quantitative PCR, which has been described as one of the most powerful tools to quantify gene expression (Schmittgen and Livak, 2008), was performed using a LightCycler 480-II machine (Roche, Barcelona, Spain) with SYBR Green Real-time qPCR master mix (Applied Biosystems, Warrington, UK) and specific primers at 300 nm concentrations (see Table 1). The melting curves analysis showed only a single clear peak, and the sizes of the PCR products were confirmed by agarose gel electrophoresis. A 10-fold dilution series of the template was used to amplify each gene to validate the efficiency of each assay and to confirm that the amplification efficiencies of the target and reference genes were comparable (indicated by a near-zero slope value for both the target and reference genes). The 18S ribosomal RNA gene was used as an internal control for normalization. The saccharin-vehicle group (the non-ethanol-treated group) was used as a calibrator (non-treated group, n = 20 after discarding the five more high extreme saccharin responses), and the 2–ΔΔCT method was used to analyse the expression data (Schmittgen and Livak, 2008).
Table 1.
Details about the base sequences of the primers used
| Gene | Name | GenBank accession no. | Direction | Primer sequence (5′-3′) | Amplicon length (nt) | |
|---|---|---|---|---|---|---|
| Internal control | 18S | 18S ribosomal RNA gene | M11188.1 | Left | GGAGCCTGAGAAACGGCTA | 64 |
| Right | TCGGGAGTGGGTAATTTGC | |||||
| Topiramate targets | Ca2 | Carbonic anhydrase 2 (Car2), mRNA | NM_019291.1 | Left | CATTACTGTCAGCAGTGAGCAGA | 91 |
| Right | CCAGTTGTCCACCATCAGTTC | |||||
| Ca4 | Carbonic anhydrase 4 (Car4), mRNA | NM_019174 | Left | GGGAAGTTAAGAACAACCAACACT | 107 | |
| Right | CAGGTGTAACTGTATGGCCTTG | |||||
| Grik1 | Glutamate receptor, ionotropic, kainate 1 (Grik1), mRNA | NM_001111117.1 | Left | TTTGAAGCCTCCCGAAGAG | 112 | |
| Right | CCAGAGCATTGCAAATAGACTGT | |||||
| RNA methyltransferases | Trdmt1 | tRNA aspartic acid methyltransferase 1 (Trdmt1), mRNA | NM_001031643.1 | Left | CCTCCTCGACATCGTTAAGC | 77 |
| Right | TCCCTTCTATGTAGCTTCCATACC | |||||
| Rnmt | RNA (guanine-7-) methyltransferase (Rnmt), mRNA | NM_001008299.2 | Left | TGAAAACAAAATGCTCTTAAAACG | 60 | |
| Right | TTCTCGTTCGCAGGGTATG | |||||
| DNA methyltransferases | Dnmt1 | DNA (cytosine-5-)-methyltransferase 1 | ENSRNOG00000039859 | Left | GTCTACCGACTGGGTGACAGT | 70 |
| Right | GGCTGGCCATTTTGATGT | |||||
| Dnmt3a | DNA (cytosine-5-)-methyltransferase 3 alpha | ENSRNOG00000026649 | Left | AACGGAAGCGGGATGAGT | 70 | |
| Right | ACTGCAATCACCTTGGCTTT | |||||
| Dnmt1 corepressors | Hdac2 | Histone deacetylase 2 (Hdac2), mRNA | NM_053447.1 | Left | GCTGTCCTCGAGCTACTGAAA | 110 |
| Right | GTCATCACGCGATCTGTTGT | |||||
| Dmap1 | DNA methyltransferase 1-associated protein 1 (Dmap1), mRNA | NM_001015006.1 | Left | CCTTTCGCCAGGTTCAATAA | 91 | |
| Right | CCTTAGTCCATGCATCATCGT | |||||
| Pcna | Pcna, mRNA | NM_022381.3 | Left | GAACTTTTTCACAAAAGCCACTC | 103 | |
| Right | GTGTCCCATGTCAGCAATTTT | |||||
| Uhrf1 | Ubiquitin-like with PHD and ring finger domains 1 (Uhrf1), mRNA | NM_001008882.1 | Left | GCTGGAGCCCTACACACTTC | 60 | |
| Right | TTGCCCTTGTCCTCCTTG | |||||
| Reward system | Drd1 | Dopamine receptor D1 | NM_012546.2 | Left | CGAACTGTATGGTGCCCTTC | 62 |
| Right | GATGGAATCGATGCAGAATG | |||||
| Drd2 | Dopamine receptor D2 | NM_012547.1 | Left | TGAACAGGCGGAGAATGG | 70 | |
| Right | CTGGTGCTTGACAGCATCTC | |||||
| Th | Tyrosine hydroxylase | ENSRNOT00000027682 | Left | TCTCCCTGAGGGGTACAAAA | 77 | |
| Right | GTGAATTTTGGCTTCAAATGTCT | |||||
| Oprm1 | μ-Opioid receptor1 | NM_013071.2 | Left | GTGGATCGAACTAACCACCAG | 69 | |
| Right | GAGACCCAGTTAGGGCAATG |
The animals were killed by decapitation immediately before the ethanol self-administration session (after chronic topiramate treatment). The prefrontal cortex, including the frontal association cortex and the more rostral/anterior regions of the lateral-ventral-dorsal-medial areas of the orbital cortex, prelimbic cortex and secondary motor cortex (Paxinos and Watson, 1998) were immediately dissected on ice, and were quickly frozen on dry ice at −80°C. Total RNA was isolated using Tripure Isolation Reagent (Roche) and was stored at −80°C. One microgram of total RNA was reverse transcribed into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche).
Experimental design
Experiment 1: the effects of increasing the dose of topiramate on the response to ethanol and co-administration of cocaine
Here, we aimed to establish a dose–response curve for topiramate (2.5–40 mg·kg−1) on operant ethanol self-administration and cocaine co-administration (i.p.) (Figure 1A). Because topiramate often causes uncomfortable CNS side effects, such as sedation, and according to the dosage and topiramate administration protocols in clinical studies (Kampman et al., 2004; ClinicalTrials.gov, US NIH, 2009), the doses of topiramate were progressively increased every 3 days, and each dose was divided into two doses (morning and afternoon dosing). Before the pharmacological treatments, the animals were matched and distributed among groups according to the number of ethanol responses exhibited at baseline.
Figure 1.
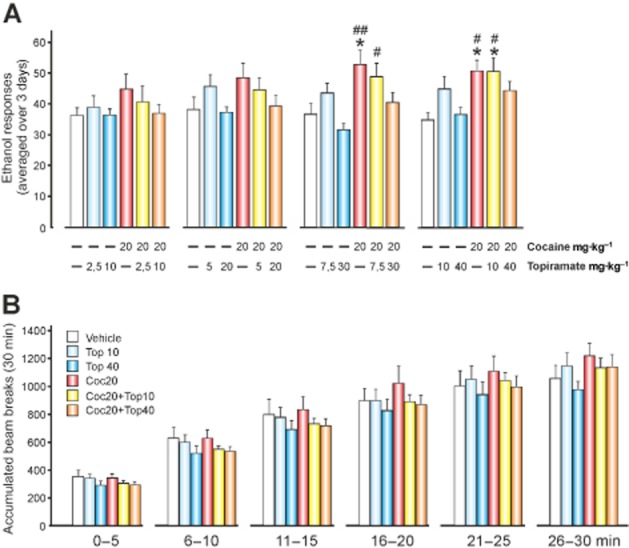
The effects of increasing the dose of topiramate on the response to ethanol and co-administration of cocaine. The doses of topiramate were progressively increased every 3 consecutive days, and each dose was divided into two doses (morning and afternoon dosing). During the morning, topiramate was administered 120 min before access to ethanol. During the afternoon, topiramate was administered 120 min after cocaine administration. (A) The mean ± SEM of the operant responses for 10% v v-1 ethanol averaged over 3 days (n = 9–11 per group). *P < 0.05 compared with the vehicle group; #P < 0.05 and ##P < 0.01 compared with 30/40 mg·kg−1 of topiramate. (B) Motor activity. Mean ± SEM of the accumulated beam breaks observed for 30 min before the operant ethanol self-administration session. There were no significant differences between the groups.
To discard any of the sedative effects of topiramate from the highest doses, which could confound the results of the operant ethanol self-administration, we monitored the locomotor activity of the animals for 30 min before the animals gained access to the operant ethanol chambers (Figure 1B). The locomotor activity was examined in 5-min periods in a single assay, and the animals were fully counterbalanced.
Experiment 2: the effect of topiramate on the behavioural effects of cocaine
We conducted experiment 2 after a washout period of 3 days from experiment 1. This experiment was conducted to investigate whether the preventative effects of topiramate on the cocaine-induced increase in responses to ethanol could be determined at the moment of topiramate administration before cocaine injection or before ethanol self-administration (Figure 2A). In the first case, before cocaine injection, it might be assumed that topiramate would block the acquisition of the effects of cocaine on the response to ethanol, whereas in the second case, before ethanol self-administration, it may be assumed that topiramate would block the expression of the effects of cocaine on the response to ethanol. Therefore, the animals were treated with a dose of 40 mg·kg−1 (p.o.) topiramate, which was found, in experiment 1, to be the most effective dose at preventing the increase in ethanol responses induced by cocaine and without motor impairment. In experiment 1, each dose was divided into two (morning and afternoon dosing). In experiment 2, topiramate was given either 120 min before the cocaine injection or 120 min before the operant ethanol session for 3 consecutive days. We used four group of rats (n = 9–11), that they were fully counterbalanced; one group received the topiramate treatment before cocaine injection for 3 days and the other received the topiramate before ethanol access for another 3 days. There was a washout period of 3 days between treatments. Thus, all animals were treated with topiramate before cocaine and before ethanol, but in a different order, except the vehicle group that was never treated with either topiramate or cocaine.
Figure 2.
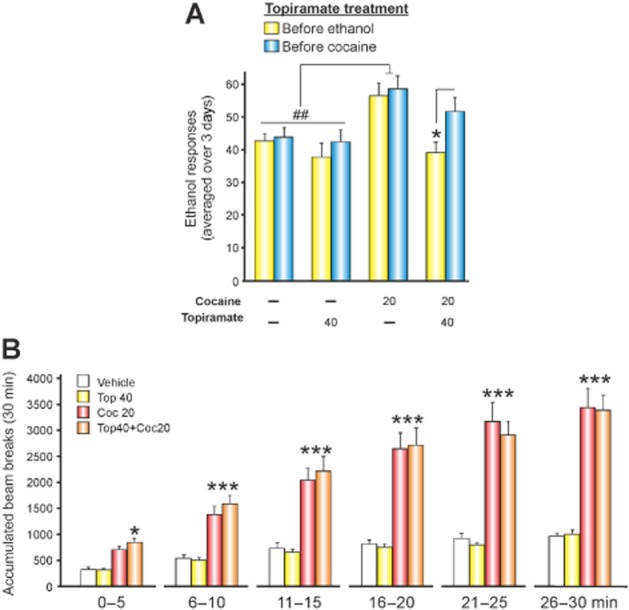
The effect of topiramate on the behavioural effects of cocaine. (A) A single dose of topiramate was administered either 120 min before the operant self-administration session or 120 min before the cocaine injection. Mean ± SEM operant responses for 10% v v−1 ethanol was averaged over three days (n = 9–11 per group). ##P < 0.01 compared with the two cocaine-groups (20 mg·kg−1). *P < 0.05 compared with the group treated with topiramate before cocaine injection. Only topiramate before ethanol was effective at reducing cocaine-induced responses to ethanol. (B) Motor activity. Mean ± SEM of the accumulated beam breaks for the 30 min after cocaine injection. Topiramate was administered 120 min before the cocaine injection. *P < 0.05 and ***P < 0.001 compared with the either the vehicle or the topiramate 40 mg·kg−1 group.
In addition, we examined the effects of topiramate on the cocaine-induced psychostimulant motor activity (Figure 2B); 120 min after the administration of topiramate, the animals were treated with cocaine and were introduced immediately into the locomotor activity apparatus for 30 min. The activity of the animal was monitored in 5-min periods. The locomotor activity was examined in a single assay, and the animals were fully counterbalanced.
Experiment 3: the effects of chronic treatment with topiramate on the response to ethanol, the metabolism of cocaine and blood ethanol levels
We conducted experiment 3 after a washout period of 3 days from experiment 2. To study the effects of chronic treatment with topiramate on responses to ethanol and according to the results from the previous experiments, we decided to treat the animals with topiramate (40 mg·kg−1, p.o.) 120 min before operant ethanol self-administration for 8 consecutive days (Figure 3A). Additionally, we expected that with this chronic treatment regimen, we would be able to highlight the differences among groups for the ethanol/cocaine blood analysis and the subsequent genetic expression experiments. Blood for the analysis of ethanol was collected from the tail of the rats immediately after the operant ethanol self-administration session 2 days before they were killed. Blood for the analysis of the cocaine metabolite benzoylecgonine was collected from the rat trunk immediately before operant ethanol self-administration as they were killed.
Figure 3.

The effects of chronic topiramate treatment on the response to ethanol, cocaine metabolism and blood ethanol levels. (A) Mean ± SEM operant responses for 10% v v−1 ethanol averaged over 8 consecutive days (n = 9–11 per group). **P < 0.01 compared with the cocaine group (20 mg·kg−1). (B) Scatter plot of reinforcements obtained by the rats during the 30 min ethanol session and blood ethanol levels (mg%) determined immediately after this test session. Reinforcements were significantly correlated with the blood ethanol levels. (C) Topiramate administered 120 min before the operant self-administration reduced the blood ethanol level (mg%) independently of cocaine treatment. ***P < 0.001 compared with the cocaine group (20 mg·kg−1). #P < 0.05 and ##P < 0.01 compared with the vehicle group. (D) Benzoylecgonine (ng·mL−1) was examined 18 h after the last cocaine administration and after alcohol self-administration. Topiramate did not change the metabolism of the cocaine.
Experiment 4: the effects of chronic topiramate treatment on the gene expression in the rat prefrontal cortex
The goal of this experiment was to investigate the changes in gene expression in the prefrontal cortex associated with our behavioural results (Figure 4A–E). For this purpose, we assessed the genetic expression of: (i) two DNA methyltransferases (Dnmts), Dnmt1 and Dnmt3a, which methylate DNA; (ii) The RNA methyltransferases Trdmt1 (formerly known as Dnmt2) and Rnmt enzymes, which methylate tRNA and mRNA, respectively; (iii); the proteins that form Dnmt1 corepressor complexes, Hdac2, Dmap1, Pcna and Uhrf1, which are described in the Introduction; (iv) key protein receptors targeted by topiramate, which include the kainate receptor containing the GluK1 subunit (Grik1) and the carbonic anhydrase enzymes types II and IV (Ca2 and Ca4); and (v) proteins that regulate importantly the function of the reward system: the dopamine D1 and D2 receptors (for nomenclature see Alexander et al., 2013b), the tyrosine hydroxylase enzyme and the μ-opioid receptor.
Figure 4.
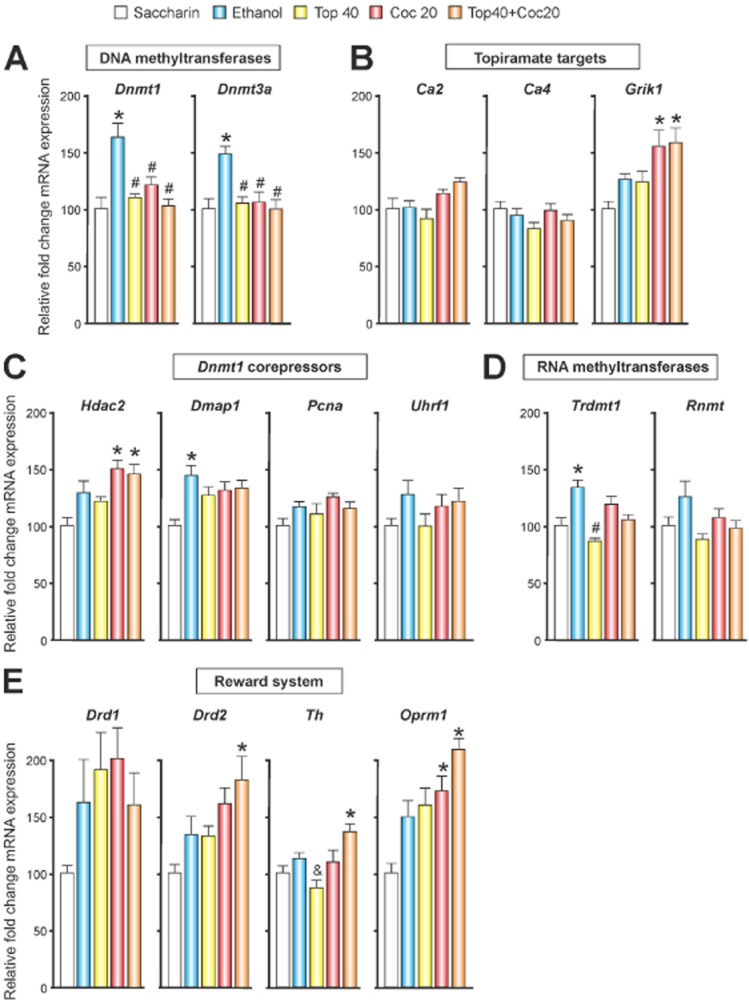
The effects of chronic treatment with topiramate on gene expression in the rat prefrontal cortex. Mean ± SEM the relative fold change using the 2ΔCt method (see Methods section). The 18S ribosomal RNA gene was used as an internal control for the normalization and gene expression of the operant saccharine self-administration group, which was used as the control (calibrator). (A) Ethanol increased the expression of the DNA methyltransferase genes, and topiramate or cocaine blocked this effect. (B) No changes were observed in the expression of carbonic anhydrase enzymes type II and IV (Ca2 and Ca4), but cocaine increased the gene expression of the kainate receptor containing the GluK1 subunit (Grik1). (C) Dnmt1 DNA methyltransferase corepressors showed heterogeneous results. Gene expression of Hdac2 increased after cocaine treatment, and Dmap1 increased after ethanol self-administration. (D) Ethanol increased the gene expression of the enzyme that methylates tRNA (Trdmt1, formerly known as Dnmt2). (E) The co-administration of topiramate and cocaine increased Drd2, Th and Oprm1 gene expression and cocaine alone increased the Oprm1 gene expression. *P < 0.01 compared with the saccharine-control group. #P < 0.01 compared with the ethanol group. &P < 0.01 compared with the topiramate + cocaine group.
We focused on the prefrontal cortex because of its contribution to addictive behaviour (Lüscher and Malenka, 2011), the involvement in regulating cognitive behaviour in rodents and in humans (Dayas et al., 2007; Vengeliene et al., 2009; Abernathy et al., 2010), and the susceptibility to the effects of topiramate on genetic expression (Navarrete et al., 2012).
Experiment 5: effects of topiramate on episodic-like memory
As topiramate has an effect on cognition in humans (e.g. Sommer et al., 2013), we decided to investigate its effect on episodic memory. With this aim, we used the novel object recognition test, which has been proven useful for evaluating this type of declarative memory in animal models (Winters et al., 2008). A group of 32 rats were divided as follows: (i) chronic group, which received every 3 days an increasing dose of topiramate p.o. (10, 20, 30 and 40 mg·kg−1), divided into two doses (morning and afternoon dosing) as described in experiment 1; and (ii) acute and (iii) vehicle groups, which followed the same experimental manipulations as the chronic group but were treated with vehicle. Then, on the 13th day, whereas the chronic and acute groups were treated with topiramate 40 mg·kg−1 120 min before the novel object recognition test (sample session), the vehicle group was treated with vehicle.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) statistical software package (version 20.0) for Windows (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. The data from experiment 1 were analysed using a three-way ANOVA [increasing the doses (×4) or the time intervals (×6) as the within-subject factors and drug treatments (×2) as the between-subject factors]. The results from experiment 2 were analysed using a two-way ANOVA (with order and drug treatment as the between-subject factors). The motor data were analysed using a two-way ANOVA [with time intervals (×6) as the within-subject factor and the drug treatment as the between-subject factor]. The results from experiment 3, chronic topiramate treatment, were analysed using a one-way ANOVA (with drug treatment as the between-subject factor). To test the relationship between blood ethanol levels and ethanol reinforcement, Pearson's correlation coefficient was used. The effects of topiramate on cocaine metabolites and blood ethanol levels were analysed using a one-way ANOVA. The data from experiment 4 were analysed using a one-way ANOVA (with the drug treatment as the between-subject factor). Here, Bonferroni's correction for multiple testing was applied, and only P-values ≤ 0.01 were considered statistically significant. The data from experiment 5 were analysed using a two-way ANOVA (with old/new object and drug treatment as the between-subject factors).
Results
Experiment 1: the effects of increasing the dose of topiramate on the response to ethanol and co-administration of cocaine
The ANOVA and post hoc data analyses indicated that topiramate did not reduce the response to ethanol, that cocaine (20 mg·kg−1, i.p.) increased the responses to ethanol, and that topiramate blocked the cocaine-induced increase on the response to ethanol in a dose-dependent manner [topiramate F(3,162) = 4.91, P < 0.005; cocaine F(1,54) = 6.57, P < 0.05; interaction F(3,162) = 3.34, P < 0.05], as shown in Figure 1A. There were no significant differences in the activity towards the inactive lever. Additionally, the effects of cocaine on operant ethanol self-administration were shown after repeated injections (7–9th days).
The locomotor activity of the animal was monitored during the 30 min prior to the introduction into the operant ethanol self-administration chamber. Figure 1B shows that there were no significant depressant/stimulant effects of topiramate among groups throughout this 30-min period [cocaine F(2,54) = 0.32, not significant (NS); topiramate F(2,54) = 0.49, NS]. These experiments allowed us to select the dose of 40 mg·kg−1 of topiramate for future experiments and discard the two groups of rats treated with the 10 mg·kg−1 dose of topiramate.
Experiment 2: effects of topiramate on cocaine's behavioural effects
Figure 2A shows that topiramate administration prior to the cocaine injection failed to reduce the higher response rates to ethanol induced by cocaine. However, topiramate administration 120 min before ethanol access fully prevented the cocaine-induced higher response rates for ethanol [treatment F(3,72) = 9.22, P < 0.001; order F(1,72) = 4.02, P < 0.05; interaction F(3,72) = 1.04, NS].
We also evaluated the effects of topiramate on cocaine-induced psychostimulant motor activity. Figure 2B shows that cocaine caused a robust increase in the locomotor activity of the animal, and administering topiramate 120 min before the cocaine injection was not able to prevent this increase and did not show any effect on the spontaneous locomotor activity [treatment F(3,37) = 16.94, P < 0.001; time F(5,185) = 161.83, P < 0.001; interaction F(15,185) = 16.32, P < 0.001].
Experiment 3: the effects of chronic treatment with topiramate on the response to ethanol, cocaine metabolism and blood ethanol levels
As expected from the previous experiments, the chronic treatment with topiramate 120 min prior to the operant ethanol self-administration session fully prevented the increased response to ethanol induced by cocaine (Figure 3A). However, chronic topiramate treatment failed to significantly reduce the ethanol response by itself, without cocaine [drug treatment F(3,39) = 13.39, P < 0.001].
We found a positive correlation between the blood ethanol levels and the number of ethanol reinforcements received by the animals (Figure 3B) (r = 0.48, P < 0.005). The blood ethanol levels were significantly different as a function of the treatment group: topiramate either alone or in co-administration with cocaine reduced the ethanol concentration in the blood, and cocaine alone did not produce any significant change (Figure 3C) [drug treatment F(3,39) = 15.04, P < 0.001].
Benzoylecgonine, one of the two primary metabolites of cocaine, was detected approximately 18 h after the last cocaine administration (Figure 3D) [drug treatment F(3,36) = 59.75, P < 0.001]. However, topiramate did not change the metabolism of cocaine, and the benzoylecgonine levels were similar in the group only treated with cocaine and in the topiramate-cocaine group.
Experiment 4: the effects of chronic topiramate treatment on gene expression in the rat prefrontal cortex
Figures 4A and 5 show that operant ethanol self-administration caused an increase in the expression of DNA methyltransferases, and this effect was prevented by either topiramate or cocaine [FDnmt1 (4,56) = 12.95, P < 0.001; FDnmt3a (4,61) = 4.60, P < 0.005] (Figure 4A). Cocaine, independently of topiramate treatment, increased the genetic expression of glutamate receptor kainate-1 (Grik1) [F(4,56) = 2.73, P < 0.05] (Figure 4B), which is targeted by topiramate, and Hdac2 [F(4,54) = 6.27, P < 0.001] (Figure 4C), which is a Dnmt1 corepressor. We also found that operant ethanol self-administration increased the mRNA levels of the Dnmt1 corepressor Dmap1 [F(4,58) = 5.53, P < 0.005] (Figure 4C) and the RNA methyltransferase Trdmt1 [F(4,55) = 8.83, P < 0.001] (Figure 4D), which methylates tRNA. The co-administration of topiramate and cocaine resulted in an increase of Drd2 [F(4,64) = 5.40, P < 0.005], Th [F(4,54) = 6.61, P < 0.001] and Oprm1 [F(4,58) = 10.61, P < 0.001] gene expression; and cocaine alone induced an increase of Oprm1 gene expression (Figure 4E).
Figure 5.
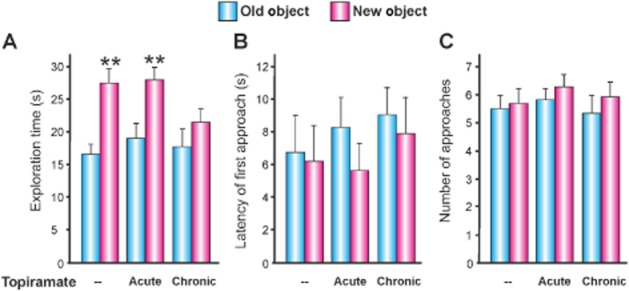
The effects of topiramate on episodic-like memory. (A) Mean ± SEM total duration of approaches to an object over the three min of the test session. **P < 0.01 compared within-treatments. There were no significant differences between-treatments. (B) Mean ± SEM latency of the first approach to an object and (C) mean ± SEM frequency of approaches to an object over the three min of the test session. For the chronic group, the dose of topiramate (10, 20, 30 and 40 mg·kg−1) was progressively increased every 3 consecutive days. The acute group was treated only once with topiramate, and the vehicle group was only treated with vehicle. Topiramate 40 mg·kg−1 or vehicle was administered on the 13th day, 120 min before the sample session.
Experiment 5: effects of topiramate on episodic-like memory
The two-way ANOVA revealed significant differences in total time exploring rats the new object through the test session (Figure 5A), but there were no significant differences caused by topiramate or its interaction with the new/old objects [objects F(1,64) = 18.47, P < 0.001; treatment F(2,64) = 1.59, P = 0.21, NS; interaction F(2,61) = 1.32, P ≤ 0.28, NS]. Nevertheless, the individual analysis within-treatment showed that there were significant differences between the old and new objects in the acute and vehicle groups (P = 0.01), but not in the group treated chronically with topiramate. There were no significant differences in the latency of first approach and frequency of approaches (Figure 5B and C).
Discussion
These experiments provide valuable information about the role of topiramate on ethanol–cocaine interactions. We report five major findings: (i) topiramate prevented the cocaine-induced increase in the response to ethanol in a dose-dependent manner without motor impairments; (ii) the preventative effects of topiramate on the high response rates to ethanol were explained by the suppression of the expression of the effects of cocaine rather than the blockade of the acquisition of the effects of cocaine; (iii) topiramate reduced blood ethanol levels independently of the co-administration of cocaine, but did not change the metabolism of cocaine; (iv) operant ethanol self-administration increased the gene expression of DNA methyltransferases and the RNA methyltransferase Trdmt1, and this effect was fully blocked by either topiramate or cocaine; and (v) cocaine increased the genetic expression of Grik1 and Hdac2, and topiramate did not inhibit this increase.
Although there are ongoing clinical trials and a recent study regarding the effects of topiramate on the dual dependence of ethanol and cocaine has been published (ClinicalTrials.gov, US NIH, 2009; Kampman et al., 2013), this is the first preclinical study demonstrating the effects of topiramate treatment on the co-administration of ethanol and cocaine. However, topiramate alone was not able to reduce the response to ethanol. This result agrees with those from previous studies that showed either that topiramate did not alter the responses of mice to ethanol (Navarrete et al., 2012), or that topiramate was ineffective in reducing ethanol consumption in Wistar rats (Breslin et al., 2010; Lynch et al., 2011). However, our results contrast with other reports showing that topiramate reduced ethanol consumption and reduced the motivation to lick for beer in animal models (Hargreaves and McGregor, 2007; Knapp et al., 2007; Nguyen et al., 2007), and reduced ethanol consumption, craving and increased the number of abstinent days from ethanol use in humans (Johnson et al., 2003; Rubio et al., 2004; Shinn and Greenfield, 2010). One plausible hypothesis for these discrepancies is that the effects of topiramate on ethanol-related behaviour are dependent upon the amounts of ethanol used by the subjects. This hypothesis predicts that the probability of topiramate having an effect on alcohol intake would be greater in subjects with higher levels of ethanol consumption than in subjects with lower levels. Indeed, the Johnson group demonstrated in at least two studies that the severity of drinking is a predictor of the efficacy of topiramate (Breslin et al., 2010; Lynch et al., 2011). These authors found that topiramate reduced ethanol consumption and relapse in ethanol-preferring rats, which are characterized by excessive ethanol drinking, whereas topiramate did not affect ethanol consumption in Wistar rats. In the present study, topiramate was effective at reducing the increased response to ethanol induced by cocaine. The same response has also been observed for cocaine response. Subjects with more severe cocaine withdrawal symptoms responded better to topiramate (Kampman et al., 2013).
Our results also show that the preventative effects of topiramate were not explained by any motor impairment, and according to previous reports (Echeverry-Alzate et al., 2012), the cocaine-induced increase in the response to ethanol is a result of chronic cocaine exposure rather than an acute consequence, as these effects appear after the sixth to seventh day of cocaine treatment. The question remained whether topiramate blocks the acquisition of the effects of the cocaine treatment or whether topiramate blocks the expression of the effects of the cocaine treatment on operant ethanol self-administration. Repeated exposure to cocaine causes behavioural sensitization, progressively increasing psychostimulant motor activity, and neuronal sensitization of glutamatergic projections from the prefrontal cortex to mesolimbic structures (Ghasemzadeh et al., 2009; Liu and Steketee, 2011). In the present study, administering topiramate 120 min before cocaine did not change the cocaine-induced psychostimulant motor activity and did not prevent the increased response to ethanol. Both results suggest that topiramate is not altering the main effects of cocaine in the rat brain. However, administering topiramate 120 min before the operant ethanol self-administration session, acute or chronically, fully prevented the increased response to ethanol induced by cocaine. Therefore, it seems more reasonable to assume that topiramate is blocking the effects of cocaine on ethanol self-administration. It has been proposed that topiramate normalizes neuronal sensitization and reduces the symptoms of cocaine withdrawal (Johnson, 2005). For instance, because there is an increase in the number of glutamate receptors in the nucleus accumbens after a short period of cocaine withdrawal (Dobi et al., 2011) and the glutamatergic projections from the prefrontal cortex modulate the nucleus accumbens (Parsegian and See, 2014), our results raise the possibility that topiramate might be adjusting the cortico-mesolimibic activity, and as a consequence, reducing the craving or incentive salience for ethanol. This explanation agrees with the mechanism of action suggested by Johnson (2004), which is that topiramate antagonizes the ability of drugs of abuse to increase cortico-mesolimbic dopamine activity by facilitating GABA suppression and reducing the excitatory effects of glutamatergic receptors in the nucleus accumbens. Although we cannot provide an unequivocal answer to whether topiramate reduces the craving for ethanol, blocks cocaine withdrawal or reduces the salience of ethanol within the drinking occasion, our results provide supporting evidence about the efficacy of topiramate in terms of the dual dependence of ethanol and cocaine.
We have shown that topiramate interfered with ethanol metabolism independently of the cocaine treatment, resulting in a reduction in blood ethanol levels. This is an interesting result, as the reduction in blood ethanol levels was not linked to a reduction in operant ethanol self-administration. This might suggest that the reducing effects of topiramate on the cocaine group is likely more associated to psychological/emotional aspects of drug addiction, such as craving and incentive salience for ethanol, rather than the necessity of animals to reach fixed blood ethanol levels. According to FDA (2012), the side effects of topiramate include metabolic acidosis, which is due to the inhibitory effect of topiramate on carbonic anhydrase enzymes types II and IV. Additionally, it has been suggested that carbonic anhydrase inhibitors target the NADH oxidoreductase enzyme, which uses NADH as a substrate (Innocenti et al., 2005). This is relevant because, in addition to acetaldehyde, ethanol consumption leads to an accumulation of NADH, which is detected by the ethanol assay kit that we used here to analyse blood samples. Therefore, our results suggest that the reduction in blood ethanol levels caused by topiramate could be related to the interference with the activity of the NADH oxidoreductase enzyme. Further studies are needed to identify the mechanism of this interaction with additional biochemistry techniques. In contrast to ethanol, benzoylecgonine, one of the two primary metabolites of cocaine (Schindler and Goldberg, 2012), did not show significant differences among the groups after 18 h of cocaine administration, indicating that topiramate does not directly affect cocaine metabolism.
We obtained heterogeneous results in the genetic expression studies. We found that the expression of the RNA/DNA methyltransferases Trmdt1, Dnmt1 and Dnmt3a was increased in a similar way after operant ethanol self-administration. However, Dnmt1 acts essentially on hemimethylated DNA and is implicated in the maintenance of DNA methylation patterns during DNA replication, and Dnmt3a shows de novo methyltransferase activity (Okano et al., 1999; Bestor, 2000). Therefore, a logical conclusion would be that ethanol alters the activity of the maintenance and de novo patterns of DNA methylation in the rat prefrontal cortex. A recent report has demonstrated that either pre- or post-natal ethanol exposure results in an increase of Dnmt1 and Dnmt3a expression in the rat hippocampus (Perkins et al., 2013). Despite the lack of a clear explanation, this is the first study that shows that topiramate and cocaine prevent the increase of DNA methyltransferase gene expression induced by ethanol. Our initial hypothesis was that the expression of Dnmt1 complex corepressors (Hdac2, Dmap1, Pcna and Uhrf1) would respond in a similar manner as Dnmt1 did after operant ethanol self-administration. However, only the expression of the Dmap1 exhibited an increase similar to Dnmt1. This result suggests that the recruitment of gene silencing corepressors by DNMT1 might be specific for the stimuli that cause the change in activity, which are ethanol and cocaine in the present study. Interestingly, despite that, Hdac2 is recruited by DNMT1 as a corepressor of gene transcription, HDAC2 has transcriptional silencing activity by itself. HDAC2 is responsible for the removal of acetyl groups from specific histones, which results in gene transcription silencing. In this study, cocaine administration resulted in an increase in the gene expression of Hdac2, and this result agrees with recent work (Host et al., 2011). This result indicates that cocaine administration is associated with an increase of gene silencing in the prefrontal cortex because of the stimulation of the Hdac2 gene. Another unexpected result is that there was not a significant decrease on the genetic expression of the genes coding for the main proteins targeted for topiramate after chronic treatment, which include the carbonic anhydrase enzymes types II (Ca2) and IV (Ca4) and Grik1. However, cocaine increased the genetic expression of Grik1. This last finding is not surprising, considering all of the evidence linking cocaine glutamatergic signalling and cocaine addiction (see Schmidt and Pierce, 2010, for a review). Three of the four genes assessed, Drd2, Th and Oprm1, related to the reward system were up-regulated in the group treated with topiramate and cocaine. Previous studies have demonstrated in animal models that cocaine increased the expression of these three genes (Balda et al., 2009; Kreek et al., 2012; Lawhorn et al., 2013). Nevertheless, our results demonstrate, for the first time, that there is a clear effect with the co-administration of topiramate, but the underlying mechanism of action is unknown.
We expected to reduce the side effects of topiramate increasing the dose of topiramate progressively and dividing them into two doses (morning and afternoon dosing) as it is recommended in the clinical setting (Kampman et al., 2004; ClinicalTrials.gov, US NIH, 2009). However, and surprisingly, those animals did not show significant differences exploring the new object (suggesting impaired episodic-like memory), whereas the animals treated acutely with topiramate 40 mg·kg−1 and those treated merely with vehicle, showed a significant increase over the time spent with the new object (suggesting intact episodic-like memory). Therefore, our results may indicate that either low doses of topiramate should be considered in further studies or that it should be carefully evaluated in the risk–benefit ratio of using moderate/high doses of topiramate for the treatment of co-abuse of ethanol and cocaine.
In conclusion, we provided evidence for the efficacy of topiramate in the context of the dual dependence of ethanol and cocaine. Furthermore, we provided novel information regarding the effects of topiramate on cocaine- and ethanol-related behaviours and metabolism, and we presented novel insights regarding the changes in the expression of the genes controlling the epigenetic mechanisms (epigenetic genes) and genes related directly to the reward system in the prefrontal cortex.
Acknowledgments
This work was supported by the Fondo de Investigación Sanitaria (Red de Trastornos Adictivos, FEDER, RD12/0028/0015 to J.A.L.M., RD12/0028/001 to F.R. de F., RD12/0028/0014 to R.N.), Ministerio de Ciencia e Innovación (SAF2011-26818 to J.A.L.M.), and the European Foundation for Alcohol Research (EA 12 21 to J.A.L.M.).
Glossary
- Ca2, Ca4
carbonic anhydrase enzymes types II and IV
- DMAP1
DNA methyltransferase 1-associated protein
- DNMT1
DNA methyltransferase
- Grik1
kainate receptor gene containing the GluK1 subunit
- HDAC2
histone deacetylase-2
- TRDMT1
tRNA aspartic acid methyltransferase 1
Conflict of interest
None.
References
- Abernathy K, Chandler LJ, Woodward JJ. Alcohol and the prefrontal cortex. Int Rev Neurobiol. 2010;91:289–320. doi: 10.1016/S0074-7742(10)91009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AEMPS (Spanish Agency for Medicines and Health Products) 2012. Topamax data sheet. Available at: http://www.aemps.gob.es/cima/pdfs/es/ft/63960/FT_63960.pdf (accessed 6/8/2013)
- Alén F, Gómez R, González-Cuevas G, Navarro M, López-Moreno JA. Nicotine causes opposite effects on alcohol intake: evidence in an animal experimental model of abstinence and relapse from alcohol. Nicotine Tob Res. 2009;11:304–311. doi: 10.1093/ntr/ntp139. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: G-protein couple receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1607. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–196. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. The neuronal nitric oxide synthase (nNOS) gene contributes to the regulation of tyrosine hydroxylase (TH) by cocaine. Neurosci Lett. 2009;457:120–124. doi: 10.1016/j.neulet.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Botia B, Legastelois R, Alaux-Cantin S, Naassila M. Expression of ethanol-induced behavioral sensitization is associated with alteration of chromatin remodeling in mice. PLoS ONE. 2012;7:e47527. doi: 10.1371/journal.pone.0047527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Li H, Rogawski MA. Topiramate reduces excitability in the basolateral amygdala by selectively inhibiting GluK1 (GluR5) kainate receptors on interneurons and positively modulating GABAA receptors on principal neurons. J Pharmacol ExpTher. 2009;330:558–566. doi: 10.1124/jpet.109.153908. [DOI] [PubMed] [Google Scholar]
- Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology (Berl) 2010;207:529–534. doi: 10.1007/s00213-009-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Baicy KJ, Olsen RW. Topiramate attenuates withdrawal signs after chronic intermittent ethanol in rats. Neuroreport. 2004;15:207–210. doi: 10.1097/00001756-200401190-00040. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Liu X, Simms JA, Weiss F. Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry. 2007;61:979–989. doi: 10.1016/j.biopsych.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry-Alzate V, Tuda-Arízcun M, Bühler KM, Santos Á, Giné E, Olmos P, et al. Cocaine reverses the naltrexone-induced reduction in operant ethanol self-administration: the effects on immediate-early gene expression in the rat prefrontal cortex. Neuropharmacology. 2012;63:927–935. doi: 10.1016/j.neuropharm.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A, Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res. 2005;159:247–266. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration) 1995. Topamax Review and Evaluation of Pharmacology and Toxicology. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020505_000_Pharm-toxicology_rvw.pdf (accessed 6/8/2013)
- FDA (Food and Drug Administration) 1996. Topamax tablets approval letter. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020505s000_Topamax.cfm (accessed 6/8/2013)
- FDA (Food and Drug Administration) 2012. Medication guide. Available at: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails (accessed 6/8/2013)
- Gasior M, Ungard JT, Witkin JM. Preclinical evaluation of newly approved and potential antiepileptic drugs against cocaine-induced seizures. J Pharmacol Exp Ther. 1999;290:1148–1156. [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C. Locomotor sensitization to cocaine is associated with distinct pattern of glutamate receptor trafficking to the postsynaptic density in prefrontal cortex: early versus late withdrawal effects. Pharmacol Biochem Behav. 2009;92:383–392. doi: 10.1016/j.pbb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, McGregor IS. Topiramate moderately reduces the motivation to consume alcohol and has a marked antidepressant effect in rats. Alcohol Clin Exp Res. 2007;31:1900–1907. doi: 10.1111/j.1530-0277.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Hervouet E, Vallette FM, Cartron PF. Dnmt1/transcription factor interactions: an alternative mechanism of DNA methylation inheritance. Genes Cancer. 2010;1:434–443. doi: 10.1177/1947601910373794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Host L, Dietrich JB, Carouge D, Aunis D, Zwiller J. Cocaine self-administration alters the expression of chromatin-remodelling proteins; modulation by histone deacetylase inhibition. J Psychopharmacol. 2011;25:222–229. doi: 10.1177/0269881109348173. [DOI] [PubMed] [Google Scholar]
- Innocenti A, Villar R, Martinez-Merino V, Gil MJ, Scozzafava A, Vullo D, et al. Carbonic anhydrase inhibitors: inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with benzo[b]thiophene 1,1-dioxide sulphonamides. Bioorg Med Chem Lett. 2005;15:4872–4876. doi: 10.1016/j.bmcl.2005.04.078. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Uses of topiramate in the treatment of alcohol dependence. Expert Rev Neurother. 2004;4:751–758. doi: 10.1586/14737175.4.5.751. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K. Oral topiramate for treatment of alcohol dependence: a randomized controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Gunderson EW, Haughey HM, Wang XQ, et al. Topiramate's effects on cocaine-induced subjective mood, craving and preference for money over drug taking. Addict Biol. 2013;18:405–416. doi: 10.1111/j.1369-1600.2012.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Spratt K, Wierzbicki MR, O'Brien CP. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133:94–99. doi: 10.1016/j.drugalcdep.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp CM, Mercado M, Markley TL, Crosby S, Ciraulo DA, Kornetsky C. Zonisamide decreases ethanol intake in rats and mice. Pharmacol Biochem Behav. 2007;87:65–72. doi: 10.1016/j.pbb.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Levran O, Reed B, Schlussman SD, Zhou Y, Butelman ER. Opiate addiction and cocaine addiction: underlying molecular neurobiology and genetics. J Clin Invest. 2012;122:3387–3393. doi: 10.1172/JCI60390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhorn C, Edusei E, Zhou Y, Ho A, Kreek MJ. Acute binge pattern cocaine administration induces region-specific effects in D1-r- and D2-r-expressing cells in eGFP transgenic mice. Neuroscience. 2013;253:123–131. doi: 10.1016/j.neuroscience.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Justinova Z, Wertheim CE, Barnes C, Goldberg SR. Topiramate does not alter nicotine or cocaine discrimination in rats. Behav Pharmacol. 2008;19:13–20. doi: 10.1097/FBP.0b013e3282f3cf84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Steketee JD. Repeated exposure to cocaine alters medial prefrontal cortex dopamine D2-like receptor modulation of glutamate and dopamine neurotransmission within the mesocorticolimbic system. J Neurochem. 2011;119:332–341. doi: 10.1111/j.1471-4159.2011.07362.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Gao Q, Li P, Zhao Q, Zhang J, Li J, et al. UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat Commun. 2013;4:1563. doi: 10.1038/ncomms2562. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Bond C, Breslin FJ, Johnson BA. Severity of drinking as a predictor of efficacy of the combination of ondansetron and topiramate in rat models of ethanol consumption and relapse. Psychopharmacology (Berl) 2011;217:3–12. doi: 10.1007/s00213-011-2253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maryanoff BE, Gardocki JF. 1985. Anticonvulsant sulfamate derivatives. US Patent no. 4 513 006, USA.
- Maryanoff BE, Nortey SO, Gardocki JF, Shank RP, Dodgson SP. Anticonvulsant O-alkyl sulfamates. 2,3:4,5-bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate and related compounds. J Med Chem. 1987;30:880–887. doi: 10.1021/jm00388a023. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Hiyoshi T, Kudo T, Yagi K, Seino M. Anticonvulsant effect of topiramate [2,3:4,5-bis-O-(1-methylethylidene-beta-D-fructopyranose sulfate] on amygdaloid kindled seizures in the cat. Jpn J Psychiatry Neurol. 1993;47:394–395. doi: 10.1111/j.1440-1819.1993.tb02119.x. [DOI] [PubMed] [Google Scholar]
- Navarrete F, Pérez-Ortiz JM, Manzanares J. Pregabalin- and topiramate-mediated regulation of cognitive and motor impulsivity in DBA/2 mice. Br J Pharmacol. 2012;167:183–195. doi: 10.1111/j.1476-5381.2012.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SA, Malcolm R, Middaugh LD. Topiramate reduces ethanol consumption by C57BL/6 mice. Synapse. 2007;61:150–156. doi: 10.1002/syn.20350. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39:811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Perkins A, Lehmann C, Lawrence RC, Kelly SJ. Alcohol exposure during development: impact on the epigenome. Int J Dev Neurosci. 2013;31:391–397. doi: 10.1016/j.ijdevneu.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis AD, Castro LA, Faria R, Laranjeira R. Craving decrease with topiramate in outpatient treatment for cocaine dependence: an open label trial. Rev Bras Psiquiatr. 2008;30:132–135. doi: 10.1590/s1516-44462008005000012. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rountree MR, Bachman KE, Baylin SB. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat Genet. 2000;25:269–277. doi: 10.1038/77023. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Jiménez-Arriero MA, Palomo T, Manzanares J, Ferre F. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37:37–40. doi: 10.1055/s-2004-815473. [DOI] [PubMed] [Google Scholar]
- Schermelleh L, Haemmer A, Spada F, Rösing N, Meilinger D, Rothbauer U, et al. Dynamics of Dnmt1 interaction with the replication machinery and its role in postreplicative maintenance of DNA methylation. Nucleic Acids Res. 2007;35:4301–4312. doi: 10.1093/nar/gkm432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Goldberg SR. Accelerating cocaine metabolism as an approach to the treatment of cocaine abuse and toxicity. Future Med Chem. 2012;4:163–175. doi: 10.4155/fmc.11.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider K, Fuchs C, Dobay A, Rottach A, Qin W, Wolf P, et al. Dissection of cell cycle-dependent dynamics of Dnmt1 by FRAP and diffusion-coupled modeling. Nucleic Acids Res. 2013;41:4860–4876. doi: 10.1093/nar/gkt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn AK, Greenfield SF. Topiramate in the treatment of substance-related disorders: a critical review of the literature. J Clin Psychiatry. 2010;71:634–648. doi: 10.4088/JCP.08r04062gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer BR, Mitchell EL, Wroolie TE. Topiramate: effects on cognition in patients with epilepsy, migraine headache and obesity. Ther Adv Neurol Disord. 2013;6:211–227. doi: 10.1177/1756285613481257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA. The effect of cocaine on the expression of motor activity and conditioned place preference in high and low alcohol-preferring Wistar rats. Pharmacol Biochem Behav. 2005;82:314–319. doi: 10.1016/j.pbb.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Tatum WO, Kaplan PW, Jallon P. Epilepsy A to Z: A Concise Encyclopedia. 2nd edn. New York: Demos Medical Pub; 2009. [Google Scholar]
- US NIH (US National Institutes of Health) 2009. ClinicalTrials.gov. Available at: http://www.clinicaltrials.gov (accessed 6/8/2013)
- Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addict Biol. 2009;14:384–396. doi: 10.1111/j.1369-1600.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- Warnault V, Darcq E, Levine A, Barak S, Ron D. Chromatin remodeling – a novel strategy to control excessive alcohol drinking. Transl Psychiatry. 2013;3:e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Gorska D, Dyr W, Czarnecka E. Effect of repeated treatment with topiramate on the beta-endorphin plasma level in rats selectively bred for high and low alcohol preference. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:525–528. doi: 10.1016/j.pnpbp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Bajer B, Gorska D, Andrzejczak D, Dyr W, Bieńkowski P. Effect of repeated treatment with topiramate on voluntary alcohol intake and beta-endorphin plasma level in Warsaw alcohol high-preferring rats. Psychopharmacology (Berl) 2013;225:275–281. doi: 10.1007/s00213-012-2812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


