Abstract
BACKGROUND AND PURPOSE
A growing number of studies have demonstrated that oxytocin (OT) plays an analgesic role in modulation of nociception and pain. Most work to date has focused on the central mechanisms of OT analgesia, but little is known about whether peripheral mechanisms are also involved. Acid-sensing ion channels (ASICs) are distributed in peripheral sensory neurons and participate in nociception. Here, we investigated the effects of OT on the activity of ASICs in dorsal root ganglion (DRG) neurons.
EXPERIMENTAL APPROACH
Electrophysiological experiments were performed on neurons from rat DRG. Nociceptive behaviour was induced by acetic acid in rats and mice lacking vasopressin, V1A receptors.
KEY RESULTS
OT inhibited the functional activity of native ASICs. Firstly, OT dose-dependently decreased the amplitude of ASIC currents in DRG neurons. Secondly, OT inhibition of ASIC currents was mimicked by arginine vasopressin (AVP) and completely blocked by the V1A receptor antagonist SR49059, but not by the OT receptor antagonist L-368899. Thirdly, OT altered acidosis-evoked membrane excitability of DRG neurons and significantly decreased the amplitude of the depolarization and number of action potentials induced by acid stimuli. Finally, peripherally administered OT or AVP inhibited nociceptive responses to intraplantar injection of acetic acid in rats. Both OT and AVP also induced an analgesic effect on acidosis-evoked pain in wild-type mice, but not in V1A receptor knockout mice.
CONCLUSIONS AND IMPLICATIONS
These results reveal a novel peripheral mechanism for the analgesic effect of OT involving the modulation of native ASICs in primary sensory neurons mediated by V1A receptors.
Keywords: oxytocin, acid-sensing ion channel, proton-gated current, vasopressin, V1A receptor, dorsal root ganglion neuron, electrophysiology, pain
Introduction
Acid-sensing ion channels (ASICs) are members of proton-gated cation channels and are expressed in both central and peripheral nervous systems (Waldmann et al., 1997b). In peripheral sensory neurons, ASICs have been found on cell bodies and sensory terminals, where they have been suggested to be important for nociception (Alvarez de la Rosa et al., 2002; Benson et al., 2002; Wemmie et al., 2013). As pH sensors, ASICs are activated by a decrease in extracellular pH and depolarize the terminals of nociceptive primary sensory neurons to trigger pain sensation. Direct perfusion of acidic solutions into the skin causes pain in humans (Steen et al., 1995; Ugawa et al., 2002; Jones et al., 2004). Protons, canonical ligands for ASICs, are released and cause tissue acidosis under multiple pathological conditions such as inflammation, tissue injury, ischaemic stroke and cancer (Deval et al., 2010). It is well known that the local extracellular pH levels drop to 5.4 in acute inflammation and 6.3 or lower in severe ischaemia (Smith et al., 1986; Steen et al., 1992; Kweon and Suh, 2013). The accumulating protons are sufficient to activate nociceptors by activating proton-sensitive ion channels such as ASICs and transient receptor potential vanilloid receptors type 1 (TRPV1: for nomenclature see Alexander et al., 2013b) (Steen et al., 1992; Frey Law et al., 2008). Studies have suggested that ASICs, rather than TRPV1, mainly mediate pain sensation induced by acid injection, because the pain sensation is significantly attenuated by the non-selective ASIC inhibitor amiloride (Ugawa et al., 2002; Wemmie et al., 2006; Deval et al., 2008). It has been shown that inflammation and tissue injury increase the expression levels of ASIC mRNA in dorsal root ganglion (DRG) neurons, which contribute to hyperalgesia (Voilley et al., 2001; Hori et al., 2010; Chen et al., 2011). Together, increasing evidence has shown that ASICs play an important role in various pain conditions such as inflammatory pain and post-operative pain (Deval et al., 2011; Wemmie et al., 2013).
Oxytocin (OT) is a nine amino acid neuropeptide that is synthesized in the paraventricular and supraoptic nuclei of the hypothalamus (Sofroniew, 1983; Viero et al., 2010). OT is released into the CNS as a modulator of neuronal transmission. Additionally, it is also transported down axons of the posterior pituitary and is secreted into bloodstream as a neurohormone. OT is best known for its roles in female parturition and lactation (Gimpl and Fahrenholz, 2001). Some evidence has suggested that OT plays an important role in pain modulation and nociceptive transmission (Rash et al., 2013). OT has been found to have analgesic effects in a variety of pain tests when administered into the brain and the spinal cord (Yu et al., 2003; Gao and Yu, 2004; Miranda-Cardenas et al., 2006; Condes-Lara et al., 2007). Up to now, most studies have focused on the spinal cord as the site of OT analgesia. In the spinal cord, OT may inhibit nociceptive neuronal responses indirectly by activating inhibitory GABA interneurons, or directly by inhibiting second-order neurons (Robinson et al., 2002; Rojas-Piloni et al., 2007; Breton et al., 2009; Condes-Lara et al., 2009). Recently, Hobo et al. (2012) reported that OT targets central terminals of primary sensory afferents and may reduce neurotransmitter release. It has been shown that spinal release or intrathecal injection of OT can relieve both acute and chronic pain in human and rodent species (Rash et al., 2013). OT also exerts an analgesic effect after systemic administration (Lundeberg et al., 1994; Schorscher-Petcu et al., 2010). However, it is not known whether the hormonal effect of OT on nociceptive processing occurs in peripheral terminals of primary sensory afferents.
We report here that OT inhibited the activity of ASICs in DRG neurons and relieved acidosis-evoked pain when administered peripherally. Moreover, the effects of OT on ASICs were mimicked by arginine vasopressin (AVP) and mediated by the vasopressin type1A V1A receptor (for nomenclature see Alexander et al., 2013a), but not by the OT receptor. These conclusions were further confirmed by transgenic knockout mice lacking V1aR. Peripherally administered OT and AVP failed to influence nociceptive behaviours induced by acidosis in V1aR knockout (V1a–/–) mice.
Methods
Isolation of the DRG neurons
The experimental protocol was approved by the animal research ethics committee of Hubei University of Science and Technology. All procedures conformed to international guidelines on the ethical use of animals, and every effort was made to minimize the number of animals used and their suffering. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). Ten- to 12-week-old Sprague-Dawley female rats were anaesthetized with ethyl ether and then decapitated. The DRGs were taken out and transferred immediately into DMEM (Sigma-Aldrich, St. Louis, MO, USA) at pH 7.4. After the removal of the surrounding connective tissues, the DRGs were minced with fine spring scissors and the ganglion fragments were placed in a flask containing 5 mL of DMEM in which trypsin (type II-S, Sigma-Aldrich) 0.5 mg·mL−1, collagenase (type I-A, Sigma-Aldrich) 1.0 mg·mL−1 and DNase (type IV, Sigma-Aldrich) 0.1 mg·mL−1 had been dissolved, and incubated at 35°C in a shaking water bath for 25–30 min. Soybean trypsin inhibitor (type II-S, Sigma-Aldrich) 1.25 mg·mL−1 was then added to stop trypsin digestion. Dissociated neurons were placed into a 35 mm Petri dish and kept for at least another 60 min before electrophysiological recordings. The neurons selected for the electrophysiological experiments were 15–35 μm in diameter.
Electrophysiological recordings
Whole-cell patch-clamp and voltage-clamp recordings were carried out at room temperature (22–25°C) using a MultiClamp-700B amplifier and Digidata-1440A A/D converter (Axon Instruments, Foster City, CA, USA). Recording pipettes were pulled using a Sutter P-97 puller (Sutter Instruments, Novato, CA, USA). The micropipettes were filled with internal solution containing KCl 140 mM, MgCl2 2.5 mM, HEPES 10 mM, EGTA 11 mM and ATP 5 mM; its pH was adjusted to 7.2 with KOH, and osmolarity was adjusted to 310 mOsm·L−1 with sucrose. Cells were bathed in an external solution containing NaCl 150 mM, KCl 5 mM, CaCl2 2.5 mM, MgCl2 2 mM, HEPES 10 mM and d-glucose 10 mM; its osmolarity was adjusted to 330 mOsm·L−1 with sucrose and pH to 7.4. The resistance of the recording pipette was in the range of 3–6 MΩ. A small patch of membrane underneath the tip of the pipette was aspirated to form a gigaseal and then negative pressure was applied to rupture it, thus establishing a whole-cell configuration. The adjustment of capacitance compensation and series resistance compensation was performed before recording the membrane currents. The membrane voltage was maintained at −60 mV in all voltage-clamp experiments unless otherwise specified. Current-clamp recordings were obtained by switching to current-clamp mode after a stable whole-cell configuration was formed in voltage-clamp mode. Only cells with a stable resting membrane potential (more negative than −50 mV) were used in the study. Signals were sampled at 10–50 kHz and filtered at 2–10 kHz, and the data were stored in compatible personal computer (PC) for off/online analysis using the pCLAMP 10 acquisition software (Axon Instruments).
Drug application
All chemicals were purchased from Sigma-Aldrich. The drugs used were hydrochloric acid, OT, AVP, amiloride, APETx2, SR49059, L-368899, YM-254890, capsazepine and tetrodotoxin (TTX). Stocks of drugs were made up and diluted daily in the external solution at a minimum of 1:1000 to a final working concentration. Next, they were held in a linear array of fused silica tubes (o.d./i.d. = 500/200 μm) connected to a series of independent reservoirs. The application pipette tips were positioned 30 μm away from the recorded neurons. The application of each drug was driven by gravity and controlled by the corresponding valve, and rapid solution exchange could be achieved within about 100 ms by shifting the tubes horizontally with a PC-controlled micromanipulator. Cells were constantly bathed in normal external solution flowing from one tube connected to a larger reservoir between drug applications. To functionally characterize ASIC activity, we used capsazepine (10 μM) to block TRPV1 channels (Poirot et al., 2006).
Animals and nociceptive behaviour induced by acetic acid
All behavioural measurements were performed using Sprague-Dawley rats (female, 10–12 weeks old), littermate wild type (WT) and V1A–/– mice (female, 6–8 weeks old, and in C57BL/6 background). The generation of V1A–/– mice by homologous recombination has been described previously (Hu et al., 2003). All animals were kept on a 12 h light/dark cycle at a temperature of 22–24°C and humidity of 50–60%; and with ad libitum access to food and water. Animals were placed in a 30 × 30 × 30 cm3 Plexiglas chamber (ProBeCare,Wuhan, China) and allowed to habituate for at least 30 min before nociceptive behaviour experiments. After pretreatment with 10 μL capsazepine (100 μM), a double-blind experiment was carried out. In rats, 20 μL of acetic acid solution (0.6%) together with 20 μL of external solution, amiloride, OT, AVP and SR 49059 were coded, and the other experimenters administered them s.c. into the dorsal face of the hind paw using a 30-gauge needle connected to a 100 μL Hamilton syringe (Hamilton Co., Reno, NV, USA). In mice, the volume per injection was 10 μL. Nociceptive behaviour (i.e. number of flinches) was counted over a 5 min period starting immediately after the injection (Deval et al., 2008; Omori et al., 2008).
Data analysis
Data were statistically compared using Student's t-test or anova, followed by Bonferroni's post hoc test. Statistical analysis of concentration–response data was performed using non-linear curve-fitting program ALLFIT. Data are expressed as mean ± SEM.
Results
OT decreased ASIC currents in rat DRG neurons
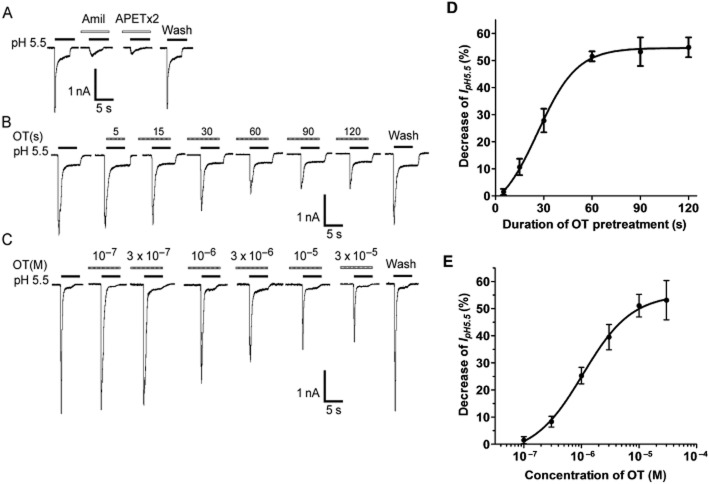
All current measurements in this study were performed in small and medium diameter (15–35 μm) acutely isolated DRG neurons of adult female rats. To functionally characterize ASIC currents, we measured proton-gated currents (IpH) in the presence of capsazepine (10 μM) to block proton-induced TRPV1 activation in the whole-cell patch-clamp configuration (Gavva et al., 2005; Poirot et al., 2006). As our previous report showed (Qiu et al., 2012), a rapid reduction of extracellular pH from 7.4 to 5.5 for 5 s evoked an inward current (IpH 5.5) in most native DRG neurons (79.41%, 135/170). ASICs are the only known channels that mediate the IpH in the presence of the TRPV1 inhibitor capsazepine, as all observed IpH could be completely blocked by 100 μM of amiloride, a broad-spectrum ASIC channel blocker (Figure 1A). We thus considered them to be ASIC currents because they existed in the presence of capsazepine to block proton-induced TRPV1 activation. Most (72.59%, 98/135) of these acid-evoked currents may be ASIC3-like currents (Figure 1A), which can be characterized by a large transient current followed by fast inactivation and then a small sustained current with no or very slow inactivation (Wang et al., 2013). APETx2, a sea anemone peptide, blocks ASIC3 homomeric and heteromeric channels both in transfected cells and rat primary sensory neuron cultures (Diochot et al., 2004). We found that the ASIC3-like currents were also completely blocked by 3 μM of APETx2, further supporting them as ASIC3-like current (Figure 1A). We mainly observed the ASIC3-like currents in this study.
Figure 1.
Inhibition of ASIC currents by OT in rat DRG neurons. (A) Representative current traces were evoked by extracellular application of a pH 5.5 solution for 5 s in the presence of capsazepine (10 μM) to block proton-induced TRPV1 activation. The proton-induced current could be completely blocked by 100 μM amiloride (Amil), a broad-spectrum ASIC channel blocker. It was also blocked by 3 μM APETx2, an ASIC3 blocker. (B and D) Representative current traces and summary data show the effect of OT pretreatment duration on IpH. Current traces illustrate the effect of OT (10−5 M) pretreatment duration on IpH in a DRG cell. The graph shows that the inhibitory effect of OT (10−5 M) increased as OT pretreatment duration increased from 15 to 120 s (n = 8). (C and E) Representative current traces and summary data show a concentration-dependent inhibition of the peak amplitude of IpH by OT. The sequential current traces illustrate the inhibition of IpH by different concentrations of OT (10−7 M − 3 × 10−5 M). All data were obtained from a single DRG neuron. The graph shows OT decreased IpH in a concentration-dependent manner. Each point represents the mean ± SEM of seven to nine neurons. DRG neurons with membrane potential clamped at −60 mV.
Application of OT (10−5 M) prior to application of acid solution decreased IpH 5.5 in the majority of acid-sensitive neurons (63.27%, 62/98). The level of OT inhibition of IpH 5.5 was correlated with the duration of OT pretreatment. As shown in Figure 1B and D, the level of inhibition of IpH 5.5 gradually increased as the duration of OT pretreatment increased from 15 to 60 s. The inhibitory effect of OT reached its maximum (51.52 ± 1.82%, n = 8) at 60 s of pretreatment, after which longer durations had no further effect. However, no inhibitory effects were observed when OT and acidosis of pH 5.5 were co-applied for only 5 s. These results indicate that the inhibition of ASIC currents was dependent on the duration of OT pretreatment.
We next investigated whether the inhibition of ASIC currents was dependent on the concentration of OT. Figure 1C shows that the amplitudes of IpH 5.5 decreased as concentration of OT increased from 10−7 M to 3 × 10−5 M. Figure 1E shows the dose–response curve for OT in the inhibition of IpH. OT had a maximum effect (53.10 ± 7.26%, n = 8) at a concentration of 3 × 10−5 M. The EC50 value of the dose–response curve for OT was 1.06 ± 0.19 μM.
The V1A receptor is involved in OT inhibition of ASIC currents
The inhibition of ASIC currents by OT may involve intracellular signal transduction because this inhibition is a time-consuming process and there was no inhibitory effect observed when OT and acidosis were co-applied. The slow onset of OT inhibition may point to a role of GPCRs in modulating IpH 5.5. First, we observed the effect of YM-254890, a specific Gq/11 inhibitor, on the inhibition of OT (Uemura et al., 2006). In 5 μM YM-254890-pretreated neurons, OT only produced an inhibition of 8.36 ± 0.94% on ASIC currents, which is significantly less than 52.12 ± 4.17% of control conditions (P < 0.01, unpaired t-test, n = 9). The results indicated that Gq/11 protein couple receptors are involved in blocking OT inhibition. We further verified whether the inhibition of IpH 5.5 by OT was mediated by OT receptors. Contrary to our expectations, addition of L-368899 (3 × 10−5 M), a non-peptide OT receptor antagonist (Vrachnis et al., 2011), failed to block the OT inhibition of IpH 5.5 (Figure 2A). However, the OT inhibition of IpH 5.5 was completely blocked by co-treatment of OT with SR49059 (3 × 10−5 M), a selective V1A receptor antagonist (Figure 2B) (Serradeil-Le Gal et al., 1993). In addition to OT, we further observed whether other V1A receptor agonists also decreased ASIC currents. AVP, another V1A receptor agonist, was added before the acid solution and also exerted a similar inhibitory effect on IpH 5.5 (Figure 2C). The amplitude of IpH 5.5 decreased to 43.78 ± 3.77% of control condition after 10−5 M AVP pretreatment for 60 s (n = 8, P < 0.01, pairing t-test). Likewise, the V1A receptor antagonist SR49059 (3 × 10−5 M) completely blocked the AVP inhibition of IpH 5.5 (Figure 2C). Together, these results indicate that the OT inhibition of ASIC currents is mediated by V1A receptors, but not by OT receptors.
Figure 2.
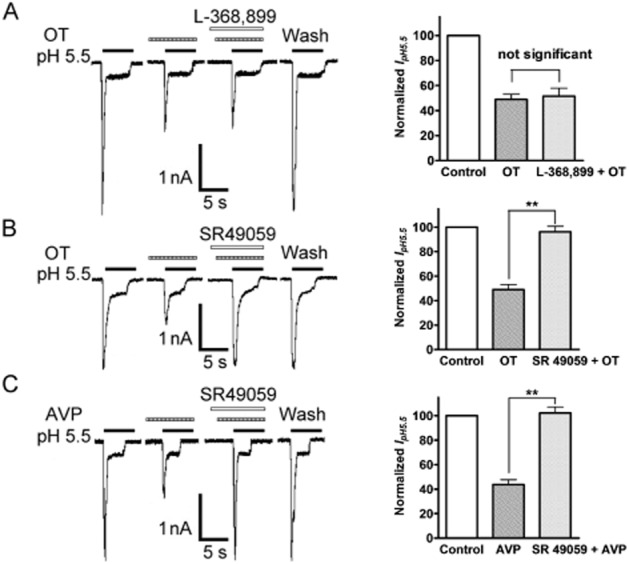
Involvement of the V1A receptor in OT inhibition of ASIC currents. The current traces in A and B show that the inhibition of IpH 5.5 by OT (10−5 M) pre-applied alone was abolished by the co-application of OT and SR49059 (3 × 10−5 M), a selective V1A receptor antagonist, but not by addition of L-368899 (3 × 10−5 M), a non-peptide OT receptor antagonist. The current traces in (C) show that AVP has a similar inhibitory effect on IpH 5.5, and the inhibition was also blocked by SR49059. Bar graphs in the right panel show currents normalized to control (100%). Data in all bar graphs are shown relative to control. Error bars show ±SEM. Statistical tests were performed on raw data using paired t-test, and significance is shown as follows: *P < 0.05, **P < 0.01. n = 8 in each column.
OT decreased ASIC currents in a pH-dependent manner
We then investigated whether the inhibition of OT was dependent on pH. Figure 3A shows the effect of OT pretreatment for 60 s on currents evoked by different pHs. The decrease in current caused by OT pretreatment was most pronounced at pH 6.0 and 5.5, whereas at pH 4.5 there was no difference between currents recorded in the presence and absence of OT pretreatment. This is illustrated in Figure 3B, which shows the concentration–response curve to protons in the presence and absence of OT. The pH0.5 value of curve changed from 5.93 ± 0.04 to 5.43 ± 0.04 in the presence of OT (n = 8, P < 0.05, Bonferroni's post hoc test). However, the maximal current response to protons and the threshold pH values of both curves had no significant difference in the presence and absence of OT. These results indicated that OT decreased ASIC currents in a pH-dependent manner.
Figure 3.
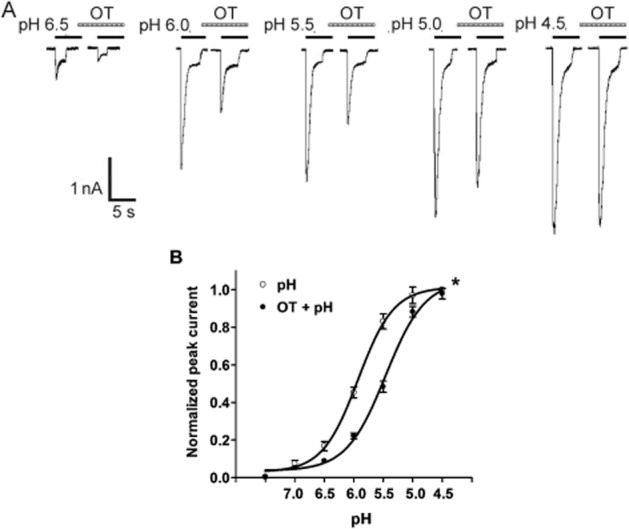
Concentration–response relationship for protons with or without the pre-application of OT. (A) Sequential currents evoked by different pHs in the absence or presence of OT. (B) The concentration–response curves for protons with or without 10−5 M OT as a pretreatment. Each point represents the mean ± SEM of 7–11 neurons. All current values were normalized to the current response induced by pH 4.5 applied alone (marked with asterisk). The curves shown are a best fit of the data to the logistic equation I = Imax/[1 + (pH0.5/C)nH], where C is the concentration of protons, I is the normalized current response value, pH0.5 is the proton concentration that produced half the maximal current response to protons, and nH is the Hill coefficient. The curves for protons without and with OT pretreatment were drawn according to the equation described above.
OT decreased proton-induced membrane excitability of rat DRG neurons
Activation of ASICs by protons induces sodium influx, resulting in membrane depolarization and neuronal excitation. Further experiments were performed to record DRG neuron excitability in current-clamp model in the presence of capsazepine (10 μM) to block proton-induced TRPV1 activation. As shown in Figure 4A, a steep pH drop from 7.4 to 5.5 for 5 s could trigger bursts of action potentials under current-clamp conditions in the tested neuron, whereas the whole-cell inward current was also induced by pH 5.5 in the same cell with voltage-clamp recording. Importantly, pretreatment of OT (10−5 M) for 60 s decreased the number of action potentials evoked by acidosis. The mean number of action potentials decreased from 12.57 ± 1.30 of control condition to 8.39 ± 1.08 with pretreatment of OT in the 10 neurons tested (P < 0.05, paired t-test) (Figure 4C). After a washout of OT for 10 min, the mean number of action potentials evoked by acidosis was 11.59 ± 1.96, which was not significantly different from the control condition (12.57 ± 1.30, paired t-test, P > 0.1, n = 10; Figure 4A and C).
Figure 4.
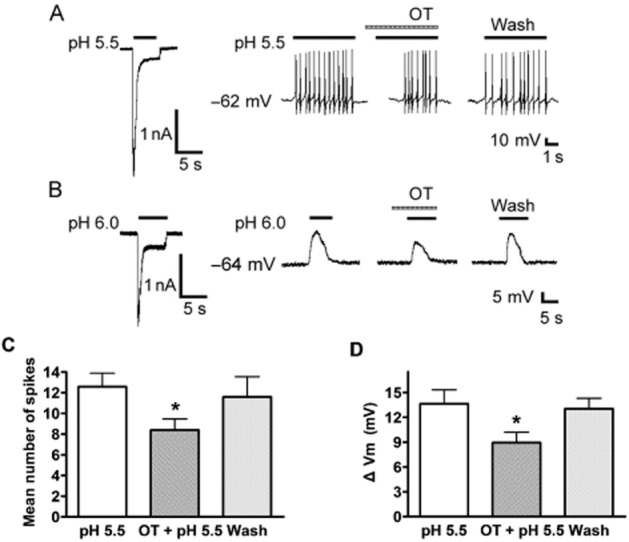
Effect of OT on proton-evoked membrane excitability of rat DRG neurons. (A) Original current and spikes recordings from the same DRG neuron. Left panel: a pH 5.5 acid stimulus induced an inward current with voltage-clamp recording. Right panel: the pH 5.5 acid stimulus produced cell spikes with current-clamp recording in the same neuron in the presence of the TRPV1 inhibitor capsazepine (10 μM). The treatment with OT (10−5 M) for 60 s inhibited the acidosis-induced spiking activity. (B) Original current and membrane potential recordings from the same DRG neuron. Left panel: voltage-clamp recording of current induced by a pH 5.5 acid stimulus. Holding potential was −60 mV. Right panel: current-clamp recording (I = 0 pA) of the depolarization evoked by the pH 5.5 acid stimulus from the same neuron as the left panel. The treatment of OT (10−5 M) for 60 s decreased the acidosis-induced membrane depolarization. No action potential was triggered by the membrane depolarization in the neuron tested in the presence of capsazepine (10 μM) and TTX (1 μM) to block proton-induced TRPV1 activation and Na+ channel-mediated action potentials respectively. (C and D) Bar graphs show the effects of OT on the number of spikes and membrane potential depolarization produced by pH 5.5. The acidosis-evoked depolarization and spikes recovered to control condition after washout of OT for 10 min. *P < 0.05, paired t-test, compared with pH alone, n = 10 in each column.
It was shown that TTX is not effective in blocking the IpH, although it blocks the majority of voltage-gated Na+ currents (Lilley et al., 2004). We then observed the membrane potential of DRG neurons in the presence of capsazepine (10 μM) and TTX (1 μM) to block proton-induced TRPV1 activation and Na+ channel-mediated action potentials respectively. A pH 5.5 acid stimulus induced an inward current in a DRG neuron tested with voltage-clamp recording, whereas it also produced a depolarization of the resting membrane potential in the same cell under current-clamp recording (I = 0 pA) conditions (Figure 4B). In this particular cell, pretreatment of OT (10−5 M) for 60 s decreased the depolarization of the membrane potential evoked by pH 5.5 acid stimuli (Figure 4B). In 10 neurons tested, membrane potential depolarized from 61.58 ± 5.33 to 47.95 ± 4.92 mV after exposure to pH 5.5. In contrast, OT decreased the magnitude of acidosis-induced depolarization from 13.63 ± 1.69 to 8.94 ± 1.27 mV (paired t-test, P < 0.05, n = 10) (Figure 4D). After washout of OT for 10 min, the acidosis-evoked depolarization of membrane potential recovered to control condition (13.02 ± 1.28 mV, paired t-test, P > 0.1, compared with 13.63 ± 1.69 mV of control condition, n = 10; Figure 4B and D). Collectively, these results indicated that OT reversibly decreased proton-induced membrane excitability of rat DRG neurons.
OT and AVP decreased nociceptive responses to intraplantar injection of acetic acid in rats
Intraplantar injection of acetic acid elicited an intense flinch/shaking response in rats (Deval et al., 2008; Omori et al., 2008). The flinch response mainly occurred during 0–5 min after injection of acetic acid. In the present study, intraplantar injection of acetic acid solution (0.6%, 20 μL) in the presence of the TRPV1 inhibitor capsazepine (100 μM) caused an intense flinch/shaking response in female rats. The acidosis-evoked pain may be mediated by ASIC3, as it was potently blocked by treatment with 200 μM amiloride and 20 μM APETx2 (Figure 5A). Administration of OT significantly decreased flinching behaviour induced by acetic acid in a dose-dependent manner. Quantitative analysis showed that OT decreased the number of flinches from 11.56 ± 1.25 of vehicle treatment to 10.62 ± 0.91, 8.06 ± 0.62 and 6.68 ± 0.55 at dose of 0.1, 1 and 10 μM, respectively (n = 10, P < 0.05 or 0.01, post hoc Bonferroni's test) (Figure 5B). In contrast, the analgesic effect of OT on acetic acid-induced pain behaviour was not observed when OT was co-injected together with SR49059, a selective V1A receptor antagonist. And the number of flinches induced by acetic acid (12.73 ± 1.67) was not significantly different from those induced by vehicle treatment (P > 0.1, post hoc Bonferroni's test, n = 10). Moreover, administration of AVP also significantly decreased flinching behaviour induced by acetic acid from 11.56 ± 1.25 of vehicle treatment to 6.18 ± 1.14 (P < 0.05, post hoc Bonferroni's test, n = 10) (Figure 5C). Likewise, the V1A receptor antagonist SR49059 completely blocked the analgesic effect of AVP on acetic acid-induced pain (Figure 5C). These results indicate that the acidosis-evoked pain was relieved by the activation of V1A receptors by OT or AVP at the periphery.
Figure 5.
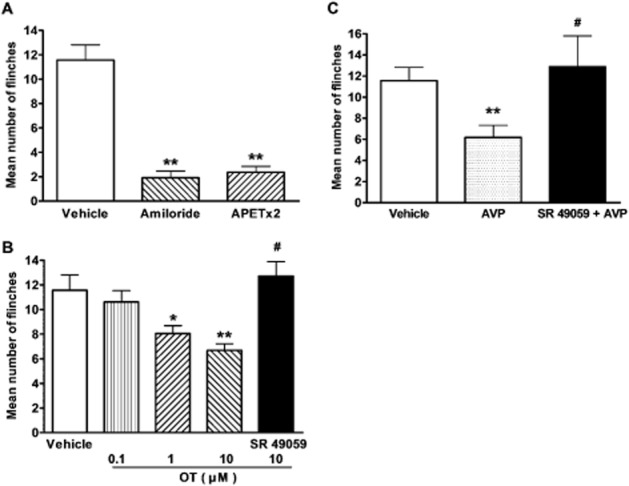
Effects of OT and AVP on nociceptive responses to intraplantar injection of acetic acid in rats. Intraplantar injection of acetic acid (0.6%, 20 μL) evoked a flinch/shaking response in female rats. The bar graph in (A) shows acidosis-evoked pain was blocked by pretreatment with 200 μM amiloride and 20 μM APETx2. **P < 0.01, unpaired t-test, compared with vehicle column. n = 10 in each column. The bar graph in (B) shows that pretreatment with OT decreased flinching behaviour induced by acetic acid in a dose-dependent manner. The effect of OT was blocked by SR49059, a selective V1A receptor antagonist. *P < 0.05, **P < 0.01, one-way anova followed by post hoc Bonferroni's test, compared with vehicle column; #P < 0.01, post hoc Bonferroni's test, compared with OT (10 μM) column. n = 10 in each column. The bar graph in (C) shows that pretreatment with 10 μM AVP also decreased flinching behaviour induced by acetic acid, and SR49059 completely blocked the analgesia of AVP on acetic acid-induced pain. **P < 0.01, one-way anova followed by post hoc Bonferroni's test, compared with vehicle column; #P < 0.01, post hoc Bonferroni's test, compared with AVP column. n = 10 in each column. Flinch/shaking of paw was recorded as the number of flinches per observation period (5 min).
OT and AVP failed to decrease nociceptive behaviours induced by acetic acid in mice lacking V1A receptors
To further address the finding that the OT and AVP analgesic effects on acidosis-evoked pain were mediated by the V1A receptor, we investigated the roles of OT and AVP in specific V1A–/– mice. Intraplantar injection of acetic acid solution (0.6%, 10 μL) in the presence of the TRPV1 inhibitor capsazepine (100 μM) also caused an intense flinch/shaking response in female mice. There was no significant difference in flinching behaviour induced by acetic acid between V1A–/– mice and WT littermates. Similar to that observed in rats, administration of 10 μM OT or AVP significantly decreased flinching behaviour induced by acetic acid from 29.56 ± 2.03 of vehicle treatment to 16.89 ± 1.06 and 15.50 ± 1.68, respectively, in V1A receptor WT littermates (P < 0.1, post hoc Bonferroni's test, n = 10) (Figure 6). However, OT or AVP failed to decrease flinching behaviour induced by acetic acid in V1A–/– mice (Figure 6). After treatment of OT or AVP, the number of flinches induced by acetic acid in V1A–/– mice were 33.46 ± 5.37 and 26.92 ± 1.61, respectively, which were not significantly different from 28.76 ± 1.43 of vehicle treatment (P > 0.1, post hoc Bonferroni's test, n = 10). These results suggest that the OT- and AVP-induced analgesic effects on acidosis-evoked pain were absent in V1A–/– mice, but present in WT littermates.
Figure 6.
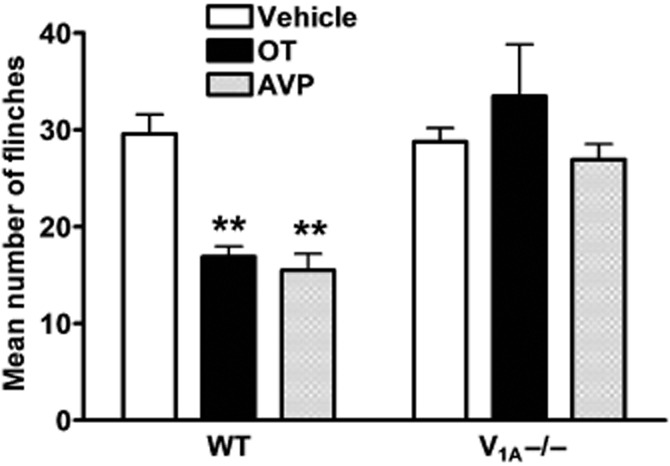
Effects OT and AVP on nociceptive behaviours induced by acetic acid in V1A–/– mice. Intraplantar injection pf acetic acid (0.6%, 10 μL) evoked a flinch/shaking response in female V1A–/– mice and WT littermates. Administration of 10 μM OT or AVP decreased flinching behaviour induced by acetic acid in WT, but not V1A–/– mice. **P < 0.01, one-way anova followed by post hoc Bonferroni's test, compared with vehicle column. n = 10 in each column. Flinch/shaking of paw was recorded as the number of flinches per observation period (5 min).
Discussion
This study demonstrates that OT can exert an inhibitory effect on the functional activity of ASICs. OT decreased the amplitude of ASIC currents and acidosis-evoked membrane excitability in dissociated rat DRG neurons. Peripherally administered OT inhibited nociceptive responses to intraplantar injection of acetic acid in vivo. Effects of OT on ASICs could be mimicked by AVP and mediated by V1A receptors, but not by OT receptors. The conclusion was further supported by the data that OT or AVP-induced analgesic effect on acidosis-evoked pain was completely absent in V1A–/– mice, but present in WT littermates.
A rapid drop in the extracellular pH from 7.4 to 5.5 for 5 s was found to evoke an inward current in most native DRG neurons. The acidosis-evoked currents may be involved in the activation of ASIC and TRPV1 channels (Bevan and Geppetti, 1994). The contribution of TRPV1 was ruled out as capsazepine was used to block proton-induced TRPV1 activation (Gavva et al., 2005; Poirot et al., 2006). Our previous work showed that the acid-induced currents are not blocked by the TRPV1 blocker AMG 9810 (Qiu et al., 2012). Importantly, these acid-induced currents could be completely blocked by amiloride, a broad-spectrum ASIC channel blocker, suggested the acid currents were mediated by ASICs. To date, at least four genes encoding seven ASIC subunits have been cloned in mammals (Krishtal, 2003). All ASICs besides ASIC4 are present in DRG neurons (Alvarez de la Rosa et al., 2002; Benson et al., 2002). ASIC3 have emerged as critical pH sensors predominantly expressed in nociceptors (Price et al., 2001; Voilley et al., 2001; Deval et al., 2008). The present acid currents were completely blocked by ASIC3 channel blocker APETx2 and characterized by a large transient current followed by fast inactivation and then a small sustained current (Wang et al., 2013). They may be ASIC3-like currents, although we cannot rule out the possibility of other ASIC subunits. We considered the acid currents as ASIC3 currents in the present study.
Our study showed that OT exerted an inhibitory effect on ASIC currents in a dose-dependent manner in rat DRG neurons. Inhibition of OT was due to reduction of the affinity of ASICs to protons, as shown by a decrease in the pH0.5. ASICs are extracellular pH sensors and are selectively permeable to cations (Wemmie et al., 2013). Activation of ASICs by a rapid drop in pH induces an inward current, which causes a depolarization of the resting membrane potential and triggers bursts of action potentials (Mamet et al., 2002). The current-clamp experiments showed that OT decreased the amplitude of the depolarization and the number of action potentials induced by extracellular acid stimuli. Effect of OT on acidosis-evoked neuronal excitability of DRG neurons appeared to be related to their inhibitory effect on the ASIC current amplitude in voltage-clamp experiments. Consistent with the electrophysiological results, behavioural experiments showed that OT relieved acidosis-evoked pain in a dose-dependent manner. Altogether, the present study indicated that OT inhibited the functional activity of ASICs in primary sensory neurons.
The central and peripheral effects of OT are thought to be mediated by OT's binding to a single isoform of the OT receptor (Gimpl and Fahrenholz, 2001). OT receptors are densely expressed in pain-relevant laminae I and II of the dorsal horn and are a likely target of spinal oxytocinergic projections from the paraventricular nucleus of the hypothalamus (Reiter et al., 1994; Puder and Papka, 2001; Condes-Lara et al., 2007). However, the presence of OT receptors in DRG is controversial. In the mouse, OT receptor mRNA was only barely expressed in this area (Schorscher-Petcu et al., 2010). Instead, it was reported recently that OT receptors are expressed in C-fibre cell bodies, but not in skin nociceptive terminals in rats (Moreno-Lopez et al., 2013). Both OT and AVP, as well as the OT and V1A receptor, display a high degree of sequence homology, and both peptides can activate both receptors (Chini and Manning, 2007). Similar to OT, systemic injections of AVP also lead to analgesia (Mogil et al., 2011). AVP analgesia is believed to be mediated by V1A receptors, as V1A receptor mRNA is reported to be abundantly expressed in mouse DRGs, and V1A receptor-positive neurons were predominantly of small and medium diameter (Schorscher-Petcu et al., 2010). Both OT and AVP are also expressed in the DRG and trigeminal ganglia of the rat and cause an increase in the accumulation of inositol phosphates (IP) through V1A receptors (Kai-Kai et al., 1985; Horn and Lightman, 1987; Kai-Kai and Che, 1995). A recent study showed that i.v. injection of a low dose of OT causes an OT receptor-mediated antinociceptive effect whereas a high dose of OT exerts a V1A receptor-mediated pronociceptive effect in rats (Juif and Poisbeau, 2013). In contrast, a study in transgenic mice lacking the OT or V1A receptor indicates that OT-induced antinociception was mediated by V1A receptors, but not by OT receptors, in mice (Schorscher-Petcu et al., 2010). The study showed that i.p. injection of OT still produces mechanical and thermal analgesia in OT receptor-knockout mice as well as in WT littermates, but does not alter nociceptive threshold in V1A–/– mice (Schorscher-Petcu et al., 2010). In the present study, OT inhibition of ASICs appeared to involve V1A receptors, but not OT receptors. Firstly, OT inhibition of ASIC currents was blocked by the V1A receptor antagonist SR49059, but not by OT receptor antagonist L-368899. Secondly, the effect of OT on ASICs was mimicked by AVP. Thirdly, behavioural experiments showed that analgesia mediated by OT and AVP on acetic acid-induced pain were completely blocked by SR49059. Finally, OT and AVP failed to relieve acidosis-evoked pain in V1A–/– mice, whereas they significantly decreased nociceptive behaviours induced by acetic acid in WT littermates.
The present study showed that the inhibitory effect of OT was mediated by V1A receptors. It was reported that activation of the V1A receptor by AVP could induce hyperpolarization in most DRG neuronal membranes and increase the membrane conductance of the DRG neurons (Hu et al., 2004). But we observed that the baselines of original membrane current and potential did not change when DRG neurons were treated with OT and AVP in both voltage-clamp and current-clamp recordings, as seen in Figures 1 to 4. ASIC3 is a voltage-insensitive and sodium-selective ion channel, and the effect of OT on ASIC3 currents may not be due to the hyperpolarization. As for the mechanistic link between V1A receptors and ASIC3, one plausible explanation may be the involvement of calcineurin signalling, a pathway-modulating calcium-dependent protein phosphorylation. The activation of V1A receptors seems to trigger a multifaceted and complex response in which the calcineurin pathway is stimulated (Scicchitano et al., 2005; Toschi et al., 2011). Calcineurin has been reported to be involved in the regulation of ASICs (Chai et al., 2007). Cyclosporin A, a specific inhibitor of calcineurin, induces an increase in ASIC current amplitude in mouse cultured cortical neurons (Chai et al., 2007). This suggests that calcineurin-dependent dephosphorylation could play an important role for ASICs, as these channels have been reported to be in a highly phosphorylated state at basal level (Leonard et al., 2003). Thus, we speculate that the activation of V1A receptors by OT or AVP up-regulated calcineurin, which induced calcineurin-dependent dephosphorylation of ASICs and resulted in a decrease in ASIC current amplitude.
Extracellular acidosis is a common feature in pain-generating pathological conditions such as inflammation, tissue injury, ischaemic stroke, infections and cancer (Wemmie et al., 2013). Although both ASICs and TRPV1 could be involved, ASICs are believed to be the primary mediators of pain caused by extracellular acidification (Wemmie et al., 2006; Deval et al., 2008). It has been shown that acidosis-induced pain is significantly attenuated by the non-selective ASIC inhibitor amiloride, whereas the TRPV1 antagonist capsazepine failed to influence C-fibre excitability induced by an acidic solution (Habelt et al., 2000; Ugawa et al., 2002). Intraplantar injection of acetic acid elicited an intense flinch/shaking response in rats (Deval et al., 2008; Omori et al., 2008). In the present study, the acidosis-evoked pain was also mediated by ASICs, as it was potently blocked by the ASIC channel blocker amiloride. Among ASICs, ASIC3 is expressed almost exclusively expressed in sensory neurons and predominantly in nocifensive sensory neurons (Waldmann et al., 1997a; Waldmann and Lazdunski, 1998; Krishtal, 2003; Deval et al., 2008). Recently, it was reported that peripheral ASIC3 plays a significant role in post-operative pain (Deval et al., 2011). Pharmacological inhibition of ASIC3 channels with the toxin APETx2 or in vivo knockdown of the ASIC3 protein significantly reduced the post-operative spontaneous, thermal and postural pain behaviours (Deval et al., 2011). OT and AVP do not cross the blood–brain barrier (Ermisch et al., 1985). So far, it is not known whether the hormonal role of OT on nociceptive processing occurs in peripheral terminals of primary sensory afferents. One study has shown that OT inhibits ATP-activated currents in rat DRG neurons (Yang et al., 2002). OT is also found to inhibit intracellular calcium increases in capsaicin-sensitive DRG neurons (Hobo et al., 2012). However, it was recently reported that OT activates intracellular calcium signalling in cultured rat primary sensory neurons through a PKC-dependent mechanism (Ayar et al., 2014). In this work, we used the cell body of DRG neurons as a simple and accessible model to examine the characteristics of the membrane of peripheral terminals. Histochemical analysis reveals the presence of ASIC3 in cutaneous nerve endings (Price et al., 2001; Deval et al., 2008). OT inhibition of ASICs hinted that a novel peripheral analgesic mechanism of OT occurs in peripheral terminals of primary sensory neurons. This view was further supported by behavioural experiments that peripheral application of OT relieved acidosis-evoked pain in a dose-dependent manner.
In conclusion, this study shows that OT inhibits the functional activity of ASICs in DRG neurons. The OT inhibition of ASICs was mediated by V1A receptors, but not by OT receptors. OT relieved acidosis-evoked pain when administered peripherally, whereas OT analgesia was absent in mice lacking V1A receptors. Our results reveal a novel peripheral mechanism of OT analgesic action by modulating native ASICs in primary sensory neurons. This indicates that OT released while giving birth may have multiple pathways of analgesic effects.
Acknowledgments
We thank Dr Shuang-Bao Hu (National Institute of Mental Health, Bethesda, MD, USA) for providing V1A receptor knockout mice. We also thank Dr Weizheng Wei (University of California, Los Angeles, Los Angeles, CA, USA) for helpful comments on the manuscript. This work was supported by the National Natural Science Foundation of China (No. 81171039 and No. 30970944), Program for New Century Excellent Talents in University (NCET-11-0967) and Team of Outstanding Young Scientific and Technological Innovation of the Higher Education Institutions of Hubei Province (T201113).
Glossary
- ASIC
acid-sensing ion channels
- AVP
arginine vasopressin
- DRG
dorsal root ganglion
- IpH
proton-gated current
- OT
oxytocin
- TRPV1
transient receptor potential vanilloid channel type 1
- TTX
tetrodotoxin
- V1A receptor
vasopressin type 1A receptor
- V1A–/– mice
V1A receptor knockout mice
- WT
wild-type
Conflict of interest
We have no conflict of interest to declare.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: G-protein couple receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: Ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci U S A. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayar A, Ozcan M, Alcin E, Serhatlioglu I, Ozcan S, Kutlu S, et al. Oxytocin activates calcium signaling in rat sensory neurons through a protein kinase C-dependent mechanism. J Physiol Biochem. 2014;70:43–48. doi: 10.1007/s13105-013-0278-z. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, et al. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Breton JD, Poisbeau P, Darbon P. Antinociceptive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol Pain. 2009;5:63. doi: 10.1186/1744-8069-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S, Li M, Lan J, Xiong ZG, Saugstad JA, Simon RP. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J Biol Chem. 2007;282:22668–22677. doi: 10.1074/jbc.M703624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WH, Hsieh CL, Huang CP, Lin TJ, Tzen JT, Ho TY, et al. Acid-sensing ion channel 3 mediates peripheral anti-hyperalgesia effects of acupuncture in mice inflammatory pain. J Biomed Sci. 2011;18:82. doi: 10.1186/1423-0127-18-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Manning M. Agonist selectivity in the oxytocin/vasopressin receptor family: new insights and challenges. Biochem Soc Trans. 2007;35((Pt 4)):737–741. doi: 10.1042/BST0350737. [DOI] [PubMed] [Google Scholar]
- Condes-Lara M, Martinez-Lorenzana G, Rojas-Piloni G, Rodriguez-Jimenez J. Branched oxytocinergic innervations from the paraventricular hypothalamic nuclei to superficial layers in the spinal cord. Brain Res. 2007;1160:20–29. doi: 10.1016/j.brainres.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Condes-Lara M, Rojas-Piloni G, Martinez-Lorenzana G, Lopez-Hidalgo M, Rodriguez-Jimenez J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 2009;1247:38–49. doi: 10.1016/j.brainres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Deval E, Noel J, Lay N, Alloui A, Diochot S, Friend V, et al. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008;27:3047–3055. doi: 10.1038/emboj.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noel J, Salinas M, Baron A, Diochot S, et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther. 2010;128:549–558. doi: 10.1016/j.pharmthera.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Deval E, Noel J, Gasull X, Delaunay A, Alloui A, Friend V, et al. Acid-sensing ion channels in postoperative pain. J Neurosci. 2011;31:6059–6066. doi: 10.1523/JNEUROSCI.5266-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23:1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermisch A, Ruhle HJ, Landgraf R, Hess J. Blood–brain barrier and peptides. J Cereb Blood Flow Metab. 1985;5:350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Yu LC. Involvement of opioid receptors in the oxytocin-induced antinociception in the central nervous system of rats. Regul Pept. 2004;120:53–58. doi: 10.1016/j.regpep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Tamir R, Qu Y, Klionsky L, Zhang TJ, Immke D, et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J Pharmacol Exp Ther. 2005;313:474–484. doi: 10.1124/jpet.104.079855. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Habelt C, Kessler F, Distler C, Kress M, Reeh PW. Interactions of inflammatory mediators and low pH not influenced by capsazepine in rat cutaneous nociceptors. Neuroreport. 2000;11:973–976. doi: 10.1097/00001756-200004070-00015. [DOI] [PubMed] [Google Scholar]
- Hobo S, Hayashida K, Eisenach JC. Oxytocin inhibits the membrane depolarization-induced increase in intracellular calcium in capsaicin sensitive sensory neurons: a peripheral mechanism of analgesic action. Anesth Analg. 2012;114:442–449. doi: 10.1213/ANE.0b013e31823b1bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Ozaki N, Suzuki S, Sugiura Y. Upregulations of P2X(3) and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats. Pain. 2010;149:393–405. doi: 10.1016/j.pain.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Horn AM, Lightman SL. Vasopressin-induced turnover of phosphatidylinositol in the sensory nervous system of the rat. Exp Brain Res. 1987;68:299–304. doi: 10.1007/BF00248795. [DOI] [PubMed] [Google Scholar]
- Hu HY, Sun ZP, Zhao YM, Si JQ, Zheng Y. Effect of arginine vasopressin on membrane potential of dorsal root ganglion neurons in rats. Sheng Li Xue Bao. 2004;56:107–111. [PubMed] [Google Scholar]
- Hu SB, Zhao ZS, Yhap C, Grinberg A, Huang SP, Westphal H, et al. Vasopressin receptor 1a-mediated negative regulation of B cell receptor signaling. J Neuroimmunol. 2003;135:72–81. doi: 10.1016/s0165-5728(02)00442-3. [DOI] [PubMed] [Google Scholar]
- Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24:10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juif PE, Poisbeau P. Neurohormonal effects of oxytocin and vasopressin receptor agonists on spinal pain processing in male rats. Pain. 2013;154:1449–1456. doi: 10.1016/j.pain.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Kai-Kai MA, Che YM. Distribution of arginine-vasopressin in the trigeminal, dorsal root ganglia and spinal cord of the rat; depletion by capsaicin. Comp Biochem Physiol A Physiol. 1995;110:71–78. doi: 10.1016/0300-9629(94)00145-j. [DOI] [PubMed] [Google Scholar]
- Kai-Kai MA, Swann RW, Keen P. Localization of chromatographically characterized oxytocin and arginine-vasopressin to sensory neurones in the rat. Neurosci Lett. 1985;55:83–88. doi: 10.1016/0304-3940(85)90316-7. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Kweon HJ, Suh BC. Acid-sensing ion channels (ASICs): therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013;46:295–304. doi: 10.5483/BMBRep.2013.46.6.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard AS, Yermolaieva O, Hruska-Hageman A, Askwith CC, Price MP, Wemmie JA, et al. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci U S A. 2003;100:2029–2034. doi: 10.1073/pnas.252782799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley S, LeTissier P, Robbins J. The discovery and characterization of a proton-gated sodium current in rat retinal ganglion cells. J Neurosci. 2004;24:1013–1022. doi: 10.1523/JNEUROSCI.3191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeberg T, Uvnas-Moberg K, Agren G, Bruzelius G. Anti-nociceptive effects of oxytocin in rats and mice. Neurosci Lett. 1994;170:153–157. doi: 10.1016/0304-3940(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Cardenas Y, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Lopez-Hidalgo M, Freund-Mercier MJ, et al. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122:182–189. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Sorge RE, LaCroix-Fralish ML, Smith SB, Fortin A, Sotocinal SG, et al. Pain sensitivity and vasopressin analgesia are mediated by a gene-sex-environment interaction. Nat Neurosci. 2011;14:1569–1573. doi: 10.1038/nn.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez Y, Martinez-Lorenzana G, Condes-Lara M, Rojas-Piloni G. Identification of oxytocin receptor in the dorsal horn and nociceptive dorsal root ganglion neurons. Neuropeptides. 2013;47:117–123. doi: 10.1016/j.npep.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Omori M, Yokoyama M, Matsuoka Y, Kobayashi H, Mizobuchi S, Itano Y, et al. Effects of selective spinal nerve ligation on acetic acid-induced nociceptive responses and ASIC3 immunoreactivity in the rat dorsal root ganglion. Brain Res. 2008;1219:26–31. doi: 10.1016/j.brainres.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576((Pt 1)):215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Puder BA, Papka RE. Hypothalamic paraventricular axons projecting to the female rat lumbosacral spinal cord contain oxytocin immunoreactivity. J Neurosci Res. 2001;64:53–60. doi: 10.1002/jnr.1053. [DOI] [PubMed] [Google Scholar]
- Qiu F, Qiu CY, Liu YQ, Wu D, Li JD, Hu WP. Potentiation of acid-sensing ion channel activity by the activation of 5-HT(2) receptors in rat dorsal root ganglion neurons. Neuropharmacology. 2012;63:494–500. doi: 10.1016/j.neuropharm.2012.04.034. [DOI] [PubMed] [Google Scholar]
- Rash JA, Aguirre-Camacho A, Campbell TS. Oxytocin and pain: a systematic review and synthesis of findings. Clin J Pain. 2013 doi: 10.1097/AJP.0b013e31829f57df. [Epub ahead of print] doi: 10.1097/AJP.0b013e31829f57df. [DOI] [PubMed] [Google Scholar]
- Reiter MK, Kremarik P, Freund-Mercier MJ, Stoeckel ME, Desaulles E, Feltz P. Localization of oxytocin binding sites in the thoracic and upper lumbar spinal cord of the adult and postnatal rat: a histoautoradiographic study. Eur J Neurosci. 1994;6:98–104. doi: 10.1111/j.1460-9568.1994.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, et al. Oxytocin mediates stress-induced analgesia in adult mice. J Physiol. 2002;540((Pt 2)):593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Piloni G, Lopez-Hidalgo M, Martinez-Lorenzana G, Rodriguez-Jimenez J, Condes-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137:69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano BM, Spath L, Musarò A, Molinaro M, Rosenthal N, Nervi C, et al. Vasopressin-dependent myogenic cell differentiation is mediated by both Ca2+/calmodulin-dependent kinase and calcineurin pathways. Mol Biol Cell. 2005;16:3632–3641. doi: 10.1091/mbc.E05-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Garcia C, Lacour C, Guiraudou P, Christophe B, et al. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, von Hanwehr R, Siesjo BK. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J Cereb Blood Flow Metab. 1986;6:574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen KH, Issberner U, Reeh PW. Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci Lett. 1995;199:29–32. doi: 10.1016/0304-3940(95)12002-l. [DOI] [PubMed] [Google Scholar]
- Toschi A, Severi A, Coletti D, Catizone A, Musarò A, Molinaro M, et al. Skeletal muscle regeneration in mice is stimulated by local overexpression of V1a-vasopressin receptor. Mol Endocrinol. 2011;25:1661–1673. doi: 10.1210/me.2011-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Takamatsu H, Kawasaki T, Taniguchi M, Yamamoto E, Tomura Y, et al. Effect of YM-254890, a specific Galphaq/11 inhibitor, on experimental peripheral arterial disease in rats. Eur J Pharmacol. 2006;536:154–161. doi: 10.1016/j.ejphar.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, et al. Review: oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16:e138–e156. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrachnis N, Malamas FM, Sifakis S, Deligeoroglou E, Iliodromiti Z. The oxytocin–oxytocin receptor system and its antagonists as tocolytic agents. Int J Endocrinol. 2011;2011:350546. doi: 10.1155/2011/350546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Wang X, Li WG, Yu Y, Xiao X, Cheng J, Zeng WZ, et al. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci. 2013;33:4265–4279. doi: 10.1523/JNEUROSCI.3376-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci. 2013;14:461–471. doi: 10.1038/nrn3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Wu ZZ, Li X, Li ZW, Wei JB, Hu QS. Modulation by oxytocin of ATP-activated currents in rat dorsal root ganglion neurons. Neuropharmacology. 2002;43:910–916. doi: 10.1016/s0028-3908(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Yu SQ, Lundeberg T, Yu LC. Involvement of oxytocin in spinal antinociception in rats with inflammation. Brain Res. 2003;983:13–22. doi: 10.1016/s0006-8993(03)03019-1. [DOI] [PubMed] [Google Scholar]