Abstract
BACKGROUND AND PURPOSE
CYP2D6 metabolizes many centrally acting drugs, neurotoxins and endogenous neurochemicals, and differences in brain levels of CYP2D have been associated with brain function and drug response. Alcohol consumers and smokers have higher levels of CYP2D6 in brain, but not liver, suggesting ethanol and/or nicotine may induce human brain CYP2D6. We investigated the independent and combined effects of chronic ethanol self-administration and nicotine treatment on CYP2D expression in African green monkeys.
EXPERIMENTAL APPROACH
Forty monkeys were randomized into control, ethanol-only, nicotine-only and ethanol + nicotine groups. Two groups voluntarily self-administered 10% ethanol in sucrose solution for 4 h·day−1, whereas two groups consumed sucrose solution on the same schedule. Two groups received daily s.c. injections of 0.5 mg·kg−1 nicotine in saline bid, whereas two groups were injected with saline on the same schedule.
KEY RESULTS
Both nicotine and ethanol dose-dependently increased CYP2D in brain; brain mRNA was unaffected, and neither drug altered hepatic CYP2D protein or mRNA. The combination of ethanol and nicotine increased brain CYP2D protein levels to a greater extent than either drug alone (1.2–2.2-fold, P < 0.05 among the eight brain regions assessed). Immunohistochemistry revealed the induction of brain CYP2D protein within specific cell types and regions in the treatment groups.
CONCLUSIONS AND IMPLICATIONS
Ethanol and nicotine increase brain CYP2D protein levels in monkeys, in a region and treatment-specific manner, suggesting that CNS drug responses, neurodegeneration and personality may be affected among people who consume alcohol and/or nicotine.
Keywords: CYP2D, brain, liver, nicotine, ethanol, induction, monkey
Introduction
Cytochrome P450 2D6 (CYP2D6; see Alexander et al., 2013) is an enzyme that is involved in the metabolism of a wide range of centrally acting drugs (e.g. amphetamines, antidepressants, analgesics, antipsychotics), neurotoxins [e.g. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)] and endogenous neurochemicals (e.g. dopamine, 5-HT) (Gilham et al., 1997; Miksys and Tyndale, 2002). CYP2D is expressed in the liver and extra-hepatic tissues, including the brain (Guengerich, 2003; Dutheil et al., 2008). Hepatic CYP2D is not generally susceptible to induction (Lewis, 1998), whereas CYP2D in the brain can be induced by a variety of CNS-acting drugs in a brain region-specific manner (Hedlund et al., 1996; Miksys et al., 2002; Mann et al., 2008).
Human smokers and alcohol consumers have higher levels of brain CYP2D6 in various brain regions in comparison with non-smokers/non-alcohol consumers (Miksys et al., 2002; Mann et al., 2008); however, it is not known whether the increase is due to nicotine or ethanol specifically, as other components of smoke or other associated variables, may contribute to the higher levels of brain CYP2D6. Additionally, human post-mortem data are confounded by individuals being exposed to both alcohol and cigarettes, making it difficult to identify the effects of the individual drugs. Chronic nicotine treatment increases brain CYP2D protein levels in rodents (Yue et al., 2008) and African green monkeys (AGMs, vervets, Chlorocebus sabaeus) (Mann et al., 2008) without affecting hepatic CYP2D levels, suggesting that CYP2D6 may be regulated via a drug- and organ-specific mechanism.
Mann et al. (2012) observed that human brain CYP2D6 expression increased with age. An increase in brain CYP2D6 levels may result in an altered therapeutic effect of clinical drugs, and individuals who are smokers, or older, may experience less effect from antidepressants inactivated by CYP2D6 relative to non-smokers and younger individuals (Nelson et al., 1995; George et al., 2008). Brain CYP2D6 is localized to specific brain regions and cells including those that are affected by Parkinson's disease (PD) (Gilham et al., 1997; Mann et al., 2008); elevated levels of CYP2D6 may play a protective role by inactivating neurotoxins such as MPTP reducing the risk for PD (Miksys and Tyndale, 2006; Mann and Tyndale, 2010). Furthermore, CYP2D6 metabolizes endogenous compounds, for example, rat brain membranes and yeast cells expressing CYP2D6 can convert tyramine to dopamine (Hiroi et al., 1998; Bromek et al., 2010). Thus, variations in brain CYP2D6 may alter the levels of biogenic amines and subsequently affect personality and behaviour; an association of CYP2D6 genotype with personality phenotypes and brain function has been observed (Kirchheiner et al., 2006; 2010; Penas-Lledo et al., 2009). Overall, these findings suggest that the induction of CYP2D6 in the brain may contribute to varied CNS drug response, localized neurotoxin inactivation and personality among individuals.
Alcohol and nicotine are commonly consumed together, and both drugs are easily accessible and legally available (Funk et al., 2006). Several studies have reported a strong positive association between cigarette smoking and alcohol use. Individuals who smoke cigarettes are more likely to drink alcohol, and individuals who drink alcohol are more likely to smoke cigarettes (Bien and Burge, 1990; Difranza and Guerrera, 1990). The combined effects of ethanol and nicotine on CYP2D6 levels remain unknown. This was assessed in monkey brains, as their neuroanatomy closely resembles human brain (Mann et al., 2008). Monkeys also possess similar CYP enzyme expression and activity to humans (Uno et al., 2011), and willingly consume ethanol at levels comparable with human consumption (Ervin et al., 1990; Palmour et al., 1997).
We investigated whether chronic ethanol self-administration, like chronic nicotine administration, induces brain CYP2D in monkeys, and if exposure to the combination of ethanol and nicotine would result in a further increase in brain CYP2D protein levels. We also investigated whether the effects of ethanol self-administration and nicotine treatment on CYP2D protein levels were dependent on the level of alcohol intake and nicotine dose respectively.
Methods
Animals
Adult (6–8 years-old) male African green monkeys (AGMs) were housed outdoors in social groups at the Behavioural Sciences Foundation (St. Kitts) (Palmour et al., 1997), given monkey chow supplemented with fresh fruit and vegetables, and drinking water ad libitum. Experimental protocols were approved by the Institutional Review Board of the St. Kitts Behavioural Sciences Foundation and the University of Toronto Animal Care Committee. All procedures were conducted in accordance with the guidelines of the Canadian Council on Animal Care. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Treatment
The study timeline (Supporting Information Figure S1) has been previously described in detail (Ferguson et al., 2011). During the ethanol preference screening (first 14 days), monkeys had access to 10% v.v−1 alcohol in 0.5% w.v−1 sucrose solution in addition to water for 4 h·day−1. Typically, 30–40% of monkeys will consume alcohol in preference to sucrose, and 14–20% will consistently consume alcohol at levels greater than 1 g·kg−1·day−1 (Ervin et al., 1990). Forty monkeys that voluntarily consumed more than 1 g of ethanol kg−1·day−1 were randomized into four groups, based on their daily ethanol consumption. During the washout period (days 15–28) monkeys had no drug exposure. During phase II (days 29–42), the ethanol-only and the ethanol + nicotine groups self-administered 10% v.v−1 alcohol in 0.5% w.v−1 sucrose solution for 4 h·day−1. The remaining groups had access to 0.5% w.v−1 sucrose solution on the same schedule. During phase III (days 43–63), in addition to sucrose or alcohol, the nicotine-only and ethanol + nicotine groups received s.c. injections of nicotine bitartrate (calculated as nicotine base; Sigma-Aldrich Canada Co., Oakville, Canada) in saline (pH 7.0) at doses of 0.05 mg·kg−1 bid on day 43, 0.1 mg·kg−1 bid on day 44, 0.25 mg·kg−1 bid on day 45 and 0.5 mg·kg−1 bid for the subsequent 19 days. The ethanol-only and control groups received saline s.c. injections on the same schedule. On day 64, animals were killed by an overdose of sodium pentobarbital and subsequent bilateral thoracotomy, and livers and brains were fixed with phosphate-buffered 4% paraformaldehyde for immunohistochemistry, stored in RNAlater (Life Technologies, Inc., Burlington, Canada) or stored at −80°C until further use.
Membrane preparation and immunoblotting
Monkey brain membranes and liver microsomes were prepared, their protein content assayed and stored at −80°C as previously described (Miksys et al., 2002; Mann et al., 2008; Ferguson et al., 2011).
Brain membranes (25 μg) and liver microsomes (1 μg) were separated by SDS-PAGE, using 4% stacking and 10% separating gels and immunoblotted as previously described (Mann et al., 2008), with some minor modifications. For brain, Coomassie Blue R-250 (Sigma-Aldrich, St Louis, MO, USA) staining was used to determine equal loading of protein among lanes. Blots were blocked for 2 h in 5% w v−1 skim milk powder, and 1% normal horse serum (NHS) in 50 mM Tris-buffered saline (Bioshop Canada Inc., Burlington, Canada) with 0.1% w v−1 BSA and 0.1% Triton X-100 (TBS-T) (VWR International, Mississauga, Canada), incubated for 3 h at room temperature or overnight at 4°C with rabbit anti-CYP2D6 polyclonal antibody (Sigma-Aldrich) diluted 1:2000 in 0.05% NHS in TBS-T, re-blocked, then incubated with peroxidase-conjugated anti-rabbit secondary antibody (Millipore, Temecula, CA, USA) diluted 1:10 000–1:15 000 in 0.05% NHS in TBS-T for 1.5 h. For liver, blots were blocked for 1 h in 1% skim milk in TBS-T, incubated for 2 h at room temperature with sheep anti-CYP2D6 polyclonal antibody (Biomol International, Plymouth Meeting, PA, USA) diluted 1:1000 in TBS-T, re-blocked, then incubated with peroxidase-conjugated anti-sheep secondary antibody (Millipore) diluted 1:5000 in TBS-T for 1 h. To assess equal loading of protein among lanes, liver blots were re-probed with mouse anti-actin monoclonal antibody (1:3600; Sigma-Aldrich) followed by peroxidase-conjugated anti-mouse secondary antibody (1:7500; Fisher Scientific, Ottawa, Canada). Protein was visualized using chemiluminescence (Fisher Scientific) followed by exposure to autoradiography film (UltiDent, St. Laurent, Canada). Films were digitized and analysed with MCID software (Interfocus Imaging, Ltd., Linton, UK). Brainstem membranes and liver microsomes from control monkeys were serially diluted to generate standard curves to establish the linear range of detection for the assays (Supporting Information Figure S2A,B). Two immunoreactive protein bands were detected in monkey brain (Supporting Information Figure S2); the lower band that co-migrated with monkey liver and cDNA-expressed human CYP2D6 (52 kDa) (Supporting Information Figure S2C) was analysed.
Monkey brain CYP2D after different doses of ethanol and nicotine
Monkeys were separated into low and high ethanol consumers by a median split based on their mean daily ethanol consumption during phases II and III (see Ferguson et al., 2011). Putamen membranes (25 μg) from low (34.2 mL·kg−1·day−1) and high (41.2 mL·kg−1·day−1) ethanol consumers (n = 8–9 per group) were assessed together by immunoblotting. Putamen membranes (25 μg) from a previous study (treated with saline or 0.3 mg·kg−1 s.c. bid of nicotine; n = 6 per group; Mann et al., 2008) and from this study (sucrose + saline and 0.5 mg·kg−1 s.c. bid of nicotine; n = 10 per group) were assessed together by immunoblotting.
Immunohistochemistry
Frozen coronal sections of fixed tissue (14 μm thick) were immunostained for CYP2D as previously described (Miksys et al., 2002). Briefly, free-floating sections were blocked at room temperature for 1 h in 3% w.v−1 skimmed milk, and 5% v.v−1 NHS in 10 mM PBS with 0.5% w.v−1 BSA, and 0.01% v.v−1 Triton X-100 (PBST), incubated for 48 h at 4°C in rabbit anti-human CYP2D6 polyclonal antibody (Sigma-Aldrich; diluted 1:1000 in PBST with 2% NHS), then visualized using biotinylated goat anti-rabbit immunoglobulin (Millipore; diluted 1 μL·mL−1 in PBST with 2% NHS) followed by the avidin–biotin complex technique and reaction with diaminobenzidine and hydrogen peroxide (Vector, Burlington, Canada). Negative control sections were incubated without primary antibody. CYP2D staining intensity was scored semi-quantitatively and independently by two individuals, blinded to treatment group, as intense (++++), strong (+++), moderate (++), weak (+) or absent (−).
RNA quantification
RNA isolation, quantification, cDNA synthesis and PCR amplification were performed as previously described (Ferguson et al., 2011) with minor modifications. The mRNA sequence of the CYP2D gene for the AGM is not available; therefore, primer design and specificity were based on alignment of human CYP2D6 mRNA sequence with rhesus macaque (Macaca mulatta) CYP2D17 mRNA sequence. Primers for real-time PCR amplification of CYP2D and β-actin (ACTB) cDNAs were as follows: CYP2D forward primer (CYP2D6ex2/3) 5′-CCC GCC TGT GCC CAT CAA-3′, CYP2D reverse primer (CYP2D6ex3) 5′-ATG GGT CAC CGA GGA GGC-3′, β-actin forward primer (ACTBFex3) 5′-CAG AGC AAG AGA GGC ATC CT-3′ and β-actin reverse primer (ACTBRex4) 5′-GGT CTC AAA CAT GAT CTG GGT C-3′. After PCR amplification, the expected size DNA product was detected and conformed by sequencing as unique and highly homologous to both human and macaque (Supporting Information Figure S4).
Data analysis
Two-way anova (nicotine treatment × ethanol self-administration) followed by one-way anova and Bonferroni's multiple comparison post hoc test were used to test group differences in brain and in hepatic CYP2D protein and mRNA levels. One-way anova followed by post hoc tests (Bonferroni's multiple comparison post test and test for linear trend) were used to test dose-dependent group differences in CYP2D protein levels. Outliers within groups were identified when greater than two times the SD from the group average.
Results
The independent and combined impact of ethanol self-administration and nicotine treatment on brain and liver CYP2D
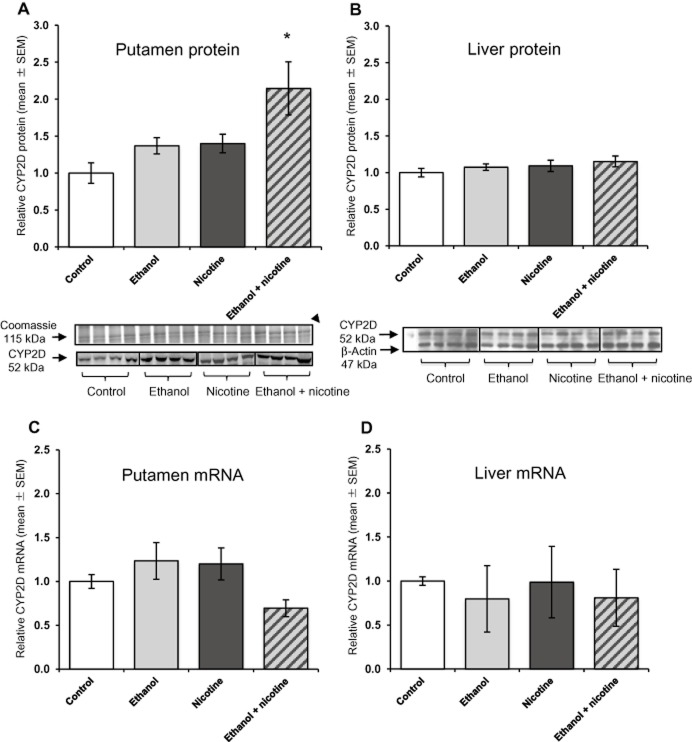
A significant main effect of ethanol self-administration [F(1, 29) = 1.18, P = 0.030] and of nicotine treatment [F(1, 29) = 1.32, P = 0.023] was observed in the putamen (Figure 1A), with no interaction between treatments [F(1, 29) = 0.13, P = 0.448]. Compared with the control group, monkeys in the combined ethanol + nicotine group had a 2.1-fold (P < 0.05) increase in brain CYP2D protein. In contrast, there was no significant main effect of ethanol self-administration [F(1, 36) = 1.06, P = 0.31] or nicotine treatment [F(1, 36) = 1.77, P = 0.19] on hepatic CYP2D protein levels (Figure 1B), and no interaction between treatments [F(1, 36) = 0.012, P = 0.91].
Figure 1.
Brain CYP2D is altered by ethanol self-administration and nicotine treatment. (A) Putamen CYP2D protein was increased by ethanol and by nicotine. Representative immunoblot of brain CYP2D protein (52 kDa) from the putamen of monkeys in the control, ethanol-only, nicotine-only and ethanol + nicotine treatment groups. Coomassie Blue staining (115 kDa) was used to confirm equal protein loading among lanes (n = 4 shown of 10 per group). (B) Liver CYP2D protein was not altered by ethanol or nicotine. Representative immunoblot of hepatic CYP2D protein (52 kDa) from monkeys in the control, ethanol-only, nicotine-only and ethanol + nicotine treatment groups (n = 4 shown of 10 per group analysed). β-Actin (47 kDa) was used to confirm equal hepatic protein loading among lanes. (C) Putamen CYP2D mRNA levels were not altered by ethanol or nicotine. Mean CYP2D mRNA levels were normalized to β-actin. (D) Hepatic CYP2D mRNA levels were not altered by ethanol or nicotine. Mean CYP2D mRNA levels were normalized to β-actin. Results are expressed as mean ± SEM of 6–10 monkeys per group. All data are presented relative to the control group. Two-way anova used to determine differences in brain and hepatic CYP2D protein and mRNA levels. Significant difference indicated by *P < 0.05, compared with control group.
All putamen samples tested were within the linear range of the CYP2D immunoblotting assay; this was reassessed by loading either more or less protein than the control sample (25 μg) based on whether their CYP2D levels fell below or above the median value respectively (Supporting Information Figure S3).
There were no differences in CYP2D mRNA levels between the four treatment groups in either putamen or liver (Figure 1C and D). There was no significant main effect of ethanol self-administration in the putamen [F(1, 25) = 0.96, P = 0.34] or the liver [F(1, 35) = 0.00021, P = 0.99], or of nicotine treatment in the putamen [F(1, 25) = 1.45, P = 0.24] or the liver [F(1, 35) = 2.63, P = 0.11].
Regional induction patterns of brain CYP2D in monkeys exposed to ethanol self-administration and nicotine treatment
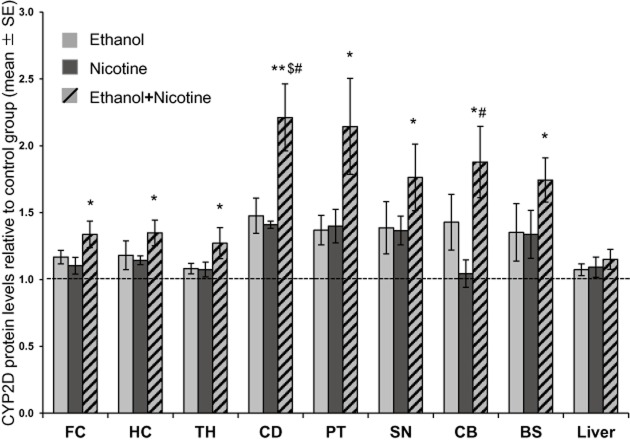
The expression of CYP2D in the control group among the eight brain regions was similar to that seen in a previous study (r = 0.97; Mann et al., 2008). There was a significant main effect of ethanol self-administration on brain CYP2D levels in all eight brain regions examined [frontal cortex, F(1,36) = 7.94, P = 0.008; hippocampus, F(1, 34) = 5.90, P = 0.021; thalamus, F(1, 36) = 4.14, P = 0.049; caudate, F(1, 35) = 18.57, P = 0.0001; putamen, F(1, 29) = 1.18, P = 0.030; substantia nigra, F(1, 33) = 5.51, P = 0.025; cerebellum, F(1, 35) = 12.65, P = 0.0011; brainstem, F(1, 33) = 4.80, P = 0.036]. There was also a significant main effect of nicotine treatment on brain CYP2D levels in four of the brain regions examined [caudate, F(1, 35) = 14.91, P = 0.001; putamen, F(1, 29) = 1.32, P = 0.023; substantia nigra, F(1,33) = 4.97, P = 0.033; brainstem, F(1, 33) = 4.45, P = 0.043]. There were no significant interaction effects between ethanol self-administration and nicotine treatment in any brain region. Compared with the control group, monkeys in the combined ethanol and nicotine group had higher levels of CYP2D protein in all eight brain regions investigated ranging from 1.2 to 2.2-fold (P < 0.05; Figure 2). In the combined ethanol and nicotine group, protein levels in caudate (P < 0.05) and cerebellum (P < 0.05) CYP2D were significantly higher compared with the nicotine-only group, and caudate (P < 0.05) was significantly higher compared with the ethanol-only group (Figure 2).
Figure 2.
Ethanol self-administration and nicotine treatment alter CYP2D in monkey brain but not liver. Relative increases in CYP2D protein levels compared with the control group. Results are expressed as mean ± SEM of 8–10 monkeys per group, with the assay repeated three to six times. Two-way anova, followed by a one-way anova and Bonferroni's multiple comparison post hoc test were used to determine differences in brain CYP2D protein levels. Significant differences indicated: *P < 0.05, **P < 0.001 compared with control group; $P < 0.05 compared with ethanol-only group; #P < 0.05 compared with nicotine-only group. The dashed line at 1 represents levels of CYP2D in control (sucrose + saline) animals. BS, brainstem; CB, cerebellum; CD, caudate; FC, frontal cortex; HC, hippocampus; PT, putamen; SN, substantia nigra; TH, thalamus.
Dose-dependent effect of ethanol self-administration and nicotine treatment on brain CYP2D
High (41.2 mL·kg−1·day−1, n = 10) and low (34.2 mL·kg−1·day−1, n = 10) ethanol consumers had significantly different ethanol intake (two-tailed, unpaired Student's t-test, P < 0.05; Ferguson et al., 2011). Putamen CYP2D levels were ethanol dose specific (ptrend = 0.005; Figure 3A). Relative to controls, high ethanol consumers had 2.2-fold higher [F(2, 22) = 4.95, P < 0.05; Figure 3A] and low ethanol consumers had 1.5-fold higher (non-significant) CYP2D levels.
Figure 3.
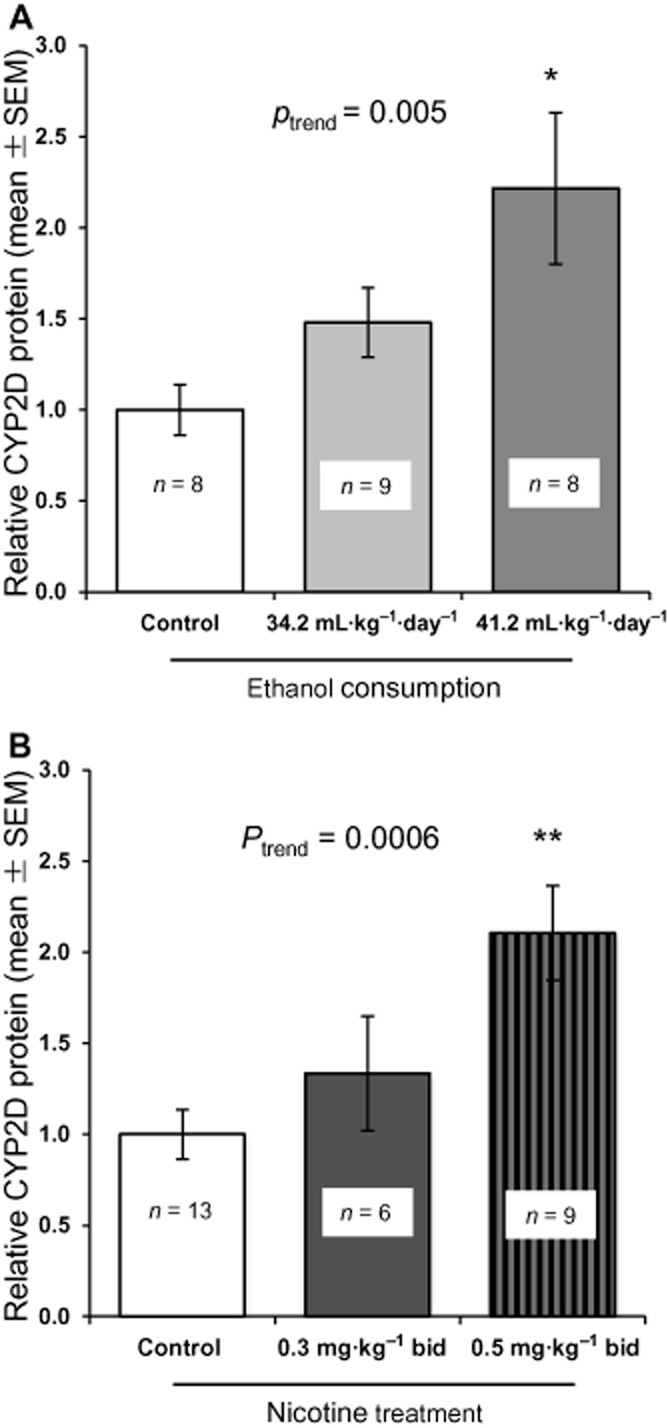
Ethanol self-administration and nicotine treatment have a dose-specific effect on brain CYP2D in monkeys. (A) Ethanol had a dose-specific effect on putamen CYP2D levels. (B) Nicotine had a dose-specific effect on putamen CYP2D levels. Results are expressed as mean ± SEM of 8–10 monkeys per group, with the assay repeated three to six times. Significant difference indicated: *P < 0.05, **P < 0.001 compared with the control group [saline animals (previous study) combined with sucrose + saline (our study)].
Control (sucrose + saline) putamen CYP2D levels were not significantly different from control (saline) putamen CYP2D levels from our previously published study (Mann et al., 2008) (data not shown). Putamen CYP2D levels were nicotine dose specific (ptrend = 0.0006; Figure 3B). Relative to controls, 0.5 mg·kg−1 bid treated monkeys had 2.1-fold higher (P < 0.001; Figure 3B) and 0.3 mg·kg−1 bid treated monkeys (previous study) had 1.3-fold higher (non-significant) CYP2D levels.
CYP2D protein is induced in monkey brain by ethanol self-administration and nicotine treatment, as assessed by immunohistochemistry
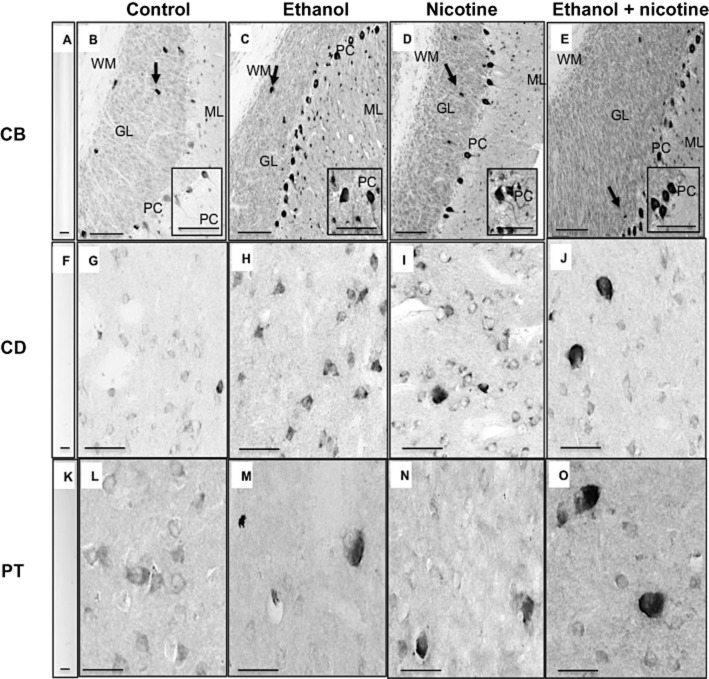
The intensity of region- and cell-specific immunohistochemistry (Table 1) was consistent with our immunoblotting data (Figure 2). Ethanol self-administration induced CYP2D protein expression, for example, in the frontal cortex (e.g. layers III and V), hippocampus (e.g. pyramidal cells and subiculum), caudate (e.g. neuronal cells) and cerebellum (e.g. Purkinje cells) (Table 1; Figure 4). Nicotine treatment induced CYP2D protein expression, for example, in the frontal cortex (layers III and V), caudate and putamen (neuronal cells), and cerebellum (e.g. Purkinje cells) (Table 1; Figure 4). In some regions, for example, cerebellum, immunoblotting detected no differences in CYP2D protein levels between nicotine-only and control groups (Figure 2); however, cerebellar Purkinje cells, molecular and granular layers stained more intensely in nicotine-only compared with control groups (Table 1; Figure 4D). In brain regions where ethanol self-administration and nicotine treatment independently induced CYP2D cellular staining (e.g. putamen), the combined ethanol + nicotine group appeared to have greater CYP2D immunostaining, suggesting a greater effect of the combination of ethanol + nicotine when compared with each individual drug alone (Table 1; Figures 2 and 4). In control animals, the cellular distribution and intensity of CYP2D immunostaining varied across the brain regions (Table 1), consistent with the varied levels of brain CYP2D detected with immunoblotting (Figure 2; Table 1). For example, in cerebellum Purkinje cell bodies and dendritic processes stained moderately, granular cell layer stained lightly, and there was little to no staining in cells of the molecular layer (Figure 4B). In the striatum, neuronal cell staining was weak (Figure 4G and L). There was no staining in control sections incubated without primary antibody (Figure 4A, F, K).
Table 1.
Immunohistochemical staining for CYP2D protein in monkey brain
| CYP2D | ||||
|---|---|---|---|---|
| Brain region | Control | Ethanol | Nicotine | Ethanol + nicotine |
| Frontal cortex | ||||
| Layer I | − | − | − | − |
| Layer II | + | +/++ | ++ | + |
| Layer III | ++/+++ | +++ | +++/++++ | +++/++++ |
| Layer IV | +/++ | +/++ | + | + |
| Layer V | ++/+++ | +++/++++ | ++++ | +++/++++ |
| Layer VI | + | +/++ | + | + |
| White matter | −/+ | −/+ | −/+ | −/+ |
| Hippocampus | ||||
| Dentate gyrus | +/++ | ++ | +/++ | +/++ |
| CA1–CA3 | −/+ | +/++ | +/++ | +/++ |
| Polymorphic layer | ++ | +++ | ++ | ++ |
| Pyramidal cells | ++/+++ | +++ | ++++ | ++ |
| Molecular layer | −/+ | + | + | + |
| Subiculum | ++ | ++/+++ | ++ | ++/+++ |
| Striatum | ||||
| Caudate | ++ | +++/++++ | +++ | ++/+++ |
| Neurons | + | ++/+++ | ++/+++ | +++/++++ |
| Putamen | ++ | ++ | ++ | ++ |
| Neurons | + | ++ | ++ | +++/++++ |
| Cerebellum | ||||
| Molecular layer | −/+ | +/++ | +/++ | +/++ |
| Purkinje cells | ++ | +++/++++ | ++++ | ++++ |
| Granular layer | + | ++ | ++/+++ | ++/+++ |
| Golgi cells | +++ | +++ | +++ | +++ |
| White matter | − | − | − | − |
Key: intense (++++), strong (+++), moderate (++), weak (+) and no staining (−).
Figure 4.
Induction of CYP2D protein expression by ethanol self-administration and nicotine treatment in monkey brain assessed by immunohistochemistry. In the granular layer (GL) and molecular layer (ML) of the cerebellum, CYP2D staining was very weak in the control group (B), and moderately strong in the ethanol-only (C), nicotine-only (D) and the combination ethanol + nicotine group (E). In Purkinje cells (PC) and their cell bodies and dendritic processes of the cerebellum, there was some staining of CYP2D in the control group (B), and intense staining in the ethanol-only (C), nicotine-only (D) and the combination ethanol + nicotine group (E). Within the cerebellum, the four treatment groups exhibited strong staining of golgi cells (arrow) within the GL and no staining was observed in the white matter (WM) (B–E). In the caudate, cellular staining for CYP2D was weak in the control group (G), moderately strong in the ethanol-only group (H), strong in the nicotine-only group (I) and intense in the combination ethanol + nicotine group (J). In the putamen, cellular staining for CYP2D was weak in the control group (L), moderately strong in the ethanol-only group (M), the nicotine-only group (N) and intense in the combination ethanol + nicotine group (O). CYP2D immunoreactivity was not detectable in the absence of primary antibody in the cerebellum (A), caudate (F) and the putamen (K). Scale bar in A, F, K = 20 μm; scale bar in B–E = 100 μm; inset scale bar for B–E = 200 μm; scale bar in G–J, L–O = 200 μm. CB, cerebellum; CD, caudate; PT, putamen.
Discussion
Monkeys treated with ethanol and nicotine, both alone and in combination, had higher brain CYP2D levels compared with controls in both brain regions and at the cellular level, consistent with our previous observations in humans, where smokers and alcohol consumers had higher brain CYP2D levels than non-smokers and non-alcohol consumers (Miksys et al., 2002; Mann et al., 2008). As in humans, the magnitude of increase in monkey brain CYP2D varied by brain region and inducer (Figure 2); individually, both drugs showed evidence of a dose-dependent increase in brain CYP2D. There were even greater increases in brain CYP2D protein after giving both drugs in combination; however, there was no statistical interaction between ethanol and nicotine treatments, suggesting a lack of synergism. Neither ethanol nor nicotine altered hepatic CYP2D protein levels either alone or in combination.
This is the first report on the induction of CYP2D by ethanol self-administration in monkeys; ethanol induced CYP2D dose dependently, and in most brain regions, consistent with the higher levels observed in human alcohol consumers (Miksys et al., 2002). The comparable observations in drug-treated monkeys and in levels of CYP2D6 in smokers and/or alcohol consumers (Miksys et al., 2002; Mann et al., 2008) suggest that ethanol and nicotine are likely the responsible agents. We saw some minor differences compared with our previous studies in monkeys and humans (Miksys et al., 2002; Mann et al., 2008) that may be due to differences in dose, and/or method and duration of drug exposure. For most brain regions, cellular increases in CYP2D by ethanol and nicotine were consistent with differences detected by immunoblotting (Table 1; Figures 2 and 4). In the frontal cortex and the cerebellum, there were no differences detected by immunoblotting between treatment groups (Figure 2), but there were obvious differences in cellular staining (Table 1; Figure 4), highlighting one advantage of assessing the induction of brain CYP2D by both qualitative (immunohistochemistry) and quantitative (immunoblotting) techniques.
In control monkeys, expression of CYP2D protein varied across brain regions, similar to previously published findings in monkeys and human non-smokers, and non-alcohol consumers (Miksys et al., 2002; Mann et al., 2008). As previously observed, CYP2D6 was expressed in neuronal cells and their projections (e.g. pyramidal neurons and granular neurons), and in non-neuronal cells (e.g. glial cells and astrocytes) (Siegle et al., 2001; Miksys and Tyndale, 2002; Mann et al., 2008), and the regional variation in expression is probably due to the heterogeneous cellular make-up across brain regions.
There were no differences in CYP2D mRNA between treatment and control groups (Figure 1C) as we had seen previously in rats (Joshi, 2006; Yue et al., 2008). Possible mechanisms of induction may include increased translational efficiency or protein stabilization, inhibition of ubiquitination and subsequent degradation of brain CYP2D protein (Kane et al., 2004). Hepatic CYP2E1 can be induced by ethanol without changes in mRNA levels; the protein is protected from degradation (Roberts et al., 1995). Nicotine can down-regulate ubiquitinating proteins in the hypothalamus (Kane et al., 2004), which may decrease routing of CYP2D for degradation.
We have shown that brain CYPs are active in situ (Miksys and Tyndale, 2009) and that altering brain CYP levels and activity can impact local drug metabolism and behavioural and pharmacological response to centrally acting drugs (Ferguson and Tyndale, 2011; Khokhar and Tyndale, 2011; 2012; Miksys and Tyndale, 2013; Zhou et al., 2013). CYP2D6 metabolizes a variety of centrally acting drugs such as antidepressants, endogenous neurochemicals and toxins (Gilham et al., 1997; Miksys and Tyndale, 2002); therefore, having altered levels of CYP2D in the brain may affect local drug metabolism and drug response, neurotransmitter levels, and/or contribute to neuroprotection/toxicity (Ferguson and Tyndale, 2011). Here behaviourally relevant doses of ethanol and nicotine were chosen to model moderate-to-heavy consumption in humans (Ferguson et al., 2011). The dose relationship between brain CYP2D levels and ethanol and nicotine suggests that high levels of human alcohol consumption and smoking may increase brain CYP2D more than low levels of consumption, and even more so when consumed together. Individuals with elevated brain CYP2D6 may have altered response to centrally acting drugs, which may be greater in those with high levels of nicotine and alcohol consumption and co-consumption. For example, variability in CYP2D6 expression may be associated with various drug outcomes, such as adverse drug reactions or altered effects of a drug. Brain CYP2D6 may also increase with age (Mann et al., 2012); therefore, the impact of brain CYP2D6 on drug metabolism may be elevated in the elderly population; desipramine, an antidepressant inactivated by CYP2D6, requires higher doses in older patients (Nelson et al., 1995). Having altered brain CYP2D may also affect the abuse liability of a drug. For example, we have shown that brain CYP2D activates codeine to morphine and is responsible for the initial phases of codeine-induced analgesia (Zhou et al., 2013). Individuals with high brain CYP2D levels, such as smokers and alcohol consumers, may activate codeine to morphine more quickly, experience increased analgesia during the initial phases (Zhou et al., 2013) and be at increased risk for codeine's abuse liability as the reinforcing effects would be faster.
The ability of CYP2D6 to synthesize and metabolize a range of endogenous neurochemicals (Funae et al., 2003) suggests that CYP2D6 may play a role in normal brain function, in addition to xenobiotic metabolism. CYP2D can catalyse the conversion of tyramine and 5-methoxytryptamine to their respective neurotransmitters, dopamine and 5-HT (Bromek et al., 2010; 2011; Cheng et al., 2013; Haduch et al., 2013), and altered levels of brain CYP2D6 may affect mood and personality (Funae et al., 2003; Yu et al., 2003a; Bromek et al., 2010). In addition, human resting brain activity varies with CYP2D6 genotype, and genetic variation in CYP2D6 has been associated with differing personality phenotypes (Llerena et al., 1993; Kirchheiner et al., 2006; 2010; Gonzalez et al., 2008). Thus, variation in brain CYP2D activity may alter neurotransmitter levels and normal brain function resulting in small differences in behaviour among those with higher, versus lower, levels of CYP2D6. CYP2D6 also metabolizes neurosteroids including progesterone, allopregnanolone and anandamide (Hiroi et al., 2001; Kishimoto, 2004; Niwa et al., 2008; Snider et al., 2008). These neurosteroids can regulate neurotransmitter release and alter mood and behaviour (Hlatky et al., 2002; Pluchino et al., 2006; Bambico and Gobbi, 2008). These differences in levels of brain CYP2D6 may be the result of genetic variation and/or the result of nicotine and ethanol consumption. Alternatively, consumption of nicotine and ethanol may occur, at least in part, to modulate CYP2D6 levels and resulting dopamine and 5-HT levels (Yu et al., 2003b; Gonzalez et al., 2008).
CYP2D6 genetic poor metabolizers are at increased risk for some brain disorders including PD and tardive dyskinesia (McCann et al., 1997; Singh et al., 2010; Fleeman et al., 2011), which may be due to the reduced ability to inactivate neurotoxin substrates (Quik et al., 2006a,b; Vance et al., 2010). CYP2D6 can inactivate Parkinson's inducing neurotoxins such as MPTP (Modi et al., 1997) and 1-methyl-4-phenylpyridinium (MPP+) (Matoh et al., 2003; Mann and Tyndale, 2010), and we have shown that inhibiting CYP2D in a human neuronal cell line increases toxicity of MPTP and MPP+ (Mann and Tyndale, 2010), whereas overexpression of CYP2D6 in PC12 cells protects against MPP+ cytotoxicity (Matoh et al., 2003). Similarly, there is an inverse correlation between smoking and the onset of PD, suggesting that higher brain CYP2D6 in smokers may be protective (Mihailescu and Drucker-Colin, 2000). Consistent with higher levels of brain CYP2D6 being protective, and lower levels being a risk factor, PD patients expressed lower levels of brain CYP2D6 compared with age-matched controls (Mann et al., 2012).
In summary, this was the first study to show the induction of CYP2D in monkey brain by ethanol and the combination of ethanol and nicotine. The induction was organ (not liver), brain region and cell specific, and does not appear to involve transcriptional mechanisms. The higher levels of human brain CYP2D6 seen in smokers and/or alcohol consumers may be due, at least in part, to nicotine and ethanol, agents found in cigarettes and alcohol respectively. Chronic exposure to ethanol and/or nicotine may contribute to variation in the expression of brain CYP2D and may influence CNS-acting drug response, neurotransmitter levels, resulting behaviour and neurotoxicity.
Acknowledgments
We would like to thank the staff of the Behavioral Science Foundation, St. Kitts, for their dedication and care in conducting all aspects of the animal experiments. Funding for this work was provided by the Centre for Addiction and Mental Health (CAMH) and the CAMH Foundation, Canadian Institutes for Health Research (MOP97751), the Canadian Foundation for Innovation (grant #20289 and #16014), the Ontario Ministry of Research and Innovation, Ontario Graduate Scholarship (RTM) and the Endowed Chair in Addiction for the Department of Psychiatry (RFT).
Glossary
- AGM
African green monkey
- CNS
central nervous system
- CYP
cytochrome P450
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NHS
normal horse serum
- PD
Parkinson's disease
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TBS-T
tris buffered saline with Triton X-100
Authors contributions
S M and R F T participated in research design. R T M, S M and E H conducted the experiments. R T M performed data analysis. R T M and R F T wrote or contributed to the writing of the manuscript.
Conflict of interests
R. F. T. has participated in 1-day advisory meetings for Novartis and McNeil.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12652
Figure S1 Study timeline.
Figure S2 Detection of brain and liver CYP2D protein in monkey is linear and quantifiable.
Figure S3 Confirmation of quantification of CYP2D protein levels.
Figure S4 African green monkey CYP2D cDNA-amplified sequence.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Gobbi G. The cannabinoid CB1 receptor and the endocannabinoid anandamide: possible antidepressant targets. Expert Opin Ther Targets. 2008;12:1347–1366. doi: 10.1517/14728222.12.11.1347. [DOI] [PubMed] [Google Scholar]
- Bien TH, Burge R. Smoking and drinking – a review of the literature. Int J Addict. 1990;25:1429–1454. doi: 10.3109/10826089009056229. [DOI] [PubMed] [Google Scholar]
- Bromek E, Haduch A, Daniel WA. The ability of cytochrome P450 2D isoforms to synthesize dopamine in the brain: an in vitro study. Eur J Pharmacol. 2010;626:171–178. doi: 10.1016/j.ejphar.2009.09.062. [DOI] [PubMed] [Google Scholar]
- Bromek E, Haduch A, Gołembiowska K, Daniel WA. Cytochrome P450 mediates dopamine formation in the brain in vivo. J Neurochem. 2011;118:806–815. doi: 10.1111/j.1471-4159.2011.07339.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Zhen Y, Miksys S, Beyoğlu D, Krausz KW, Tyndale RF, et al. Potential role of CYP2D6 in the central nervous system. Xenobiotica. 2013;11:973–984. doi: 10.3109/00498254.2013.791410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Dutheil F, Beaune P, Loriot MA. Xenobiotic metabolizing enzymes in the central nervous system: contribution of cytochrome P450 enzymes in normal and pathological human brain. Biochimie. 2008;90:426–436. doi: 10.1016/j.biochi.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Ervin FR, Palmour RM, Young SN, Guzman-Flores C, Juarez J. Voluntary consumption of beverage alcohol by vervet monkeys: population screening, descriptive behavior and biochemical measures. Pharmacol Biochem Behav. 1990;36:367–373. doi: 10.1016/0091-3057(90)90417-g. [DOI] [PubMed] [Google Scholar]
- Ferguson CS, Tyndale RF. Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol Sci. 2011;32:708–714. doi: 10.1016/j.tips.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CS, Miksys S, Palmour R, Tyndale RF. Independent and combined effects of ethanol self-administration and nicotine treatment on hepatic CYP2E1 in African green monkeys. Drug Metab Dispos. 2011;39:2233–2241. doi: 10.1124/dmd.111.040378. [DOI] [PubMed] [Google Scholar]
- Fleeman N, Dundar Y, Dickson R, Jorgensen A, Pushpakom S, McLeod C, et al. Cytochrome P450 testing for prescribing antipsychotics in adults with schizophrenia: systematic review and meta-analyses. Pharmacogenomics J. 2011;11:1–14. doi: 10.1038/tpj.2010.73. [DOI] [PubMed] [Google Scholar]
- Funae Y, Kishimoto W, Cho T, Niwa T, Hiroi T. CYP2D in the brain. Drug Metab Pharmacokinet. 2003;18:337–349. doi: 10.2133/dmpk.18.337. [DOI] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor–refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- Gilham DE, Cairns W, Paine MJ, Modi S, Poulsom R, Roberts GC, et al. Metabolism of MPTP by cytochrome P4502D6 and the demonstration of 2D6 mRNA in human foetal and adult brain by in situ hybridization. Xenobiotica. 1997;27:111–125. doi: 10.1080/004982597240802. [DOI] [PubMed] [Google Scholar]
- Gonzalez I, Penas-Lledo EM, Perez B, Dorado P, Alvarez M, LLerena A. Relation between CYP2D6 phenotype and genotype and personality in healthy volunteers. Pharmacogenomics. 2008;9:833–840. doi: 10.2217/14622416.9.7.833. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochromes P450, drugs, and diseases. Mol Interv. 2003;3:194–204. doi: 10.1124/mi.3.4.194. [DOI] [PubMed] [Google Scholar]
- Haduch A, Bromek E, Sadakierska-Chudy A, Wójcikowski J, Daniel WA. The catalytic competence of cytochrome P450 in the synthesis of serotonin from 5-methoxytryptamine in the brain: an in vitro study. Pharmacol Res. 2013;67:53–59. doi: 10.1016/j.phrs.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Hedlund E, Wyss A, Kainu T, Backlund M, Kohler C, Pelto-Huikko M, et al. Cytochrome P4502D4 in the brain: specific neuronal regulation by clozapine and toluene. Mol Pharmacol. 1996;50:342–350. [PubMed] [Google Scholar]
- Hiroi T, Imaoka S, Funae Y. Dopamine formation from tyramine by CYP2D6. Biochem Biophys Res Commun. 1998;249:838–843. doi: 10.1006/bbrc.1998.9232. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Kishimoto W, Chow T, Imaoka S, Igarashi T, Funae Y. Progesterone oxidation by cytochrome P450 2D isoforms in the brain. Endocrinology. 2001;142:3901–3908. doi: 10.1210/endo.142.9.8363. [DOI] [PubMed] [Google Scholar]
- Hlatky MA, Boothroyd D, Vittinghoff E, Sharp P, Whooley MA, Grp HR. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy – results from the Heart and Estrogen/Progestin Replacement Study (HERS) trial. JAMA. 2002;287:591–597. doi: 10.1001/jama.287.5.591. [DOI] [PubMed] [Google Scholar]
- Joshi M. Induction and recovery time course of rat brain cyp2e1 after nicotine treatment. Drug Metab Dispos. 2006;34:647–652. doi: 10.1124/dmd.105.008029. [DOI] [PubMed] [Google Scholar]
- Kane JK, Konu O, Ma JZ, Li MD. Nicotine coregulates multiple pathways involved in protein modification/degradation in rat brain. Brain Res Mol Brain Res. 2004;132:181–191. doi: 10.1016/j.molbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Khokhar JY, Tyndale RF. Drug metabolism within the brain changes drug response: selective manipulation of brain CYP2B alters propofol effects. Neuropsychopharmacology. 2011;36:692–700. doi: 10.1038/npp.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar JY, Tyndale RF. Rat brain CYP2B-enzymatic activation of chlorpyrifos to the oxon mediates cholinergic neurotoxicity. Toxicol Sci. 2012;126:325–335. doi: 10.1093/toxsci/kfs029. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheiner J, Lang U, Stamm T, Sander T, Gallinat J. Association of CYP2D6 genotypes and personality traits in healthy individuals. J Clin Psychopharmacol. 2006;26:440–442. doi: 10.1097/01.jcp.0000229484.52955.22. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J, Seeringer A, Godoy AL, Ohmle B, Maier C, Beschoner P, et al. CYP2D6 in the brain: genotype effects on resting brain perfusion. Mol Psychiatry. 2010;16:333–341. doi: 10.1038/mp.2010.42. [DOI] [PubMed] [Google Scholar]
- Kishimoto W. Cytochrome P450 2D catalyze steroid 21-hydroxylation in the brain. Endocrinology. 2004;145:699–705. doi: 10.1210/en.2003-1109. [DOI] [PubMed] [Google Scholar]
- Lewis DF. The CYP2 family: models, mutants and interactions. Xenobiotica. 1998;28:617–661. doi: 10.1080/004982598239236. [DOI] [PubMed] [Google Scholar]
- Llerena A, Edman G, Cobaleda J, Benitez J, Schalling D, Bertilsson L. Relationship between personality and debrisoquine hydroxylation capacity. Suggestion of an endogenous neuroactive substrate or product of the cytochrome P4502D6. Acta Psychiatr Scand. 1993;87:23–28. doi: 10.1111/j.1600-0447.1993.tb03325.x. [DOI] [PubMed] [Google Scholar]
- Mann A, Tyndale RF. Cytochrome P450 2D6 enzyme neuroprotects against 1-methyl-4-phenylpyridinium toxicity in SH-SY5Y neuronal cells. Eur J Neurosci. 2010;31:1185–1193. doi: 10.1111/j.1460-9568.2010.07142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann A, Miksys S, Lee A, Mash DC, Tyndale RF. Induction of the drug metabolizing enzyme CYP2D in monkey brain by chronic nicotine treatment. Neuropharmacology. 2008;55:1147–1155. doi: 10.1016/j.neuropharm.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann A, Miksys SL, Gaedigk A, Kish SJ, Mash DC, Tyndale RF. The neuroprotective enzyme CYP2D6 increases in the brain with age and is lower in Parkinson's disease patients. Neurobiol Aging. 2012;33:2160–2171. doi: 10.1016/j.neurobiolaging.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Matoh N, Tanaka S, Takehashi M, Banasik M, Stedeford T, Masliah E, et al. Overexpression of CYP2D6 attenuates the toxicity of MPP+ in actively dividing and differentiated PC12 cells. Gene Expr. 2003;11:117–124. doi: 10.3727/000000003108749017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SJ, Pond SM, James KM, Le Couteur DG. The association between polymorphisms in the cytochrome P-450 2D6 gene and Parkinson's disease: a case-control study and meta-analysis. J Neurol Sci. 1997;153:50–53. doi: 10.1016/s0022-510x(97)00179-2. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailescu S, Drucker-Colin R. Nicotine, brain nicotinic receptors, and neuropsychiatric disorders. Arch Med Res. 2000;31:131–144. doi: 10.1016/s0188-4409(99)00087-9. [DOI] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. Nicotine induces brain CYP enzymes: relevance to Parkinson's disease. J Neural Transm Suppl. 2006;70:177–180. doi: 10.1007/978-3-211-45295-0_28. [DOI] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. Brain drug-metabolizing cytochrome P450 enzymes are active in vivo, demonstrated by mechanism-based enzyme inhibition. Neuropsychopharmacology. 2009;34:634–640. doi: 10.1038/npp.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksys S, Tyndale RF. Cytochrome P450-mediated drug metabolism in the brain. J Psychiatry Neurosci. 2013;38:152–163. doi: 10.1503/jpn.120133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksys S, Rao Y, Hoffmann E, Mash DC, Tyndale RF. Regional and cellular expression of CYP2D6 in human brain: higher levels in alcoholics. J Neurochem. 2002;82:1376–1387. doi: 10.1046/j.1471-4159.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- Miksys SL, Tyndale RF. Drug-metabolizing cytochrome P450s in the brain. J Psychiatry Neurosci. 2002;27:406–415. [PMC free article] [PubMed] [Google Scholar]
- Modi S, Gilham DE, Sutcliffe MJ, Lian LY, Primrose WU, Wolf CR, et al. 1- methyl-4-phenyl-1,2,3,6-tetrahydropyridine as a substrate of cytochrome P450 2D6: allosteric effects of NADPH-cytochrome P450 reductase. Biochemistry. 1997;36:4461–4470. doi: 10.1021/bi962633p. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Mazure CM, Jatlow PI. Desipramine treatment of major depression in patients over 75 years of age. J Clin Psychopharmacol. 1995;15:99–105. doi: 10.1097/00004714-199504000-00004. [DOI] [PubMed] [Google Scholar]
- Niwa T, Okada K, Hiroi T, Imaoka S, Narimatsu S, Funae Y. Effect of psychotropic drugs on the 21-hydroxylation of neurosteroids, progesterone and allopregnanolone, catalyzed by rat CYP2D4 and human CYP2D6 in the brain. Biol Pharm Bull. 2008;31:348–351. doi: 10.1248/bpb.31.348. [DOI] [PubMed] [Google Scholar]
- Palmour RM, Mulligan J, Howbert JJ, Ervin F. Of monkeys and men: vervets and the genetics of human-like behaviors. Am J Hum Genet. 1997;61:481–488. doi: 10.1086/515526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penas-Lledo EM, Dorado P, Pacheco R, Gonzalez I, Llerena A. Relation between CYP2D6 genotype, personality, neurocognition and overall psychopathology in healthy volunteers. Pharmacogenomics. 2009;10:1111–1120. doi: 10.2217/pgs.09.75. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Luisi M, Lenzi E, Centofanti M, Begliuomini S, Freschi L, et al. Progesterone and progestins: effects on brain, allopregnanolone and beta- endorphin. J Steroid Biochem Mol Biol. 2006;102:205–213. doi: 10.1016/j.jsbmb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Quik M, Chen L, Parameswaran N, Xie X, Langston JW, McCallum SE. Chronic oral nicotine normalizes dopaminergic function and synaptic plasticity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned primates. J Neurosci. 2006a;26:4681–4689. doi: 10.1523/JNEUROSCI.0215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, et al. Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem. 2006b;98:1866–1875. doi: 10.1111/j.1471-4159.2006.04078.x. [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Shoaf SE, Song BJ. Rapid changes in cytochrome P4502E1 (CYP2E1) activity and other P450 isozymes following ethanol withdrawal in rats. Biochem Pharmacol. 1995;49:1665–1673. doi: 10.1016/0006-2952(95)00098-k. [DOI] [PubMed] [Google Scholar]
- Siegle I, Fritz P, Eckhardt K, Zanger UM, Eichelbaum M. Cellular localization and regional distribution of CYP2D6 mRNA and protein expression in human brain. Pharmacogenetics. 2001;11:237–245. doi: 10.1097/00008571-200104000-00007. [DOI] [PubMed] [Google Scholar]
- Singh M, Khanna VK, Shukla R, Parmar D. Association of polymorphism in cytochrome P450 2D6 and N-acetyltransferase-2 with Parkinson's disease. Dis Markers. 2010;28:87–93. doi: 10.3233/DMA-2010-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Sikora MJ, Sridar C, Feuerstein TJ, Rae JM, Hollenberg PF. The endocannabinoid anandamide is a substrate for the human polymorphic cytochrome P450 2D6. J Pharmacol Exp Ther. 2008;327:538–545. doi: 10.1124/jpet.108.141796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Iwasaki K, Yamazaki H, Nelson DR. Macaque cytochromes P450: nomenclature, transcript, gene, genomic structure, and function. Drug Metab Rev. 2011;43:346–361. doi: 10.3109/03602532.2010.549492. [DOI] [PubMed] [Google Scholar]
- Vance JM, Ali S, Bradley WG, Singer C, Di Monte DA. Gene–environment interactions in Parkinson's disease and other forms of parkinsonism. Neurotoxicology. 2010;31:598–602. doi: 10.1016/j.neuro.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Yu AM, Granvil CP, Haining RL, Krausz KW, Corchero J, Kupfer A, et al. The relative contribution of monoamine oxidase and cytochrome p450 isozymes to the metabolic deamination of the trace amine tryptamine. J Pharmacol Exp Ther. 2003a;304:539–546. doi: 10.1124/jpet.102.043786. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Byrd LG, Krausz KW, Kupfer A, Gonzalez FJ. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics. 2003b;13:173–181. doi: 10.1097/01.fpc.0000054066.98065.7b. [DOI] [PubMed] [Google Scholar]
- Yue J, Miksys S, Hoffmann E, Tyndale RF. Chronic nicotine treatment induces rat CYP2D in the brain but not in the liver: an investigation of induction and time course. J Psychiatry Neurosci. 2008;33:54–63. [PMC free article] [PubMed] [Google Scholar]
- Zhou K, Khokhar JY, Zhao B, Tyndale RF. First demonstration that brain CYP2D-mediated opiate metabolic activation alters analgesia in vivo. Biochem Pharmacol. 2013;85:1848–1855. doi: 10.1016/j.bcp.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.