Abstract
Artemisia species have been extensively used for the management of diabetes in folklore medicine. The current study was designed to investigate the antidiabetic and antihyperlipidemic effects of Artemisia amygdalina. Petroleum ether, ethyl acetate, methanol, and hydroethanolic extracts of Artemisia amygdalina were tested for their antidiabetic potentials in diabetic rats. The effect of extracts was observed by checking the biochemical, physiological, and histopathological parameters in diabetic rats. The hydroethanolic and methanolic extracts each at doses of 250 and 500 mg/kg b. w significantly reduced glucose levels in diabetic rats. The other biochemical parameters like cholesterol, triglycerides, low density lipoproteins (LDL), serum creatinine, serum glutamate pyruvate transaminase (SGPT), serum glutamate oxaloacetate transaminase (SGOT), and alkaline phosphatise (ALP), were found to be reduced by the hydroethanolic and methanolic extracts. The extracts also showed reduction in the feed and water consumption of diabetic rats when compared with the diabetic control. The histopathological results of treated groups showed the regenerative/protective effect on β-cells of pancreas in diabetic rats. The current study revealed the antidiabetic potential of Artemisia amygdalina being effective in hyperglycemia and that it can effectively protect against other metabolic aberrations caused by diabetes in rats, which seems to validate its therapeutic traditional use.
1. Introduction
Diabetes mellitus is a metabolic disorder characterized by a loss of glucose homeostasis, with disturbances of carbohydrate, fat, and protein metabolism resulting from defects in insulin secretion, insulin action, or both [1]. Diabetes mellitus is represented by hyperglycemia, lipidaemia, and oxidative stress; it predisposes affected individuals to long-term complications affecting the eyes, skin, kidneys, nerves, and blood vessels [2]. Diabetesis prevalent in all parts of the world and rapidly increasing worldwide. The estimated number of adults living with diabetes has soared to more than 371 million, having a prevalence of 8.3% [3]. India has the more than 63 million of diabetic persons [3]. Despite considerable progress in the treatment of diabetes by oral hypoglycaemic agents, search for newer drugs continues because the existing synthetic drugs have several limitations and harmful effects [4, 5]. Therefore, managing diabetes without any side effects is still a challenging task for health care providers [6]. Hence, the studies are being conducted for finding more efficient, safer, and less expensive hypoglycaemic agents. Herbal medicines have ever been used and claimed as antidiabetic agents but very less are available on commercially formulated forms [7].
Ethnomedicine is a promising field of research in Kashmir, as the valley grows varied medicinal and aromatic plants including those used in curing various diseases [8, 9]. It has been reported that there are 220 medicinal plant species, belonging to 178 genera distributed over 77 families being used in Kashmir and there are many plants which are not being paid due attention [10]. Artemisia is a widespread and varied genus of the family Asteraceae with great therapeutic and economic importance. It has greater than 500 species [11, 12]. Artemisia amygdalina is a species of the family Asteraceae having great therapeutic and economic importance. Artemisia amygdalina commonly known as “Veer Teethwan” is an erect, up to 1.5 m tall perennial herb. Many stems arise from the base which are shallow to deeply grooved, glabrous, with hairy younger shoots in this plant [13]. Artemisia amygdalina is a valuable ethnomedicinal angiosperm of Kashmiri ecosystem; it is confined to specific belts of subalpine region in Kashmir [14]. Various antidiabetic plants have been worked out and one such medicinal plant having folklore claims being used in diabetes is Artemisia amygdalina. Artemisia amygdalina is a widely used medicinal plant in folk medicine [11, 15]. The plant has been reported to have antioxidant potential [16], free radical scavenging activity [13], and anti-inflammatory activity [17]. It also has other pharmacological actions, such as protecting liver,lowering the blood pressure, eliminating fever and sedation, and is used for gastrointestinal ailments [15, 18]. The hexane fraction of Artemisia amygdalina has been reported to have potent cytotoxic activity [19]. The active principles in this plant are the terpenes, p-cymene, and 1,8-cineole [20]. Six cytotoxic constituents, namely, ergostadien-3-ol (1), ludartin (2), 5-hydroxy-6,7,3,4-tetramethoxyflavone (3) (from shoot) and trans-matricaria ester (4), diacetylenic spiroenol ether (5), and cis-matricaria ester (6) (from root), have been isolated [19]. In view of its wide ethnomedicinal values, folklore claims, and reported activities, the plant was validated for its antidiabetic activity.
2. Materials and Methodology
2.1. Plant Material and Extraction
The plant material was collected from the local areas of Kashmir and was identified by the Centre of Taxonomy, University of Kashmir. Sample specimen (voucher specimen number 1803-KASH) was deposited in the herbarium of Centre of Taxonomy, University of Kashmir. The whole plant was used for the extraction. The plant material (whole plant) was completely shade-dried and coarsely ground. The extracts were prepared by continuous hot extraction using petroleum ether, ethyl acetate, methanol and hydroethanol (1 : 1) as solvents. Extracts obtained were concentrated, dried and kept in desiccators for further use. The yields of the petroleum ether, ethyl acetate, methanol, and hydroethanolic extracts were 2.8, 3.2, 5.8, and 6.3% (w/w), respectively.
2.2. Experimental Animals
Albino Wistar rats weighing 120–150 g of either sex were selected for the study. They were fed a standard rat pellet and water from Reverse Osmosis Purifier (Kent). Research on animals was conducted in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) as the institute has CPCSEA registration (Reg. number 927/GO/c/06/CPCSEA). The experimental protocol was approved by the Institutional Animal Ethics Committee of the Regional Research Institute of Unani Medicine, Srinagar, Jammu and Kashmir, India.
2.3. Acute Oral Toxicity Study of the Crude Extracts of Artemisia amygdalina
Acute oral toxicity study was performed for the extracts of Artemisia amygdalina according to the guidelines of the Organisation for Economic Cooperation and Development (OECD) [21]. The rats were kept on fasting overnight, being provided only water prior to oral dosing. Then the extract was administered orally at different dose levels, that is, 100, 200, 500, 1000, 1500, and 2000 mg/kg of body weight. The rats were observed continuously for 24 h for behavioural and any adverse change and thereafter for any lethality.
2.4. Experimental Design
All the animals were randomly divided into several groups with five animals in each, serving as normal (nondiabetic), diabetic control, diabetic treated with different extracts, and diabetic reference control, that is, glibenclamide. Glibenclamide was given at a dose of 3 mg/kg of body weight [22, 23]. The oral administration of crude extracts (different extracts with different concentrations) was continued once daily at the same time for 14 days. Body weight and blood glucose levels were estimated on the 0th, 7th, and 14th day of treatment [24].
2.5. Induction of Diabetes
Diabetes was induced in albino Wistar rats by a single intraperitoneal (i.p.) injection of 50 mg/kg of streptozotocin (STZ), reconstituted in freshly prepared normal saline (0.9% W/V) after overnight fasting. After 72 h of STZ administration, glucose levels were measured in blood samples collected from retroorbital sinus of rats. Rats with fasting serum glucose levels more than 200 mg/dL were considered diabetic and selected for further study [25, 26].
2.6. Assessment of Effects of Extracts on Biochemical Parameters
Oral administration with plant extracts was started 72 h after streptozotocin injection in diabetic rats while normal group and diabetic control group were administered only with vehicle. The rats were sacrificed after 14 days after anesthetising them using isoflurane and blood was collected on the termination day from dorsal vena cava by opening the abdomen. Serum was collected and analyzed for glucose, cholesterol, triglycerides, LDL, SGOT, SGPT, ALP, creatinine, and total protein estimation by using Automatic Biochemistry Analyser (Erba; XL-640).
2.7. Effect of Extracts on Body Weight, Feed Consumption, and Water Consumption
The effect of the extracts (hydroethanolic and methanolic) on parameters like body weight, feed consumption, and water consumption were determined and recorded during the study period.
2.8. Histopathological Studies
On the 14th day, all the animals were sacrificed and pancreas was removed. Pancreatic samples were taken for histopathology and the tissue samples were processed in tissue processor (Leica made). Pancreatic sections were stained with haematoxylin and eosin Y (H/E) dyes. The sections of pancreas were observed under light microscope (Olympus) for histopathological study.
2.9. Oral Glucose Tolerance Test
The oral glucose tolerance test was performed in overnight fasted normal rats [27]. Healthy rats were randomly selected and distributed into six groups (n = 5). One group (normal) was administered R.O. water, four groups were given orally extracts of Artemisia amygdalina (petroleum ether, ethyl acetate, methanolic, and hydroethanolic each at a dose level of 500 mg/kg of b. w, resp.) and the sixth group was given glibenclamide (3 mg/kg). Glucose (2 g/kg) was fed 1 h after the administration of R.O. water, extracts, and glibenclamide. Blood was collected from the retroorbital sinus under isoflurane inhalation at 0, 30, 60, 90, and 120 minutes (min) of glucose administration and glucose levels were estimated.
2.10. Preliminary Phytochemical Tests
The crude methanolic extract, hydroethanolic, ethyl acetate and petroleum ether fractions of Artemisia amygdalina were subjected to qualitative tests for identification of different constituents like alkaloids, flavonoids, terpenoids, phenolics, anthraquinones, glycosides, saponins and tannins by using standard qualitative methods described by Trease and Evans [28].
2.11. Statistical Analysis
All the values of body weight, fasting serum glucose, and biochemical estimations were expressed as mean ± SEM and ANOVA was carried out followed by post-Dunnett's t-test using SPSS 16.0 statistical software. Differences between groups were considered significant at P < 0.01 levels.
3. Results
3.1. Preliminary Phytochemical Analysis of Different Fractions of Artemisia amygdalina
Preliminary screening of methanolic extracts revealed the presence of alkaloids, phenolics, and glycosides. The hydroethanolic extract mainly showed the presence of flavonoids along with phenolics, tannins, and alkaloids while petroleum ether extract showed the presence of terpenes and steroids. The ethyl acetate extract was found to have contents of carbohydrates, glycosides, flavonoids, and terpenoids.
3.2. Acute Oral Toxicity Testing
The extracts were found to be safe up to the dose level of 2000 mg/kg of body weight in rats. The extracts did not induce any toxicological effect in any rat. There was no lethality found by oral administration of any extracts of Artemisia amygdalina.
3.3. Antihyperglycaemic Effect of Artemisia amygdalina
The effect of extracts of Artemisia amygdalina and glibenclamide on serum glucose levels in normal, diabetic, and extract treated rats is presented in Table 1(a). The highest percent variation in fasting glucose levels was shown by hydroethanolic extract (54.07%), followed by methanolic extract (43.93%). The standard reference drug glibenclamide (3 mg/kg b. w) was found to decrease fasting glucose levels by 48.09% after 14 days of treatment. The other two extracts, that is, pet. ether and ethyl acetate, also showed inhibitory effect on glucose levels but were not considered for further studies. The highly bioactive fractions were tested for dose dependence and were observed to show increased antihyperglycaemic activity with increase in dose. The hydroethanolic fraction showed a more significant effect in decreasing blood glucose levels than the methanolic fraction and the maximum % variation observed at 500 mg/kg b. w was 56.88 while the % variation at the same dose in methanolic fraction was 45.09 (Table 1(b)).
Table 1.
(a) Effect of extracts of Artemisia amygdalina and glibenclamideon fasting blood glucose levels of rats. (b) Dose-dependent effect of hydroethanolic and methanolic extracts of Artemisia amygdalina and glibenclamideon fasting blood glucose levels of rats.
(a)
| Groups | Serum glucose (mg/dL) | |||
|---|---|---|---|---|
| 0th day | 7th day | 14th day | % variation | |
| Normal | 82.32 ± 0.99 | 85.43 ± 1.39 | 83.57 ± 2.22 | −1.51 |
| Diabetic control | 360.14 ± 5.06 | 387.58 ± 3.28 | 410.13 ± 2.48 | −13.88 |
| Pet Ether extract (500 mg/kg b. w) | 350.55 ± 3.26 | 309.87 ± 2.85 | 266.57 ± 4.10 | 23.95 |
| Hydroethanolic extract (500 mg/kg b. w) | 386.47 ± 5.51 | 232.51 ± 3.82** | 177.49 ± 2.00** | 54.07 |
| Methanolic extract (500 mg/kg b. w) | 359.54 ± 3.33 | 284.65 ± 2.83** | 201.59 ± 3.38** | 43.93 |
| Ethyl Acetate extract (500 mg/kg b. w) | 371.35 ± 2.92 | 325.43 ± 6.43 | 260.53 ± 2.94 | 29.84 |
| Glibenclamide (3 mg/kg b. w) | 365.9 ± 3.97 | 248.78 ± 4.16** | 189.92 ± 3.07** | 48.09 |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with normal at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
(b)
| Groups | Serum Glucose (mg/dL) | |||
|---|---|---|---|---|
| 0th day | 7th day | 14th day | % variation | |
| Normal | 85.66 ± 1.09 | 84.86 ± 1.13 | 84.12 ± 2.25 | 1.80 |
| Diabetic control | 375.41 ± 4.62 | 390.82 ± 4.75 | 419.52 ± 3.94 | −11.75 |
| Hydroethanolic extract (50 mg/kg b. w) | 380.76 ± 5.24 | 366.27 ± 4.55 | 352.78 ± 5.84 | 7.35 |
| Hydroethanolic extract (100 mg/kg b. w) | 355.15 ± 4.48 | 330.85 ± 6.3 | 288.54 ± 3.96 | 18.76 |
| Hydroethanolic extract (250 mg/kg b. w) | 370.59 ± 5.23 | 290.36 ± 6.34** | 238.45 ± 7.46** | 35.66 |
| Hydroethanolic extract (500 mg/kg b. w) | 377.81 ± 5.45 | 227.55 ± 4.36** | 162.92 ± 3.85** | 56.88 |
| Methanolic extract (50 mg/kg b. w) | 358.96 ± 6.94 | 350.12 ± 7.24 | 344.4 ± 5.74 | 4.06 |
| Methanolic extract (100 mg/kg b. w) | 366.85 ± 5.89 | 340.53 ± 7.84 | 328.12 ± 4.85 | 10.56 |
| Methanolic extract (250 mg/kg b. w) | 390.61 ± 6.37 | 358.24 ± 5.67 | 295.48 ± 6.13 | 24.35 |
| Methanolic extract (500 mg/kg b. w) | 365.37 ± 8.7 | 278.42 ± 4.75** | 200.64 ± 5.82** | 45.09 |
| Glibenclamide (3 mg/kg b. w) | 372.27 ± 4.86 | 258.87 ± 5.64** | 195.25 ± 4.37** | 47.55 |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with normal at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
3.4. Effect of Extracts of Artemisia amygdalina and Glibenclamide on Various Biochemical Parameters in Rats
The extracts of Artemisia amygdalina hydroethanolic extract (500 mg/kg b. w) and methanolic extract (500 mg/kg b. w) significantly lowered the levels of cholesterol, triglycerides, LDL, and creatinine in diabetic rats when compared with the diabetic control group (Table 2). Total protein was found to be lowered in diabetic control group, while it was found to be elevated in the extract- and glibenclamide treated diabetic rats. The extracts were also shown to significantly lower the enzymatic activity of liver marker enzymes (SGPT, SGOT, and ALP) in diabetic rats as represented in Table 3.
Table 2.
Effect of extracts of Artemisia amygdalina and glibenclamide on various biochemical parameters in rats.
| Groups | Cholesterol (mg/dL) |
Triglycerides (mg/dL) |
LDL (mg/dL) |
Total protein (g/dL) |
Creatinine (mg/dL) |
|---|---|---|---|---|---|
| Normal | 50.59 ± 2.77 | 62.83 ± 2.46 | 83.35 ± 3.22 | 7.11 ± 0.30 | 0.69 ± 0.05 |
| Diabetic control | 149.33 ± 6.93 | 120.83 ± 4.96 | 189.38 ± 5.61 | 4.20 ± 0.44 | 1.98 ± 0.09 |
| Hydroethanolic extract (250 mg/kg b. w) | 84.32 ± 3.67 | 95.74 ± 5.54 | 132.45 ± 3.35 | 5.75 ± 0.29 | 1.18 ± 0.05 |
| Hydroethanolic extract (500 mg/kg b. w) | 64.83 ± 2.19** | 75.66 ± 4.87** | 101.36 ± 2.95** | 6.12 ± 0.32 | 0.88 ± 0.08** |
| Methanolic extract (250 mg/kg b. w) | 88.12 ± 3.79 | 98.27 ± 5.28 | 142.77 ± 4.23 | 5.15 ± 0.26 | 1.07 ± 0.06 |
| Methanolic extract (500 mg/kg b. w) | 73.35 ± 2.92** | 84.43 ± 6.44** | 128.54 ± 3.46** | 6.67 ± 0.28** | 0.97 ± 0.07** |
| Glibenclamide (3 mg/kg b. w) | 60.83 ± 2.73** | 78.00 ± 2.73** | 92.37 ± 3.67** | 7.03 ± 0.21** | 0.78 ± 0.04** |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with diabetic control at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
Table 3.
Effect of extracts of Artemisia amygdalina and glibenclamideon liver marker enzymes of streptozotocin induced diabetic rats.
| Groups | SGOT (U/L) | SGPT (U/L) | ALP (U/L) |
|---|---|---|---|
| Normal | 115.00 ± 5.14 | 95.23 ± 6.75 | 175.67 ± 8.68 |
| Diabetic control | 243.00 ± 9.57 | 209.16 ± 6.35 | 296.17 ± 7.85 |
| Hydroethanolic extract (250 mg/kg b. w) | 164.31 ± 6.53 | 148.76 ± 7.69 | 232.17 ± 6.82 |
| Hydroethanolic extract (500 mg/kg b. w) | 129.66 ± 8.08** | 112.83 ± 7.11** | 201.66 ± 7.94** |
| Methanolic extract (250 mg/kg b. w) | 174.76 ± 4.83 | 177.62 ± 7.71 | 229.77 ± 6.18 |
| Methanolic extract (500 mg/kg b. w) | 138.53 ± 2.94** | 121.85 ± 6.69** | 219.53 ± 7.37 |
| Glibenclamide (3 mg/kg b. w) | 119.50 ± 4.43** | 103.73 ± 3.00** | 198.50 ± 7.14** |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with diabetic control at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
3.5. Effect of Extracts of Artemisia amygdalina and Glibenclamide on Feed Consumption and Water Consumption in Rats
The extract treated rats (hydroethanolic and methanolic extract at a dose level of 500 mg/kg b. w) and glibenclamide treated rats (3 mg/kg b. w) significantly overcame the symptoms of diabetes, that is, polyphagia and polydipsia. The extract- and glibenclamide treated rats consumed less water and feed when compared with the diabetic control ones. Table 4 shows the effect of extracts and glibenclamide on feed and water consumption in rats.
Table 4.
Effect of the extracts of Artemisia amygdalina and glibenclamideon feed intake and fluid intake of the rats.
| Groups | Water consumption (mL/day) |
Food consumption (g/day) |
|---|---|---|
| Normal (water) | 24 ± 4.02 | 19.34 ± 2.36 |
| Diabetic (water) | 65 ± 7.60 | 29.67 ± 1.23 |
| Hydroethanolic extract (250 mg/kg b. w) | 46 ± 5.37 | 25.33 ± 3.30 |
| Hydroethanolic extract (500 mg/kg b. w) | 39 ± 7.16** | 23.56 ± 3.42** |
| Methanol extract (250 mg/kg b. w) | 49 ± 4.02 | 24.24 ± 2.60 |
| Methanol extract (500 mg/kg b. w) | 44 ± 4.92** | 22.87 ± 2.01** |
| Glibenclamide (3 mg/kg b. w) | 44 ± 6.71** | 22.33 ± 1.23** |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with diabetic control at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
3.6. Effect of Extracts of Artemisia amygdalina and Glibenclamide on Body Weight in Rats
The body weight of rats belonging to diabetic control group was drastically decreased upon the induction of diabetes. The extract- and glibenclamide treated rats were found to gain body weight significantly when compared with the diabetic control group as shown in Table 5.
Table 5.
Effect of extracts of Artemisia amygdalina and glibenclamideon body weight in streptozotocin induced diabetic rats.
| Groups | Body weight (g) | |||
|---|---|---|---|---|
| 0th day | 7th day | 14th day | % variation | |
| Normal | 120 ± 1.79 | 135 ± 3.13 | 155 ± 2.68 | 29.16 |
| Diabetic control | 125 ± 1.34 | 118 ± 1.79 | 107 ± 3.13 | −14.4 |
| Hydroethanolic extract (250 mg/kg b. w) | 127 ± 2.24 | 132 ± 2.24 | 139 ± 3.58** | 9.44 |
| Hydroethanolic extract (500 mg/kg b. w) | 122 ± 2.68 | 128 ± 3.13 | 135 ± 2.24** | 10.65 |
| Methanolic extract (250 mg/kg b. w) | 125 ± 3.13 | 130 ± 3.58 | 137 ± 4.92** | 9.6 |
| Methanolic extract (500 mg/kg b. w) | 123 ± 2.24 | 130 ± 3.58 | 140 ± 5.37** | 13.82 |
| Glibenclamide (3 mg/kg b. w) | 125 ± 1.79 | 133 ± 2.68 | 142 ± 3.13** | 13.6 |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with diabetic control at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
3.7. Histopathology
Administration of streptozotocin decreased the number of β-cells. With the result, the observed mean pancreatic weight of untreated diabetic group was less compared to the mean weight of pancreas of normal (nondiabetic) and diabetic treated groups. The sections from the untreated diabetic group demonstrated shrunken islets of Langerhans with degenerative necrosis. There was an increased vacuolation (Figure 1(b)). In the extract treated rats (Figures 1(c), 1(d), 1(e), and 1(f)) islets of Langerhans appeared less shrunken as compared to those of untreated diabetic group. The histological appearance of the pancreatic islet cells of the control was normal (Figure 1(a)). The morphology of the pancreas of Artemisia amygdalina extracts (hydroethanolic and methanolic) treated diabetic rats revealed remarkable improvement in the islets of Langerhans. There was an increase in the islet cellular density, with an increase in granulation, and vacuolation was reduced or absent in many islets (Figures 1(c), 1(d), 1(e), and 1(f)). The histological study of pancreatic sections of glibenclamide treated group resembled extract treated groups in shape, size, and texture (Figure 1(g)).
Figure 1.
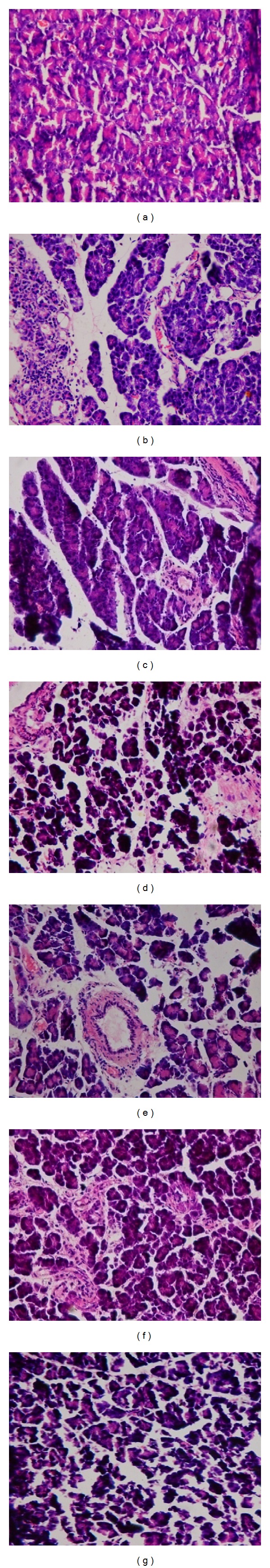
(a) Normal acini and normal cellular population in the islets of Langerhans in pancreas of vehicle-treated rats. (b) Extensive damage to the islets of Langerhans and reduced dimensions of islets. (g) Restoration of normal cellular population size of islets with hyperplasia by glibenclamide. The partial restoration of normal cellular population and enlarged size of β-cells with hyperplasia are shown by hydroethanolic extract (250 mg/kg and 500 kg/kg—(c) and (d), resp.) and methanolic extracts (250 mg/kg and 500 mg/kg—(e) and (f), resp.).
3.8. Oral Glucose Tolerance Test (OGTT)
Table 6(a) shows the OGTT study; blood glucose concentration in all groups reached peak levels after 30 min of glucose administration (2 g/kg) and then began to decrease. As compared to normal group, the glucose levels of experimental rats treated with extracts and glibenclamide showed a very steep reduction. Hydroethanolic extract (500 mg/kg of b. w) showed more significant antihyperglycemic activity than other extracts and glibenclamide treated rats. Hydroethanolic and methanolic extracts were found to lower the glucose levels in a dose-dependent pattern (Table 6(b)).
Table 6.
(a) Effect of extracts of Artemisia amygdalina and glibenclamide on glucose tolerance of rats. (b) Dose-dependent effect of hydroethanolic and methanolic extracts of Artemisia amygdalina on glucose tolerance of rats.
(a)
| Groups | Blood glucose level (mg/dL) | |||||
|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | % variation | |
| Normal | 82.35 ± 3.44 | 147.58 ± 4.11 | 133.60 ± 5.05 | 125.34 ± 6.40 | 118.36 ± 3.53 | 43.72 |
| Pet. ether extract (500 mg/kg b. w) | 83.14 ± 2.82 | 138.58 ± 6.84 | 127.13 ± 2.46 | 118.93 ± 5.72 | 90.57 ± 3.44 | 8.93 |
| Ethyl acetate extract (500 mg/kg b. w) | 80.76 ± 2.10 | 142.48 ± 3.53 | 125.71 ± 6.40 | 112.91 ± 5.90 | 94.64 ± 6.35 | 17.18 |
| Hydroethanolic extract (500 mg/kg b. w) | 83.245 ± 3.71 | 110.68 ± 3.00** | 97.79 ± 3.49** | 89.51 ± 4.20** | 79.51 ± 4.52** | -4.48 |
| Methanolic extract (500 mg/kg b. w) | 80.47 ± 3.22 | 122.27 ± 4.20 | 112.72 ± 4.38 | 98.61 ± 5.99 | 86.44 ± 4.65** | 7.414 |
| Glibenclamide (3 mg/kg b. w) | 81.89 ± 3.00 | 118.82 ± 3.85** | 107.82 ± 3.67** | 98.24 ± 4.29** | 82.56 ± 4.11** | 0.81 |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with normal at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
(b)
| Groups | Blood glucose level (mg/dL) | |||||
|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | % variation | |
| Normal | 81.25 ± 2.97 | 149.49 ± 3.24 | 136.64 ± 4.22 | 128.44 ± 5.53 | 115.42 ± 6.30 | 42.05 |
| Hydroethanolic extract (50 mg/kg b. w) | 82.41 ± 3.23 | 145.45 ± 4.67 | 131.24 ± 2.35 | 122.46 ± 4.86 | 112.25 ± 4.33 | 36.20 |
| Hydroethanolic extract (100 mg/kg b. w) | 83.67 ± 4.15 | 132.56 ± 4.32 | 120.18 ± 4.50 | 110.16 ± 4.34 | 101.14 ± 5.36 | 20.88 |
| Hydroethanolic extract (250 mg/kg b. w) | 81.54 ± 5.25 | 121.24 ± 2.96** | 107.73 ± 4.39** | 96.64 ± 3.74** | 87.15 ± 2.54** | 6.88 |
| Hydroethanolic extract (500 mg/kg b. w) | 82.42 ± 4.64 | 108.68 ± 3.38** | 96.21 ± 3.44** | 83.31 ± 4.72** | 72.15 ± 5.42** | 221212.46 |
| Methanolic extract (50 mg/kg b. w) | 83.66 ± 4.12 | 150.52 ± 3.35 | 140.88 ± 3.48 | 128.25 ± 2.78 | 118.26 ± 3.35 | 41.35 |
| Methanolic extract (100 mg/kg b. w) | 82.52 ± 4.45 | 141.65 ± 3.22 | 129.42 ± 3.68 | 116.27 ± 3.85 | 105.51 ± 5.22 | 27.86 |
| Methanolic extract (250 mg/kg b. w) | 83.24 ± 5.24 | 131.57 ± 4.38 | 116.21 ± 5.12 | 106.35 ± 4.62 | 95.23 ± 4.95** | 14.40 |
| Methanolic extract (500 mg/kg b. w) | 82.57 ± 6.35 | 120.09 ± 5.14 | 103.41 ± 2.56 | 92.35 ± 3.44 | 81.13 ± 2.55** | 1.74 |
| Glibenclamide (3 mg/kg b. w) | 80.68 ± 2.75 | 116.78 ± 5.83** | 104.23 ± 6.73** | 94.16 ± 5.25** | 83.25 ± 4.75** | 3.18 |
The values are expressed as mean ± SEM; n = 5 in each group. **P < 0.01 as compared with normal at the same time (one-way ANOVA followed by Dunnett's multiple comparison test).
4. Discussion
Many Indian medicinal plants are reported to be useful in diabetes mellitus and many species of genus Artemisia have been reported to have antidiabetic activity [29]. In Artemisia indica the hydromethanolic crude extract at a dose of 200 and 400 mg/kg b. w and chloroform fraction (200 mg/kg b. w) administered orally for 15 days showed a significant reduction in blood glucose level [30]. Similar results were observed in our study that was carried out on Artemisia amygdalina where hydroethanolic and methanolic extract produced a significantdecrease in the serum glucose level at a dose of 500 mg/kg. The extracts also showed increased dose-dependent antihyperglycaemic effect. The results also correlate with the study carried on Artemisia judaica, where the bioactive principles found were the flavonoids [31]. In other similar studies carried out on Artemisia sieberi, Artemisia pallens, and Artemisia herba-alba, treatment of diabetic rats with the fractions obtained from these plants significantly reduced the blood glucose levels [30, 32–34]. These studies suggest the commonness of bioactive principles among these related species, thus making them effective in the treatment of diabetes.
Since liver is the central metabolic organ in body responsible for glucose and lipid homeostasis, diabetes leads to hepatic dysfunction. Streptozotocin induced diabetes in rats causes the elevated activities of liver marker enzymes (SGPT, SGOT, and ALP) due to the reported destruction of hepatocytes [35]. Rats orally treated with the extracts of Artemisia amygdalina (hydroethanolic and methanolic extracts each at a dose level of 500 mg/kg of b. w) were found to have significantly reduced activities of these enzymes, thus indicating less damage to hepatocytes.
Streptozotocin induced diabetic rats are associated with hyperlipidemia and increased levels of serum creatinine [36]. However the extract treated rats (hydroethanolic and methanolic extracts each at a dose level of 500 mg/kg of b. w) showed reduced levels of cholesterol, triglycerides, LDL, and serum creatinine when compared with the diabetic control group. The lowering of these lipid substances and serum creatinine in the blood of treated rats is presumed mainly to be a manifestation of lowering of blood glucose level.
The serum protein levels in diabetic rats were reduced as compared to normal group, while the serum protein levels of treated rats (hydroethanolic and methanolic extracts each at a dose level of 500 mg/kg of b. w) were found to be higher than those of diabetic group indicating lowered protein degradation. There was a significant reduction in the feed and water consumption in diabetic rats treated with hydroethanolic and methanolic extracts of Artemisia amygdalina (each at a dose level of 500 mg/kg of b. w) when compared with the diabetic control group. The decrease in feed and water consumption may be attributed to the decrease in protein degradation and improved glycemic control as is obvious from the results stated earlier. The body weight among the rats administered with the extracts of Artemisia amygdalina was found to be in an increasing fashion possibly due to the reduction in lowering of glucose levels thus sparing the body fat and muscle protein which otherwise are utilised in diabetic rats.
Streptozotocin (STZ) administration generally causes the destruction of β-cells after three days and reaches its peak at three to four weeks in rats [37]. β-cells are particularly sensitive to damage by nitric oxide and free radicals because of their low levels of free radical scavenging enzymes [38]. The results of this present study indicate that the extracts of Artemisia amygdalina may lead to the regeneration/proliferation of the pancreatic β-cells possibly due to the prevention of free radical formation induced by STZ. Since pancreas contains stable (quiescent) β-cells which have the regenerative capacity, the surviving cells proliferate by replication to supplicate the lost cells [39]. New pancreatic β-cells can be formed by neogenesis or by replication of the preexisting differentiated cells [40]; hence it is assumed from the study that the extracts of Artemisia amygdalina are also responsible for the proliferation of β-cells, as there are already reports showing extracts of other medicinal plants which have a β-cell regenerative potential [41, 42].
The antidiabetic potential of the different extracts of Artemisia amygdalina may be due the presence of any of the secondary metabolites (flavonoids, alkaloids, phenolics, glycosides, and terpenes) present in variedconcentrations in Artemisia amygdalina. These secondary metabolites are reported to possess antidiabetic potential differently and are the suggested reason for the difference in activities of these extracts [43–48]. Many of the flavonoids have been found to possess the antidiabetic potential [49]. We also suggest the potent antidiabetic potential of hydroethanolic extract because of the presence of flavonoid compound(s) in it.
The improved glycemic control in oral glucose tolerance tests by the extracts of Artemisia amygdalina shows that the extracts also lower the blood glucose levels even in normal rats. The effect of lowering blood glucose levels in normal rats may be due to the increased efficiency of the peripheral tissues for the uptake of glucose from blood. Thus the extracts can also be useful in patients with type II diabetes and need further detailed studies for the validation of these results.
5. Conclusion
The common symptoms of diabetes, that is, polyphagia, polydipsia, and weight loss, were found to be lessened by the extracts of Artemisia amygdalina (hydroethanolic and methanolic extracts each at a dose level of 500 mg/kg of b. w) in diabetic rats. The extracts significantly reduced fasting glucose levels in diabetic rats and also reduced the lipid profile parameters in diabetic rats. The extracts were found significantly decreasing the activities of SGPT, SGOT, and ALP in diabetic rats. In conclusion, our histopathological investigation along with the biochemical evaluations suggests the strong antidiabetic potential of Artemisia amygdalina. The results observed show the effect on both the pancreatic β-cells and the blood glucose level. Further mechanistic studies are required to suggest the appropriate mechanism for the antidiabetic effect of the plant.
Acknowledgments
The authors are grateful to the Central Council for Research in Unani Medicine, New Delhi, India, for financial assistance. They are also highly thankful to Mr. Showkat Ahmad Teli, Mr. Shafiq Ahmad Khan, Mr. Bashir Ahmad Bhat, and Mr. Ashiq Ahmad Bhat for their valuable contribution to plant collection, allocation of animals, and maintenance of animal house.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Imam K. Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. Journal of Advances in Experimental Medicine and Biology. 2012;771:340–355. doi: 10.1007/978-1-4614-5441-0_25. [DOI] [PubMed] [Google Scholar]
- 2.Elosta A, Ghous T, Ahmed N. Natural products as Anti-glycation agents: possible therapeutic potential for diabetic complications. Current Diabetes Reviews. 2012;8(2):92–108. doi: 10.2174/157339912799424528. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation IDF. Diabetes Atlas. 5th edition. 2012. [PubMed] [Google Scholar]
- 4.Spiller HA, Sawyer TS. Toxicology of oral antidiabetic medications. American Journal of Health-System Pharmacy. 2006;63(10):929–938. doi: 10.2146/ajhp050500. [DOI] [PubMed] [Google Scholar]
- 5.Eurich DT, McAlister FA, Blackburn DF, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. British Medical Journal. 2007;335(7618):497–501. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena A, Vikram NK. Role of selected Indian plants in management of type 2 diabetes: a review. The Journal of Alternative and Complementary Medicine. 2004;10(2):369–378. doi: 10.1089/107555304323062365. [DOI] [PubMed] [Google Scholar]
- 7.Jung M, Park M, Lee HC, Kan Y-H, Kang ES, Kim SK. Antidiabetic agents from medicinal plants. Current Medicinal Chemistry. 2006;13(10):1203–1218. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- 8.Kachroo P, Nahvi IM. Forest Flora of Srinagar and Plants of Neighbourhood. Dehra Dun, India: Bishen Singh Mahendra Pal Singh; 1987. Ethnobotany of Kashmiris. [Google Scholar]
- 9.Dhar U, Kachroo P. Alpine Flora of Kashmir Himalaya. Jodhpur, India: Scientific publishers; 1983. [Google Scholar]
- 10.Guna GA. Medicinal flora of Kashmir valley [Ph.D. thesis] Srinagar, India: University of Kashmir; 2006. [Google Scholar]
- 11.Ashraf M, Hayat MQ, Jabeen S, Shaheen N, Khan MA, Yasmin G. Artemisia L. species recognized by the local community of northern areas of Pakistan as folk therapeutic plants. Journal of Medicinal Plant Research. 2010;4(2):112–119. [Google Scholar]
- 12.Willcox M. Artemisia species: from traditional medicines to modern antimalarials—and back again. The Journal of Alternative and Complementary Medicine. 2009;15(2):101–109. doi: 10.1089/acm.2008.0327. [DOI] [PubMed] [Google Scholar]
- 13.Rasool R, Ganai BA, Akbar S, Kamili AN. Free radical scavenging potential of in vitro raised and greenhouse acclimatized plants of Artemisia amygdalina . Chinese Journal of Natural Medicines. 2013;4(11):377–384. doi: 10.1016/S1875-5364(13)60055-2. [DOI] [PubMed] [Google Scholar]
- 14.Dar AR, Dar GH, Zafar R. Conservation of Artemisia amygdalina—acritically endangered endemic plant species of Kashmir Himalaya. Endangered Species Update. 2006;23(1):34–39. [Google Scholar]
- 15.Sivagnanam SK, Rao RK, Mudiganti, Dar UM, Jeelani PG. Preliminary phytochemical analysis of Artemisia amygdalina, Nerium odorum and Strychnos potatorum . Journal of Pharmacy Research. 2012;5(7):3734–3739. [Google Scholar]
- 16.Rasool R, Ganai BA, Kamili AN, Akbar S. Antioxidant potential in callus culture of Artemisia amygdalina Decne. Natural Product Research. 2012;26(22):2103–2106. doi: 10.1080/14786419.2011.617749. [DOI] [PubMed] [Google Scholar]
- 17.Mubashir K, Ganai BA, Ghazanfar K, Akbar S, Malik AH, Masood A. Evaluation of Artemisia amygdalina D. for anti-Inflammatory and immunomodulatory potential. ISRN Inflammation. 2013;2013:5 pages. doi: 10.1155/2013/483646.483646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qaisar M. Asteraceae. Flora of Pakistan. 2006;207:120–121. [Google Scholar]
- 19.Lone SH, Bhat KA, Naseer S, Rather RA, Khuroo MA, Tasduq SA. Isolation, cytotoxicity evaluation and HPLC-quantification of the chemical constituents from Artemisia amygdalina Decne. Journal of Chromatography B. 2013;940:135–141. doi: 10.1016/j.jchromb.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 20.Rather MA, Ganai BA, Kamili AN, et al. Comparative GC-FID and GC-MS analysis of the mono and sesquiterpene secondary metabolites produced by the field grown and micropropagated plants of Artemisia amygdalina Decne. Acta Physiologiae Plantarum. 2012;34(3):885–890. [Google Scholar]
- 21.OECD. OECD guidelines for the testing of chemicals. Test. 2008;(425)
- 22.Thakkar NV, Patel JA. Pharmacological evaluation of “glyoherb”: a polyherbal formulation on streptozotocin-induced diabetic rats. International Journal of Diabetes in Developing Countries. 2010;30(1):1–7. doi: 10.4103/0973-3930.60001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabitha V, Ramachandran S, Naveen KR, Panneerselvam K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. Journal of Pharmacy and Bioallied Sciences. 2011;3(3):397–402. doi: 10.4103/0975-7406.84447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad W, Khan I, Khan MA, Ahmad M, Subhan F, Karim N. Evaluation of antidiabetic and antihyperlipidemic activity of Artemisia indica linn (aeriel parts) in Streptozotocin induced diabetic rats. Journal of Ethnopharmacology. 2014;151(1):618–623. doi: 10.1016/j.jep.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Mohan Y, Jesuthankaraj GN, Thangavelu NR. Antidiabetic and antioxidant properties of Triticum aestivum in streptozotocin-induced diabetic rats. Advances in Pharmacolological Sciences. 2013;2013:9 pages. doi: 10.1155/2013/716073.716073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzeng TF, Liou SS, Chang CJ, Liu IM. The ethanol extract of Lonicera japonica (Japanese Honeysuckle) attenuates diabetic nephropathy by inhibiting p-8 MAPK activity in streptozotocin-induced diabetic rats. Planta Medica. 2014;80(2-3):121–139. doi: 10.1055/s-0033-1360196. [DOI] [PubMed] [Google Scholar]
- 27.Lee CW, Lee HS, Cha YJ, Joo WH, Kang DO, Moon JY. In vivo Investigation of anti-diabetic properties of ripe onion juice in normal and streptozotocin-induced diabetic rats. Preventive Nutrition and Food Science. 2013;18(3):169–174. doi: 10.3746/pnf.2013.18.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trease GE, Evans WC. Textbook of Pharmacognosy. Vol. 12. London, UK: Balliese Tindall and Company Publisher; 1983. [Google Scholar]
- 29.Nadkarni KM, Nadkarni AK. Indian Materia Medica. Vol. 1. Bombay, India: Popular Prakashan; 1976. [Google Scholar]
- 30.Ahmad W, Khan I, Khan MA, Ahmad M, Subhan F, Karim N. Evaluation of antidiabetic and antihyperlipidemic activity of Artemisia indica linn. (aeriel parts) in Streptozotocin induced diabetic rats. Journal of Ethnopharmacology. 2013;151:618–623. doi: 10.1016/j.jep.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Nofa SM, Mahmoud SS, Ramadan A, Soliman GA, Fawzy R. Anti-diabetic effect of Artemisia judaica extracts. Research Journal of Medicine and Medical Sciences. 2009;4(1):42–48. [Google Scholar]
- 32.Al-Shamaony L, Al-Khazraji SM, Twaij HAA. Hypoglycaemic effect of Artemisia herba alba. II. Effect of a valuable extract on some blood parameters in diabetic animals. Journal of Ethnopharmacology. 1994;43(3):167–171. doi: 10.1016/0378-8741(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 33.Subramoniam A, Pushpangadan P, Rajasekharan S, Evans DA, Latha PG, Valsaraj R. Effects of Artemisia pallens Wall. on blood glucose levels in normal and alloxan-induced diabetic rats. Journal of Ethnopharmacology. 1996;50(1):13–17. doi: 10.1016/0378-8741(95)01329-6. [DOI] [PubMed] [Google Scholar]
- 34.Mansi K, Amneh M, Nasr H. The hypolipidemic effects of Artemisia sieberi (A. herba-alba) in alloxan induced diabetic rats. International Journal of Pharmacology. 2007;3(6):487–491. [Google Scholar]
- 35.Daisy P, Eliza J, Ignacimuthu S. Influence of Costus speciosus (Koen.) sm. rhizome extracts on biochemical parameters in streptozotocin induced diabetic rats. Journal of Health Science. 2008;54(6):675–681. [Google Scholar]
- 36.Prisilla DH, Balamurugan R, Shah HR. Antidiabetic activity of methanol extract of Acorus calamus in STZ induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine. 2012;2(2):941–946. [Google Scholar]
- 37.Adeghate E, Ponery AS. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue and Cell. 2002;34(1):1–6. doi: 10.1054/tice.2002.0217. [DOI] [PubMed] [Google Scholar]
- 38.Spinas GA. The dual role of nitric oxide in islet β-cells. News in Physiological Sciences. 1999;14(2):49–54. doi: 10.1152/physiologyonline.1999.14.2.49. [DOI] [PubMed] [Google Scholar]
- 39.Kumar V, Cotran R, Robbins S. Basic Pathology. Vol. 5. Philadelphia, Pa, USA: WB Saunders; 1992. [Google Scholar]
- 40.Govan AT, Macfarlane PS, Callander R. Pathology Illustrated. Vol. 2. New York, NY, USA: Churchill Levingstone; 1986. [Google Scholar]
- 41.Gupta RK, Kumar D, Chaudhary AK, Maithani M, Singh R. Antidiabetic activity of Passiflora incarnata Linn. in streptozotocin- induced diabetes in mice. Journal of Ethnopharmacology. 2012;139(3):801–806. doi: 10.1016/j.jep.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Yadav SK, Nagori BP, Kumar DP. Pharmacological characterization of different fractions of Calotropis procera (Asclepiadaceae) in streptozotocin induced experimental model of diabetic neuropathy. Journal of Ethnopharmacology. 2014;152(2):349–357. doi: 10.1016/j.jep.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Brahmachari G. Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. 2011. Six Bio-flavonoids with promising antidiabetic potentials: a critical survey; pp. 187–212. [Google Scholar]
- 44.Arif T, Sharma B, Gahlaut A, Kumar V, Dabur R. Anti- diabetic agents from medicinal plants: a review. Chemical Biological Letters. 2014;1(1):1–13. [Google Scholar]
- 45.Deutschländer MS, Lall N, van de Venter M, Hussein AA. Hypoglycemic evaluation of a new triterpene and other compounds isolated from Euclea undulata Thunb. var. myrtina (Ebenaceae) root bark. Journal of Ethnopharmacology. 2011;133(3):1091–1095. doi: 10.1016/j.jep.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 46.Sharma VJ, Shah UD. Antihyperglycemic activity of flavonoids from methanolic extract of aerial parts of Scoparia dulcis in streptozotocin induced diabetic rats. International Journal of ChemTech Research. 2010;2(1):214–218. [Google Scholar]
- 47.Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. Journal of Medicinal Plant Research. 2011;5(31):6697–6703. [Google Scholar]
- 48.Tan M-J, Ye J-M, Turner N, et al. Antidiabetic Activities of Triterpenoids Isolated from Bitter Melon Associated with Activation of the AMPK Pathway. Chemistry and Biology. 2008;15(3):263–273. doi: 10.1016/j.chembiol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Hussain SA, Marouf BH. Flavonoids as alternatives in treatment of type 2 diabetes mellitus. Academia Journal of Medicinal Plants. 2013;1(2):31–36. [Google Scholar]


