Abstract
A very high prevalence of microfilaremia of 42.68 per cent out of 164 canine blood samples examined was observed in Cherthala (of Alappuzha district of Kerala state), a known human Brugia malayi endemic area of south India. The species of canine microfilariae were identified as Dirofilaria repens, Brugia malayi, and Acanthocheilonema reconditum. D. repens was the most commonly detected species followed by B. pahangi. D. immitis was not detected in any of the samples examined. Based on molecular techniques, microfilariae with histochemical staining pattern of “local staining at anal pore and diffuse staining at central body” was identified as D. repens in addition to those showing acid phosphatase activity only at the anal pore. Even though B. malayi like acid phosphatase activity was observed in few dogs examined, they were identified as genetically closer to B. pahangi. Hence, the possibility of dogs acting as reservoirs of human B. malayi in this area was ruled out.
1. Introduction
Major filarial parasites of dogs are Acanthocheilonema reconditum, Acanthocheilonema dracunculoides, Brugia malayi, Brugia pahangi, Brugia ceylonensis, Brugia patei, Cercopithifilaria grassii, Dirofilaria immitis, and Dirofilaria repens [1–4]. Microfilariae of Brugia spp. are sheathed while those of others are not sheathed.
Canine filariosis was reported from various parts of India mainly from states like Kerala, Tamil Nadu, Karnataka, Orissa, West Bengal, Bihar, Uttar Pradesh, and Maharashtra. The species of microfilariae detected from these states include D. immitis from Kerala [5], C. grassi from Tamil Nadu [6], D. immitis from Himalayas [7], D. immitis and A. reconditum from West Bengal [8], D. immitis and D. repens from Orissa [9], D. repens from Kerala [10], D. repens and A. reconditum from Karnataka [11], A. reconditum, D. immitis, and D. repens from Maharashtra and New Delhi, and Microfilaria auquieri and a novel species of Acanthocheilonema from Ladakh, India [12]. In general, it is believed that D. immitis is mostly prevalent in north eastern India [13] while D. repens is confined to southern parts of the country [14, 15].
Currently, there is paucity of information on the prevalence of filarial worms of dogs in the genus Brugia from India. The disease caused in humans by subperiodic B. malayi, in Malaysia and Indonesia, is considered zoonotic due to the existence of animal reservoir hosts like cats and dogs [16]. Brugian filariosis is endemic in Alappuzha district of Kerala State [17–19] and thus the presence of B. malayi in dogs is always suspected. Even though B. malayi like microfilariae [20, 21] were detected from cats and dogs from India, they were not confirmed as B. malayi. The present study focuses on the detection and differentiation of microfilariae of domestic canines inhabiting a B. malayi endemic area of southern India (Alappuzha district of Kerala state) based on histochemical staining and molecular techniques.
2. Materials and Methods
2.1. Ethics Statement
All study procedures and protocols were approved by the institutional animal ethics committee of College of Veterinary and Animal Sciences, Pookode, Kerala (which follows committee for the purpose of control and supervision of experiments on animals (CPCSEA) guidelines, Ministry of Environment and Forest, Government of India). The committee did not deem it necessary for this research group to obtain formal approval to conduct this study. National Centre for Diseases Control, directly under Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India, is authorized to collect microfilaria positive human blood samples. Written informed consent was obtained from the human patients and owners of dogs before collection of samples.
2.2. Preliminary Screening and Collection of Samples
Samples were collected from dogs by collaborating veterinarians at “prophylactic antirabies vaccination and blood parasite detection camps” in the south Indian panchayats of Kerala state, namely, Kuthiathode (KUT), Kadakkarappally (KAD), and Pattanakkad (PKT). Brugian filariosis is endemic in this area [17–19]. Before sample collection data regarding the age, sex, and breed of these dogs were recorded.
Blood samples were collected from the saphenous vein. A drop of blood was examined by wet film technique at the camp site itself. Thick smears were prepared, fixed with methanol, stained using Giemsa, and examined for the presence of microfilariae with or without sheath. Whole nonheparinized (1 mL) and heparinized (1 mL) blood samples were also collected from the saphenous vein of all these dogs. A total of 164 blood samples of dogs (37 from Kadakkarappally, 57 from Kuthiathode, and 70 from Pattanakkad) were collected.
Blood sample of a known B. malayi infected human patient from Alappuzha, the endemic district of Kerala, was also collected to serve as positive control for histochemical staining and PCR techniques.
2.3. Histochemical Differentiation
The nonheparinized blood was allowed to clot and the serum fraction was centrifuged in an Eppendorf tube for 5 minutes at 1000 rpm. After discarding the supernatant, the sediment containing the microfilariae was resuspended in the remaining serum and a drop of this fluid was used for preparation of smears. They were air dried, fixed in absolute chilled acetone for 1 minute, further air dried, and stored in −20°C until used. These smears were used for histochemical staining within two weeks after preparation.
Histochemical staining was performed [22] to study the differences in the acid phosphatase enzyme activity in microfilariae for identification of the species [22–26]. Briefly, 20 mL of solution I (Michaelis Veronal Acetate Buffer, pH 10.0) was mixed with 50 mL of distilled water and 4 mL of solution II (0.05 g Naphthol AS-TR phosphate, sodium salt (Sigma) in 5 mL of N, N-dimethyl formamide) was added to it in a beaker. In another beaker, 3.2 mL each of solutions III (1.0 g Pararosaniline hydrochloride, 5.0 mL concentrated hydrochloric acid, and 20.0 mL distilled water) and IV (4 per cent sodium nitrite) was mixed and then added to the mixture in the first beaker. The pH of the mixture was adjusted to 5.0 with 0.1 N sodium hydroxide solution. The final solution was prepared fresh every time before the staining procedure.
The air dried smears were incubated in the substrate for 1 hour at 37°C, rinsed in distilled water, counterstained in solution V (mixture of 77.1 mL of 0.2 M sodium phosphate, 122.9 mL 0.1 M nitric acid, and 2 g methyl green) for 5 minutes, and rinsed in distilled water. The slides were then dehydrated in 95 per cent and absolute ethyl alcohol, respectively, rinsed in xylene, and mounted using DPX. The smears were examined for the precipitated red azo dye indicating acid phosphatase activity.
2.4. Polymerase Chain Reaction
DNA isolation from the heparinised whole blood samples of dogs and human was based on phenol-chloroform isoamyl alcohol (PCI) method [27]. The leukocyte DNA from a three-day-old pup served as negative control. For the Brugia specific PCR, the DNA isolated from a known B. malayi infected human patient's blood served as positive control. Canine blood samples with heavy (one microfilaria in every low power field) monoinfection with D. repens and A. reconditum (confirmed by histochemical staining) served as positive controls for these species. The reactions were carried out in a thermal cycler (Eppendorf, Germany); the products were visualized (Alpha Innotech, USA) after electrophoresis on a 1.5 per cent agarose gel and documented.
2.4.1. D. repens Specific PCR
PCR assay for the amplification of 246 bp direct tandem repeats of D. repens using specific primers [28] 5′-CCGGTAGACCATGGCATTAT-3′ (Forward) and 5′-CGGTCTTGGACGTTTGGTTA-3′ (Reverse) custom synthesized from IDT, USA, was standardized. The PCR amplification was performed in 25 μL reaction volume containing 2.5 μL 10x PCR buffer, 1 μL (0.25 mM) dNTP, and 30 pmol of each primer, 1.5 U Taq polymerase, and 5 μL of template DNA. Reaction conditions were as follows: after the initial denaturation at 94°C for 5 minutes, 40 cycles each with 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 25 seconds were run. A final extension at 72°C for 5 minutes was also given. One product of D. repens specific PCR was directly sent to Chromos Biotech, Bangalore, for sequencing.
2.4.2. A. reconditum Specific PCR
PCR assay for the amplification of 348 bp ITS2 fragment of A. reconditum using primers [29] 5′-CAGGTGATGGTTTGATGTGC-3′ (Forward) and 5′-CACTCGCACTGCTTCACTTC-3′ (Reverse) custom synthesized from IDT, USA, was standardized. The PCR amplification was performed in 25 μL reaction volume containing 2.5 μL 10x PCR buffer, 1 μL (0.25 mM) dNTP, and 0.3 mM of each primer, 2.5 U Taq polymerase, and 5 μL of template DNA. The PCR cycling consisted of a denaturation step at 94°C for 3 minutes and 30 cycles each with denaturing at 94°C for 3 minutes, annealing at 63°C for 1 minute, extension at 72°C for 30 seconds, and final extension at 72°C for 7 minutes. One product of A. reconditum specific PCR was directly sent to Chromos Biotech, Bangalore, for sequencing.
2.4.3. Brugia Specific PCR
PCR amplification of a 322 bp Hha1 fragment [30] using specific primers 5′-GCGCATAAATTCATCAGC-3′ (Forward) and 5′-GCGCAAAACTTAATTACAAAAGC-3′ (Reverse) constituted the genetic diagnostic criterion for Brugia infection in the study dogs. The PCR amplification was performed in 25 μL reaction volume containing 2.5 μL 10x PCR buffer, 1 μL (0.25 mM) dNTP, and 10 pmol of each primer, 2.5 U Taq polymerase, and 5 μL of template DNA. The PCR procedure consisted of an initial denaturation step at 94°C for 5 minutes and 35 cycles each of denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, extension at 72°C for 1 minute, and final extension at 72°C for 10 minutes. PCR products were cloned and sequenced.
2.4.4. Amplification of 5.8S-ITS2-28S
In order to confirm the identity of microfilariae with new acid phosphatase staining pattern, PCR assay for the amplification of 5.8S-ITS2-28S fragment of rDNA gene ofsuspected samplesusing specific primers [3], 5′-AGTGCGAATTGCAGACGCATTGAG-3′ (Forward), 5′-AGCGGGTAATCACGACTGAGTTGA-3′ (Reverse), was standardized. The PCR amplification was performed in 25 μL reaction volume containing 2.5 μL 10x PCR buffer, 1 μL (0.25 mM) dNTP, and 100 pmol of each primers, 1.5 U Taq polymerase, and 5 μL of template DNA. Reaction conditions were as follows: after the initial denaturation at 94°C for 2 minutes, 32 cycles each with 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds were run. A final extension at 72°C for 7 minutes was also given. PCR products were cloned and sequenced.
2.5. Cloning and Sequencing of PCR Products
PCR products were eluted from the gel using QIA quick gel extraction kit (QIAgen, Germany) based on manufacturer's protocol. The eluted product (8 μL) was used for cloning into a pTZ57R/T vector (InsTAclone PCR cloning kit, Fermentas, USA) which was used to transform the JM107 strain of E. coli (Fermentas, USA). The transformed cells were plated on an LB agar with ampicillin (50 μg/mL) as the selective antibiotic. After overnight incubation at 37°C, positive white colonies were selected and subcultured in LB broth overnight. The presence of the insert was confirmed by restriction digestion of the isolated plasmid from the subculture using EcoR1 and Sal I enzymes (GeneJET plasmid mini prep kit, Fermentas, USA). The stab culture of the colony containing the insert was sent for automated sequencing to Chromos Biotech, Bangalore. In order to verify mixed infection or recombination, 2 clones from each PCR products were sequenced.
2.6. Sequence Analysis
The sequence analysis was done using ClustalV method of MegAlign programme (DNASTAR, USA).
3. Results
3.1. Preliminary Screening
A total of 164 blood samples of dogs were collected from Kadakkarappally (37), Kuthiathode (57), and Pattanakkad (70). Out of 164 dogs, 114 were from nondescript animals while 50 were from exotic breeds (Spitz, German shepherd, Dachshund, and Doberman).
Wet film examination revealed microfilariae in 11 (29.72 per cent) samples from Kadakkarappally, 23 (40.35 per cent) from Kuthiathode and 17 (24.29 per cent) from Pattanakkad (Table 1). The overall prevalence of microfilaremia based on wet film examination was 31.17 per cent.
Table 1.
Prevalence of canine microfilariae in a human B. malayi endemic area of Kerala, India, based on wet film examination, Giemsa staining, histochemical staining, and polymerase chain reaction.
| Place | Samples examined | W. f | G | Histochemical reaction/acid phosphatase reaction | Polymerase chain reaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D. repens | A. reconditum | B. pahangi | New reaction |
B. malayi
like |
D. repens | A. reconditum | Brugia sp. (Hha1) | ||||
| Kadakkarappally | 37 | 11 | 16 | 11 | 0 | 3 | 11 | 3 | 10 | 0 | 6 |
| Kuthiathode | 57 | 23 | 29 | 18 | 2 | 3 | 28 | 0 | 28 | 2 | 7 |
| Pattanakkad | 70 | 17 | 25 | 16 | 0 | 12 | 19 | 3 | 26 | 0 | 10 |
|
| |||||||||||
| Total | 164 | 51 | 70 | 45 | 2 | 18 | 58 | 6 | 64 | 2 | 23 |
W. f: wet film examination; G: Giemsa staining.
Giemsa staining technique revealed microfilariae in 16 (43.24 per cent) samples from Kadakkarappally, 29 (50.87 per cent) from Kuthiathode, and 25 (35.71 per cent) from Pattanakkad. Overall, the prevalence of microfilaremia based on Giemsa staining was 42.68 per cent. Four dogs revealed microfilariae with sheath.
Fifty two per cent of exotic breeds and 38 per cent of nondescript breeds of dogs harboured microfilariae. Prevalence of microfilaraemia was more common in dogs above two years of age. Also, male dogs exhibited higher prevalence (Table 2).
Table 2.
Microfilaraemia in different breeds, age groups, and sexes of dogs in a B. malayi endemic area of Kerala, India, based on Giemsa staining technique.
| Blood smears | Area | Breed | Age group | Sex | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ND | Exotic | ||||||||||
| Spitz | G.S.D | Dach | Dob | <2 years | >2 years | Male | Female | ||||
| Examined |
Kadakkarappally | 29 | 6 | 2 | 0 | 0 | 14 | 23 | 33 | 4 | 37 |
| Kuthiathode | 36 | 10 | 5 | 4 | 2 | 13 | 44 | 39 | 18 | 57 | |
| Pattanakkad | 49 | 17 | 2 | 1 | 1 | 31 | 39 | 56 | 14 | 70 | |
| Total | 114 | 33 | 9 | 5 | 3 | 58 | 106 | 128 | 36 | 164 | |
| Positive | Kadakkarappally | 11 | 3 | 2 | 0 | 0 | 4 | 12 | 16 | 0 | 16 (43.24%) |
| Kuthiathode | 16 | 6 | 2 | 3 | 2 | 4 | 25 | 24 | 5 | 29 (50.87%) | |
| Pattanakkad | 17 | 6 | 1 | 0 | 1 | 3 | 22 | 20 | 5 | 25 (35.71%) | |
| Total | 44 (38.6%) | 15 (45.46%) | 5 (55.56%) | 3 (60%) | 3 (100%) | 11 (18.97%) | 59 (55.66%) | 60 (46.88%) | 10 (27.78%) | 70 (42.68%) | |
ND: Nondescript, G.S.D: German shepherd, Dach: Dachshund, Dob: Doberman.
3.2. Histochemical Differentiation
The D. repens microfilariae showed single locus of intense acid phosphatase staining in the region of the anal pore (Figure 1). This type of reaction was observed in 27.4 per cent of dogs.
Figure 1.
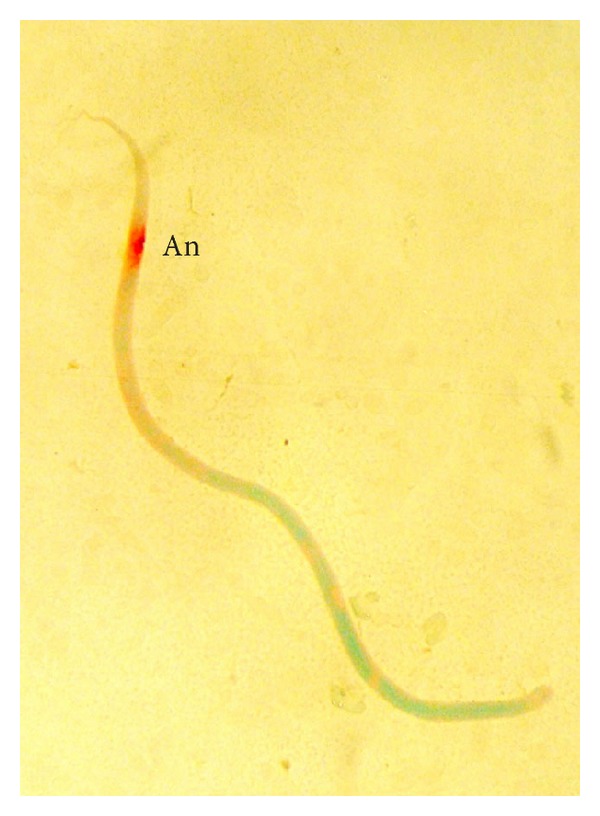
D. repens microfilaria showing acid phosphatase activity at the anal pore (An).
The entire body, especially between the excretory and anal pores, was stained bright red in microfilariae of A. reconditum (Figure 2). Only two out of 164 smears examined revealed this type of reaction.
Figure 2.
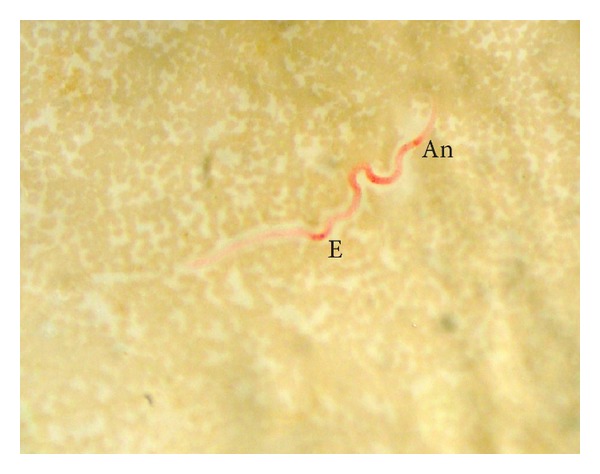
A. reconditum microfilaria showing acid phosphatase activity throughout the entire body, especially between the excretory (E) and anal pores (An).
For B. pahangi microfilariae, the heavy and diffuse acid phosphatase activity was observed along the entire body length, although the excretory and anal pores were still recognizable (Figure 3). A total of 18 smears (11 per cent) showed this type of reaction.
Figure 3.
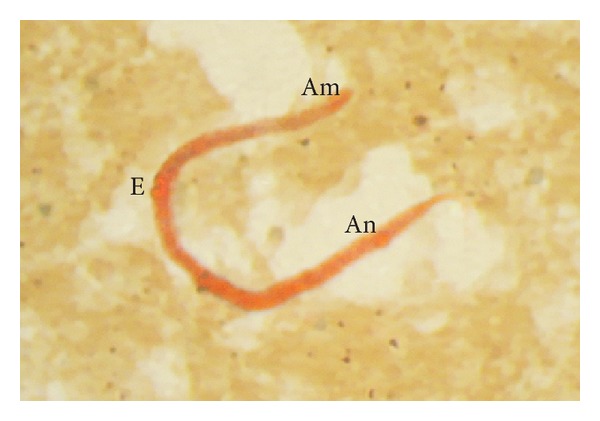
B. pahangi microfilaria showing heavy and diffuse acid phosphatase activity along the entire body length. The excretory (E) and anal pores (An) are recognizable.
The acid phosphatase staining pattern, with local staining at the anal pore and diffuse staining in the central body (Figure 4), was observed in 58 cases (35.4 per cent). Most of the samples contained a mixture of D. repens and this type of microfilariae.
Figure 4.
Microfilaria showing acid phosphatase activity with red spot at anal pore (An) region and diffuse red staining at the central body.
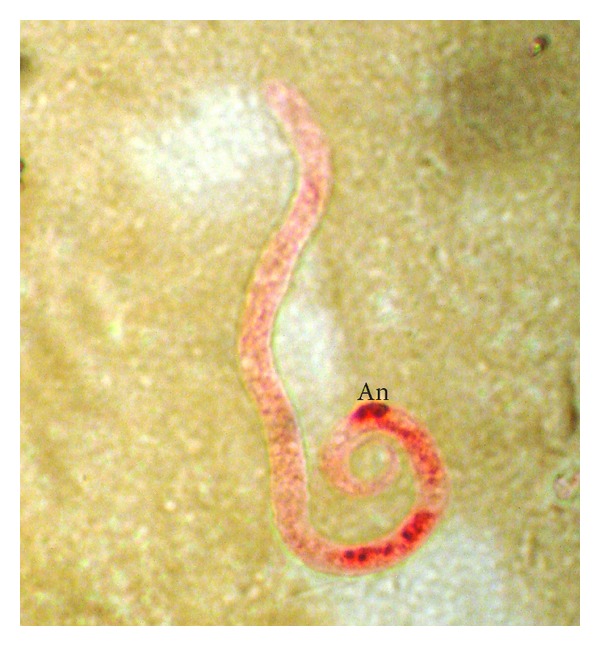
Blood smears prepared from human patients with confirmed B. malayi infection contained microfilariae with loci of acid phosphatase activity in the areas of the amphids, the excretory pore, the anal pore, and the phasmids (Figure 5). Out of 164 dogs tested, 6 harboured microfilariae with histochemical reaction similar to B. malayi (Figure 6).
Figure 5.
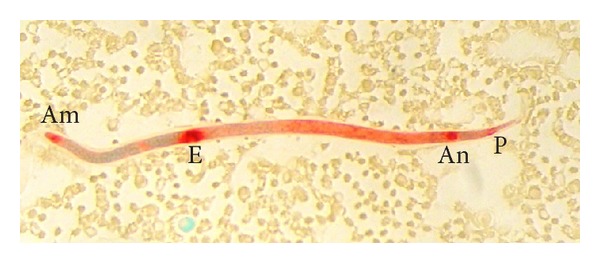
Human B. malayi microfilaria showing acid phosphatase activity in the areas of amphid (Am), excretory pore (E), anal pore (An), and phasmid (P).
Figure 6.
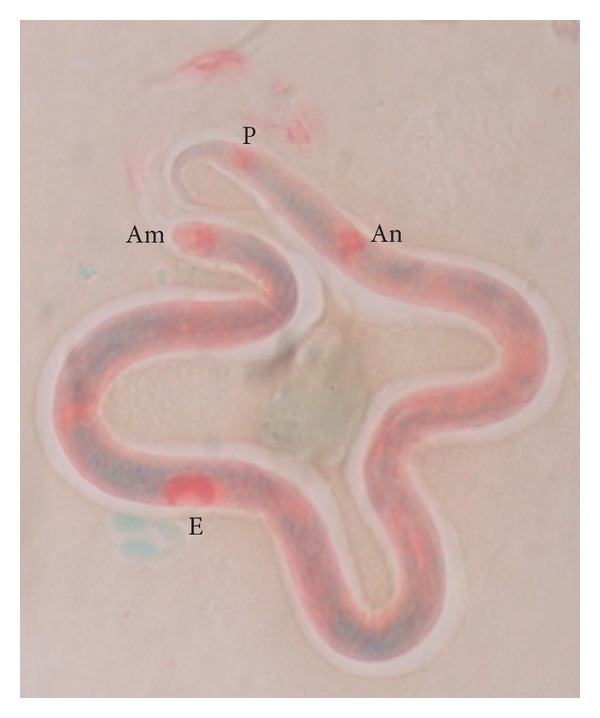
Canine B. malayi like microfilaria showing acid phosphatase activity in the areas of amphid (Am), excretory pore (E), anal pore (An), and phasmid (P).
3.3. Polymerase Chain Reaction
3.3.1. D. repens
PCR detected the 246 bp D. repens-specific product in 64 out of 164 samples. PCR product from a sample that contained microfilariae showing a single locus of staining in the region of the anal pore was sequenced for confirmation (accession number JN830762).
3.3.2. A. reconditum
PCR with primers specific for A. reconditum amplified the expected 348 bp product from two samples containing microfilariae with histochemical reactions typical of A. reconditum. The partial sequence of the PCR product was submitted to GenBank (accession number JQ039745).
3.3.3. Brugia Species
PCR with primers specific for Brugia sp. amplified the expected 322 bp product from 23 (14 per cent) samples.
One Hha1 PCR product each from samples showing only B. pahangi (PKT 94) (accession number JN601135), human B. malayi (accession number JN413104), and B. malayi like parasite of dog (two clones from KAD 19) (accession numbers JN601136 and JN601137) were cloned and sequenced. The phylogenetic tree (Figure 7) and distance matrix (Figure 8) plotted based on ClustalV method of MegAlign programme (DNASTAR, USA) demonstrated that the microfilariae with histochemical reaction similar to B. malayi were genetically closer to B. pahangi of dogs.
Figure 7.
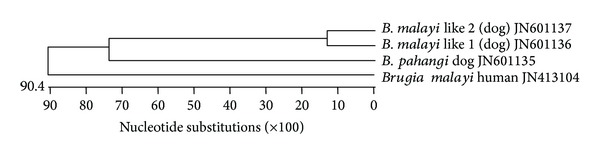
Phylogenetic tree constructed based on Hha1 sequence of different Brugia species.
Figure 8.
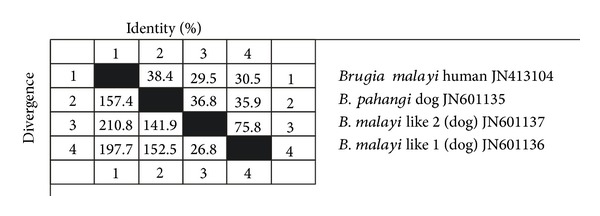
Distance matrix based on Hha1 sequence of different Brugia species.
3.3.4. Amplification of 5.8S-ITS2-28S
For confirmation of the species of microfilariae with the acid phosphatase staining reaction pattern “local staining at the anal pore and diffuse staining in the central body,” sequence analysis of PCR product of 5.8S-ITS2-28S fragment of rDNA gene was resorted to. The 584 bp fragment was sent for sequencing. When submitted to NCBI-BLAST, the 492/498 bp stretches of the 584 bp product (accession numbers JQ039743 and JQ039744) shared 97 per cent identity with the previously reported D. repens sequence AY 693808.
4. Discussion
Filarial infections in dogs can be diagnosed through morphological observations of the circulating microfilariae, detection of circulating antigens, histochemical staining of circulating microfilariae, or through molecular approaches. Proper identification of circulating microfilariae based on morphology requires the involvement of a well-trained parasitologist. Filarial infection with multiple species and morphological alterations of microfilariae due to incorrect preventive treatment of dogs are not easily differentiated morphologically even by trained persons [31].
Histochemical staining to detect acid phosphatase activity can overcome most of these problems; however, this technique requires fresh samples to yield optimal results. Besides being time consuming and labour intensive, both staining methods require expertise to identify and confirm the species [32]. Molecular methods based on species specific PCRs are simple and easy to perform. Use of properly designed primers and control template DNAs can make PCR assays species specific. PCR assays are very sensitive due to the exponential amplification of target DNA. Additionally, it may be possible with a PCR to detect circulating DNA liberated from host destroyed microfilariae or from adult worms [30].
Microscopical examination of Giemsa stained blood smears were used previously for detection of the presence of microfilaremia in dogs of Thrissur, Kerala. The prevalence varied from 7 to 26.5 per cent [10, 12, 33]. In the present study, using the same technique, the overall prevalence of microfilaremia in dogs was 42.68 per cent and the highest prevalence (50.87 per cent) was observed in Kuthiathode. Only two samples revealed sheathed microfilariae. Other techniques (histochemical staining and PCR) employed in the study detected more number of dogs positive for microfilariae especially Brugia species.
Even though the most pathogenic canine filaria, D. immitis, was reported previously from the Kerala state [5], it was not identified in any of the dogs tested in the present study. Unambiguous identification of D. repens and A. reconditum in dogs from the study population by histochemical staining and sequencing of PCR products was possible in this study.
Recently, A. reconditum (9.3 per cent) was identified as the most common species in North India [12] followed by D. repens (6.7 per cent) and D. immitis (1.5 per cent). In the present study, the microfilariae with a new histochemical staining pattern (local staining at the anal pore and diffuse staining at the central body) were more common. Based on 97 per cent identity of 5.8S-ITS2-28S region of rDNA gene [3] of this form of microfilariae with that of D. repens, the species was confirmed as D. repens. Therefore, D. repens is identified as the most common species of filarial worm in the study area. However, 97 per cent identity may also indicate an interspecific difference and therefore the possibility of a new species could not be ruled out.
Another important finding of the study was the presence of Brugia species in the dogs of the area. The most prevalent Brugia species identified in the study area was B. pahangi. Out of 164 dogs examined, 18 (11 per cent) harboured microfilariae with acid phosphatase staining pattern specific to that parasite. Histochemical staining pattern similar to B. malayi was observed in 6 out of 164 dogs examined. PCR specific for Brugia sp. (Hha1) revealed the diagnostic 322 bp product in 23 samples that included B. malayi like microfilariae and B. pahangi as indicated by histochemical staining. Hha1 PCR products amplified in samples of dogs showing only either of these parasites based on histochemical staining were selected for sequencing. Similarly, the Hha1 fragment of the B. malayi (human) was also sequenced. Phylogenetic analysis of the sequences revealed that the suspected B. malayi like parasite was genetically closer to B. pahangi (dogs) than to B. malayi (human). Therefore, the results of the present study did not indicate potential of dogs acting as reservoirs of B. malayi.
However, a recent study conducted at Thrissur, Kerala, (100 km away from the study area) revealed that out of 100 dogs with symptoms of filariosis (fever, anorexia, conjunctivitis, oedema of limb, and scrotum) circulating microfilariae were detected in 80 cases, of which all the 16 cases with sheathed microfilariae were identified as B. malayi based on histochemical staining and PCR [20]. The primers specific for amplification of 294 bp trans-spliced exon 1 (SLX) region (5S r RNA) of Brugia species [16] followed by sequence analysis (90 bases with query coverage of 32 per cent) was used for confirming the identification of B. malayi in dogs. The authors concluded that the high prevalence of B. malayi in Thrissur emphasized the possible role of dogs in the transmission of human filariosis. The results of the present study are contrary to the above report.
Lymphatic filariosis is one of the major diseases of mankind in tropical and subtropical countries and the global burden of this type of disease is 119.1 million cases [34] of which 12.9 million are affected by Brugian filariosis. In India, B. malayi was reported from a few foci only, the largest single tract being Travancore-Cochin state (present study area) [17]. B. malayi is known to occur in two forms, periodic and subperiodic. The disease caused by subperiodic B. malayi, in Malaysia and Indonesia, is considered zoonotic due to the existence of animal reservoir hosts like cats and dogs [16, 35]. Previously, two out of 57 cats examined in Orissa state, India, were found infected with B. malayi like microfilariae [21].
Other epidemiological factors also should be considered before confirming the absence of reservoir status in dogs for B. malayi in Cherthala. The zoonotic subperiodic B. malayi is transmitted in nature by Mansonia bonnae while M. dives, M. annulata, and M. uniformis are efficient laboratory vectors. The Indian situation is different. Microfilariae of B. malayi in India are purely nocturnal in their periodicity [21]. In Cherthala “Taluk” (an administrative subdivision of a district) of Alappuzha district of Kerala, Brugian filariosis is transmitted by M. annulifera, M. uniformis, and M. Indiana [17–19]. The efficient vector (M. bonnae) for the transmission of zoonotic Brugian filariosis is not reported from this area. Also, a 90.7 per cent decline in the trend of disease rate for human filarial infections in Cherthala Taluk was also reported [36]. If animal reservoirs like dogs were there in existence, there would have been further increase in the number of outbreaks of the disease because of the availability of reservoirs (including stray dog population) and absence of microfilaricidal therapy in them. Also, when B. malayi could not be detected from Cherthala, the endemic hotspot, it is difficult to detect the parasite from a place which is 100 km away from the endemic area. So, the possibility for dogs acting as reservoirs of B. malayi in these areas is very remote. In addition, the clinical symptoms reported in dogs of Thrissur, Kerala [20], were similar to that of dogs infected with B. pahangi [37] which include varying levels of microfilaraemia, episodic lymphadenopathy, lymphangitis, and limb oedema. For these reasons, the detection of B. malayi in dogs of Thrissur might be a false detection.
The microfilariae of three species, namely, B. tupiae (reported previously from Malaysia, Thailand, and Vietnam), B. ceylonensis, and B. pahangi, are liable to be confused with those of B. malayi [38]. B. ceylonensis was first described in lymphatics of dogs in 1962 from Sri Lanka [39]. This parasite, which is transmitted by Aedes aegypti, was reported from Kerala too as early in 1974, but there is no recent documentation of this parasite from the state. By contrast, B. ceylonensis was recently reported from the conjunctiva of a human patient in Sri Lanka [40]. Also, a Sri Lankan survey of 65 dogs revealed 7 per cent prevalence of single infection with B. ceylonensis [41]. The present study could not differentiate the B. malayi like microfilariae of dogs from B. ceylonensis due to the absence of reports on the typical histochemical staining reaction of B. ceylonensis microfilariae or gene accessions. The B. malayi like microfilariae observed in dogs of the study area could be a new Brugia species, B. ceylonensis, or a genetic variant of B. pahangi.
5. Conclusion
Microfilariae of D. repens, A. reconditum, and B. pahangi occur in dogs of Cherthala Taluk, Alappuzha district, Kerala, the human B. malayi endemic area of south India. Sequence analysis of 5.8S-ITS2-28S fragment of rDNA gene of microfilariae with the acid phosphatase staining pattern of local staining at the anal pore and diffuse staining in the central body revealed 97 per cent homology with D. repens. The B. malayi like microfilariae observed in dogs of the study area could be a new Brugia species, B. ceylonensis, or a genetic variant of B. pahangi.
Acknowledgments
Financial support from Kerala State Council for Science, Technology and Environment through the research project entitled “Investigation on the role of dogs in the transmission of Brugian filarial infections to humans and molecular epidemiology of filarial infections (020/SRSAGR/2006/CSTE)” is thankfully acknowledged. The help rendered by veterinarians working in Alapuzha district especially Dr. Rani Bharathan, Dr. Sangeeth Narayanan, and Dr. Jayachandra Kamath is also thankfully acknowledged.
Abbreviations
- DNA:
Deoxyribonucleic acid
- PCR:
Polymerase chain reaction
- rDNA:
Ribosomal DNA
- DPX:
Distyrene, plasticiser (dibutyl phthalate), and xylene.
Conflict of Interests
The authors declare that there is no professional or financial conflict of interests related to this paper.
References
- 1.Irwin PJ. Companion animal parasitology: a clinical perspective. International Journal for Parasitology. 2002;32(5):581–593. doi: 10.1016/s0020-7519(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JD. Current Veterinary Therapy VII. Philadelphia, Pa, USA: W. B. Saunders; 1979. Canine heart worm disease; pp. 326–335. [Google Scholar]
- 3.Rishniw M, Barr SC, Simpson KW, Frongillo MF, Franz M, Dominguez Alpizar JL. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Veterinary Parasitology. 2006;135(3-4):303–314. doi: 10.1016/j.vetpar.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Simón F, Kramer LH, Román A, et al. Immunopathology of Dirofilaria immitis infection. Veterinary Research Communications. 2007;31(2):161–171. doi: 10.1007/s11259-006-3387-0. [DOI] [PubMed] [Google Scholar]
- 5.Valsala KV, Bhaskaran R. Dirofilariosis in dogs. Kerala Journal of Veterinary Science. 1974;5:74–77. [Google Scholar]
- 6.Balasubramaniam G, Anandan R, Alwar VS. On the occurrence of Dipetalonema grassi (Noe, 1907) from dogs in India. Indian Veterinary Journal. 1975;52:513–516. [Google Scholar]
- 7.Sarkar P, Barak DK, Bhattarchajee HM. Pathology of Dirofilaria immitis infection in dogs. Indian Veterinary Journal. 1976;53:55–57. [Google Scholar]
- 8.Chakrabarthi A, Choudhury MN. Studies on canine filariasis in West Bengal. Indian Journal of Animal Health. 1983;22:151–155. [Google Scholar]
- 9.Patnaik MM. On filarial nematodes in domestic animals in Orissa. Indian Veterinary Journal. 1989;66:573–574. [Google Scholar]
- 10.Radhika R. Prevalence, clinical pathology and treatment of microfilariasis in dogs [M.S. thesis] Thrissur, India: Kerala Agricultural University; 1997. [Google Scholar]
- 11.Ananda KJ. Studies on microfilariosis in dogs of Mangalore and Bangalore regions in Karnataka [M.S. thesis] Bangalore, India: University of Agricultural Sciences; 2003. [Google Scholar]
- 12.Megat Abd Rani PA, Irwin PJ, Gatne M, Coleman GT, McInnes LM, Traub RJ. A survey of canine filarial diseases of veterinary and public health significance in India. Parasites & Vectors. 2010;3(1):p. 30. doi: 10.1186/1756-3305-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bortharkur SK, Sarmah K, Rajakhowa TK, Das MR, Rahman S. Dirofilaria immitis infection in a dog. Journal of Veterinary Parasitology. 2006;20:167–169. [Google Scholar]
- 14.Ananda KJ, D'Souza PE, Jaganath MS. Methods for identification of microfilaria of Dirofilaria repens and Dipetalonema reconditum . Journal of Veterinary Parasitology. 2006;20(1):45–47. [Google Scholar]
- 15.Sabu L, Devada K, Subramanian H. Dirofilariosis in dogs and humans in Kerala. Indian Journal of Medical Research. 2005;121(5):691–693. [PubMed] [Google Scholar]
- 16.Chansiri K, Tejangkura T, Kwaosak P, Sarataphan N, Phantana S, Sukhumsirichart W. PCR based method for identification of zoonostic Brugia malayi microfilariae in domestic cats. Molecular and Cellular Probes. 2002;16(2):129–135. doi: 10.1006/mcpr.2001.0402. [DOI] [PubMed] [Google Scholar]
- 17.Iyengar MOT. Studies on the epidemiology of filariasis in Travancore. Indian Medical Research Memoirs. 1938;30:1–179. [Google Scholar]
- 18.Rajagopalan PK, Panicker KN, Pani SP. Impact of 50 years of vector control on the prevalence of Brugia malayi in Shertallai area of Kerala state. Indian Journal of Medical Research A:Infectious Diseases. 1989;89:418–425. [PubMed] [Google Scholar]
- 19.Sabesan S, Kumar NP, Krishnamoorthy K, Panicker KN. Seasonal abundance and biting behaviour of Mansonia annulifera, M. uniformis and M. indiana and their relative role in the transmission of malayan filariasis in Shertallai (Kerala state) Indian Journal of Medical Research A: Infectious Diseases. 1991;93:253–258. [PubMed] [Google Scholar]
- 20.Ambily VR, Pillai UN, Arun R, Pramod S, Jayakumar KM. Detection of human filarial parasite Brugia malayi in dogs by histochemical staining and molecular techniques. Veterinary Parasitology. 2011;181(2–4):210–214. doi: 10.1016/j.vetpar.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 21.Edeson JFB. The epidemiology and treatment of infection due to Brugia malayi . Bulletin of World Health Organization. 1962;27:529–541. [PMC free article] [PubMed] [Google Scholar]
- 22.Chalifoux L, Hunt RD. Histochemical differentiation of Dirofilaria immitis and Dipetalonema reconditum . Journal of the American Veterinary Medical Association. 1971;158(5):601–605. [PubMed] [Google Scholar]
- 23.Acevedo RA, Theis JH, Kraus JF, Longhurst WM. Combination of filtration and histochemical stain for detection and differentiation of Dirofilaria immitis and Dipetalonema reconditum in the dog. American Journal of Veterinary Research. 1981;42(3):537–540. [PubMed] [Google Scholar]
- 24.Lee SE, Song KH, Liu J, et al. Comparison of the acid-phosphatase staining and polymerase chain reaction for detection of Dirofilaria repens infection in dogs in Korea. Journal of Veterinary Medical Science. 2004;66(9):1087–1089. doi: 10.1292/jvms.66.1087. [DOI] [PubMed] [Google Scholar]
- 25.Ortega-Mora LM, Gomez-Bautista M, Rojo-Vazquez FA. The acid phosphatase activity and morphological characteristics of Dipetalonema dracunculoides (cobbold, 1870) microfilariae. Veterinary Parasitology. 1989;33(2):187–190. doi: 10.1016/0304-4017(89)90066-6. [DOI] [PubMed] [Google Scholar]
- 26.Redington BC, Montgomery CA, Jervis HR, Hockmeyer WT. Histochemical differentiation of the microfilariae of Brugia pahangi and sub periodic Brugia malayi . Annals of Tropical Medicine and Parasitology. 1975;69(4):489–492. [Google Scholar]
- 27.Sambrook J, Russell DW. A Laboratory Manual. 3rd edition. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 2001. Molecular cloning; pp. 6.1–6.12. [Google Scholar]
- 28.Vakalis N, Spanakos G, Patsoula E, Vamvakopoulos NC. Improved detection of Dirofilaria repens DNA by direct polymerase chain reaction. Parasitology International. 1999;48(2):145–150. doi: 10.1016/s1383-5769(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 29.Mar PH, Yang I, Chang GN, Fei ACY. Specific polymerase chain reaction for differential diagnosis of Dirofilaria immitis and Dipetalonema reconditum using primers derived from internal transcribed spacer region 2 (ITS2) Veterinary Parasitology. 2002;106(3):243–252. doi: 10.1016/s0304-4017(02)00032-8. [DOI] [PubMed] [Google Scholar]
- 30.Lizotte MR, Supall T, Partono F, Williams SA. A polymerase chain reaction assay for the detection of Brugia malayi in blood. American Journal of Tropical Medicine and Hygiene. 1994;51(3):314–321. doi: 10.4269/ajtmh.1994.51.314. [DOI] [PubMed] [Google Scholar]
- 31.Casiraghi M, Bazzocchi C, Mortarino M, Ottina E, Genchi C. A simple molecular method for discriminating common filarial nematodes of dogs (Canis familiaris) Veterinary Parasitology. 2006;141(3-4):368–372. doi: 10.1016/j.vetpar.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Nuchprayoon S, Junpee A, Poovorawan Y, Scott AL. Detection and differentiation of filarial parasites by universal primers and polymerase chain reaction-restriction fragment length polymorphism analysis. American Journal of Tropical Medicine and Hygiene. 2005;73(5):895–900. [PubMed] [Google Scholar]
- 33.Saseendranath MR, Varghese CG, Jayakumar KM. ‘Incidence of canine dirofilariosis’ in Trichur, Kerala. Indian Journal of Veterinary Medicine. 1986;6:p. 139. [Google Scholar]
- 34.World Health Organization. Initial Assessment, Monitoring and Certification. Vol. 99. Atlanta, Ga, USA: WHO; 1998. Epidemiologic approaches to lymphatic filariasis elimination; pp. 1–35. [Google Scholar]
- 35.Laing ABC, Edeson JFB, Wharton RH. Studies on filariasis in Malaya: further experiments on the transmission of Brugia malayi and Wuchereria bancrofti . Annals of Tropical Medicine and Parasitology. 1961;55:86–92. doi: 10.1080/00034983.1961.11686022. [DOI] [PubMed] [Google Scholar]
- 36.Regu K, Rajendran R, Ali MKS, Koya SM, Dhariwal AC, Lal S. Decline of Brugian filariasis in Cherthala taluk, Alappuzha district, Kerala. Journal of Communicable Diseases. 2005;37(3):209–218. [PubMed] [Google Scholar]
- 37.Snowden KF, Hammerberg B. The lymphatic pathology of chronic Brugia pahangi infection in the dog. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1989;83(5):670–678. doi: 10.1016/0035-9203(89)90394-5. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. WHO Technical Report Series. 821. Geneva, Switzerland: WHO; 1992. Lymphatic filariasis, the disease and its control. [PubMed] [Google Scholar]
- 39.Jayewardene LG. On two filarial parasites from dogs in Ceylon, Brugia ceylonensis n. sp. and Dipetalonema sp.inq. Journal of Helminthology. 1962;36:269–280. doi: 10.1017/s0022149x00023944. [DOI] [PubMed] [Google Scholar]
- 40.Dissanaike AS, Jayaweera Bandara CD, Padmini HH, Ihalamulla RL, Naotunne TDS. Recovery of a species of Brugia, probably B. ceylonensis, from the conjunctiva of a patient in Sri Lanka. Annals of Tropical Medicine and Parasitology. 2000;94(1):83–86. [PubMed] [Google Scholar]
- 41.Rajapakshe R, Perera WSR, Ihalamulla RL, et al. Study of dirofilariasis in a selected area in the Western Province. Ceylon Medical Journal. 2005;50(2):58–61. doi: 10.4038/cmj.v50i2.1570. [DOI] [PubMed] [Google Scholar]


