Zebrafish have four tubulin deglutamylases: Ccp1, Ccp2, Ccp5, and Ccp6. Except for ccp1, all deglutamylase genes are expressed during ciliogenesis in zebrafish. Only loss of ccp5 induces cilia hyperglutamylation and the complete spectrum of ciliopathy phenotype. ccp5 knockdown can bypass Fleer/Ift70 or Ift88 deficiency in zebrafish to form multicilia.
Abstract
Glutamylation is a functionally important tubulin posttranslational modification enriched on stable microtubules of neuronal axons, mitotic spindles, centrioles, and cilia. In vertebrates, balanced activities of tubulin glutamyl ligase and cytoplasmic carboxypeptidase deglutamylase enzymes maintain organelle- and cell type–specific tubulin glutamylation patterns. Tubulin glutamylation in cilia is regulated via restricted subcellular localization or expression of tubulin glutamyl ligases (ttlls) and nonenzymatic proteins, including the zebrafish TPR repeat protein Fleer/Ift70. Here we analyze the expression patterns of ccp deglutamylase genes during zebrafish development and the effects of ccp gene knockdown on cilia formation, morphology, and tubulin glutamylation. The deglutamylases ccp2, ccp5, and ccp6 are expressed in ciliated cells, whereas ccp1 expression is restricted to the nervous system. Only ccp5 knockdown increases cilia tubulin glutamylation, induces ciliopathy phenotypes, including axis curvature, hydrocephalus, and pronephric cysts, and disrupts multicilia motility, suggesting that Ccp5 is the principal tubulin deglutamylase that maintains functional levels of cilia tubulin glutamylation. The ability of ccp5 knockdown to restore cilia tubulin glutamylation in fleer/ift70 mutants and rescue pronephric multicilia formation in both fleer- and ift88-deficient zebrafish indicates that tubulin glutamylation is a key driver of ciliogenesis.
INTRODUCTION
Glutamylation is an evolutionarily conserved posttranslational modification that occurs prominently on α- and β-tubulin associated with stable microtubules of mitotic spindles, neuronal axons, centrioles, and cilia (Edde et al., 1990). Functionally, tubulin glutamylation regulates cytoskeletal processes by modulating microtubule–protein interactions, such as those with neuronal kinesin motor KIF-1A during neurite extension (Ikegami et al., 2007) and with septins during polarized vesicle transport (Spiliotis et al., 2008). In addition, glutamylation stimulates the activity of the microtubule-severing protein spastin during microtubule disassembly (Lacroix et al., 2010) and modulates the activities of inner arm dyneins to regulate cilia motility (Kubo et al., 2010; Suryavanshi et al., 2010). Variations in tubulin glutamylation patterns contribute to the structural and functional heterogeneity of microtubules in distinct organelles of vertebrate organisms (Verhey and Gaertig, 2007). Cytoplasmic microtubules are not glutamylated (Bobinnec et al., 1998), whereas cilia transition zone microtubules are monoglutamylated, and neuronal and axonemal microtubules are polyglutamylated (Edde et al., 1991; Fouquet et al., 1994; Huitorel et al., 2002; Kann et al., 2003). Biochemically, glutamylation is a reversible process. Glutamate side chains are linked to genetically encoded glutamate(s) within C-termini of α- or β-tubulin by tubulin glutamyl ligase members of the tubulin tyrosine ligase–like (TTLL) protein family (Janke et al., 2005; van Dijk et al., 2007). Conversely, side chain glutamates are removed by tubulin deglutamylase members of the cytoplasmic carboxy peptidase (CCP) protein family (Rogowski et al., 2010). It is believed that the diversity of microtubule subtypes generated by tubulin posttranslational modifications is maintained by balanced activities of TTLL glutamylases and CCP deglutamylases; however, the functional repertoire of ttll and ccp genes in different organisms is not fully known.
The protein localization and expression patterns of vertebrate tubulin glutamyl ligases and deglutamylases suggest unique roles for different ttll or ccp gene family members (Pathak et al., 2011; Bosch Grau et al., 2013; Lyons et al., 2013). Green fluorescent protein (GFP) fusion proteins of TTLL1, TTLL9, and TTLL11 show preferential localization to cilia basal bodies, whereas those of TTLL5, TTLL6, and TTLL7 localize along ciliary axonemes (Janke et al., 2005; van Dijk et al., 2007). Functional studies reveal that TTLL1 is required for respiratory cilia and sperm motility (Ikegami et al., 2010; Vogel et al., 2010), whereas TTLL5 is exclusively required for sperm motility (Lee et al., 2013). Analysis of ttll mRNA expression in developing zebrafish and mouse brain ependymal cells suggests that ttll6 is uniquely important in ciliated cells, whereas ttll1, ttll4, and ttll7 may function in both ciliated cells and neurons (Pathak et al., 2011; Bosch Grau et al., 2013). ttll6 knockdown in zebrafish embryos or cultured ependymal cells primarily affects cilia motility (Pathak et al., 2011; Bosch Grau et al., 2013), whereas ttll1 is required for glutamylation of zebrafish secondary motor neurons (Pathak et al., 2011) and TTLL7 is required for growth of MAP2-positive neurites in PC12 cells (Ikegami et al., 2006).
Tubulin deglutamylases also play important roles in cilia and neurons. In Caenorhabditis elegans, GFP fusion proteins of the tubulin deglutamylases CCPP-1 and CCPP-6 localize to amphid cilia (Kimura et al., 2010; O'Hagan et al., 2011). Mutation in C. elegans ccpp-1 causes excessive accumulation of KLP-6 kinesin and polycystin-2 in cilia and also increases the transport rate of OSM-3/KIF17 on axonemal microtubules (Kimura et al., 2010; O'Hagan et al., 2011). Deficiency of the mouse CCP1 tubulin deglutamylase increases polyglutamylation of brain neurons in pcd mice, leading to their progressive degeneration (Fernandez-Gonzalez et al., 2002; Rogowski et al., 2010). In zebrafish, whole-embryo quantitative PCR studies show that ccp1, ccp2, ccp5, and ccp6 mRNA expression increases over the first 8 d of development; however, the tissue-specific expression of zebrafish ccp genes and how expression correlates with ccp gene function have not been fully explored (Lyons et al., 2013).
In addition to changes in glutamylase and deglutamylase activity, tubulin glutamylation can be altered by mutations in putative basal body and intraflagellar transport (IFT)–associated proteins. Mutation in human CEP41 results in loss of cilia, aberrant TTLL6 localization, and consequent reduction in axonemal tubulin glutamylation (Lee et al., 2012). Mutation in the zebrafish fleer/ift70 gene causes significant reduction in axonemal tubulin glutamylation and loss of multiciliated cell ciliogenesis (Pathak et al., 2007). Fleer/Ift70 is a tetratrico peptide repeat (TPR)–rich protein related to CeDYF-1/CrIft-70 and is a component of the IFT complex (Fan et al., 2010). It is not known whether fleer mutants lack tubulin glutamyl ligase activity or mislocalize Ttll enzymes, or, alternatively, whether the Fleer protein limits the activity of deglutamylases or restricts their access to the cilia compartment.
Here we focus on the expression and function of zebrafish ccp deglutamylases in normal and fleer- or ift88-deficient cilia. We find that expression of zebrafish ccp deglutamylase genes ccp2, ccp5, and ccp6 is strongly enriched in ciliated cell types, whereas ccp1 expression is restricted to the nervous system and somites. Despite overlapping expression patterns, we find that ccp5 is the major deglutamylase in zebrafish cilia and that ccp5 deficiency can restore cilia glutamylation in the fleer mutant and improve multiciliogenesis in fleer- and ift88-deficient zebrafish.
RESULTS
Expression of tubulin deglutamylases during zebrafish development
Tubulin deglutamylases are members of the M14 CCP protein family, alternatively known as ATP/GTP-binding protein–like family. Zebrafish harbor four orthologues of mouse deglutamylase genes: Ccp1, Ccp2, Ccp5, and Ccp6 (Supplemental Figure S3A; Lyons et al., 2013). A limited analysis of zebrafish ccp gene expression found evidence for specific expression of ccp1, ccp2, and ccp5 in the pronephros and olfactory placode (Lyons et al., 2013). We undertook a complete expression analysis of ccp1, ccp2, ccp5, and ccp6 and found that ccp1 expression was confined mainly to the developing CNS and somites at all developmental stages, whereas ccp2, ccp5, and ccp6 were highly expressed in multiple ciliated cell types, including Kupffer's vesicle (KV), nephrogenic mesoderm, the pronephros, otic placode, olfactory placode, and lateral line organs (Figure 1 and Supplemental Figure S1). ccp1 and ccp5 were maternally expressed in four- to eight-cell-stage embryos (Figure 1, A and C). At the 10-somite stage, ccp1 was expressed on the periphery of KV and in axial neurogenic cells (Figure 1, E and I), whereas ccp5 and ccp6 (Figure 1, G and H) but not ccp2 (Figure 1F) were expressed in KV epithelial cells. Expression of ccp2, ccp5, and ccp6 was also observed in the nephrogenic mesoderm at this stage and persisted until 54 h postfertilization (hpf) (Figure 1, J–L and N–P, and Supplemental Figure S1, F–H and J–L), whereas ccp1 expression continued to be restricted to brain and spinal cord (Figure 1, M and Q). At 54 hpf, ccp2, ccp5, and ccp6 were all expressed in the olfactory placode (Figure 1, R–T), while ccp6 was also expressed in the retina and otic placode (Figure 1T). Overall the expression patterns of ccp2, ccp5, and ccp6 show enrichment in ciliated cells, similar to the glutamyl ligase gene ttll6 and the glutamylation regulator fleer (Pathak et al., 2007, 2011).
FIGURE 1:
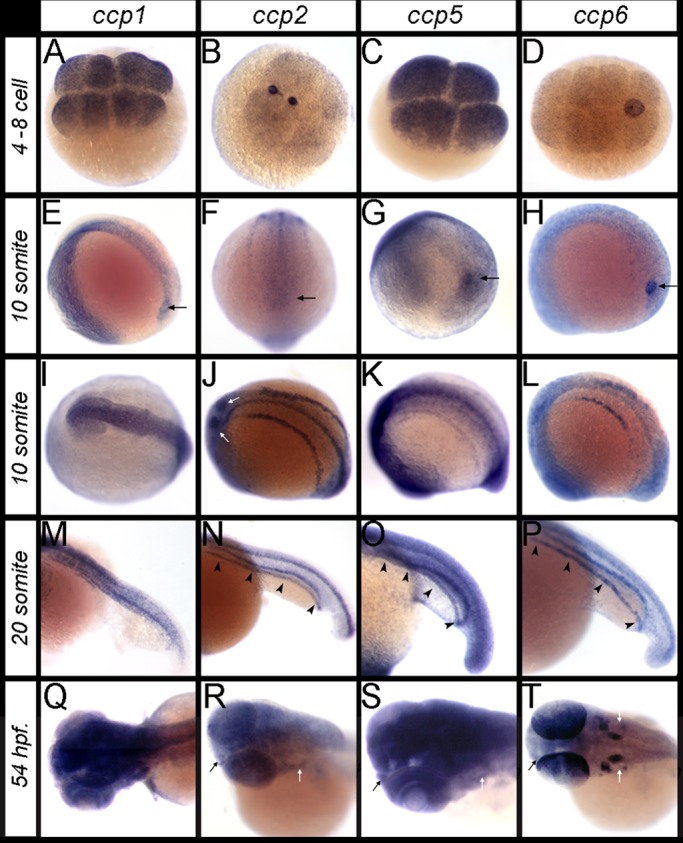
RNA in situ hybridization patterns of ccp1, ccp2, ccp5, and ccp6 during zebrafish development. (A–D) The four- to eight-cell-stage embryos, showing maternal transcripts of (A) ccp1, (B) ccp2, (C) ccp5, and (D) ccp6. (E–H) Lateral and caudal views of 10-somite embryos, showing expression of (E) ccp1, (F) ccp2, (G) ccp5, and (H) ccp6 relative to the Kupffer's vesicle (black arrows). (I–L) Dorsal views of 10-somite embryos, showing expression of (I) ccp1 in the CNS and (J) ccp2, (K) ccp5, and (L) ccp6 in the spinal canal and bilateral nephrogenic mesoderm. (M–P) Twenty-somite embryos, showing that (M) ccp1 is expressed medially in somites and spinal canal, whereas (N) ccp2, (O) ccp5, and (P) ccp6 expression is prominent medially in the spinal canal and bilaterally in the pronephroi (black arrowheads). (Q–T) Dorsal views of the head in 54-hpf larvae, showing (Q) ccp1, (R) ccp2, (S) ccp5, and (T) ccp6 are widely expressed in CNS, eye, otic placode (white arrows), and olfactory placode (black arrows). In T, note the distinct expression of ccp6 in otic placodes and posterior cell layers of the eye.
ccp5 knockdown induces ciliopathy phenotypes
To assess the function of ccp genes in ciliogenesis, we knocked down ccp1, ccp2, ccp5, and ccp6 using antisense morpholinos. Injections of splice-blocking morpholino oligos caused near-complete elimination of wild-type ccp gene mRNA (Figure 2A and Supplemental Figure S2A), resulting in truncated splice products predicted to encode proteins lacking carboxypeptidase domains (Supplemental Figure S3, B–F). Of these four genes, only ccp5 knockdown induced a typical spectrum of ciliopathy phenotypes, including axis curvature, pronephric cysts, and hydrocephalus (Figure 2B). Whereas isolated hydrocephalus was observed in ccp1 morphants (Figure 2, B and C), this was accounted for by widespread cell death in the CNS (Supplemental Figure S2B), similar to the CCP1-deficient mouse pcd mutant phenotype (Mullen et al., 1976; Rogowski et al., 2010), with subsequent obstruction of the spinal canal with cell debris (Supplemental Figure S2B). Although knockdown of ccp2 eliminated all wild-type ccp2 mRNA, no obvious embryonic phenotype was frequently observed (Supplemental Figure S2, A and B).
FIGURE 2:
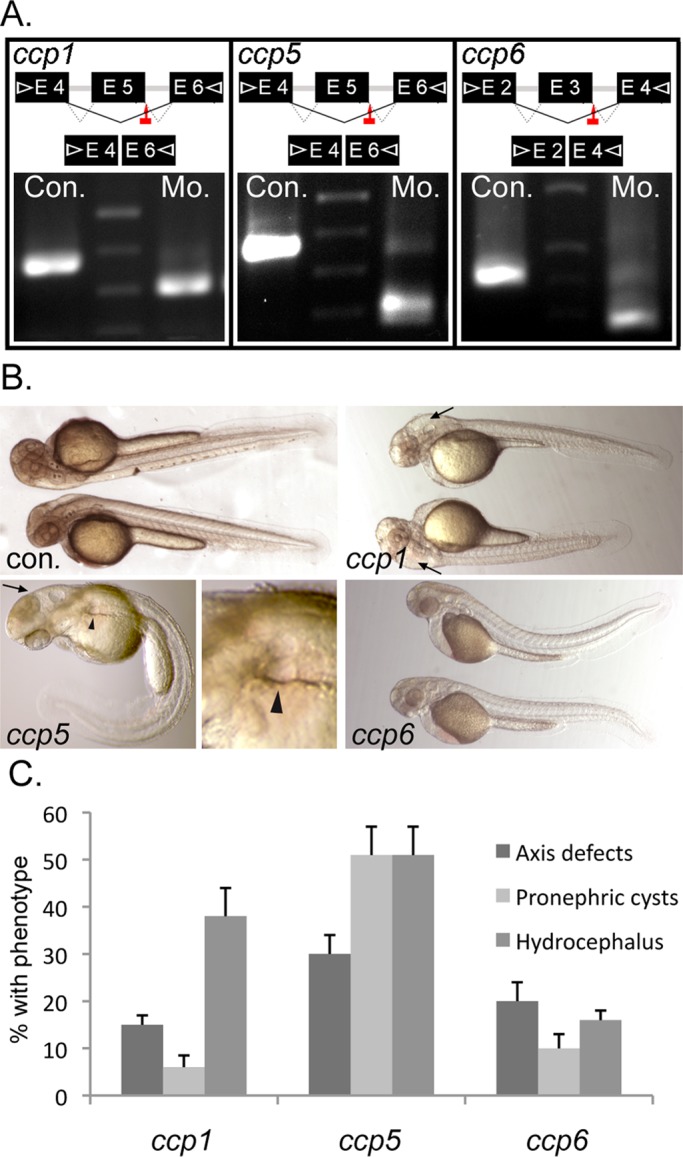
ccp5 knockdown alone induces the typical spectrum of ciliopathy phenotypes in wild-type zebrafish. (A) Splice donor sites targeted by antisense morpholinos (red arrows) in ccp1, ccp5, and ccp6 genes and agarose gel analysis showing RT-PCR amplicons of ccp1, ccp5, and ccp6 generated using primer pairs (arrowheads) in indicated exons were smaller in morphant (Mo.) embryos than in respective control (Con.) In each case, the reduced size of morphant amplicon relative to control matched the size of the deleted target exon. (B) External morphology of 2.5-d-old zebrafish injected with optimal dose of control or antisense morpholinos designed to knock down ccp1, ccp5, and ccp6 genes, respectively. (C) Frequency of phenotype associated with cilia defects. ccp1 morphants mostly exhibit isolated hydrocephalus, whereas ccp5 morphants exhibit hydrocephalus and pronephric cysts, often with acutely curved body axis. Replicates: ccp1×5Mo (n = 181 embryos/4 injections); ccp5×5Mo (n = 275 embryos/5 injections); ccp6×3Mo (n = 261embryos/5 injections).
ccp5 deficiency induces cilia microtubule hyperglutamylation and motility defects
To determine the cellular basis of ciliopathy phenotypes in ccp5-deficient embryos, we analyzed cilia in the pronephros and brain ventricles with respect to glutamylated tubulin content, length, and motility. Double immunolabeling of whole-mount embryos with monoclonal antibody (mAb) GT335 (recognizing monoglutamylated or polyglutamylated tubulin) and mAb 6-11B-1 (specific to acetylated tubulin) allowed direct assessment of cilia tubulin glutamylation in wild-type versus ccp5-deficient embryos (Figure 3). Tubulin glutamylation in control single cilia and multicilia extends along the length of the cilium (Figure 3, A and C, and Supplemental Figure S4, A and C; also see later discussions of Figures 5, A and C, and 6), decreasing from the base to the tip (Pathak et al., 2007). Relative to control cilia imaged under identical conditions, glutamylated tubulin immunoreactivity was significantly increased relative to acetylated tubulin in ccp5-deficient cilia (Figure 3, D–F, and later discussions of Figures 5, D–F, and 6), whereas it appeared unchanged in cilia of ccp1 (unpublished data) and ccp6 morphants (Figure 3, G–I). Measurements of the axonemal extent of immunolabeling of single pronephric cilia for glutamylated versus acetylated tubulin revealed a small but significant increase in the extent of tubulin glutamylation in ccp5-deficient cilia (Figure 3J), whereas overall cilia length (acetylated tubulin) was not significantly different. To quantify tubulin glutamylation in cilia, we acquired multiple images of immunolabeled multicilia under identical conditions and analyzed them using ImageJ to measure total pixel intensity for red (glutamylated tubulin) and green (acetylated tubulin) channels in the same region of interest. Expressing the intensity of the red and green channels as a ratio, we found that pronephric cilia tubulin glutamylation increased 2.5-fold (0.56) in ccp5-deficient cilia compared with control (0.20; Figure 3K), whereas the average ratio of glutamylated tubulin to acetylated tubulin in ccp6-deficient cilia was similar to control (0.165). High-speed videomicroscopy analysis of pronephric multicilia motility from 2.5-d-old control larvae revealed a synchronous waveform with an average beat amplitude of 6 μm (Figure 4, B and C, and Supplemental Movie S1). In contrast, motile multicilia in the ccp5-deficient morphants were uncoordinated with individual ccp5-deficient cilia either completely paralyzed or moving independently with significantly reduced beat amplitudes (Figure 4, B and C, and Supplemental Movie S2). Cilia tubulin hyperglutamylation thus appears to inhibit motility-associated functions of microtubules.
FIGURE 3:
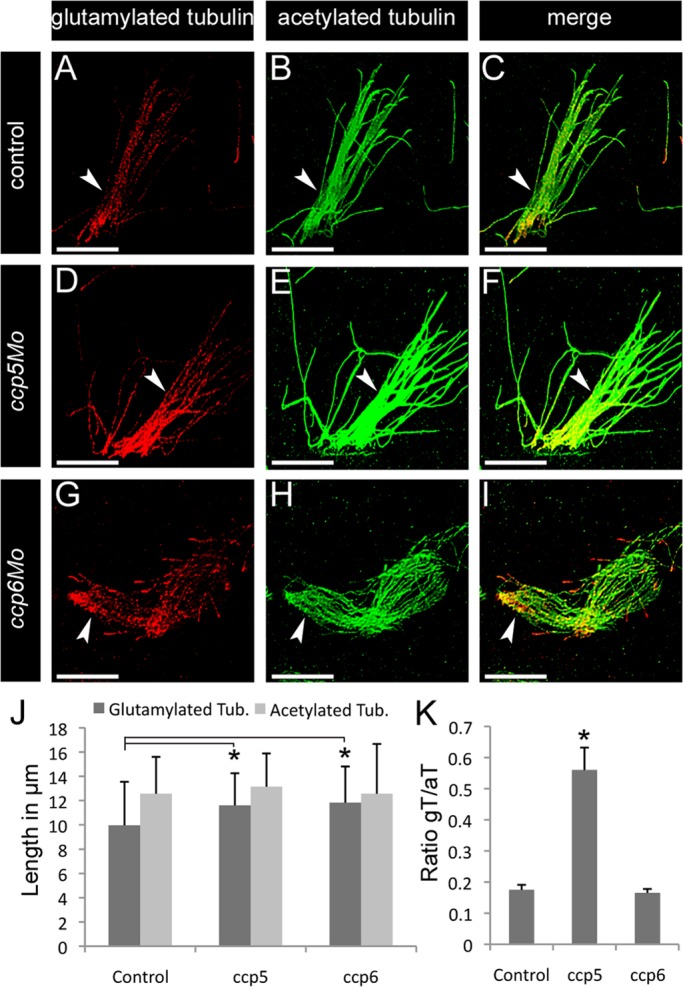
ccp5 knockdown induced hyperglutamylation of cilia microtubules. Representative images of pronephric cilia from 2.5-d-old zebrafish larvae double immunolabeled with glutamylated tubulin–specific mAb GT335 (red) and acetylated tubulin–specific mAb 6-11B-1 (green), Scale bars, 10 μm. (A–C) Pronephric multicilia (arrowheads) from control larva showing that (A) glutamylated tubulin normally decreases from the base to the tip of axonemes, in contrast to (B) uniformly distributed acetylated tubulin. (D–F) Pronephric multicilia from ccp5 morphant larva showing (D) enhanced labeling intensity of glutamylated tubulin along their axonemes marked by (E) acetylated tubulin. (G–I) Pronephric multicilia from ccp6 morphant, showing that (G) labeling intensity of glutamylated tubulin along their axonemes marked by (H) acetylated tubulin is similar to control. (J) Average length of glutamylated tubulin labeled segment increased in the pronephric cilia of ccp5 and ccp6 morphants relative to controls, although length of their acetylated tubulin-labeled segments was similar. Control (n = 33, cilia/4 larvae), ccp5 morphants (n = 42 cilia/4 larvae), ccp6 morphants (n = 31 cilia/4 larvae). Error bars, SD; t test, *p < 0.05. (K) Average intensity ratio of glutamylated tubulin relative to acetylated tubulin significantly increased in pronephric cilia of ccp5 morphants relative to control or ccp6 morphants. n = 36 cilia/4 control larvae; 33 cilia/4 ccp5 morphant larvae; 45 cilia/4 ccp6 morphant larvae. Error bars, SEM; t test, *p < 0.05.
FIGURE 5:
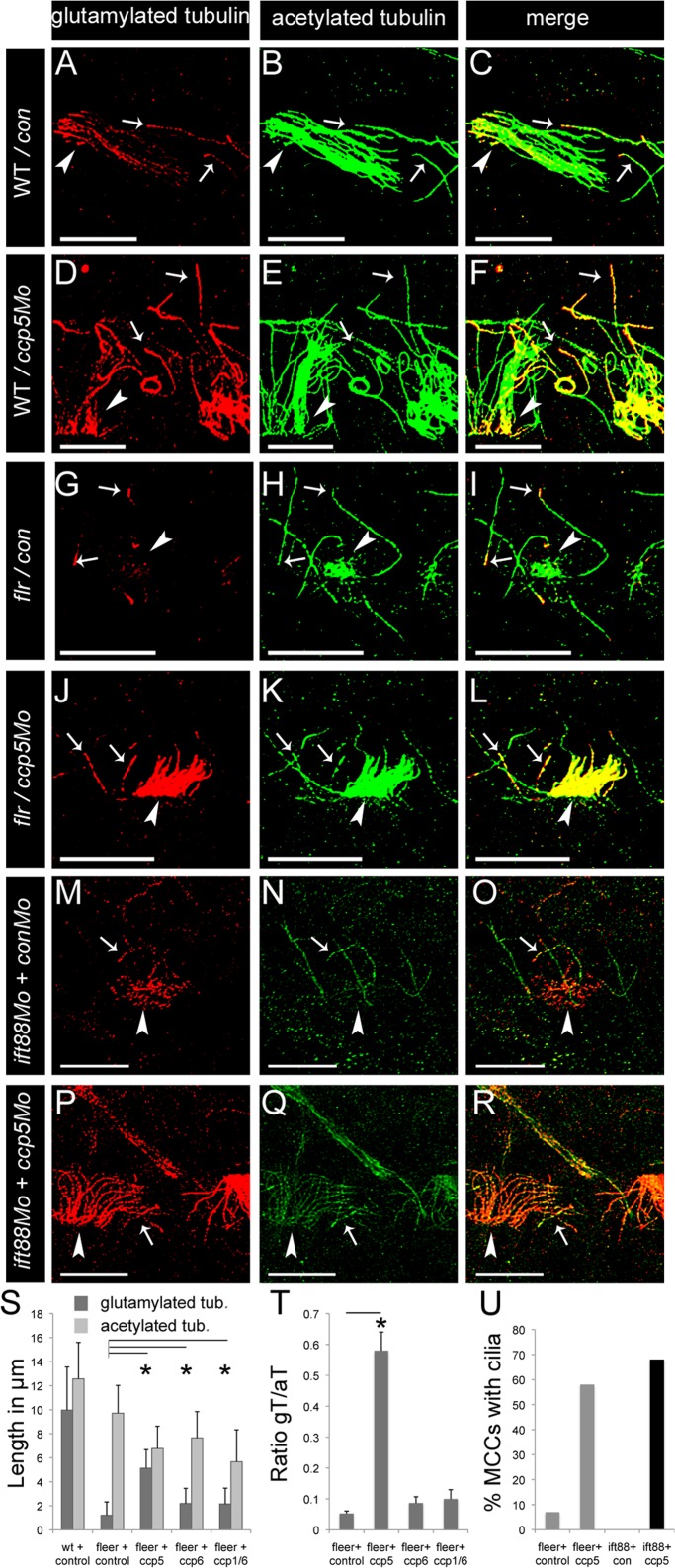
ccp5 knockdown in Fleer- and Ift88-deficient zebrafish promotes pronephric multicilia assembly. (A–R) Representative images of pronephric single cilia (white arrows) and multicilia (white arrowheads) of 2.5-d-old zebrafish larvae double immunolabeled with glutamylated tubulin–specific mAb GT335 (red) and acetylated tubulin–specific mAb 6-11B-1 (green). Scale bars, 10 μm. (A–C) Pronephric cilia in control larva showing (A) that glutamylated tubulin levels gradually decrease from base to tip of axonemes; (B) that acetylated tubulin is uniformly distributed along the entire length of axonemes; and (C) their merge. (D–F) Pronephric cilia in ccp5 morphant showing (D) glutamylated tubulin at elevated levels along the entire length of axonemes, (E) acetylated tubulin, and (F) their merge. (G–I) Pronephric cilia of fleer mutant injected with control morpholino showing (G) glutamylated tubulin restricted near the base of single cilia and absent in multicilia, (H) acetylated tubulin in axonemes of single cilia and accumulated in cytoplasm of multiciliated cells, and (I) their merge. (J–L) Pronephric cilia of fleer mutant injected with ccp5×5Mo showing (J) glutamylated tubulin at elevated levels in axonemes of both single and multicilia, (K) acetylated tubulin in axonemes of single cilia and restored multicilia (note reduced cytoplasmic accumulation of acetylated tubulin in multiciliated cells), and (L) their merge. (M–O) Pronephric cilia of ift88 morphants showing (M) glutamylated tubulin gradually decreasing along single cilia and abnormally accumulated at the base of multicilia, (N) acetylated tubulin present along single cilia but notably absent in multiciliated cells, and (O) their merge. (P–R) Pronephric cilia in double morphant of ift88 and ccp5 showing (P) glutamylated tubulin at elevated levels in axonemes of both single and restored multicilia (note reduced cytoplasmic accumulation of glutamylated tubulin in multiciliated cells), (Q) acetylated tubulin in single and restored multicilia, and (R) their merge. (S) Average length of glutamylated tubulin– and acetylated tubulin–labeled segments in pronephric cilia of WT zebrafish injected with control Mo (n = 33 cilia/4 larvae), fleer mutants injected with control Mo (n = 50 cilia/4 larvae), ccp5×5Mo (n = 55 cilia/5 larvae), ccp6×3Mo alone (n = 60 cilia/4 larvae), and the combination ccp1×5Mo/ccp6×3Mo (n = 86 cilia/5 larvae). Error bars, SD; t test, *p < 0.05. (T) Average intensity ratios of glutamylated tubulin relative to acetylated tubulin measured in individual double-immunolabeled pronephric single cilia and multicilia from fleer mutant larvae injected with control MO (n = 48 cilia/4 larvae), ccp5×5Mo (n = 78 cilia/6 larvae), ccp6×3Mo alone (n = 41 cilia/4 larvae), and the combination ccp1×5Mo/ccp6×3Mo (n = 36 cilia/4 larvae). Error bar, SEM; t test, *p < 0.05. (U) Percentage of MCCs that extend axonemes >2 μm from their apical surface in the pronephros of fleer mutants injected with control morpholino (n = 55 MCCs/6 larvae), fleer mutants injected with ccp5×5Mo (n = 48 MCCs/8 larvae), zebrafish injected with the combination ift88Mo and control Mo (n = 43 MCCs/8 larvae), and zebrafish injected with the combination ift88Mo and ccp5×5Mo (n = 41 MCCs/8 larvae).
FIGURE 6:
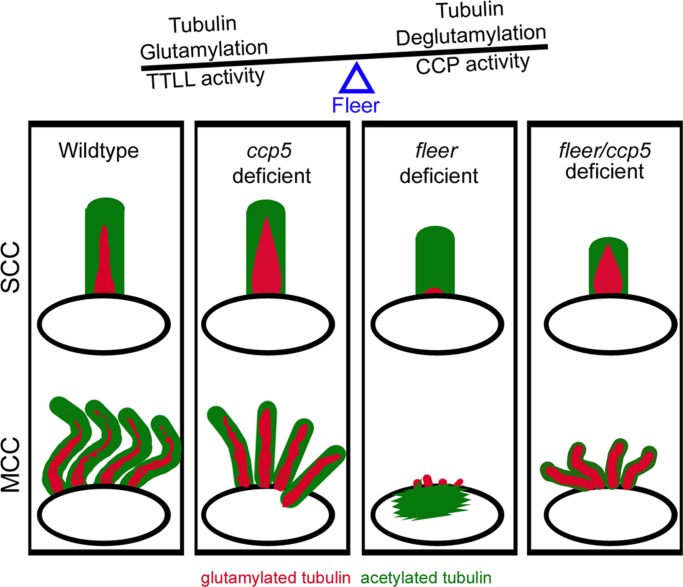
Schematic depicting ccp5 knockdown–induced changes to tubulin glutamylation in normal and flee-deficient cilia. In normal cilia of wild-type single ciliated cells (SCCs) and MCCs, glutamylated tubulin is enriched maximally at the base and gradually decreases toward the tip. Loss of ccp5 in otherwise normal cilia significantly increases the overall amount and extent of glutamylated tubulin without increasing cilia length. Hyperglutamylation inhibits efficient cilia motility. Glutamylated tubulin is significantly reduced and restricted to the base of short, single cilia that persist in the fleer mutants. Fleer-deficient cilia remain competent for tubulin glutamylation and show greater-than-normal increase in overall intensity and extent when Ccp5 deglutamylase is knocked down in the fleer mutants. Multicilia in fleer mutants are more sensitive to the loss of glutamylation; however, multiciliated cells in compound fleer/ccp5-deficient zebrafish show improved assembly of cilia upon increase in tubulin glutamylation.
FIGURE 4:
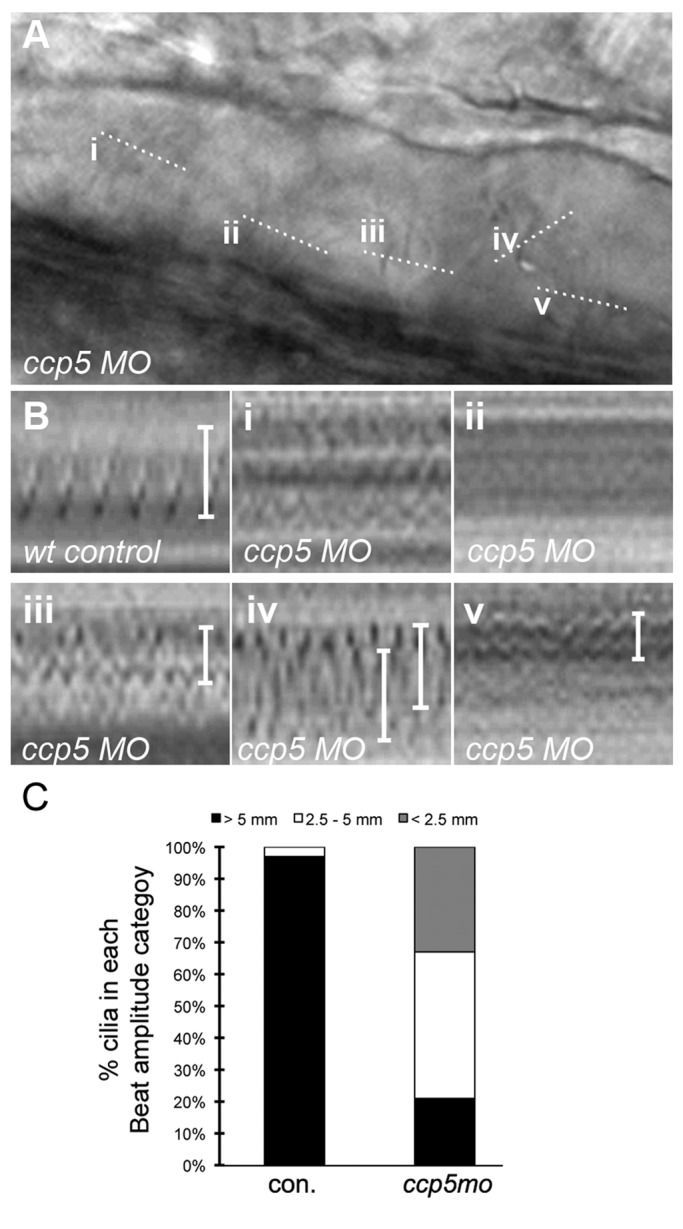
ccp5 inactivation perturbs cilia motility. (A) Frame of a movie taken of pronephric cilia from a 2.5-d-old ccp5 morphant. Lower-case Roman numerals indicate multicilia analyzed for motility defects; dotted lines indicate the plane of the line scanned through the cilium. (B) Kymograms of pronephric cilia from a wild-type control and ccp5-deficient multicilia (i–v) from the movie frame in A. (C) Quantitative representation of beat amplitude categories of pronephric multicilia observed in control embryos (n = 36 cilia from eight embryos) and ccp5 morphants (n = 33 cilia from seven embryos).
ccp5 knockdown restores tubulin glutamylation and promotes multicilia assembly in IFT-deficient zebrafish
We previously showed that mutation in the zebrafish cilia TPR protein Fleer/Ift70 significantly reduced axonemal tubulin glutamylation, cilia motility, and single cilia length and blocked ciliogenesis in multiciliated cells (Pathak et al., 2007). Given the increase in cilia glutamylation we observed with ccp5 knockdown, we tested whether compound deficiency of ccp deglutamylases and fleer would restore glutamylation in fleer mutant cilia. We injected fleerca1 in-cross clutches with control, ccp5, ccp6, and ccp1/ccp6 morpholinos and identified homozygous fleerca1−/− 2.5-d-old larvae by genotyping. We then performed double immunolabeling with mAb GT335, specific to glutamylated tubulin, and 6-11B-1, specific to acetylated tubulin. Analysis of glutamylated tubulin in control (Figure 5A) and fleer mutant pronephric single cilia (arrows in Figure 5, G and I; Figure 6) confirmed that tubulin glutamylation was significantly reduced and restricted to the cilia base in fleer larvae. Multiciliated cells in the fleer mutants do not extend axonemes or show glutamylated basal bodies (arrowhead, Figure 5G; Pathak et al., 2007) but instead have aberrant apical cytoplasmic accumulation of acetylated tubulin (arrowhead, Figure 5H; Supplemental Figure S4, E, H, and K, and Figure 6). Strikingly, compound fleer mutant/ccp5 morphants showed significantly increased intensity of glutamylated tubulin reactivity in single cilia and prominent glutamylated tubulin-positive axonemes extending from pronephric multiciliated cells (Figures 5, J–L, and 6). Restoration of glutamylation or multicilia axoneme elongation was not observed in compound fleer/ccp6 morphants (Supplemental Figure S5, G–I) or fleer/ccp1/ccp6 morphants (Supplemental Figure S5, J–L). Quantification of the extent of glutamylated and acetylated tubulin in individual cilia from fleer mutant or compound fleer/ccp-gene deficient larvae (Supplemental Figure S5) revealed that whereas glutamylated tubulin was confined to an average 1.2 μm length at the base of single cilia in fleer mutants, this increased significantly to 5.1 µm in cilia of fleer/ccp5-deficient larvae. However, the extent of acetylated tubulin in single pronephric cilia of ccp5/fleer-deficient larvae (6.8 μm) was reduced compared with cilia deficient in fleer alone (9.7 μm). Compound fleer/ccp6- and fleer/ccp1/ccp6-deficient larvae did not show a significant restoration of the axonemal extent of glutamylated tubulin reactivity (2.2 μm; Supplemental Figure S5). Assessment of the overall pixel intensity of glutamylated tubulin reactivity relative to acetylated tubulin in the same cilia (Figure 5T) revealed a low intensity ratio for glutamylated:acetylated tubulin in pronephric single cilia of fleer (0.05), fleer/ccp6-deficient (0.08), and fleer/ccp1/ccp6-deficient larvae (0.1) compared with control cilia (0.20). Cilia of fleer/ccp5-deficient larvae showed a remarkable recovery in the glutamylated:acetylated tubulin ratio intensity (0.58; Figure 5T). Recovery of tubulin glutamylation in compound fleer/ccp5 deficient multiciliated cells (MCCs) correlated with reappearance of short apical MCC cilia (Figures 5, J–L and U, and 6), indicating that ccp5 knockdown could partially rescue fleer mutant multiciliogenesis.
To test whether rescue of multiciliogenesis by ccp5 knockdown was specific to fleer mutants, we examined ift88-deficient zebrafish that similarly fail to generate cilia on MCCs (Tsujikawa and Malicki, 2004; Kramer-Zucker et al., 2005). In 2.5-d-old ift88 morphants, pronephric MCCs show glutamylated basal bodies (Figure 5M and Supplemental Figure S5A) but no acetylated tubulin (Figure 5, N and O) or Arl13b-positive cilia (Supplemental Figure S5, B and C). In contrast, in ift88/ccp5 double morphants, ∼70% of pronephric MCCs (Figure 5U) showed axonemes that were strongly labeled by glutamylated tubulin (arrowheads in Figure 5P; Supplemental Figure S5D), acetylated tubulin (Figure 5, Q and R), or Arl13b (Supplemental Figure S5, E and F). Our results demonstrate that cilia in the fleer mutant are competent for tubulin glutamylation (Figure 6) and suggest that high levels of tubulin glutamylation generated by ccp5 knockdown can exert a dominant positive effect on multiciliogenesis, even in cells deficient for Fleer/Ift70 or Ift88.
DISCUSSION
Tubulin glutamylation is an important posttranslational modification that is regulated by the balanced activities of ttll tubulin ligases and ccp tubulin deglutamylases (Janke and Bulinski, 2011). Our results show that the zebrafish ccp gene family is broadly expressed during development and Ccp5 is the key deglutamylase that maintains the functional set-point of cilia tubulin glutamylation. Tubulin glutamylation is also regulated in cilia by the Fleer/Ift70 protein, and we show that ccp5 knockdown partially overcomes multicilia elongation defects in Fleer/Ift70- and Ift88-deficient zebrafish. Our findings suggest that increased glutamylation can drive initial assembly of multicilia axonemes but that prevention of excessive tubulin glutamylation by ccp5 deglutamylase activity is essential for mature cilia motility and function.
Zebrafish ccp gene expression and function
Our analysis of ccp gene expression in zebrafish revealed that all four zebrafish ccp genes were expressed throughout embryonic development, extending a previous analysis of ccp gene expression in zebrafish (Lyons et al., 2013). Specifically, we find that in addition to ccp1 and ccp5, the tubulin deglutamylase genes ccp2 and ccp6 are expressed in multiple ciliated tissues throughout embryogenesis. Although overlapping expression of ccp2, ccp5, and ccp6 suggested the possibility of redundant deglutamylating activity in cilia, we found that ccp5 was the principal enzyme involved in regulating ciliary glutamyl tubulin levels. Previously ccp5 knockdown was shown by Western blotting to globally increase embryo glutamyl tubulin, but this did not distinguish between cilia and the abundant tubulin glutamylation in brain and spinal cord neurons (Lyons et al., 2013). In addition, in contrast to previous reports, we did not see an increase in cilia tubulin glutamylation in ccp1-deficient embryos (Lyons et al., 2013). Although this lack of effect could reflect structural defects of the axoneme similar to those seen in the C. elegans ccpp-1 mutants (O'Hagan et al., 2011), this could simply be due to restricted expression of ccp1 in the zebrafish nervous system. In addition, the reported increase in glutamyl tubulin immunoreactivity in the ccp1-knockdown pronephric duct was restricted to the cytoplasm and not cilia associated (Lyons et al., 2013). In ccp1-deficient embryos we did observe marked hydrocephalus, a phenotype often associated with ependymal cilia paralysis and impaired cerebrospinal fluid flow (Ibanez-Tallon et al., 2004; Kramer-Zucker et al., 2005). However, because the mouse Ccp1/Pcd mutant is known to exhibit a neuronal cell death phenotype, we used the apoptosis reporter annexin-GFP zebrafish transgenic (van Ham et al., 2010) to examine the possibility that cell debris from dying neurons might obstruct the spinal canal and found that this was indeed the case. This result is a cautionary note in interpreting hydrocephalus purely as a ciliopathy, since any physical obstruction of the spinal canal may produce hydrocephalus. Of the four ccp genes in zebrafish, we conclude that ccp5 is specifically required to regulate axonemal tubulin glutamylation.
ccp-dependent regulation of cilia structure and function
Our work suggests that Ccp5 is normally active in cilia and is important for maintaining the appropriate level of axonemal tubulin glutamylation, since ccp5 knockdown increased cilia tubulin glutamylation. Ccp5 may normally play a role in preventing formation of supernumerary glutamate chains that could compromise motility-associated functions of cilia microtubules. Hyperglutamylation has been shown to arrest motility of Tetrahymena cilia (Janke et al., 2005) and induce structural defects in axonemal microtubules (O'Hagan et al., 2011). Our results show that loss of ccp5 induces hyperglutamylation in zebrafish pronephric cilia and reduces cilia beat coordination and beat amplitude without affecting beat frequency. This may reflect a preferential effect of hyperglutamylation on inner dynein arm activity, consistent with previously reported effects of TTLL deficiency on axonemal microtubule sliding and the role of inner dynein arms in regulating cilia waveform (Brokaw and Kamiya, 1987; Wood et al., 2007; Suryavanshi et al., 2010). We also observed microtubule doublet B-tubule gap defects, similar to ttll loss-of-function cilia (Pathak et al., 2011), in a minority of cilia examined in ccp5 morphants (unpublished data). Because cilia were uniformly hyperglutamylated but only a minority showed ultrastructural defects, it is likely the B-subfiber gaps are not a primary effect of hyperglutamylation but instead represent a late-stage breakdown of cilia structural integrity. Unlike mutations that completely disrupt cilia structure and show fully penetrant kidney cyst formation (Sun et al., 2004; Sullivan-Brown et al., 2008; Huang and Schier, 2009), kidney cyst formation in ccp5-knockdown embryos was only partially penetrant. This may reflect the fact that kidney cilia motility was not completely disrupted by ccp5 knockdown but instead was disorganized and reduced in beat amplitude. We observed similar partial penetrance of kidney cyst formation in ttll3/6-gene knockdowns, which reduce tubulin glycylation and glutamylation (Pathak et al., 2011) and produce a similar disorganized cilia beat pattern. Overall the results indicate that hypoglutamylation or hyperglutamylation of cilia tubulin disrupts cilia function and suggests that ttll ligases and ccp glutamylases act in concert to establish a functional “set-point” for tubulin modifications.
Ccp5, tubulin hyperglutamylation, and IFT proteins in cilia
The restoration of axonemal glutamylation and multicilia formation by ccp5 knockdown in IFT protein–deficient zebrafish highlights the idea that tubulin glutamylation can have a dominant, positive effect on ciliogenesis. We chose to examine the effect of ccp5 knockdown and hyperglutamylation on the fleer-mutant phenotype because we showed previously that zebrafish fleer-mutant single cilia lack tubulin glutamylation and that fleer-mutant multicilia fail to form (Pathak et al., 2007). Because multiciliated cells contain more cilia (>20), it may be that the more severe ciliogenesis defect in these cells is due to more stringent IFT protein stoichiometric requirements for ciliogenesis. Although the exact mechanism of Fleer/Ift70 function in tubulin glutamylation is not known, there are several possibilities to account for loss of glutamylation in fleer mutants. Fleer may act to maintain the structural integrity of B-tubule protofilaments, the substrate for microtubule glutamylation (Kann et al., 1995; Multigner et al., 1996; Lechtreck and Geimer, 2000). Alternatively, it may serve as an IFT adaptor protein to facilitate axonemal transport of Ttll glutamylases and other essential axonemal proteins (Pathak et al., 2007; Fan et al., 2010; Taschner et al., 2011, 2012; Zhao and Malicki, 2011). A new hypothesis based on our present work is that Fleer may act to suppress the activity of tubulin deglutamylases like Ccp5. Our finding that fleer/ift70-mutant cilia remain competent for glutamylation in ccp5-deficient embryos, as well as the finding that C. elegans ccpp-1-mutant cilia show enhanced glutamylation that is not reduced by mutation in the fleer/ift70 orthologue Dyf-1 (O'Hagan et al., 2011), supports this idea. Whatever the mechanism of Fleer activity may be, our results show a dominant effect of tubulin hyperglutamylation on multiciliogenesis not only in fleer/ift70 mutants, but also in ift88 morphants. Given that some maternal fleer mRNA may persist in zygotic mutants and that morpholino knockdown of ift88 may not be complete, it is possible that some Fleer and Ift88 protein may be present and interact with higher affinity for hyperglutamylated axonemes. Nonetheless, our results raise the possibility that in some contexts, deficiencies of individual IFT proteins may be bypassed in ciliogenesis by enhanced recruitment of compensatory IFT proteins to forming cilia axonemes or possibly by enhanced activity of microtubule motor proteins in IFT transport. The identification and characterization of Ttll tubulin-modifying enzymes and Ccp deglutamylases should make it possible to determine the function of tubulin glutamylation in ciliogenesis.
MATERIALS AND METHODS
Zebrafish strains, maintenance, and genotyping
Wild-type TuAB, fleer mutant, transgenic CD-41-GFP, and transgenic UAS-annexin-V–yellow fluorescent protein lines were maintained according to standard procedures and used to obtain embryos for morpholino injections. Pigment formation in zebrafish larvae was suppressed by addition of (0.003%) 1-phenyl 2-thiourea to egg water after 20 and 24 hpf. The fleerca1 mutant allele encodes a truncated 186–amino acid Fleer N-terminal peptide as opposed to the full-length Fleer protein of 651 amino acids (Leshchiner et al., 2012). Embryos from in-crosses of fleerca1 heterozygotes were genotyped by polymorphism of a simple sequence repeat in the vicinity of the fleer locus on zebrafish chromosome 3. Genomic DNA from tail clips of embryos was used to obtain DNA amplicons using the following PCR primers:
nZ1071F, CAGCTGCTACAGCAACCTGA
nZ1071R, GGACGCGGTATGTAACCTGT
Zebrafish ccp clones
Full- and partial-length cDNAs of zebrafish ccp1 were amplified by reverse transcription (RT)-PCR based on their predicted sequence and cloned into pDONR 221 vector. Full-length clones of ccp2 in pME18S-FL3 (BC146747) and ccp5/agbl5 in pExpress-1 (BC080248) and a partial ccp6 EST (BC093361) in pME18S-FL3 were purchased from Open Biosystems (Huntsville, AL). Evolutionary analysis of ccp homologues is presented in Supplemental Figure S3 and was generated using clustalw (Chenna et al., 2003) and drawtree in the Phylip 3.695 software package (Felsenstein, 1997).
Morpholino knockdown of ccp genes
Antisense morpholinos were designed to block specific splice donor sites within ccp1, ccp2, ccp5, and ccp6 deglutamylase genes and purchased from Gene Tools (Philomath, OR). In the following morpholinos, the name of the targeted deglutamylase gene precedes the letter x, followed by the number of the specific target exon. The targeted splice site is underlined in the following sequences (5′–3′) of morpholinos:
Ccp1×5Mo: TTAAGAACACCAAAACTCACCGACA
Ccp2×2Mo: CCATACAGAAGTGGAGCTTACCTGA
Ccp5×5Mo: TCCTCTTAATGTGCAGATACCCGTT
Ccp5×8Mo: AAGTTTGACTCCTAGACGTACCTGT
Ccp6×3Mo: TTTACGTCCAAGTGCTTACCTGAGT
ift88(polaris)AUGMo: CTGGGACAAGATGCACATTCTCCAT (Kramer-Zucker et al., 2005)
Control injections were performed using a heterologous inverse sense morpholino against foxJ1a:
foxJ1a conMo: CGTCCATTGTGTAAAAGTGTAACCA (Hellman et al., 2010)
Morpholino oligonucleotides were dissolved in RNase-free water to a 2 mM stock concentration. For injections, morpholino dilutions were made in 100 mM KCl, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4), and 0.1% phenol red. A Nanoliter 2000 microinjector (WPI Instruments, Sarasota, Fl) was used to inject 4.6-nl volumes in two- to four- cell embryos. To detect morpholino-induced splicing defects upon injection of ccp5×5mo or ccp5×8mo, nested RT-PCR was performed on total RNA extracted from individual embryos using primers listed in Supplemental Table S1.
RNA in situ hybridization
Linearized DNA templates of ccp1 and ccp5 containing T7 promoter sites were obtained by restriction digestion of their clones with Xma1 and EcoR1, respectively. Linearized DNA templates of ccp2 and ccp6 cDNAs were generated from their pME18S-FL3 vectors by PCR, with the reverse primer engineered to contain the T7 promoter. Digoxigenin-labeled full-length antisense riboprobes of ccp1, ccp2, ccp5, and ccp6 were synthesized by in vitro transcription using the T7 polymerase. Whole-mount RNA in situ hybridization was performed as described previously (Liu et al., 2007; Thisse and Thisse, 2008). For imaging, the stained embryos were cleared in dimethyl formamide and mounted in 80% glycerol. Images were obtained using a Spot image digital camera mounted on a Leitz MZ12 stereomicroscope (Leica, Wetzlar, Germany) or Nikon E800 microscope (Nikon, Melville, NY).
Immunofluorescence: qualitative and quantitative analysis
Whole zebrafish larvae were fixed for immunolabeling in Dent's fixative (80% methanol, 20% dimethyl sulfoxide) at 4°C overnight. For visualization of pronephric cilia in control morphants, distension of the pronephric lumen was induced in the live larva by mechanical obstruction of their cloaca 1 h before fixation. The fixed specimens were rehydrated gradually and washed with phosphate-buffered saline (PBS) containing 0.5% Tween 20 (PBST) and blocked for 2 h with 5% normal goat serum before antibody labeling. For immunolabeling, the larvae were sequentially incubated with appropriate dilutions of each primary and secondary antibody overnight at 4°C. The dilutions of primary antibodies used were as follows: mAb GT335, 1:400 (Enzo Life Sciences, Plymouth Meeting, PA); mAb 6-11B-1, 1:800 (Sigma-Aldrich, St. Louis, MO); and Arl13b, 1:250 (gift from Z. Sun, Yale University). Immunoreactivity was detected using the secondary antibodies Alexa 546–conjugated goat anti rabbit (1:800), Alexa 546–conjugated goat anti-mouse, and Alexa 488–conjugated donkey anti-mouse (1:800). Immunolabeling with two mouse antibodies was performed as described earlier, with the first primary and secondary antibodies briefly fixed with 4% paraformaldehyde and their reactive sites blocked by sequential incubations in 10% unconjugated mouse serum and 5% unconjugated mouse Fab′ fragments (Pathak et al., 2007). Two-color confocal Z-series were acquired using sequential laser excitation under identical imaging conditions.
To analyze quantitatively the extent and intensities of glutamylated and acetylated tubulin in individual cilia, maximum intensity projection images were generated from deconvolved stacks using the Huygens Essential program (Scientific Volume Imaging, Hilversum, Netherlands) and saved in TIFF format. These maximum-intensity projections were viewed as composite color images using ImageJ (National Institutes of Health, Bethesda, MD), and lengths of lines drawn in separate red (glutamylated tubulin) and green (acetylated tubulin) channels were determined using the analyze function. The integrated pixel densities were similarly measured separately for red (glutamylated tubulin) and green (acetylated tubulin) channels in regions of interest drawn around individual cilia.
High-speed videomicroscopy analysis
The 54-hpf control and morpholino-injected, 1-phenyl 2-thiourea–treated embryos of wild-type TuAB or CD-41 GFP transgenic zebrafish were maintained alive and anesthetized in E3 egg water containing tricaine (1:25 dilution of 4.1% stock). The immobilized larvae were placed on 3% methyl cellulose, immersed in the anesthetic mix, and observed by Nomarski optics using a 40×/0.55 water immersion lens mounted on the Nikon E-800 microscope. Images of moving cilia in distended pronephric lumens (due to cystic phenotype or mechanical obstruction induced in the distal pronephros of controls) were obtained using the Dragonfly2 CCD camera (Point Grey Research, Richmond, Canada) at speeds between 235 and 245 frames/s. To obtain kymograms from sequential movie frames, the entire stack of 324 × 242 images in QuickTime movies (Apple, Cupertino, CA) were imported in ImageJ software, and the line function was used to slice the movie frames along a small plane orthogonal to the axis (near the center of cilium) of cilia selected for analysis. Beat amplitude information was assessed from the kymograms by determining the length of pixels between the extreme positions of the cilium and converted to a micrometer scale (1 μm = 2.7 pixels in a 324 × 242 tiff image). The beat frequency was assessed by measuring the number of peaks in the kymogram corresponding to a 1-s-long movie.
Supplementary Material
Acknowledgments
We acknowledge Mohamed Adan for technical assistance, Tjakko Van Ham for the gift of UAS:annexin-V–GFP transgenic fish, and members of the Drummond lab for critical input to the manuscript. We also thank Carsten Janke for the GT335 antibody and Zhaoxia Sun for the Arl13b antibody. This work was supported by National Institutes of Health Grants K01DK078741 (N.P.), K08DK08272 and R03DK097443 (A.V.), and RO1DK053093 (I.A.D.).
Abbreviations used:
- CCP
cytoplasmic carboxy peptidase
- TTLL
tubulin tyrosine ligase like
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-01-0033) on April 17, 2014.
Present addresses: *Journal of Visualized Experiments, 1 Alewife Center, Suite 200, Cambridge, MA 02140.
†Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., Seattle, WA 98109.
‡NYIT-College of Osteopathic Medicine, Old Westbury, NY 11568.
N.P., A.V., and I.A.D. designed the experiments; N.P. performed the experiments, with the exception of electron microscopy, which was performed by C.A.A.
REFERENCES
- Bobinnec Y, Moudjou M, Fouquet JP, Desbruyeres E, Edde B, Bornens M. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell Motil Cytoskeleton. 1998;39:223–232. doi: 10.1002/(SICI)1097-0169(1998)39:3<223::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bosch Grau M, Gonzalez Curto G, Rocha C, Magiera MM, Marques Sousa P, Giordano T, Spassky N, Janke C. Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J Cell Biol. 2013;202:441–451. doi: 10.1083/jcb.201305041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton. 1987;8:68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edde B, Rossier J, Le Caer JP, Berwald-Netter Y, Koulakoff A, Gros F, Denoulet P. A combination of posttranslational modifications is responsible for the production of neuronal alpha-tubulin heterogeneity. J Cell Biochem. 1991;46:134–142. doi: 10.1002/jcb.240460207. [DOI] [PubMed] [Google Scholar]
- Edde B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990;247:83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Fan ZC, Behal RH, Geimer S, Wang Z, Williamson SM, Zhang H, Cole DG, Qin H. Chlamydomonas IFT70/CrDYF-1 is a core component of IFT particle complex B and is required for flagellar assembly. Mol Biol Cell. 2010;21:2696–2706. doi: 10.1091/mbc.E10-03-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. An alternating least squares approach to inferring phylogenies from pairwise distances. Syst Biol. 1997;46:101–111. doi: 10.1093/sysbio/46.1.101. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, Morgan JI, Zuo J. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science. 2002;295:1904–1906. doi: 10.1126/science.1068912. [DOI] [PubMed] [Google Scholar]
- Fouquet JP, Edde B, Kann ML, Wolff A, Desbruyeres E, Denoulet P. Differential distribution of glutamylated tubulin during spermatogenesis in mammalian testis. Cell Motil Cytoskeleton. 1994;27:49–58. doi: 10.1002/cm.970270106. [DOI] [PubMed] [Google Scholar]
- Hellman NE, et al. The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch. Proc Natl Acad Sci USA. 2010;107:18499–18504. doi: 10.1073/pnas.1005998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitorel P, White D, Fouquet JP, Kann ML, Cosson J, Gagnon C. Differential distribution of glutamylated tubulin isoforms along the sea urchin sperm axoneme. Mol Reprod Dev. 2002;62:139–148. doi: 10.1002/mrd.10086. [DOI] [PubMed] [Google Scholar]
- Ibanez-Tallon I, Pagenstecher A, Fliegauf M, Olbrich H, Kispert A, Ketelsen UP, North A, Heintz N, Omran H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- Ikegami K, et al. Loss of alpha-tubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc Natl Acad Sci USA. 2007;104:3213–3218. doi: 10.1073/pnas.0611547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Mukai M, Tsuchida J, Heier RL, Macgregor GR, Setou M. TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites. J Biol Chem. 2006;281:30707–30716. doi: 10.1074/jbc.M603984200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc Natl Acad Sci USA. 2010;107:10490–10495. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Janke C, et al. Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science. 2005;308:1758–1762. doi: 10.1126/science.1113010. [DOI] [PubMed] [Google Scholar]
- Kann ML, Prigent Y, Fouquet JP. Differential distribution of glutamylated tubulin in the flagellum of mouse spermatozoa. Tissue Cell. 1995;27:323–329. doi: 10.1016/s0040-8166(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Kann ML, Soues S, Levilliers N, Fouquet JP. Glutamylated tubulin: diversity of expression and distribution of isoforms. Cell Motil Cytoskeleton. 2003;55:14–25. doi: 10.1002/cm.10107. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kurabe N, Ikegami K, Tsutsumi K, Konishi Y, Kaplan OI, Kunitomo H, Iino Y, Blacque OE, Setou M. Identification of tubulin deglutamylase among Caenorhabditis elegans and mammalian cytosolic carboxypeptidases (CCPs) J Biol Chem. 2010;285:22936–22941. doi: 10.1074/jbc.C110.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–445. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, Gerlich DW, Janke C. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol. 2010;189:945–954. doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Geimer S. Distribution of polyglutamylated tubulin in the flagellar apparatus of green flagellates. Cell Motil Cytoskeleton. 2000;47:219–235. doi: 10.1002/1097-0169(200011)47:3<219::AID-CM5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Lee GS, et al. Disruption of Ttll5/stamp gene (tubulin tyrosine ligase-like protein 5/SRC-1 and TIF2-associated modulatory protein gene) in male mice causes sperm malformation and infertility. J Biol Chem. 2013;288:15167–15180. doi: 10.1074/jbc.M113.453936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, et al. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nat Genet. 2012;44:193–199. doi: 10.1038/ng.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchiner I, et al. Mutation mapping and identification by whole-genome sequencing. Genome Res. 2012;22:1541–1548. doi: 10.1101/gr.135541.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- Lyons PJ, Sapio MR, Fricker LD. Zebrafish cytosolic carboxypeptidases 1 and 5 are essential for embryonic development. J Biol Chem. 2013;288:30454–30462. doi: 10.1074/jbc.M113.497933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci USA. 1976;73:208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multigner L, Pignot-Paintrand I, Saoudi Y, Job D, Plessmann U, Rudiger M, Weber K. The A and B tubules of the outer doublets of sea urchin sperm axonemes are composed of different tubulin variants. Biochemistry. 1996;35:10862–10871. doi: 10.1021/bi961057u. [DOI] [PubMed] [Google Scholar]
- O'Hagan R, Piasecki BP, Silva M, Phirke P, Nguyen KC, Hall DH, Swoboda P, Barr MM. The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans. Curr Biol. 2011;21:1685–1694. doi: 10.1016/j.cub.2011.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N, Austin CA, Drummond IA. Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J Biol Chem. 2011;286:11685–11695. doi: 10.1074/jbc.M110.209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–4364. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowski K, et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell. 2010;143:564–578. doi: 10.1016/j.cell.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180:295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Brown J, Schottenfeld J, Okabe N, Hostetter CL, Serluca FC, Thiberge SY, Burdine RD. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev Biol. 2008;314:261–275. doi: 10.1016/j.ydbio.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- Suryavanshi S, et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr Biol. 2010;20:435–440. doi: 10.1016/j.cub.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation. 2012;83:S12–S22. doi: 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Vetter M, Morawetz M, Lorentzen E. Biochemical mapping of interactions within the intraflagellar transport (IFT) B core complex: IFT52 binds directly to four other IFT-B subunits. J Biol Chem. 2011;286:26344–26352. doi: 10.1074/jbc.M111.254920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–716. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- van Dijk J, Rogowski K, Miro J, Lacroix B, Edde B, Janke C. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell. 2007;26:437–448. doi: 10.1016/j.molcel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- van Ham TJ, Mapes J, Kokel D, Peterson RT. Live imaging of apoptotic cells in zebrafish. FASEB J. 2010;24:4336–4342. doi: 10.1096/fj.10-161018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- Vogel P, Hansen G, Fontenot G, Read R. Tubulin tyrosine ligase-like 1 deficiency results in chronic rhinosinusitis and abnormal development of spermatid flagella in mice. Vet Pathol. 2010;47:703–712. doi: 10.1177/0300985810363485. [DOI] [PubMed] [Google Scholar]
- Wood CR, Hard R, Hennessey TM. Targeted gene disruption of dynein heavy chain 7 of Tetrahymena thermophila results in altered ciliary waveform and reduced swim speed. J Cell Sci. 2007;120:3075–3085. doi: 10.1242/jcs.007369. [DOI] [PubMed] [Google Scholar]
- Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J. 2011;30:2532–2544. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.